Bipolar disorder (BD) and schizophrenia (SZ) affect approximately 2–4% of the general population and are among the world’s top ten most disabling diseases. Current pharmacological therapies for BD and SZ are ineffective at targeting the underlying cause of these diseases and solely aid in reducing the severity of the psychotic symptoms. In order to advance the therapeutic potential for the treatment of these disorders, we need to identify the cellular and molecular mechanisms that contribute to the pathophysiology of complex neuropsychiatric disorders.
Epidemiology studies suggest the etiology of BD and SZ is largely due to genetic factors with approximately 60–80% of phenotypic variation in BD and 70–90% in SZ based on genetic heritability (1). Genome-wide association studies (GWAS) studies have identified multiple risk loci involved in the development of BD; the most consistent hits include DGKH, ZNF804A, SYNE1, ODZ4, CACNA1C, and ANK3 (2). One of the strongest and most replicated associations with BD has been the ANK3 gene, which encodes the ankyrin-G protein. In addition, ANK3 SNPs have been found to be associated with SZ; however, the significance threshold is not as compelling as that seen with BD. Although the genetic link between ANK3 and neuropsychiatric disease is strong, there are still no conclusive family data or animal data that represent a “smoking gun” proving the role of ANK3 in BD or SZ.
In this issue of Biological Psychiatry, Hughes et al. (2016) uncovered a SNP (rs41283526) in ANK3 with a strong protective effect against BD and SZ. Their experimental data indicate the presence of this SNP is associated with a decrease in the splicing of a small 54 nucleotide exon of ANK3, resulting in an odds ratio of .31 for BD and .21 for SZ. These findings strengthen the link between ANK3 and normal neuronal function; however, how alternative splicing of the ANK3 gene is involved in disease pathophysiology remains elusive. To answer this question, we need to evaluate how the splice variants of ankyrin-G regulate distinct cellular and neuronal circuit functions and how abnormalities in these processes contribute to neuropsychiatric disease.
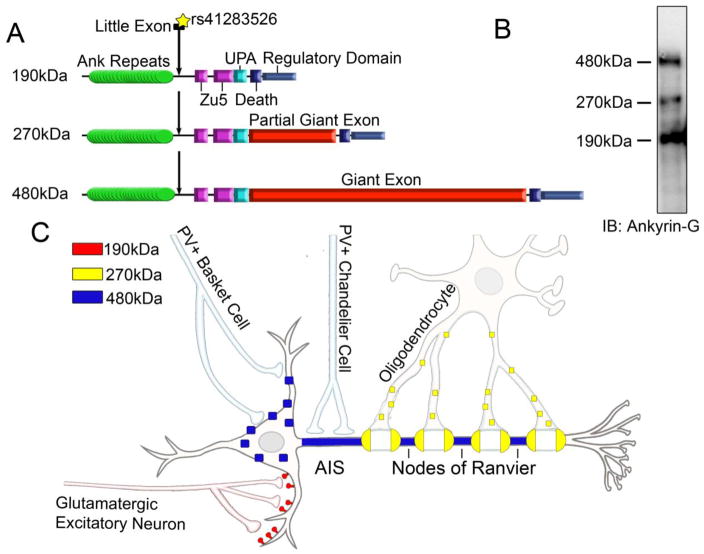
Ankyrins are a family of scaffolding proteins that organize ion channels, transporters, and cell adhesion molecules into discrete domains at the plasma membrane and link these protein complexes to the spectrin-actin cytoskeleton. Three ankyrin genes are present in vertebrates: ANK1, encoding ankyrin-R; ANK2, encoding ankyrin-B; and ANK3, encoding ankyrin-G (3). The canonical ankyrins consists of a membrane-binding domain at the N-terminus with 24 consecutive ankyrin repeats folded into a solenoid structure, followed by two ZU5 domains, a UPA domain, a death domain, and an unstructured C-terminal regulatory domain (3). Alternative splicing of the ANK3 gene gives rise to three main classes of ankyrin-G isoforms: the canonical 190kDa isoform described above, and the 270kDa isoform and giant 480kDa isoform, which arise from alternative splicing of a 7.6kb vertebrate-specific exon (Figure 1A, B) (4). In addition, there is evidence for extensive splice variation within these three main classes of transcripts, including the “little exon” described in Hughes, et al. (5). The canonical 190kDa isoform of ankyrin-G is expressed in most tissues and cell types throughout the body including brain, heart, skeletal muscle, kidney, and retina, and plays a critical role in the localization of key signaling proteins to the cardiac intercalated disc and transverse tubule, epithelial lateral membrane, and photoreceptor outer segment. In contrast, the 270kDa and 480kDa isoforms of ankyrin-G are found predominantly in the nervous system where they localize ion channels and transporters to the axon initial segment (AIS), nodes of Ranvier, and the somatodendritic membrane (4).
Figure 1.
(A) Representative domain structure of the main classes of splice variants of ankyrin-G. The position of the little exon containing the loss-of-function SNP (rs41283526) identified by Hughes et al. (2016) is depicted by arrow. (B) Western blot of whole brain lysate from adult wild-type mice probed with anti-ankyrin-G antibodies. (C) Schematic demonstrating the different subcellular localization of the 190kDa (red), 270kDa (yellow) and 480kDa (blue) variants of ankyrin-G.
In addition to tissue-specific distribution, the different isoforms of ankyrin-G display unique cellular functions (Figure 1C). A recent study demonstrated that the 190kDa isoform of ankyrin-G plays a critical role in the regulation of dendritic spine morphology and N-methyl-D-aspartate (NMDA) receptor trafficking of glutamatergic excitatory synapses (6). The 270kDa splice variant is likely important for oligodendrocyte function and enrichment of myelin sheaths surrounding the nodes of Ranvier (7). The 480kDa isoform of ankyrin-G has recently been characterized as the master organizer of the AIS and plays a critical role in formation of the nodes of Ranvier. The 480kDa isoform of ankyrin-G directly interacts with and localizes all known components of the AIS, including voltage-gated sodium channels, KCNQ2/3 channels, β4 spectrin, and the cell-adhesion molecule, neurofascin (NF186) (4). Recent work has shown a new role for the 480kDa splice variant of ankyrin-G in the stabilization of GABAA-receptors at somatodendritic sites and the formation GABAergic inhibitory synapses (8). Thus, splice variants of ankyrin-G contribute to the proper formation of both excitatory and inhibitory synapses.
The coordinated balance between excitatory and inhibitory circuits is necessary for normal brain function, and neural oscillations enable the rhythmic timing and communication between excitatory-inhibitory neuronal populations. Neural oscillations in the high frequency ranges, known as beta (13–30Hz) and gamma (30–200Hz) oscillations, are responsible for the precise synchronization of local cortical circuits. Gamma oscillations are of particular importance as impairments in these oscillations are seen in patients with SZ and BD (9). Clinical studies revealed that cortical gamma oscillations are decreased during sensory stimulation or cognitive tasks in individuals with SZ as compared with neurotypical patients (9). Furthermore, recent studies have suggested these alterations in gamma oscillations could act as potential biomarkers for abnormal neural network activity and behavior associated with BD (9). GABAergic inhibitory interneurons are of particular importance for the proper synchronization of neuronal networks, and multiple studies suggest BD and SZ involve abnormal GABAergic neurotransmission. Interneurons that express the Ca2+-binding protein parvalbumin (PV) are thought to play a crucial role in gamma oscillations due to their unique fast-spiking electrophysiological characteristics. PV+ basket cells synapse on to the proximal dendrites and soma of pyramidal neurons and PV+ chandelier cells synapse into the AIS to precisely control neuron firing and cortical circuit synchronization (Figure 1C). Alterations in GABAergic neurotransmission seen in individuals with BD and SZ are supported by findings of significantly reduced expression of GAD67, the 67kDa isoform of glutamate decarboxylase that synthesizes GABA, decreased GABA transporter expression, and increased post-synaptic GABAA receptor expression. Furthermore, these deficits in GABAergic signaling are significantly pronounced in the PV+ interneurons of BD and SZ patients (9). These changes in GABAergic circuits have been predominately observed in layers II/III of the prefrontal cortex and CA2/3 regions of the hippocampus, which are key brain regions affected in psychiatric disease (10). Recent studies have determined the 480kDa splice variant of ankyrin-G is critical for the stabilization of GABAergic synapses at somatodendritic sites of cortical neurons (Figure 1C). Future work needs to focus on how loss of ankyrin-G-dependent inhibitory synapses affects GABAergic circuits, gamma oscillations, and behavior.
Abnormalities in glutamatergic synapse plasticity and dendritic spine morphology have also been implicated in the pathogenesis of SZ and BD. Dysfunction of NMDA receptor signaling at glutamatergic synapses located on GABAergic interneurons has been shown to reduce neuronal synchronization (9). In addition, Smith et al. demonstrated that the 190kDa isoform of ankyrin-G plays a key role in NMDA receptor trafficking and dendritic spine morphology (6). These findings suggest a second mechanism by which ankyrin-G impacts GABAergic neurotransmission and gamma oscillations. Thus, mutations in the different splice variants of ankyrin-G may have a profound effect on the maintenance and function of mature cortical circuitry and disrupted neural oscillations seen in bipolar and schizophrenic individuals.
Studies evaluating human neuroanatomy suggest the precise refinement and pruning of excitatory and inhibitory synapses occur throughout adolescence, highlighting the developmental nature of these diseases. Emerging data suggest that SNPs associated with psychiatric diseases affect the intricate calibration of excitatory-inhibitory balance in the cortex (9). Interestingly, the accumulation of 480kDa ankyrin-G on the pyramidal cell soma in mice occurs at the start of adolescence (8), which correlates with the formation of inhibitory synapses and the onset of the symptoms of BD in humans. Consistent with these molecular findings, Hughes et al. present evidence that the splice variants containing the protective ankyrin-G SNP (rs41283526) display a clear upward shift in expression from infancy and childhood to adolescence and adulthood (5). These data suggest the timing of expression of ankyrin-G splice variants changes with human development and may be a key factor involved in the pathophysiology of these disorders.
Exciting new evidence has pointed to key roles for different splice variants of ankyrin-G in both excitatory and inhibitory synapses. Hughes et al. have found that a SNP in ANK3 (rs41283526) that prevents normal splicing of a “little exon” in ankyrin-G is actually protective against BD and SZ (5). It will be important in future work to determine which of the three main classes of ankyrin-G isoforms are affected by rs41283526, and to understand the mechanisms by which loss of expression of those splice variants provides protection against neuropsychiatric disease. Elucidation of the cellular and molecular mechanisms by which splice variants of ankyrin-G control neuronal circuits and how mutations affect ankyrin-G expression and function will hopefully provide insight into the mechanisms by which ANK3 mutations contribute to the etiology of BD and SZ.
Acknowledgments
A.N. was supported by the following NIH training grant: “Michigan Predoctoral Training in Genetics (T32GM007544).” P.J. was supported by the Heinz C. Prechter Bipolar Research Fund and the Richard Tam Foundation at the University of Michigan Depression Center. The authors would like to thank Dr. Kathy Ignatoski (University of Michigan) and Dr. Peter Mohler (Ohio State University) for providing helpful comments.
Footnotes
Financial Disclosures:
Both authors reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nothen MM, Nieratschker V, Cichon S, Rietschel M. New findings in the genetics of major psychoses. Dialogues Clin Neurosci. 2010;12:85–93. doi: 10.31887/DCNS.2010.12.1/mnoethen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muhleisen TW, Leber M, Schulze TG, Strohmaier J, Degenhardt F, Treutlein J, et al. Genome-wide association study reveals two new risk loci for bipolar disorder. Nat Commun. 2014;5:3339. doi: 10.1038/ncomms4339. [DOI] [PubMed] [Google Scholar]
- 3.Bennett V, Lorenzo DN. Spectrin- and ankyrin-based membrane domains and the evolution of vertebrates. Curr Top Membr. 2013;72:1–37. doi: 10.1016/B978-0-12-417027-8.00001-5. [DOI] [PubMed] [Google Scholar]
- 4.Jenkins PM, Kim N, Jones SL, Tseng WC, Svitkina TM, Yin HH, et al. Giant ankyrin-G: A critical innovation in vertebrate evolution of fast and integrated neuronal signaling. Proc Natl Acad Sci U S A. 2015;112:957–964. doi: 10.1073/pnas.1416544112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes T, Hansson L, Sonderby IE, Athanasiu L, Zuber V, Tesli M, et al. A Loss-of-Function Variant in a Minor Isoform of ANK3 Protects Against Bipolar Disorder and Schizophrenia. Biol Psychiatry. 2016 doi: 10.1016/j.biopsych.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 6.Smith KR, Kopeikina KJ, Fawcett-Patel JM, Leaderbrand K, Gao R, Schurmann B, et al. Psychiatric risk factor ANK3/ankyrin-G nanodomains regulate the structure and function of glutamatergic synapses. Neuron. 2014;84:399–415. doi: 10.1016/j.neuron.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang KJ, Zollinger DR, Susuki K, Sherman DL, Makara MA, Brophy PJ, et al. Glial ankyrins facilitate paranodal axoglial junction assembly. Nat Neurosci. 2014;17:1673–1681. doi: 10.1038/nn.3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tseng WC, Jenkins PM, Tanaka M, Mooney R, Bennett V. Giant ankyrin-G stabilizes somatodendritic GABAergic synapses through opposing endocytosis of GABAA receptors. Proc Natl Acad Sci U S A. 2015;112:1214–1219. doi: 10.1073/pnas.1417989112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]