Abstract
An acute burn induced coagulopathy develops after scald injury, which evolves into a subacute, hypercoagulable state. Microparticles, specifically platelet-derived MPs (PMPs), have been suggested as possible contributors. We first developed a model of burn-induced coagulopathy and then sought to investigate the role of platelets and PMPs in coagulation after burn. We hypothesized that changes in circulating platelet and PMP populations after injury would contribute to the post-burn, hypercoagulable state. A murine scald model with 28% TBSA full thickness burn injury was utilized and blood samples were collected at intervals after injury. Circulating MP populations, platelet counts, overall coagulation, and platelet function were determined. Burn injury led to hypercoagulability on post-burn day one (PBD1), which persisted 6 days after injury (PBD6). On PBD1, there was a significant decrease in platelet numbers and a decline in platelet contribution to clot formation with a concomitant increase in circulating procoagulant PMPs. On PBD6, there was a significant increase in platelet numbers and in platelet activation with no change in PMPs compared with sham. Further, on PBD1 decreased ADP-induced platelet activation was observed with a contrasting increase in ADP-induced platelet activation on PBD6. We therefore concluded that there was a temporal change in the mechanisms leading to a hypercoagulable state after scald injury, that PMPs are responsible for changes seen on PBD1, and finally that ADP-induced platelet activation was key to the augmented clotting mechanisms 6 days after burn.
Keywords: Burn injury, coagulation, hypercoagulability, microparticles, platelet function, thermal injury, thromboelastometry
INTRODUCTION
Thermal injury leads to the disruption of coagulation homeostasis by inducing changes in the balanced processes of clot formation and lysis. An acute burn induced coagulopathy, characterized by endothelial injury, release of acute phase reactants, and impaired coagulation and fibrinolytic pathways (1), may also be accompanied by the development of a transient consumptive coagulopathy. The severity of these early coagulation changes correlates with age, total body surface area (TBSA) burned, presence of inhalation injury, obesity, and increased number of invasive procedures (1–5). A subsequent, subacute, and persistent hypercoagulable state follows, independently contributing to increased mortality (6, 7). Although many burn patients have been shown to develop persistent hypercoagulability after injury, the rates of venous thromboembolism (VTE), pulmonary embolism (PE), and disseminated intravascular coagulation (DIC) are highly variable, leading to much debate regarding optimal mechanical and chemoprophylaxis in these patients (8–11). Although it is clear that endothelial injury from the acute insult leads to the release of pro-inflammatory and procoagulant factors that initiate these changes, the mechanisms behind the development and persistence of this hypercoagulability remain poorly understood.
Human studies have demonstrated alterations to coagulation parameters temporally after burn using conventional prothrombin time (PT) and international normalized ratio (INR), and more recently with viscoelastic coagulation testing using thrombelastography (TEG) and rotational thromboelastometry (ROTEM) (3, 5, 12). These changes are often accompanied by increased circulating procoagulant factors and decreased anticoagulant factors (3, 4, 13). In addition to impacting overall coagulation, thermal injury is also associated with an initial transient thrombocytopenia followed by normalization of platelet counts and eventual reactive thrombocytosis in both humans (14, 15) and animal models (16). Further, lower platelet counts are associated with increased morbidity and mortality (15, 17). Proposed pathways for these coagulation state changes include tissue factor release, consumptive coagulopathy from development of DIC, and platelet dysfunction. Although microparticles (MPs), and more specifically platelet-derived MPs (PMPs), have been shown to contribute to other hypercoagulable trauma models (18–20), their role in hypercoagulability and altered platelet function after scald injury have not been explored.
The goal of the current study was first to define hypercoagulability after murine scald injury and then to investigate the roles of platelets and PMPs in coagulation after burn. We hypothesized that a murine model of major full-thickness scald injury would result in a hypercoagulable state, and that changes in circulating MP populations would contribute to the coagulation changes observed.
MATERIALS AND METHODS
Scald injury model
Male CF-1 outbred mice aged 6–8 weeks from Charles River (Wilmington, Massachusetts) were subjected to a 28% full-thickness scald injury burn as previously described (21). A 28% scald injury model was used as coagulation changes can been seen with this severity of injury with a low risk of development of DIC (5). Briefly, mice were anesthetized with 3% isoflurane in oxygen, shaved over the dorsal surface, placed in a 28% total-body surface area template, immersed in a 90°C bath for 9 s, and resuscitated with 2mL sterile saline intraperitoneally. Sham-treated mice were treated similarly without water immersion. All murine experiments were performed between 8 am and 10 am using protocols approved by the Institutional Animal Care and Use Committee of the University of Cincinnati.
Thromboelastometry
Rotational thromboelastometry (ROTEM, TEM Systems Inc, Durham, North Carolina) analyses were performed to determine alterations in coagulation per manufacturer instructions. Whole blood collected via cardiac puncture from sham and scald-injured mice was anticoagulated with 10% citrate. Overall coagulation was determined using the NATEM test, extrinsic pathway coagulation using the EXTEM test, and fibrin contribution to clot using the FIBTEM test. For EXTEM and FIBTEM tests, 20µL of thromboplastin was added to 300µL of citrated blood to initiate clot formation. In addition, cytochalasin D was added to the FIBTEM samples to inhibit platelet activation. Clotting time (CT), clot formation time (CFT), clot lysis (LI30), α-angle (AA), and maximum clot firmness (MCF) were determined for each test. Platelet contribution to clot strength (%MCFPlatelet) was calculated by the equation: (EXTEMMCF − FIBTEMMCF)/EXTEMMCF, similar to the methods previously described (20, 22). All analyses were initiated within 10 min of whole blood collection.
Platelet count determination
Whole blood collected via cardiac puncture from injured mice was anticoagulated with heparin. Coulter AcT 10 Hematology Analyzer (Beckman Coulter, Brea, California) was used to determine complete blood count, including white blood cell count, hemoglobin, hematocrit, and platelet count.
Multiplate analysis
Whole blood was collected via cardiac puncture, anticoagulated with 10% hirudin, and analyzed by Multiplate software (Roche Diagnostics, Rotkreuz, Switzerland). ADPtest was used to determine ADP-induced platelet activation. Platelet aggregation velocity, total platelet aggregation (AU), and area under the curve (AUC) were measured.
Microparticle isolation, enumeration, and characterization
The MP isolation protocol used was adapted from those previously published by our laboratory (23). In short, whole blood collected via cardiac and was anticoagulated with heparin. It was then centrifuged at 450 g for 10 min, and the supernatant collected and centrifuged at 10,000 g for 10 min to remove platelets. The platelet-free supernatant containing the MPs was then diluted 1:1,000 with Roswell Park Memorial Institute media (RPMI) and labeled with 10µL/sample CD41 antibody (BD Pharmingen, Clone MWReg30, San Jose, California). Nanoparticle Tracking Analysis (Nanosight, Malvern Instruments Ltd, Worcestershire, UK) was then used to enumerate total and CD41 + MP (PMP) concentrations.
Microparticle procoagulant activity
Microparticle procoagulant activity was determined using a Zymuphen MP-Activity thrombin generation assay (Aniara, West Chester, Ohio). Whole blood was collected via cardiac puncture from sham and TBI mice 24 h after injury and anticoagulated with 10% citrate. Microparticles were isolated per protocol above, diluted 1:20, and the assay was performed per manufacturer protocol. The lower limit of detection was 0.2 nm.
Coagulation factor measurement by ELISA
Whole blood collected via cardiac puncture was placed in serum separator tubes (BD Bioscience, San Diego, California), centrifuged at 1,000 g for 10 min, and serum collected. Serum levels of fibrinogen and von Willebrand factor (vWF) were measured by ELISA according to the manufacturer’s protocol (MyBioSource, San Diego, California). The lower limit of detection for fibrinogen was 5 ng/mL and for vWF was 156 pg/mL.
Statistical analysis
Student’s t tests were used when comparisons were made between two treatment groups. One-way ANOVA with Tukey post-hoc test was used to compare multiple populations. Prism 6 (GraphPad Software, La Jolla, California) was used for all statistical analyses. Experiments containing multiple data points were used to calculate means and standard errors of the mean. A P-value of ≤0.05 was considered significant.
RESULTS
Increased clot strength is seen after scald injury
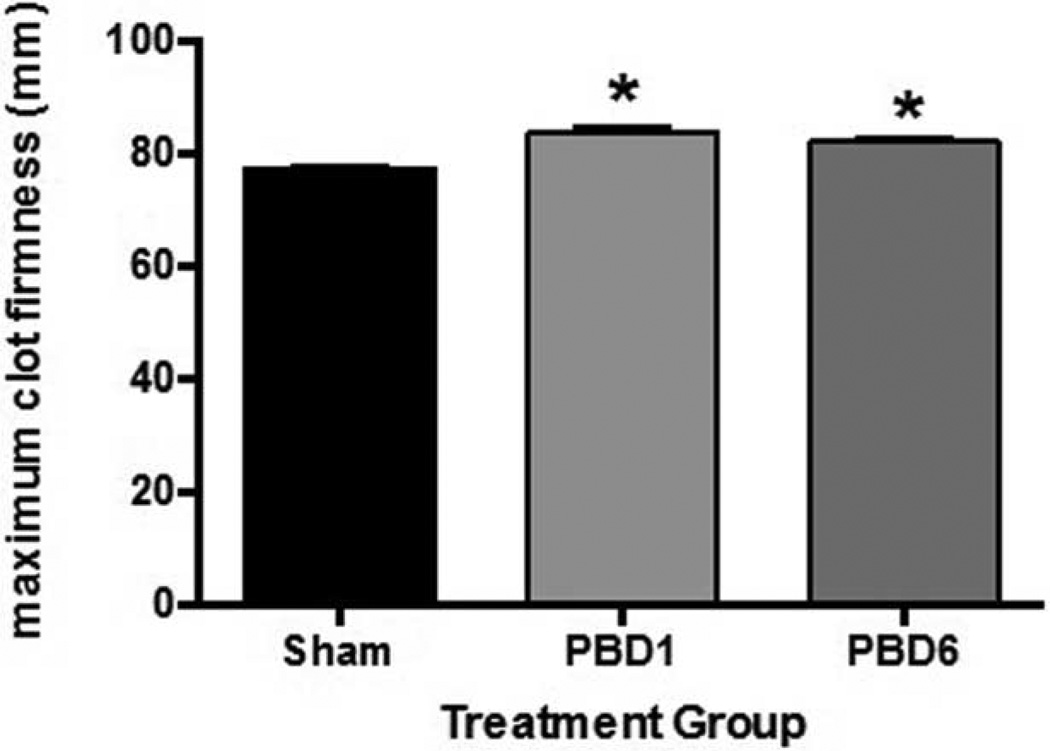
The development of a hypercoagulable state after burn injury is well documented in humans (2, 4). This hypercoagulable state has been demonstrated in various animal models (24); however, it has not yet been shown in a murine model. We therefore sought to develop a murine model of scald injury that resulted in a hypercoagulable state. We performed 28% TBSA scald injury to mice and analyzed whole blood collected temporally after injury. At both 1 and 6 days after injury, we found a significant increase in maximum clot strength on NATEM analysis in burn mice when compared with sham mice (Fig. 1).
Fig. 1. Increased clot strength is seen after scald injury.
Mice were subjected to scald injury or sham and whole blood collected temporally after injury for NATEM analysis. Sample size is 4–7 in each group. Significance was determined using ANOVA analysis with Tukey’s post-hoc test. *P < 0.01 compared with sham.
Platelet counts and platelet function are altered 1 day after scald injury
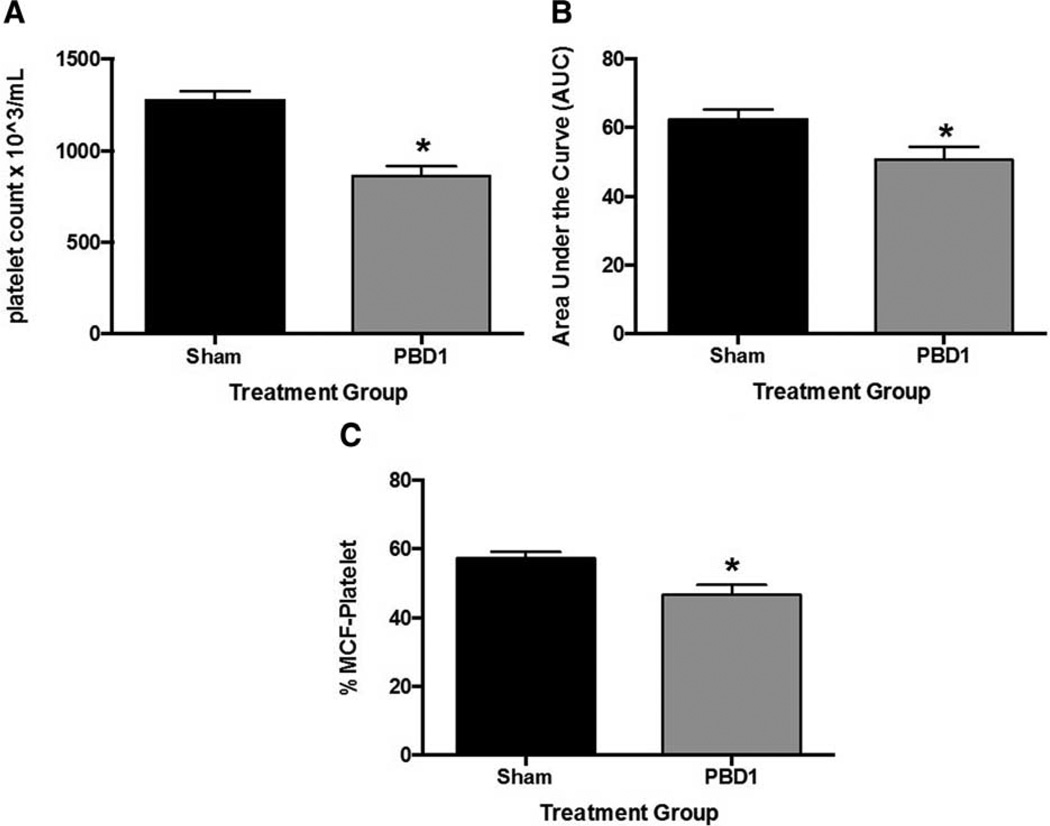
We next sought to focus on the coagulation changes seen on post-burn day one (PBD1) and to determine whether circulating platelets were impacted by scald injury. Whole blood platelet counts were significantly decreased in PBD1 mice compared with sham (Fig. 2A). Hematocrit and white blood cell counts were similar between scald and sham mice (data not shown). Further, when ADP-induced platelet function of sham and scald-injured mice was compared, there was a significant decrease in platelet activation following stimulation with this agonist (Fig. 2B). There was also a complementary decrease in the calculated platelet contribution to clot formation at this time point (Fig. 2C).
Fig. 2. Platelet counts and platelet function are altered 1 day after scald injury.
Mice were subjected to scald injury or sham and whole blood collected 1 day after injury. A, Platelet counts, B, ADP-induced platelet function, and C, platelet contribution to clot formation were then determined. Sample sizes are (A) n = 12–18, (B) n = 15–16, and (C) n = 6–10. Significance was determined using Student t test. *P < 0.05 compared with sham.
Circulating platelet-derived microparticles and microparticle procoagulant activity is increased 1 day after scald injury
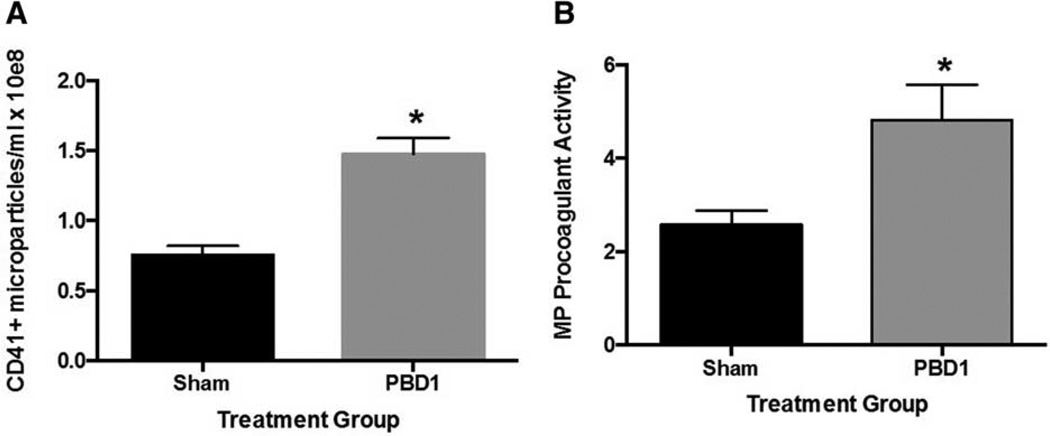
As previous studies have shown microparticles to be hypercoagulable (18–20), we next sought to determine whether MPs, and specifically PMPs, were contributing to coagulation changes seen after burn. We first enumerated circulating PMPs in whole blood. In contrast to the decrease in total serum microparticles, there was a significant increase in the concentration of PMPs in the blood of PBD1 mice compared with sham mice (Fig. 3A). Further, a significant increase in procoagulant activity of these MPs was observed at this timepoint (Fig. 3B).
Fig. 3. Circulating platelet-derived microparticles and microparticle procoagulant activity is increased 1 day after scald injury.
Mice were subjected to scald injury or sham and whole blood collected on day after injury. A, Circulating platelet-derived MP populations and B, MP procoagulant activity were then determined. Sample sizes are (A) n = 11 and (B) n = 6–10. Significance was determined using Student t test. *P < 0.05 compared with sham.
Plasma fibrinogen and von Willebrand factor levels are unchanged after scald injury
We next sought to determine whether other circulating coagulation factors could be contributing to the hypercoagulability seen after injury. As the independent contributions of fibrinogen and platelets to clot strength can alter NATEM tests, we measured plasma levels of both fibrinogen and von Willebrand factor (vWF). On PBD1, there were no changes in circulating fibrinogen (71.4 ng/mL sham vs. 46.3 ng/mL PBD1, P = 0.13) or vWF (406.5 pg/mL sham vs. 526.4 pg/mL PBD1, P = 0.54) in scald-injured mice when compared with sham mice.
Platelet counts and function are increased 6 days after scald injury
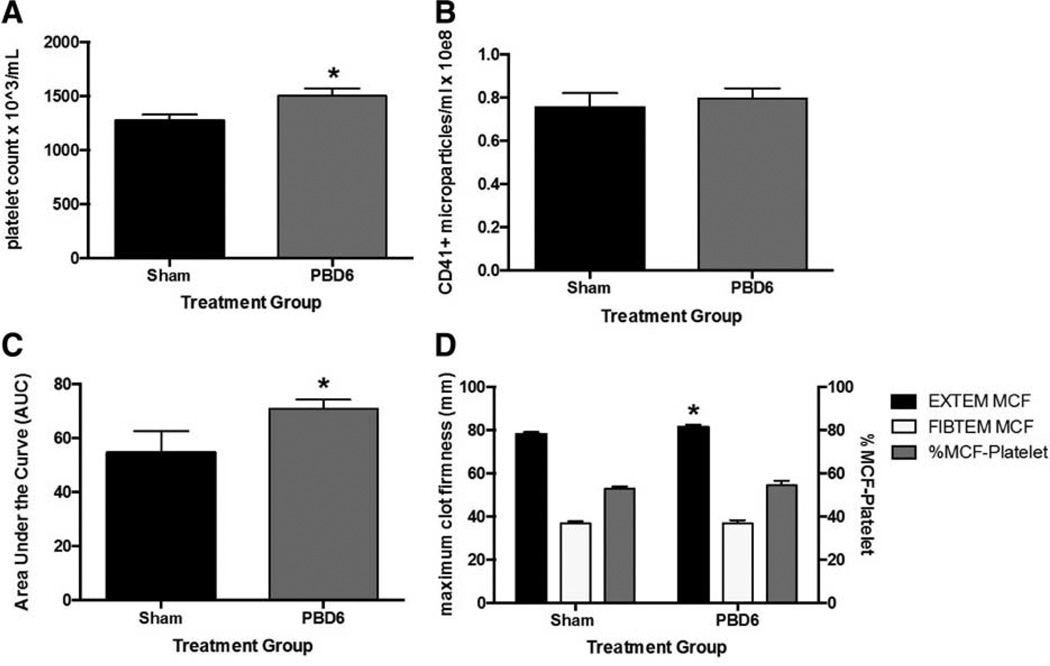
Six days after burn (PBD6), mice remained hypercoagulable as demonstrated in Figure 1. We therefore next investigated whether these coagulation changes were because of similar mechanisms as those seen on PBD1. In contrast to PBD1, when thrombocytopenia was observed, on PBD6 there was a significant increase in circulating platelet counts (Fig. 4A) with similar hematocrit and white blood cell counts compared with sham (data not shown). In addition, there was no change in PBD6 circulating PMP levels compared with sham (Fig. 4B). Further, on PBD6, a significant increase in ADP-induced platelet activation was demonstrated in scald-injured mice compared with sham mice (Fig. 4C). Finally, there was an increase in clot strength via the extrinsic pathway with no change in the relative platelet contribution to clot formation (Fig. 4D).
Fig. 4. Platelet counts and function are increased 6 days after scald injury.
Mice were subjected to scald injury or sham and whole blood collected 6 days after injury. A, Platelet counts, B, circulating platelet-derived MP populations, C, ADP-induced platelet function, and D, ROTEM analyses of EXTEM and FIBTEM tests with the calculated platelet contribution to clot formation were then determined. Sample sizes are (A) n = 12–18, (B) n = 20, (C) n = 5–10, and (D) n = 7–10. Significance was determined using Student t test. *P < 0.05 compared with sham.
DISCUSSION
In the current study, we demonstrated sustained posttraumatic hypercoagulability in a murine model of full thickness scald injury (Fig. 1). The contributing factors to this hypercoagulable state evolved over time. On examination of circulating platelet populations and platelet function 1 day after injury, we found decreased platelet counts, decreased platelet activation, and reduced platelet contribution to clot formation, suggesting that platelets were not responsible for the hypercoagulability seen at this time point (Fig. 2). There were also increased procoagulant PMPs in burn mice (Fig. 3) with no changes in circulating fibrinogen or vWF, supporting the hypothesis that PMPs are responsible for PBD1 hypercoagulability. By contrast, 6 days after injury, increased circulating platelet populations were observed with a similar number of circulating PMPs as sham mice (Fig. 4, A and B). Further, ADP-induced platelet activation was increased with a parallel increase in clot strength via the extrinsic pathway compared with sham mice. Altogether, this suggests that platelets, rather than PMPs, contribute to the hypercoagulable state on PBD6 (Fig. 4, C and D).
Our findings of hypercoagulability after scald injury in a severe burn model on PBD1 and PBD6 are consistent with previous human studies (3–5, 12) and animal models with similar trauma injuries (24, 25). Further, alterations in circulating platelet counts after injury, with an acute thrombocytopenia and subsequent recovery with thrombocytosis, are also well supported in both human (14, 15) and murine studies (16, 26, 27). Thromboelastometry has not been previously utilized in murine models of thermal injury; however, our results are consistent with increased clot strength seen in human studies (5, 12). Platelet function has also been shown to be altered after scald injury (26, 28); however, demonstration of changes in platelet aggregation over time in a murine scald injury is novel. Eurenius et al. (26) saw decreased platelet aggregation early after scald injury in rats, and hypothesized that this change in platelet function was secondary to factors extrinsic to platelets rather that because of the platelets themselves. They were unable to identify a specific factor; however, our study clearly shows PMPs are at least one factor contributing to these changes. The development of DIC has also been described in select burn populations (29); however, it is likely found in more severe burn injuries as human studies suggest that DIC mainly occurs in patients with greater than 40% TBSA burns, and may be in part because of aggressive crystalloid resuscitation (5). As our model was only a 28% TBSA burn without ongoing volume resuscitation, our finding of normal circulating fibrinogen levels would be expected.
This study is the first to assess platelet function, platelet contribution to clot formation, and the role of altered microparticle populations in these changes after scald injury. Although human and animal studies have consistently found that platelet populations are altered after injury (14–16, 26, 27), no previous models of scald injury have examined platelet function and shown temporal changes after scald injury. Although ROTEM changes have been previously described in other murine trauma models (5, 12), this is the first murine scald model that has demonstrated hypercoagulability on thromboelastometry. As previous human studies have shown that viscoelastic coagulation testing is a better predictor of resuscitation status and risk for VTE compared with PT/INR (30, 31), we believe this may be a more relevant parameter to study. Further, these results suggest that VTE prophylaxis may not be as straightforward as previously thought and that the anticoagulation and antiplatelet agents utilized may need to be tailored to the patient’s evolving state of hypercoagulability.
Understanding the mechanisms behind coagulation changes after burn is important as variable VTE rates (2) and use of anticoagulation (32) in this patient population are prevalent. The presence of hypercoagulability after burn is well established, and both the rate of VTE (11) and the need for increased chemo-prophylactic dosing (33, 34) are correlated with increased burn size. Further, post-mortem human studies show microvascular thrombosis in multiple organs, specifically lung and renal tissues, leading to multiple organ dysfunction syndrome (MODS) and death (35, 36). As MODS is the major cause of death in burn patients (37), preventing microvascular thrombosis in these patients may be life-saving. Further, although rates of VTE are heavily debated, autopsies have shown pulmonary embolism (PE) rates up to 35% (38) and PE-related mortality rates as high as 40% (11). Microparticles are known contributors to hypercoagulability in various disease processes (18, 19, 39), and previous research has demonstrated that PMPs are associated with increased procoagulant activity in fresh frozen plasma (40) and are associated with hypercoagulability in other injury models (22). The current study is the first to highlight the link between PMPs and coagulation in burns and future burn care may require targeted anticoagulation strategies based on the time course after injury, including use of agents that target platelets and PMPs specifically, to more effectively prevent thrombosis-related morbidity and mortality.
Although this study establishes a novel, murine model of hypercoagulability after scald injury with associated changes in platelet function, there are some limitations to our study. First, we utilized a single scald model with 28% TBSA burns and therefore these findings may not be applicable to minor burns or non-thermal burns. We also focused on 1 and 6 days after burn, and have yet to determine when the mechanistic changes driving hypercoagulability are occurring. In addition, we did not perform in vivo studies to further demonstrate the impact of altered microparticle populations from scald-injured mice on coagulation in naive mice. Further, whereas we were able to show alterations to platelet function, we have yet to determine whether microparticles directly impact platelet function early after burn and identify the mechanism behind increased platelet functionality seen 6 days after burn. Future studies will focus on elucidating these mechanisms, as they will allow us to better understand and manage the coagulation changes seen after scald injury.
CONCLUSION
In conclusion, acute hypercoagulability after scald injury persists 1 week after injury in a murine burn model. There is a clear temporal change in the mechanisms that lead to this hypercoagulability. Platelet-derived MPs are likely responsible for changes seen on PBD1 and platelet activation is key to the altered clotting mechanisms seen on PBD6. Better understanding of these mechanisms will allow us to target thrombosis prophylaxis to the underlying cause of hypercoagulability after injury and prevent VTE-associated morbidity and mortality.
Footnotes
None of the above authors has any disclosures.
The authors report no conflicts of interest.
REFERENCES
- 1.Lavrentieva A, Kontakiotis T, Bitzani M, Papaioannou-Gaki G, Parlapani A, Thomareis O, Tsotsolis N, Giala MA. Early coagulation disorders after severe burn injury: impact on mortality. Intensive Care Med. 2008;34:700–706. doi: 10.1007/s00134-007-0976-5. [DOI] [PubMed] [Google Scholar]
- 2.Meizoso JP, Ray JJ, Allen CJ, Van Haren RM, Ruiz G, Namias N, Schulman CI, Pizano LR, Proctor KG. Hypercoagulability and venous thromboembolism in burn patients. Semin Thromb Hemost. 2015;41:43–48. doi: 10.1055/s-0034-1398380. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Avello A, Lorente JA, Cesar-Perez J, Garcia-Frade LJ, Alvarado R, Arevalo JM, Navarro JL, Esteban A. Degree of hypercoagulability and hyperfibrinolysis is related to organ failure and prognosis after burn trauma. Thromb Res. 1998;89:59–64. doi: 10.1016/s0049-3848(97)00291-0. [DOI] [PubMed] [Google Scholar]
- 4.Van Haren RM, Thorson CM, Valle EJ, Busko AM, Guarch GA, Andrews DM, Pizano LR, Schulman CI, Namias N, Proctor KG. Hypercoagulability after burn injury. J Trauma Acute Care Surg. 2013;75:37–43. doi: 10.1097/TA.0b013e3182984911. [DOI] [PubMed] [Google Scholar]
- 5.King DR, Namias N, Andrews DM. Coagulation abnormalities following thermal injury. Blood Coagul Fibrinolysis. 2010;21:666–669. doi: 10.1097/MBC.0b013e32833ceb08. [DOI] [PubMed] [Google Scholar]
- 6.Sherren PB, Hussey J, Martin R, Kundishora T, Parker M, Emerson B. Acute burn induced coagulopathy. Burns. 2013;39:1157–1161. doi: 10.1016/j.burns.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Whittaker B, Christiaans SC, Altice JL, Chen MK, Bartolucci AA, Morgan CJ, Kerby JD, Pittet JF. Early coagulopathy is an independent predictor of mortality in children after severe trauma. Shock. 2013;39:421–426. doi: 10.1097/SHK.0b013e31828e08cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rue LW, Cioffi WG, Rush R, McManus WF, Pruitt BA. Thromboembolic complications in thermally injured patients. World J Surg. 1992;16:1151–1154. doi: 10.1007/BF02067085. [DOI] [PubMed] [Google Scholar]
- 9.Fecher AM, O’Mara MS, Goldfarb IW, Slater H, Garvin R, Birdas TJ, Caushaj PF. Analysis of deep vein thrombosis in burn patients. Burns. 2004;30:591–593. doi: 10.1016/j.burns.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 10.Barret JP, Dziewulski PG. Complications of the hypercoagulable status in burn injury. Burns. 2006;32:1005–1008. doi: 10.1016/j.burns.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 11.Harrington DT, Mozingo DW, Cancio L, Bird P, Jordan B, Goodwin CW. Thermally injured patients are at significant risk for thromboembolic complications. J Trauma. 2001;50:495–499. doi: 10.1097/00005373-200103000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Schaden E, Hoerburger D, Hacker S, Kraincuk P, Baron DM, Kozek-Langenecker S. Fibrinogen function after severe burn injury. Burns. 2012;38:77–82. doi: 10.1016/j.burns.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Niedermayr M, Schramm W, Kamolz L, Andel D, Romer W, Hoerauf K, Zimpfer M, Andel H. Antithrombin deficiency and its relationship to severe burns. Burns. 2007;33:173–178. doi: 10.1016/j.burns.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Warner P, Fields AL, Braun LC, James LE, Bailey JK, Yakuboff KP, Kagan RJ. Thrombocytopenia in the pediatric burn patient. J Burn Care Res. 2011;32:410–414. doi: 10.1097/BCR.0b013e318217f91b. [DOI] [PubMed] [Google Scholar]
- 15.Marck RE, Montagne HL, Tuinebreijer WE, Breederveld RS. Time course of thrombocytes in burn patients and its predictive value for outcome. Burns. 2013;39:714–722. doi: 10.1016/j.burns.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Fujimi S, MacConmara MP, Maung AA, Zang Y, Mannick JA, Lederer JA, Lapchak PH. Platelet depletion in mice increases mortality after thermal injury. Blood. 2006;107:4399–4406. doi: 10.1182/blood-2005-09-3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo F, Wang X, Huan J, Liang X, Chen B, Tang J, Gao C. Association of platelet counts decline and mortality in severely burnt patients. J Crit Care. 2012;27:529. doi: 10.1016/j.jcrc.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Park MS, Owen BA, Ballinger BA, Sarr MG, Schiller HJ, Zietlow SP, Jenkins DH, Ereth MH, Owen WG, Heit JA. Quantification of hypercoagulable state after blunt trauma: microparticle and thrombin generation are increased relative to injury severity, while standard markers are not. Surgery. 2012;151:831–836. doi: 10.1016/j.surg.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matijevic N, Wang YW, Wade CE, Holcomb JB, Cotton BA, Schreiber MA, Muskat P, Fox EE, Del Junco DJ, Cardenas JC, et al. Cellular microparticle and thrombogram phenotypes in the Prospective Observational Multicenter Major Trauma Transfusion (PROMMTT) study: correlation with coagulopathy. Thromb Res. 2014;134:652–658. doi: 10.1016/j.thromres.2014.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kornblith LZ, Kutcher ME, Redick BJ, Calfee CS, Vilardi RF, Cohen MJ. Fibrinogen and platelet contributions to clot formation: implications for trauma resuscitation and thromboprophylaxis. J Trauma Acute Care Surg. 2014;76:255–256. doi: 10.1097/TA.0000000000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tschop J, Martignoni A, Reid MD, Adediran SG, Gardner J, Noel GJ, Ogle CK, Neely AN, Caldwell CC. Differential immunological phenotypes are exhibited after scald and flame burns. Shock. 2009;31:157–163. doi: 10.1097/SHK.0b013e31817fbf4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Midura EF, Jernigan PL, Kuethe JW, Friend LA, Veile R, Makley AT, Caldwell CC, Goodman MD. Microparticles impact coagulation after traumatic brain injury. J Surg Res. 2015;197:25–31. doi: 10.1016/j.jss.2015.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prakash PS, Caldwell CC, Lentsch AB, Pritts TA, Robinson BR. Human microparticles generated during sepsis in patients with critical illness are neutrophil-derived and modulate the immune response. J Trauma Acute Care Surg. 2012;73:401–406. doi: 10.1097/TA.0b013e31825a776d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chai JK, Liu W, Deng HP, Cai JH, Hu QG, Zou XF, Shen CA, Yin HN, Han YF, Zhang XB, et al. A novel model of burn-blast combined injury and its phasic changes of blood coagulation in rats. Shock. 2013;40:297–302. doi: 10.1097/SHK.0b013e3182837831. [DOI] [PubMed] [Google Scholar]
- 25.Prat NJ, Montgomery R, Cap AP, Dubick MA, Sarron JC, Destombe C, May P, Magnan P. Comprehensive evaluation of coagulation in swine subjected to isolated primary blast injury. Shock. 2015;43:598–603. doi: 10.1097/SHK.0000000000000346. [DOI] [PubMed] [Google Scholar]
- 26.Eurenius E, Rothenberg J. Platelet aggregation after thermal injury. J Lab Clin Med. 1974;83:355–363. [PubMed] [Google Scholar]
- 27.Bartlett RH, Fong SW, Marrujo G, Hardeman J, Anderson W. Coagulation and platelet changes after thermal injury in man. Burns. 1979;7:370–377. [Google Scholar]
- 28.Levin GY, Egorihina MN. The role of fibrinogen in aggregation of platelets in burn injury. Burns. 2010;36:806–810. doi: 10.1016/j.burns.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Barret JP, Gomez PA. Disseminated intravascular coagulation: a rare entity in burn injury. Burns. 2005;31:354–357. doi: 10.1016/j.burns.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Park MS, Martini WZ, Dubick MA, Salinas J, Butenas S, Kheirabadi BS, Pusateri AE, Vos JA, Guymon CH, Wolf SE, et al. Thromboelastography as a better indicator of hypercoagulable state after injury than prothrombin time or activated partial thromboplastin time. J Trauma. 2009;67:266–275. doi: 10.1097/TA.0b013e3181ae6f1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kashuk JL, Moore EE, Sabel A, Barnett C, Haenel J, Le T, Pezold M, Lawrence J, Biffl WL, Cothren CC, et al. Rapid thrombelastography (r-TEG) identifies hypercoagulability and predicts thromboembolic events in surgical patients. Surgery. 2009;146:764–772. doi: 10.1016/j.surg.2009.06.054. [DOI] [PubMed] [Google Scholar]
- 32.Ferguson RE, Critchfield A, Leclaire A, Ajkay N, Vasconez HC. Current practice of thromboprophylaxis in the burn population: a survey study of 84 US burn centers. Burns. 2005;31:964–966. doi: 10.1016/j.burns.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 33.Faraklas I, Ghanem M, Brown A, Cochran A. Evaluation of an enoxaparin dosing calculator using burn size and weight. J Burn Care Res. 2013;34:621–627. doi: 10.1097/BCR.0b013e3182a2a855. [DOI] [PubMed] [Google Scholar]
- 34.Lin H, Faraklas I, Cochran A, Saffle J. Enoxaparin and antifactor Xa levels in acute burn patients. J Burn Care Res. 2011;32:1–5. doi: 10.1097/BCR.0b013e318204b346. [DOI] [PubMed] [Google Scholar]
- 35.Foley FD, Moncrief JA, Mason AD. Pathology of the lung in fatally burned patints. Ann Surg. 1968;167:251–264. doi: 10.1097/00000658-196802000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang YS, Li AO, Yang ZC. A prospective clinical study on the pathogenesis of multiple organ failure in severely burned patients. Burns. 1992;18:30–34. doi: 10.1016/0305-4179(92)90116-c. [DOI] [PubMed] [Google Scholar]
- 37.Miller SF, Bessey PQ, Schurr MJ, Browning SM, Jeng JC, Caruso DM, Gomez M, Latenser BA, Lentz CW, Saffle JR, et al. National Burn Repository 2005: a ten-year review. J Burn Care Res. 2006;27:411–436. doi: 10.1097/01.BCR.0000226260.17523.22. [DOI] [PubMed] [Google Scholar]
- 38.Coleman JB, Chang FC. Pulmonary embolism. An unrecognized event in severely burned patients. Am J Surg. 1975;130:697–699. doi: 10.1016/0002-9610(75)90423-7. [DOI] [PubMed] [Google Scholar]
- 39.Zhou L, Qi XL, Xu MX, Mao Y, Liu ML, Song HM. Microparticles: new light shed on the understanding of venous thromboembolism. Acta Pharmacol Sin. 2014;35:1103–1110. doi: 10.1038/aps.2014.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matijevic N, Wang YW, Kostousov V, Wade CE, Vijayan KV, Holcomb JB. Decline in platelet microparticles contributes to reduced hemostatic potential of stored plasma. Thromb Res. 2011;128:35–41. doi: 10.1016/j.thromres.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]