Abstract
The gut microbiome is a community of commensal organisms that are known to play a role in nutrient production as well as gut homeostasis. The composition of the gut flora can be affected by many factors; however, the impact of burn injury on the microbiome is not fully known. Here, we hypothesized that burn-induced changes to the microbiome would impact overall colon health. After scald-burn injury, cecal samples were analyzed for aerobic and anaerobic colony forming units, bacterial community, and butyrate levels. In addition, colon and total intestinal permeabilities were determined. These parameters were further determined in a germ-reduced murine model. Following both burn injury and germ reduction, we observed decreases in aerobic and anaerobic bacteria, increased colon permeability and no change to small intestinal permeability. After burn injury, we further observed a significant decrease in the butyrate producing bacteria R. Gnavus, C. Eutactus, and Roseburia species as well as decreases in colonic butyrate. Finally, in mice that underwent burn followed by fecal microbiota transplant, bacteria levels and mucosal integrity were restored. Altogether our data demonstrate that burn injury can alter the microbiome leading to decreased butyrate levels and increased colon permeability. Of interest, fecal microbiota transplant treatment was able to ameliorate the burn-induced changes in colon permeability. Thus, fecal transplantation may represent a novel therapy in restoring colon health after burn injury.
Keywords: Burn injury, gut permeability, microbiome
INTRODUCTION
The gut microbiome is in a constant state of flux due to bacterial immigration, elimination, and/or growth. In healthy individuals, the microbiome is composed of between 300 and 1000 species of bacteria, with 99% of the total mass consisting of only 40 species (1). It is the highly abundant phyla Firmicute, Proteobacteria, and Bacteroidete that most significantly function in pathogen control and gut function (2, 3). When alterations to the microbiome occur, the dysbiosis affects the regulatory environment and low abundance and pathogenic populations such as Clostridium difficile (C. diff) are able to expand, producing pathology (4, 5). The composition and biodiversity of the microbiome are affected by several factors including diet, environment, medication, and infection/inflammation, but little is known regarding the effect of burn injury (6, 7).
The relationship between the microbiome and the host is commensal (8). The bacteria produce vitamins and convert indigestible macronutrients into short chain fatty acids (SCFA), the primary nutrient of the colonocyte (3, 9). In addition to nutrition, SCFAs have also been shown to induce epithelial cell proliferation and have been implicated in preventing neoplastic transformation (10, 11). Furthermore, butyrate, a four-carbon SCFA produced by members of the Firmicute phylum has been shown to regulate T-cell function and may play a role in the systemic immune response (12). This is of particular importance as it is well known that burn patients develop a state of relative immune compromise following burn injury leaving them susceptible to nosocomial infections (13, 14). Aside from immune compromise, burn patients also develop a loss of endothelial and mucosal integrity (15). In combination, the immune compromise and “leaky” barriers can lead to massive tissue edema, bacterial translocation, and in some cases, progression to multiorgan failure and death (16, 17).
Prebiotics, probiotics, and synbiotics have all been utilized to alleviate or ameliorate alterations to the microbiome (5). Largely, these products garner only anecdotal success and likely only provide a transient effect. Fecal microbiota transplant (FMT) or stool transplant, on the other hand, has recently developed significant success as a method to restore the host microbiome for conditions ranging from inflammatory bowel disease to refractory C. diff colitis (18, 19). The method involves nasogastric or colonoscopic implantation of a healthy donor's fecal microbiota into the GI tract of a recipient. This treatment appears, not only to alleviate the acute aberration, but also to provide a lasting effect (3, 5). In this study, we hypothesized that burn injury causes alteration to the microbiome that ultimately contributes to the dysfunctional gut integrity commonly seen after burn injury. As a proof of principle, we further postulated that FMT would restore bacterial populations and gut integrity following burn injury.
METHODS
Mice
CF-1 (WT) male mice were purchased from Charles River Laboratory (Wilmington, MA). All mice were 6-weeks-old when purchased from Charles River Laboratory and allowed to acclimate for 1 to 3 weeks. All mice were housed in standard environmental conditions and were fed with a commercial pellet diet and water ad libitum. All murine experiments were performed between 8 am and 10 am using protocols approved by the Institutional Animal Care and Use Committee (IACUC, # 08-09-19-01) of the University of Cincinnati.
Materials
Amphotericin B, solubilized, was purchased from Sigma Life Sciences (St Louis, MO). Vancomycin Hydrochloride was purchased from Hospira (Lake Forest, IL). Metronidazole was purchased from Fisher (Pittsburgh, PA). Neomycin sulfate was purchased from Santa Cruz Biotechnology (Dallas, TX). Ampicillin was purchased from Sandoz Inc (Princeton, NJ). All medications were stored according to manufacturer specification and no medications were expired or beyond their effective date. Isoflurane 3% was purchased from the Henry Schein Co (Dublin, OH).
Burn injury
Mice were subjected to a 28% total body surface area (TBSA) full-thickness scald injury similar to that previously described (20). Briefly, mice were anesthetized with 3% isoflurane in oxygen, their backs shaved and placed in a 28% TBSA template. Mice were immersed in a 90°C bath for 9 s and resuscitated with 2-mL sterile saline. Sham-treated mice were treated similarly with room temperature water immersion.
Germ reduction
Mice were gavaged with an antimicrobial cocktail daily for a 10-day period leading up to the experiments, as previously described (21). The mice were initially treated with amphotericin alone at a dose of 1 mg/kg for 3 days. On days 4 to 10, the mice were gavaged with a cocktail of vancomycin (50 mg/kg), neomycin (100 mg/kg), metronidazole (100 mg/kg), and amphotericin (1 mg/kg). In addition to the gavage, ampicillin was added to their drinking water at a concentration of 1 g/L. Control mice were gavaged with ddH2O.
Fecal microbiota transplant
Fecal microbiota transplants (FMT) were prepared from cecal stool samples collected from untouched mice. Briefly, the cecal stool samples were collected from multiple mice weighed and combined. The samples were then diluted with ddH2O to a concentration of 300 mg/mL and filtered through a wire screen to remove particulate matter. Mice were then gavaged with 200 μL of FMT twice daily, once in the morning and again in the afternoon. Using fresh samples each day, the FMT was performed on PBD2 and repeated as described on PBD3 as well.
Bacterial counts
Bacterial counts were performed on stool harvested from the cecum of each mouse. Samples were serially diluted in sterile saline to the specified concentration. Aerobic bacteria were cultured on Tryptic Soy Agar pour plates (BD Pharmingen, San Jose, CA) and incubated at 37°C for 24 h (22). Anaerobic cultures were performed on CDC Anaerobe Blood Agar and incubated in BD GasPak chambers with BD GasPak EZ sachets (BD Pharmingen) at 37°C for 72 h. Following incubation, colony counts were performed on all samples.
Intestinal permeability
Mice were gavaged with 200 μL of FITC-Dextran at a concentration of 1 mg/mL versus tap water control. The mice were harvested at 4 h from the time of gavage and blood collected by cardiac puncture. The samples were placed in serum separator tubes and plasma analyzed for fluorescence levels at 520 nm by Synergy 4 Multiplate (BioTek Instruments Inc, Winooski, VT).
Colon permeability
Normally fed mice were anesthetized to effect by 2% isoflurane in oxygen via facemask. The skin was shaved and disinfected. After a 1 to 2 cm laparotomy, the ileum was ligated with a 3–0 silk tie (Ethicon, Somerville, NJ). Next, 200 uL of FIT-C Dextran (1 mg/mL) was injected into the cecum. The cecum was replaced in its original location, and the midline incision was closed in two layers with 4–0 silk suture (Schein Inc, Melville, NY). The animals were resuscitated with 1 mL of sterile saline administered subcutaneously and kept on a heating blanket for 1 h. The mice were then harvested at 4 h from the time of injection and blood was collected by cardiac puncture. The samples were placed in serum separator tubes and plasma analyzed for fluorescence levels at 520 nm by Synergy 4 Multiplate (BioTek Instruments Inc).
Bacterial species analysis
Mice were subjected to 28% burn injury as specified above. On PBD6, the mice were harvested and stool samples were collected from the cecum. These samples were processed for specific bacterial species by rRNA 16S v3 PCR analysis at the Second Genome (San Francisco, CA). Briefly, bacterial DNA was isolated from cecal samples using MoBio PowerMag Soil DNA Isolation Kit as per vendor's protocol. The frozen genomic DNA isolates were stored at −20°C. The bacterial 16S rRNA genes were amplified using the degenerate forward primer:
27F.1 5′-AGRGTTTGATCMTGGCTCAG-3′
and the nondegenerate reverse primer:
1492R.jgi 5′-GGTTACCTTGTTACGACTT-3′.
Thirty-five cycles of bacterial 16S rRNA gene PCR amplification were performed. Samples amplified to specification and were moved forward for hybridization. Bacterial 16S rRNA gene amplicons were fragmented, biotin labeled, and hybridized to the PhyloChip Array, version G3. PhyloChip arrays were washed, stained, and scanned using a GeneArray scanner (Affymetrix). Each scan was captured using standard Affymetrix software (GeneChip Micro-array Analysis Suite).
Butyrate measurement
Stool samples were collected from the cecum of burn-injured and sham mice. Samples were processed and analyzed as previously described (23). Briefly, weighed cecal specimens were diluted in 25% meta-phosphoric acid at a 1:5 ratio. Samples were then centrifuged at 16,000 × g for 15 min at 4°C, filtered through 0.45-um syringe tip filter (Thermo Fischer Scientific; Waltham, MA), and stored at −80°C until analysis. Butyrate concentrations performed by high-performance liquid chromatography using a 0.01 N sulfuric acid mobile phase (apparent pH 2.0) pumped at a flow rate of 0.7 mL/min through a Rezex ROA-organic acid H+ (8%) 300 × 7.8 mm analytical column (Phenomenex, Torrance, CA) maintained at 65°C. The effluent following a 20-μL injection was monitored with a UV detector set at 210 nm. All equipment was made by Shimadzu (Kyoto, Japan). Peak area of butyric acid was compared to standards ranging for 1 to 100 mM. Butyrate concentrations were corrected for dilution and fecal weight and expressed as μmol per gram of wet weight feces (23, 24).
Statistical analysis
Statistical comparisons were performed using Student t test (two groups), or ANOVA with Tukey's post hoc test (more than two groups). Prism 6 software (GraphPad Software, La Jolla, CA) was used for statistical analyses. A value of P<0.05 was considered statistically significant.
RESULTS
Burn injury is associated with decreased aerobic and anaerobic populations
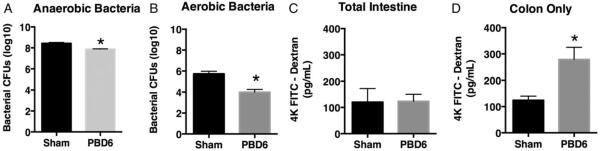
Many factors have been demonstrated to alter the microbiome with the resulting dysbiosis leading to pathologic sequelae (4, 5). To investigate whether burn injury can alter the bacterial populations of the gut, we subjected mice to 28% full thickness burn. The data demonstrate that burn injury leads to a significant decrease in anaerobic and aerobic bacteria in the gut (Fig. 1A and B). The most profound effect was noted on PBD6. Thus, we conclude that burn injury can induce an overall decrease of cultivable bacteria within the gut.
Fig. 1. Burn injury decreases gut bacterial load and increases colon permeability.
Mice were subjected to a 28% total body surface area dorsal scald-burn injury as described in the Methods. On post-burn day 6 (PBD6), cecal stool samples were cultured under (A) anaerobic or (B) aerobic conductions and CFU numbers determined. Sham or PBD6 mice underwent either (C) FITC dextran gavage to determine total intestinal permeability or (D) intestinal ligation proximal to the cecum and FITC dextran cecal injection to determine colon permeability. Sample size is 5 per group. *P<0.05, compared with sham as determined by Student t test.
Burn injury leads to increased colon permeability
It has been reported that burn patients can experience a loss of endothelial integrity associated with decreased mucosal integrity as well as absorptive capacity within the GI tract (10, 11, 25). To assess the permeability of the GI mucosa, we determined both total intestinal permeability and colon-specific permeability after burn injury. First, we observed no significant change in total intestinal permeability between sham- and scald-injured mice (Fig. 1C). However, we did observe that mice subjected to burn injury demonstrated significantly more colon permeability compared with the sham-burned control mice (Fig. 1D). Altogether these data demonstrate that burn injury leads to increased permeability within the colon with no observed difference in permeability within the small intestine.
Germ reduction leads to similar dysbiosis and colon permeability as burn injury
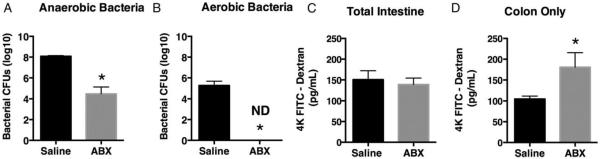
We next wanted to determine whether the loss in bacteria alone was sufficient to increase colon permeability. Noninjured mice were subjected to a 10-day antibiotic regimen specifically designed to reduce the fungal and bacterial burden in the gut. Following this germ reduction, treated mice were noted to have decreased anaerobic and aerobic bacterial populations (Fig. 2A and B). Total intestinal and colon permeability was next determined for antibody-treated and untreated mice. We observed that germ-reduced mice displayed increased colon permeability with no change in the permeability of the small intestine compared with untreated mice (Fig. 2C and D). Thus, the loss of gut microbiome can lead to increased colon permeability.
Fig. 2. Antibiotic gavage decreases gut bacterial load and increases colon permeability.
Mice underwent germ reduction by antibiotic gavage as described in the Methods. Isolated cecal stool samples from untreated or antibiotic-treated (ABX) mice were cultured under (A) anaerobic or (B) aerobic conductions and CFU numbers determined. Untreated or ABX mice underwent either (C) FITC dextran gavage to determine total intestinal permeability or (D) intestinal ligation proximal to the cecum and FITC dextran cecal injection to determine colon permeability. Sample size is 8 per group. *P<0.05, compared with saline as determined by Student t test.
Burn injury and germ reduction lead to decreased butyrate levels
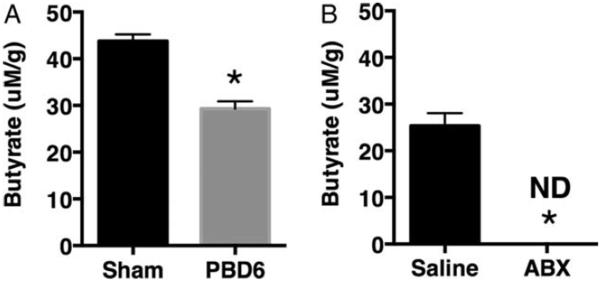
It has been demonstrated that butyrate is a major respiratory fuel of the colonic mucosa (26), whereas, in contrast, there is a decreasing usage of glutamine from jejunum to colon (27). The microbiome is the sole source of butyrate for the colon. To investigate whether burn injury and the subsequent reduction in bacteria results in altered gut butyrate levels, we subjected one cohort of mice to burn injury and another to germ reduction. Following burn injury, stool from burn-injured mice had significantly less butyrate compared with stool collected from uninjured mice (Fig. 3A). Furthermore, mice that underwent germ reduction also had significantly less butyrate when compared with control (Fig. 3B).
Fig. 3. Burn injury and antibiotic gavage decrease gut butyrate levels.
Mice were subjected to a 28% total body surface area dorsal scald-burn injury or germ reduction by antibiotic gavage as described in the Methods. Butyrate levels were determined from cecal stool samples isolated from (A) sham- or burn-injured mice (PBD6) or (B) untreated or antibiotic-treated mice. Sample size is 8 per group. *P<0.05 compared with sham as determined by Student t test.
Butyrate producing bacteria are reduced following burn injury
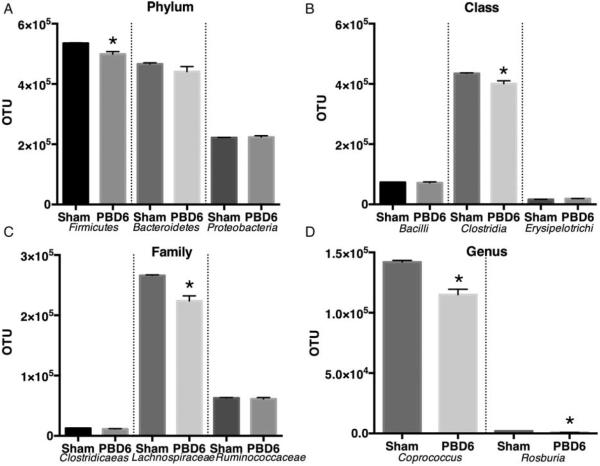
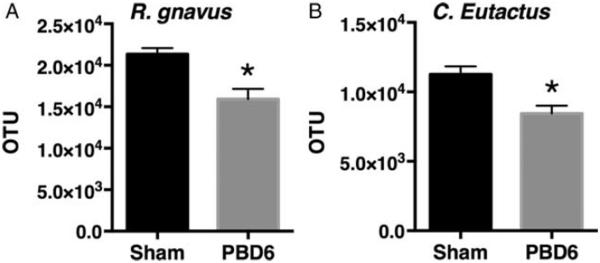
Butyrate is at least produced by bacteria from the Firmicute phylum and the Clostridiale family (10). Given the decrease in butyrate levels, we examined the bacterial abundance to determine which species were impacted by burn injury. Mice underwent burn injury and cecal samples were collected on PBD6. These samples were sent for bacterial 16S v1-3 rRNA PCR analysis. This phylogenetic analysis showed that the Firmicute phylum was significantly decreased compared with control (Fig. 4A). We continued to examine the most abundant communities from Phylum, Class, Family to Genus. The most abundant members of each classification were significantly reduced compared with control (Fig. 4A–C). Interestingly, we observed a decrease in the genus Roseburia, an anaerobic genus that encompasses a number of butyrate-producing strains. When we further narrowed our analysis, we found two species that were greatly reduced: Gnavus and Eutactus, both known butyrate producers (Fig. 5).
Fig. 4. Burn injury selectively reduces gut bacterial phylum, class, family, and genus abundance.
Mice were subjected to 28% total body surface area burn injury. On post-burn day 6, the mice were harvested and stool samples were collected from the cecum and sent for phylogenetic analysis using a sequence-specific hybridization assay of the entire 16S ribosomal RNA gene (V1–V3) to identify and measure relative abundance of >50,000 individual microbial taxa. The largest (A) phylum, (B) class, (C) family, and (D) genus from uninjured mice were compared with burn-injured mice. Sample size is 4 per group. *P<0.05 compared with sham as determined by Student t test.
Fig. 5. Burn injury selectively reduces butyrate producing bacterial strains Gnavus and Eutactus.
Mice were subjected to 28% total body surface area burn injury. On post-burn day 6, the mice were harvested and stool samples were collected from the cecum and sent for phylogenetic analysis. The (A) Ruminococcus gnavus and (B) Coprococcus eutactus from uninjured mice were compared to burn-injured mice. Sample size is 4 per group. *P<0.05 compared with sham as determined by Student t test.
FMT restored bacterial counts and mucosal integrity
Fecal microbiota transplant has been utilized in medicine for treatment of many conditions including refractory C. diff colitis (4, 28). C. diff colitis most often results from antibiotic-induced changes to the microbiome (29). Treatment for C. diff often consists of the use of probiotics and antibiotics (30, 31). To determine whether FMT could restore the composition of the host microbiome following burn injury, mice were burn- or sham-injured and then administered FMT 2 and 3 days after the procedure. Stool was collected for analysis 6 days after the procedure. Mice that received an FMT displayed bacterial counts consistent with untouched shams (data not shown). Furthermore, these mice displayed colon permeability consistent with sham mice (Fig. 6). From these observations, we conclude that FMT is able to restore mucosal integrity following burn injury.
Fig. 6. FMT restores anaerobic bacterial counts and improves colon permeability after burn injury.
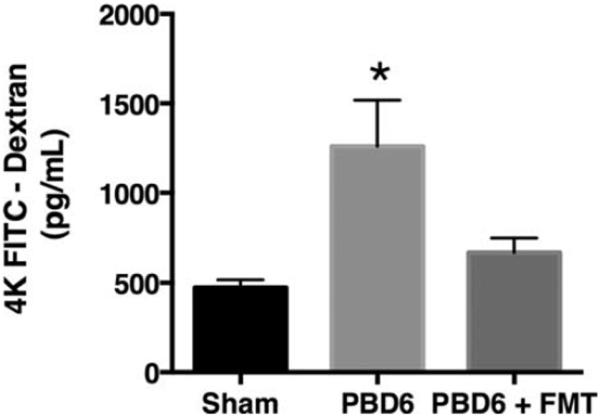
Mice were subjected to a 28% total body surface area dorsal scald-burn injury as described in the Methods. On PBD2 and on PBD3, the mice underwent FMT as described. Sham or PBD6±FMT mice underwent intestinal ligation proximal to the cecum and FITC dextran cecal injection to determine colon permeability. Sample size is 8 per group. *P<0.05 compared with sham and PBD6 + FMT as determined by ANOVA followed by the Tukey post hoc comparison test. FMT indicates fecal microbiota transplant; PBD6, post-burn day 6.
DISCUSSION
The data demonstrate the negative effects of burn injury on the overall health of the colon and the commensal organisms within it. It is well known that burn injury and the subsequent systemic inflammation lead to decreased endothelial integrity and mucosal integrity (10, 11, 25). These defects lead to massive tissue edema, bacterial translocation, and poor absorption of enteral nutrition (16, 17). What has not been well investigated is the role of the microbiome in alleviating or exacerbating these aberrations. We demonstrate that burn injury leads to a substantial decrease in the total gut bacteria, both aerobic and anaerobic. We further observed that the burn injury was associated with increased permeability and decreased butyrate levels within the colon. Utilizing a germ reduction model, we were able to show a similar decrease in butyrate and subsequent increase in colon permeability. This allowed us to focus on the “loss of function” effects associated with decreased butyrate and butyrate producing bacteria in the colon. When examined altogether, these data support our hypothesis that bacteria and/or bacterial products within the colon may be a potential mechanism driving the loss of mucosal integrity following burn injury. We conclude that burn injury leads to a loss of bacteria, specifically butyrate producing species. Without production, butyrate levels fall and there is less stimulation for the proliferation of the colonic epithelium, ultimately leading to loss of mucosal integrity. To further elucidate the mechanism behind this correlation, we performed a “gain of function” experiment and added bacteria back to the GI tract of the burned mice. We noted that mice that underwent FMT showed colon permeability and butyrate levels consistent with control mice.
It is widely reported that the gut microbiome plays a significant role in local and systemic physiology and pathology. Dysbiosis results from a variety of external and internal stimuli and can result in devastating disease states. Correcting alterations to the microflora are of key interest for this study. Both pre- and probiotics have been demonstrated to be effective in the treatment of conditions from lactose intolerance to acute gastroenteritis and even recurrent C. diff colitis (5). Despite the effectiveness in the acute period, it still remains unclear whether these interventions provide a lasting effect. In addition to probiotics, recent studies have demonstrated prebiotics like partially hydrolyzed guar gum are able to induce butyrate producing strains (32). Fecal microbiota transplant was first utilized in 1958 for treatment of pseudomembranous colitis (33) and is most often utilized for recurrent C. diff infection today. FMT is believed to restore the balanced gut flora, which provides a natural resistance against pathologic colonization. Studies investigating the duration have demonstrated that FMT restores the balance by 2 weeks and that the effect persists for 4 months or longer (34).
Two recent reports have investigated how burn injury impacts the gut microbiome. The first report investigated fecal samples from 5 patients with major burns and found that the gut microbiota was severely altered. Furthermore, butyric acid decreased to lower-than-normal levels but tended to increase after recovery in the survivors (35). The robust changes in the gut microbiome and decreased butyrate levels observed in this burned patient cohort are consistent with our data reported here. The second report also investigated fecal samples from four patients with 25% to 57% TBSA burns as well as samples from burn-injured mice and found dramatic changes in the gut microbiome characterized by gram-negative aerobic bacteria overgrowth (36). In addition, the authors observed bacterial translocation consistent with increased total intestinal permeability 1 day after burn injury. These reported changes in the microbiome from burn-injured mice are consistent with the data presented here. However, we did not observe a change in total intestinal permeability, but only a change in colon permeability despite the model of injury being very similar. A major difference between the studies is the time after burn-injury intestineal permeability was measured: 1 day in (36) and 6 days here. As we implicate butyrate and butyrate producing bacteria in the increased colon permeability, it will be of interest to determine these parameters 1 day after burn injury.
While this paper demonstrates that burn injury negatively impacts the composition of the microbiome and gut mucosal integrity, there are some limitations. First, this paper utilizes a moderate severity burn injury model and may not adequately correlate with greater severity burns that would result in greater mortality in mice but not in human patients due to increased supportive care. Additionally, our data reveal the association between a reduced gut microbiome and increased colon permeability, which is restored by FMT. While we believe that butyrate plays a key role in this observation, studies have yet to be conducted to confirm causality.
In conclusion, our study is the first to demonstrate the importance of the gut microbiome in maintaining gut integrity following burn injury. Our results indicate that restoring the microbiome by FMT, pre- or probiotics may be a potentially useful therapy in burn patients or other injured patients.
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- 2.Barcenilla A, Pryde SE, Martin JC, Duncan SH, Stewart CS, Henderson C, Flint HJ. Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl Environ Microbiol. 2000;66:1654–1661. doi: 10.1128/aem.66.4.1654-1661.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cummings JH, Macfarlane GT. Colonic microflora: nutrition and health. Nutrition. 1997;13:476–478. doi: 10.1016/s0899-9007(97)00114-7. [DOI] [PubMed] [Google Scholar]
- 4.Bien J, Palagani V, Bozko P. The intestinal microbiota dysbiosis and Clostridium difficile infection: is there a relationship with inflammatory bowel disease? Therap Adv Gastroenterol. 2013;6:53–68. doi: 10.1177/1756283X12454590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361:512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 6.Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Liggett CM, Nelson KE. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449:804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madigan MT. Brock Biology of Microorganisms. Benjamin Cummings; San Francisco: 2012. p. xxviii.p. 1043.p. 1077. [Google Scholar]
- 9.Cummings JH, Macfarlane GT. Role of intestinal bacteria in nutrient metabolism. JPEN J Parenter Enteral Nutr. 1997;21:357–365. doi: 10.1177/0148607197021006357. [DOI] [PubMed] [Google Scholar]
- 10.Frankel WL, Zhang W, Singh A, Klurfeld DM, Don S, Sakata T, Modlin I, Rombeau JL. Mediation of the trophic effects of short-chain fatty acids on the rat jejunum and colon. Gastroenterology. 1994;106:375–380. doi: 10.1016/0016-5085(94)90595-9. [DOI] [PubMed] [Google Scholar]
- 11.Gibson PR, Moeller I, Kagelari O, Folino M, Young GP. Contrasting effects of butyrate on the expression of phenotypic markers of differentiation in neoplastic and non-neoplastic colonic epithelial cells in vitro. J Gastroenterol Hepatol. 1992;7:165–172. doi: 10.1111/j.1440-1746.1992.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 12.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 13.Hanschen M, Tajima G, O'Leary F, Ikeda K, Lederer JA. Injury induces early activation of T-cell receptor signaling pathways in CD4+ regulatory T cells. Shock. 2011;35:252–257. doi: 10.1097/SHK.0b013e3181f489c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacConmara MP, Tajima G, O'Leary F, Delisle AJ, McKenna AM, Stallwood CG, Mannick JA, Lederer JA. Regulatory T cells suppress antigen-driven CD4 T cell reactivity following injury. J Leukoc Biol. 2011;89:137–147. doi: 10.1189/jlb.0210082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magnotti LJ, Deitch EA. Burns, bacterial translocation, gut barrier function, and failure. J Burn Care Rehabil. 2005;26:383–391. doi: 10.1097/01.bcr.0000176878.79267.e8. [DOI] [PubMed] [Google Scholar]
- 16.Lichtman SM. Bacterial [correction of baterial] translocation in humans. J Pediatr Gastroenterol Nutr. 2001;33:1–10. doi: 10.1097/00005176-200107000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Van Leeuwen PA, Boermeester MA, Houdijk AP, Ferwerda CC, Cuesta MA, Meyer S, Wesdorp RI. Clinical significance of translocation. Gut. 1994;35:S28–S34. doi: 10.1136/gut.35.1_suppl.s28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bagdasarian N, Rao K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA. 2015;313:398–408. doi: 10.1001/jama.2014.17103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirsch BE, Saraiya N, Poeth K, Schwartz RM, Epstein ME, Honig G. Effectiveness of fecal-derived microbiota transfer using orally administered capsules for recurrent Clostridium difficile infection. BMC Infect Dis. 2015;15:191. doi: 10.1186/s12879-015-0930-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tschop J, Martignoni A, Reid MD, Adediran SG, Gardner J, Noel GJ, Ogle CK, Neely AN, Caldwell CC. Differential immunological phenotypes are exhibited after scald and flame burns. Shock. 2009;31:157–163. doi: 10.1097/SHK.0b013e31817fbf4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Croswell A, Amir E, Teggatz P, Barman M, Salzman NH. Prolonged impact of antibiotics on intestinal microbial ecology and susceptibility to enteric Salmonella infection. Infect Immun. 2009;77:2741–2753. doi: 10.1128/IAI.00006-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martignoni A, Tschop J, Goetzman HS, Choi LG, Reid MD, Johannigman JA, Lentsch AB, Caldwell CC. CD4-expressing cells are early mediators of the innate immune system during sepsis. Shock. 2008;29:591–597. doi: 10.1097/SHK.0b013e318157f427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huda-Faujan N, Abdulamir AS, Fatimah AB, Anas OM, Shuhaimi M, Yazid AM, Loong YY. The impact of the level of the intestinal short chain fatty acids in inflammatory bowel disease patients versus healthy subjects. Open Biochem J. 2010;4:53–58. doi: 10.2174/1874091X01004010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20:159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 25.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roediger WE. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut. 1980;21:793–798. doi: 10.1136/gut.21.9.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinkus LM, Windmueller HG. Phosphate-dependent glutaminase of small intestine: localization and role in intestinal glutamine metabolism. Arch Biochem Biophys. 1977;182:506–517. doi: 10.1016/0003-9861(77)90531-8. [DOI] [PubMed] [Google Scholar]
- 28.Di Bella S, Gouliouris T, Petrosillo N. Fecal microbiota transplantation (FMT) for Clostridium difficile infection: focus on immunocompromised patients. J Infect Chemother. 2015;21:230–237. doi: 10.1016/j.jiac.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Bartlett JG, Chang TW, Gurwith M, Gorbach SL, Onderdonk AB. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978;298:531–534. doi: 10.1056/NEJM197803092981003. [DOI] [PubMed] [Google Scholar]
- 30.Johnston BC, Ma SS, Goldenberg JZ, Thorlund K, Vandvik PO, Loeb M, Guyatt GH. Probiotics for the prevention of Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Ann Intern Med. 2012;157:878–888. doi: 10.7326/0003-4819-157-12-201212180-00563. [DOI] [PubMed] [Google Scholar]
- 31.Drekonja DM, Butler M, MacDonald R, Bliss D, Filice GA, Rector TS, Wilt TJ. Comparative effectiveness of Clostridium difficile treatments: a systematic review. Ann Intern Med. 2011;155:839–847. doi: 10.7326/0003-4819-155-12-201112200-00007. [DOI] [PubMed] [Google Scholar]
- 32.Ohashi Y, Sumitani K, Tokunaga M, Ishihara N, Okubo T, Fujisawa T. Consumption of partially hydrolysed guar gum stimulates Bifidobacteria and butyrateproducing bacteria in the human large intestine. Benef Microbes. 2015;6:451–455. doi: 10.3920/BM2014.0118. [DOI] [PubMed] [Google Scholar]
- 33.Eiseman B, Silen W, Bascom GS, Kauvar AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. 1958;44:854–859. [PubMed] [Google Scholar]
- 34.Hamilton MJ, Weingarden AR, Unno T, Khoruts A, Sadowsky MJ. High-throughput DNA sequence analysis reveals stable engraftment of gut microbiota following transplantation of previously frozen fecal bacteria. Gut Microbes. 2013;4:125–135. doi: 10.4161/gmic.23571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimizu K, Ogura H, Asahara T, Nomoto K, Matsushima A, Hayakawa K, Ikegawa H, Tasaki O, Kuwagata Y, Shimazu T. Gut microbiota and environment in patients with major burns: a preliminary report. Burns. 2015;41:e28–e33. doi: 10.1016/j.burns.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 36.Earley ZM, Akhtar S, Green SJ, Naqib A, Khan O, Cannon AR, Hammer AM, Morris NL, Li X, Eberhardt JM, et al. Burn injury alters the intestinal microbiome and increases gut permeability and bacterial translocation. PLoS One. 2015;10:e0129996. doi: 10.1371/journal.pone.0129996. [DOI] [PMC free article] [PubMed] [Google Scholar]