Abstract
Frizzled proteins are the principal receptors for the Wnt family of ligands. They mediate canonical Wnt signaling together with Lrp5 and Lrp6 coreceptors. In conjunction with Celsr, Vangl, and a small number of additional membrane and membrane-associated proteins, they also play a central role in tissue polarity/planar cell polarity (PCP) signaling. Targeted mutations in 9 of the 10 mammalian Frizzled genes have revealed their roles in an extraordinarily diverse set of developmental and homeostatic processes, including morphogenetic movements responsible for palate, ventricular septum, ocular furrow, and neural tube closure; survival of thalamic neurons; bone formation; central nervous system (CNS) angiogenesis and blood–brain barrier formation and maintenance; and a wide variety of processes that orient subcellular, cellular, and multicellular structures relative to the body axes. The last group likely reflects the mammalian equivalent of tissue polarity/PCP signaling, as defined in Drosophila, and it includes CNS axon guidance, hair follicle and tongue papilla orientation, and inner ear sensory hair bundle orientation. Frizzled receptors are ubiquitous among multicellular animals and, with other signaling molecules, they very likely evolved to permit the development of the complex tissue architectures that provide multicellular animals with their enormous selective advantage.
1. INTRODUCTION
Frizzled proteins are the principal receptors for the Wnt family of signaling molecules, and they are found throughout the animal kingdom, including in the most primitive metazoa, but they are not present in plants or in simpler (single cell) eukaryotes, such as yeast (Schenkelaars, Fierro-Constain, Renard, Hill, & Borchiellini, 2015). Frizzled proteins share a common architecture: a conserved extracellular cysteine-rich domain (CRD) is followed by a domain with seven presumptive transmembrane segments. The genetic dissection of Frizzled function began in Drosophila, with the characterization of the frizzled (fz) phenotype by Gubb and Garcia-Bellido (1982). Loss-of-function mutations in fz lead to a tissue polarity phenotype, defined as a misorientation of surface structures, such as cuticular bristles and wing hairs, which normally exhibit a precise orientation relative to the body axes. This pathway is generally referred to by the not entirely accurate designation “planar cell polarity” (PCP).
The Drosophila fz gene was isolated by Vinson, Conover, and Adler (1989), and the first two vertebrate Frizzled homologues were identified several years later (Chan et al., 1992). A search for additional vertebrate Frizzled family members using degenerate PCR identified eight family members in mammals, as well as multiple family members in birds and fish (Wang et al., 1996). Two additional mammalian Frizzled genes were identified subsequently, bringing the total to 10 (Koike et al., 1999; Wang et al., 1997). The large number of vertebrate Frizzled genes together with their receptor-like structure led Wang et al. (1996) to suggest that “If Frizzled proteins act as receptors, that would imply the existence of a corresponding family of ligands. … Currently the only family of ligands known to be of this size and for which no receptors have been identified are the Wnt proteins. The availability of a large number of Frizzled genes should facilitate a biochemical test of the possibility that these two families of proteins interact directly.” Within a year, that suggestion was confirmed with the demonstration that Drosophila Wingless binds to and promotes beta-catenin stabilization via Frizzled2 (Bhanot et al., 1996). These experiments also identified the Frizzled CRD as the site of Wnt binding (Bhanot et al., 1996; Hsieh, Rattner, Smallwood, & Nathans, 1999). The recently determined structure of a Wnt–Frizzled CRD complex shows an unusual ligand–receptor interaction characterized by a relatively small protein–protein interface and a relatively large interface between the CRD and a lipid that is covalently attached to the Wnt (Janda, Waghray, Levin, Thomas, & Garcia, 2012).
The identification of Frizzleds as Wnt receptors implied that Frizzleds act in at least two distinct signaling pathways: tissue polarity/PCP signaling (which may or may not involve a Wnt ligand) and canonical Wnt signaling. The former pathway appears to involve reorganization of the cytoskeleton and is characterized by a small set of asymmetrically localized plasma membrane proteins (including Frizzled) and membrane-associated cytosolic proteins (Goodrich & Strutt, 2011). The latter pathway involves Wnt–Frizzled-dependent disinhibition of a single-pass transmembrane coreceptor (Lrp5/Lrp6 in mammals; arrow in Drosophila) leading to inhibition of beta-catenin phosphorylation and proteolysis, with a resultant migration of beta-catenin into the nucleus to regulate transcription in combination with Lef/Tcf transcription factors (MacDonald, Tamai, & He, 2009; Nusse, 2012). A third pathway, the Wnt-calcium pathway, is less well defined and has been suggested to involve a G-protein-based transduction system (Wang & Malbon, 2003).
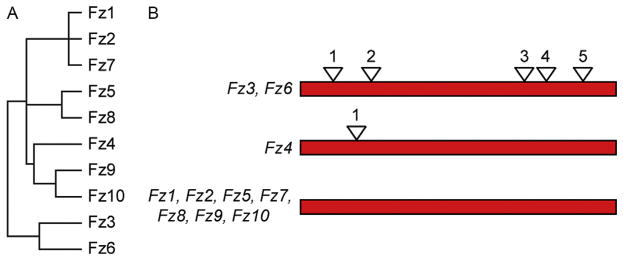
In this review, we focus on the roles of mammalian Frizzled genes in vivo, which have been largely defined by studying the effects of targeted mutations in mice. As seen in Fig. 1, a comparison of the amino acid sequences and intron–exon structures among the 10 mammalian Frizzled family members reveals branches consisting of Fz1, Fz2, and Fz7 (subfamily 1); Fz5 and Fz8 (subfamily 2); Fz9 and Fz10 (subfamily 3); Fz4 (subfamily 4); and Fz3 and Fz6 (subfamily 5, the most distant branch). Substantial genetic redundancy has been observed between pairs of Frizzled genes within subfamilies 1, 2, and 5, but, thus far, there appears to be far less redundancy between Frizzled genes in different subfamilies. However, these data should be interpreted cautiously because the redundancy data are still incomplete: many of the 45 possible pairwise combinations of Frizzled gene knockouts—especially with Frizzleds from different subfamilies—have not been tested. Moreover, in many tissues, there is overlapping expression of multiple Frizzleds, as seen, for example, in the spiral ganglion in the inner ear (Shah, Kang, Christensen, Feng, & Kollmar, 2009), so that functional redundancy may involve more than two genes. The patterns of sequence relatedness also correlate with function: Fz3 and Fz6 appear to be devoted largely or exclusively to tissue polarity signaling, whereas Fz4 is devoted largely or exclusively to canonical Wnt signaling. Some mammalian Frizzleds could signal via more than one pathway, as shown for Drosophila Frizzled, which signals through both the canonical Wnt and PCP pathways (Bhanot et al., 1999; Bhat, 1998; Müller et al., 1999).
Figure 1.
Evolutionary relationships among mammalian Frizzled proteins and genes. (A) Dendrogram showing the relatedness of the 10 mammalian Frizzled amino acid sequences. (B) Intron–exon structures of mammalian Frizzled genes, with coding regions depicted schematically as the same length. Only Fz3, Fz4, and Fz6 have introns within the coding region. The five coding introns in the Fz3 and Fz6 genes are located at identical positions. From Hua, Chang, Wang, Smallwood, and Nathans (2014).
In the text that follows, we have organized the descriptions of Frizzled gene function by subfamily. We note that most of the phenotypic analyses in mice were performed on a mixed genetic background.
2. FRIZZLED1, FRIZZLED2, AND FRIZZLED7
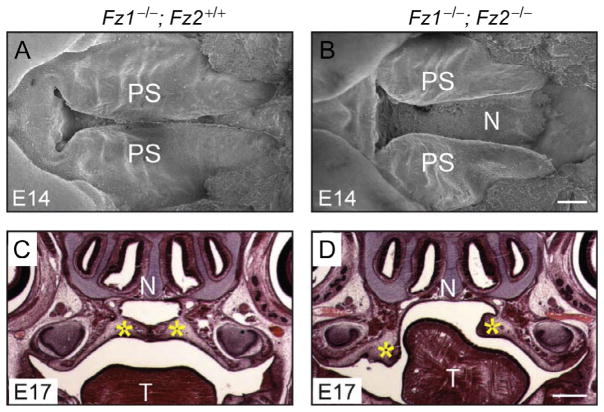
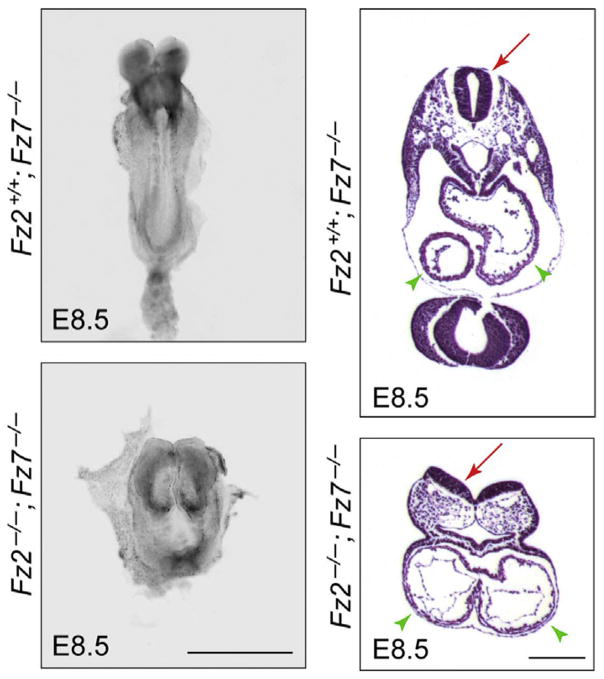
Fz1−/− mice show no apparent phenotype, whereas loss of Fz2 leads to cleft palate and neonatal lethality in ~50% of mice and runting associated with reduced olfactory function in the other ~50% (Yu et al., 2010). Approximately 15% of Fz7−/− fetuses have a ventricular septal defect (VSD), a common cardiac anomaly that results in mixing of blood between the left and the right ventricular chambers, while the remaining ~85% of Fz7−/− mice have no apparent phenotype except for a kinked tail (Yu, Ye, Guo, & Nathans, 2012). Interestingly, a variety of additional phenotypes appear when Fz1, Fz2, and Fz7 mutations are combined (Yu et al., 2010, 2012). 100% of Fz1−/−;Fz2−/− fetuses have a cleft palate (Fig. 2), and approximately two-thirds of Fz1−/−;Fz2−/− fetuses also have cardiac defects—either an isolated VSD or a combination of VSD and double outlet right ventricle, a cardiac anomaly in which the aorta is positioned partially over the right ventricular outflow tract. Fz1−/−;Fz7−/− mice have no additional defects beyond the Fz7−/− phenotype except for a gray coat color, suggesting a defect in melanocyte migration and/or development. In contrast, Fz2−/−;Fz7−/− embryos at embryonic day (E)8.5 show a severe loss of convergent extension, fail to close the neural tube, and fail to develop cardiac asymmetry (Fig. 3).
Figure 2.
Palate closure defect in Fz1−/−;Fz2−/− fetuses. (A and B) Scanning electron microscopy of the palate at E14. PS, palatal shelf. (C and D) Coronal section through the head at E17, stained with hematoxylin and eosin. N, nasal septum; T, tongue; Yellow asterisks, palatal shelves. Scale bars: B, 200 μm; D, 500 μm. From Yu et al. (2010).
Figure 3.
Convergent extension, neural tube closure, and cardiac asymmetry defects in Fz2−/−;Fz7−/− embryos. E8.5 embryos are shown intact in a dorsal view (left) and in paraffin sections stained with hematoxylin and eosin (right). Left panels, the Fz2−/−;Fz7−/− embryo is shorter and wider than the Fz2+/+;Fz7−/− control due to a failure of convergent extension. Right panels, in cross section, the Fz2−/−;Fz7−/− embryo shows an open neural tube (red arrows) and symmetric cardiac chambers (green arrowheads). The Fz2+/+;Fz7−/− embryo is phenotypically WT. Scale bars: left panels, 1 mm; right panels, 200 μm. From Yu et al. (2012).
Given that Frizzleds control several signaling pathways (PCP, canonical Wnt, and Wnt-calcium signaling), it is of interest to explore the relationship between signaling pathways affected and developmental phenotypes observed. To this end, Yu et al. (2010, 2012) examined genetic interactions between mutations in Fz1, Fz2, and Fz7 and in known PCP genes, such as Vangl2 [one of the two mammalian homologues of the Drosophila core PCP gene Van Gogh/Strabismus (Vang/Stbm)]. When various combinations of Fz1, Fz2, or Fz7 knockout (KO) alleles were examined in combination with one copy of the semidominant Looptail allele of Vangl2 (Vangl2Lp/+), there was an enhanced frequency of neural tube closure and cleft palate defects. Similarly, the frequency of cardiac defects (mostly VSDs) was enhanced when the Fz2+/−;Fz7−/− genotype was combined with one mutant copy of Wnt3a (canonical signaling), Wnt11 (noncanonical signaling), or Dvl3 (both pathways). While these data show that Fz1, Fz2, and Fz7 synergize with other canonical and noncanonical pathway components, they do not definitively indicate which pathway(s) is/are affected by the different Frizzled mutations. Palate, ventricular septum, and neural tube closures are known to involve multiple signaling pathways (Biggs, Goudy, & Dunnwald, 2015), and it is therefore plausible that defects in more than one Frizzled-based pathway combine to produce a developmental defect.
In humans, defects in tissue closure processes represent some of the most common congenital anatomic anomalies. Defects in closing the ventricular septum, palate, and neural tube occur, respectively, in ~0.4%, ~0.1%, and ~0.1% of live births, and a defect in closing the ventral urethra occurs in ~0.4% of newborn males (Au, Ashley-Koch, & Northrup, 2010; CDC, 1997; Dolk, Loane, & Garne, 2010). Congenital defects in uterine ligament, ocular furrow, and diaphragm closure are less common. All of these processes involve the directed migration and fusion of tissues, so it is not implausible that they might utilize similar or overlapping cell signaling strategies. In mice, defects in two or more canonical Wnt and/or PCP signaling components from among the Celsr, Dvl, Frizzled, Lrp, Vangl, and Wnt families have been implicated in palate, ventricular septum, neural tube, and ocular furrow closure defects (summarized in Yu et al., 2010). In humans, neural tube closure defects are associated with sequence variants in VANGL1, VANGL2, CELSR1, and FZD6 in a small fraction of cases (Allache, De Marco, Merello, Capra, & Kibar, 2012; De Marco et al., 2012; Kibar et al., 2009, 2011, 2007). These data suggest that deleterious sequence variants in genes that either code for or control the expression of canonical Wnt and/or PCP signaling components may represent genetic risk factors for a wide variety of tissue closure defects in humans.
3. FRIZZLED5 AND FRIZZLED8
Homozygous deletion of Fz5 leads to embryonic lethality at midgestation secondary to placental insufficiency (Ishikawa et al., 2001). When the placental phenotype is bypassed by conditionally ablating Fz5 in embryonic but not extraembryonic tissues (Fz5CKO/CKO;Sox2-Cre), the resulting mice survive to adulthood and exhibit interesting phenotypes in the eye and the thalamus (CKO, conditional knockout). The ocular phenotype is ~50% penetrant and is characterized by increased cell death in the ventral retina and failure to close the ventral furrow (coloboma), an excess of mesenchymal cells in the vitreous cavity, an excess of astrocytes on the vitreal face of the retina, and persistence of the vascular network within the vitreous cavity (the hyaloid vasculature) into adulthood (Liu & Nathans, 2008). The same ocular phenotype is seen when Fz5 is selectively ablated in the retina and in retinal astrocytes but not in vascular endothelial cells (ECs; Zhang, Fuhrmann, & Vetter, 2008). In the normal course of ocular development, the hyaloid vasculature regresses as the retinal vasculature matures, leaving an optically clear path between the lens and the retina. In humans, persistence of a hyaloid vascular remnant is a relatively common ocular anomaly (Duke-Elder, 1964; Goldberg, 1997).
In the brain, Fz5 is expressed selectively in neurons in the parafascicular nucleus (PFN) of the thalamus, and Fz5CKO/CKO;Sox2-Cre mice show a progressive loss of PFN neurons over the first several months of postnatal life (Liu, Wang, Smallwood, & Nathans, 2008). Genetic mosaic experiments in which Fz5−/− cells are marked by expression of a reporter show that this loss is cell autonomous. Numerous changes in gene expression in PFN neurons precede neuronal loss, including downregulation of the genes coding for beta-catenin, Wnt4, and Wnt9b, suggesting that a Fz5-dependent positive feedback loop maintains canonical Wnt signaling in the PFN. The physiological role of the PFN is not well understood, but the work of Liu et al. (2008) implicates the PFN in sensorimotor function because loss of PFN neurons in Fz5CKO/CKO;Sox2-Cre mice is accompanied by impaired motor coordination.
Fz8−/− mice have no apparent phenotype. However, loss of both Fz8 and Fz5 in embryonic tissues leads to mid-gestational lethality, and loss of one Fz8 allele in combination with loss of Fz5 in embryonic tissues leads to 100% penetrance and greater severity of the Fz5 ocular phenotypes and to a reduction in retinal progenitor proliferation (Liu, Bakeri, Li, & Swaroop, 2012). Fz8 also exhibits a genetic interaction Fz4 in the with context of kidney development (Ye, Wang, Rattner, & Nathans, 2011). Fz4−/−;Fz8−/− fetuses show reduced growth of ureteric buds and smaller kidneys, a phenotype similar to that described earlier for Wnt11−/− mice (Chi et al., 2004; Majumdar, Vainio, Kispert, McMahon, & McMahon, 2003). The genetic mosaic experiments of Ye, Wang, et al. (2011) revealed strong competition between cells within the ureteric bud, which leads to a dramatic underrepresentation of Fz4−/− or Fz4−/−;Fz8−/− cells relative to wild-type cells when both were initially present in roughly equal numbers. Intriguingly, in Fz4−/−;Fz8−/− embryos, in which all cells share the same genotype, the reduction in kidney size is more modest than would be predicted from the almost complete elimination of mutant cells in the mosaic kidney. This last observation suggests the possibility of (1) a feedback signal that enhances cell proliferation and/or survival when overall kidney growth is reduced and/or (2) an interaction between WT and mutant cells that selectively eliminates mutant cells.
4. FRIZZLED9 AND FRIZZLED10
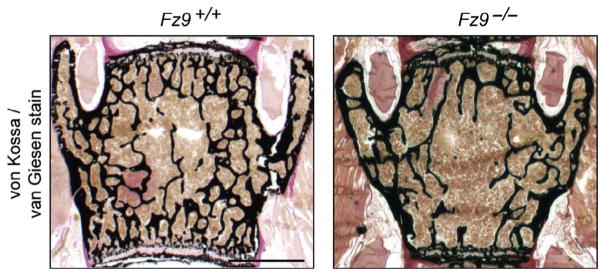
Fz9 has long intrigued human geneticists because it is one of ~20 genes in the chromosome 7 region that is present in a hemizygous state in Williams–Beuren syndrome, a complex multisystem disorder (OMIM 194050). Although Fz9−/− mice are viable and fertile, in-depth studies by three research groups have identified: (1) a subtle abnormality in B-cell development (Ranheim et al., 2005), (2) an increase in cell death in the dentate gyrus and a defect in visuo-spatial learning (Zhao et al., 2005), and (3) reduced bone mass and a reduction in newly formed bone in response to fractures, due to impaired osteoblast function (Fig. 4; Albers et al., 2011; Heilmann et al., 2013). Curiously, the bone phenotypes do not appear to be associated with alterations in canonical Wnt signaling, as judged by a comparison of the transcriptomes of Fz9−/− and Fz9+/+ osteoblasts, a cell type in which transcriptome changes in response to canonical Wnt signaling are well defined. This finding contrasts with the well-established role of Lrp5-dependent canonical Wnt signaling in promoting bone formation in mice and humans (Baron & Kneissel, 2013; Joeng, Schumacher, Zylstra-Diegel, Long, & Williams, 2011). Albers et al. (2011) have provided evidence that, in osteoblasts, Fz9 responds to Wnt5a and acts through a noncanonical pathway to control a distinct set of transcripts, including several coding for chemokines.
Figure 4.
Reduced bone density in Fz9−/− mice. The vertebral body shows reduced bone density in a 52-week-old Fz9−/− mouse (right) compared to an age- and sex-matched control (left). Scale bar, 250 μm. From Albers et al. (2011).
Fz10 is the most enigmatic of the Frizzled family members. The Fz10 gene is expressed widely in the CNS, including in multiple thalamic nuclei (Zhao, Guan, & Pleasure, 2006), but no Fz10 loss-of-function phenotypes have been reported thus far.
5. FRIZZLED4
Among the Frizzleds that are known or suspected to activate the canonical Wnt pathway, Fz4 is the best understood in terms of its biological function, its interactions with ligands and other receptor components, and its role in human disease. Fz4−/− mice exhibit a severe defect in retinal vascularization, progressive hearing loss, and a slowly progressive cerebellar degeneration that is associated with loss of the blood–brain barrier (BBB) in the cerebellum (Wang, Huso, Cahill, Ryugo, & Nathans, 2001; Wang et al., 2012; Xu et al., 2004; Ye et al., 2009). In humans, heterozygosity for loss-of-function mutations in FZD4 leads to a variable degree of retinal hypovascularization, a syndrome referred to as familial exudative vitreoretinopathy (FEVR; Nikopoulos, Venselaar, et al., 2010; Qin et al., 2005; Robitaille et al., 2002, 2011; Salvo et al., 2015; Toomes et al., 2004).
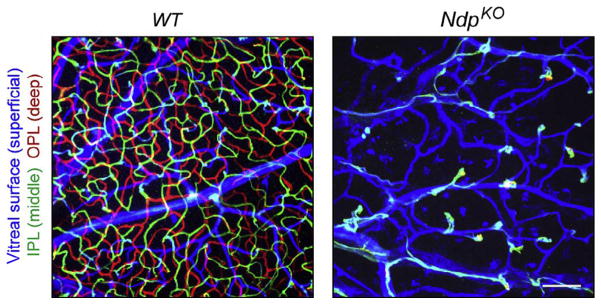
In WT mice during the first week of postnatal life, ECs emerge from the optic disc and migrate radially outward across the vitreal face of the retina to form a superficial vascular plexus. During the second postnatal week, ECs migrate vertically into the retina and form two additional tiers of capillaries, one immediately proximal and the other immediately distal to the central layer of neuronal cell bodies (the inner nuclear layer; Fruttiger, 2007). The mature retinal vasculature in mice, as in humans, consists of these three tiers of vessels. In Fz4−/− mice, EC invasion into the retina is aborted after only a short distance, resulting in a complete absence of the two intraretinal capillary beds.
A critical clue to the biochemical mechanism of Fz4 action came from studies of Norrie disease, an X-linked disorder characterized by congenital blindness and slowly progressive hearing loss (Berger & Ropers, 2001). The gene responsible for Norrie disease, NDP, codes for a distant relative of the transforming growth factor beta (TGF-beta) family, a dimeric cysteine knot protein with no sequence similarity to Wnts (Berger et al., 1992; Chen et al., 1992). Strikingly, NdpKO mice (either Ndp−/− females or Ndp−/Y males) exhibit exactly the same retinal phenotype as Fz4−/− mice (Fig. 5), providing strong evidence that the congenital blindness of Norrie disease patients most likely reflects a severe retinal hypovascularization (Luhmann et al., 2005; Rehm et al., 2002). This identity in retinal vascular phenotypes suggested that Norrin (the protein product of the Ndp gene) might be a ligand for Fz4. Indeed, this is the case: dimeric Norrin binds a pair of Fz4 CRDs with several nanomolar affinity, it activates canonical Wnt signaling in concert with Fz4 and either Lrp5 or Lrp6 coreceptors, and the NdpKO retinal vascular phenotype can be fully rescued by artificially stabilizing beta-catenin in developing ECs, thereby circumventing the signaling defect at the level of ligand and receptor (Xu et al., 2004; Zhou et al., 2014).
Figure 5.
Defective retinal vascularization in NdpKO mice. Z-stacked confocal images of flat mount adult WT and NdpKO retinas, with ECs visualized with GS-lectin. The depth of the different vascular structures has been color coded as indicated at left. The NdpKO retina has only a superficial vascular plexus from which numerous EC clusters invade a short distance into the retina. IPL, inner plexiform layer; OPL, outer plexiform layer. Scale bar, 100 μm. From Wang et al. (2012).
Recent structural and biochemical experiments have revealed the precise three-dimensional architecture of the complex between dimeric Norrin and a pair of symmetrically bound Fz4 CRDs and defined the sequence determinants of ligand–receptor recognition (Chang et al., 2015; Ke et al., 2013; Smallwood, Williams, Xu, Leahy, & Nathans, 2007). The dimeric nature of the Norrin–Fz4 CRD complex suggests that Fz4 may normally signal as a dimer, perhaps interacting symmetrically with dimeric Lrp5 or Lrp6 (Liu, Bafico, Harris, & Aaronson, 2003). If a pair of Wnt–Fz complexes is required for signaling, this would create the potential for a nonlinear dependence of signal amplitude on Wnt concentration and for a sharpening of the spatio-temporal boundaries of Wnt action.
Fz4 is expressed in all ECs throughout the body from the earliest times in vascular development through adulthood, and Ndp is expressed in selective anatomic regions of the prenatal CNS and in glia throughout the postnatal CNS: Muller glia in the retina, Bergman glia in the cerebellum, and astroglia elsewhere in the brain and spinal cord (Ye, Smallwood, et al., 2011; Ye et al., 2009). Experiments with conditional Fz4 knockout alleles show that eliminating Fz4 in ECs produces the same vascular phenotype as global elimination of Fz4.
An additional component of the Norrin/Fz4/Lrp signaling complex, Tspan12, was identified in a screen of knockout mice for ocular phenotypes (Junge et al., 2009). Tspan12 has four putative transmembrane segments and is a member of the large and diverse tetraspanin family. In cell culture, Tspan12 acts in conjunction with Fz4 and Lrp5 to specifically enhance Norrin signaling. In humans, TSPAN12 and LRP5 mutations are found in a subset of FEVR patients (Nikopoulos, Gilissen, et al., 2010; Poulter et al., 2010, 2012; Salvo et al., 2015). In summary, retinal angiogenesis is controlled by a glial ligand (Norrin) that is sensed by an EC receptor (Fz4), coreceptor (Lrp5), and coactivator (Tspan12).
In the mature retinal and cerebellar vasculatures, Norrin/Fz4 signaling is required for maintenance of the blood–retina barrier and the BBB, respectively (Wang et al., 2012; Ye et al., 2009). This barrier consists of a series of cellular and molecular specializations that distinguishes the CNS and peripheral vasculatures. In CNS ECs, these specializations include the presence of tight junctions, suppression of transcytosis, an absence of fenestrations, and expression of a variety of extrusion pumps and transporters (Daneman & Prat, 2015). The gene expression program that leads to CNS barrier competence is induced and maintained by canonical Wnt signaling (Daneman et al., 2009; Liebner et al., 2008; Stenman et al., 2008; Wang et al., 2012; Zhou et al., 2014).
Experiments in which beta-catenin, Fz4, or Norrin production is either activated or extinguished in the mature vasculature show that CNS barrier integrity is plastic (Liebner et al., 2008; Wang et al., 2012; Zhou et al., 2014). As demonstrated both by expression of molecular markers and by directly assaying the leakiness of CNS vessels, loss of canonical Wnt signaling leads within days to a loss of barrier competence. Conversely, a congenital deficiency in barrier function due to reduced canonical Wnt signaling can be reversed by restoring signaling later in life. Interestingly, the choroid plexus, a highly permeable CNS vascular bed that is the site of cerebrospinal fluid production, shows a cell-autonomous conversion to the barrier competent state (as judged by molecular markers) following EC-specific expression of a stabilized beta-catenin (Zhou et al., 2014). Loss-of-function experiments reveal CNS region-specific variations in ligands (Norrin, Wnt7a, Wnt7b, and possibly other Wnts), receptors (Fz4 and other Frizzleds), and coreceptors (Lrp5 and Lrp6), and the extent of redundancy among these components (Zhou et al., 2014). Taken together, the data reveal a system in which multiple canonical Wnt ligands derived from the CNS parenchyma control vascular barrier integrity.
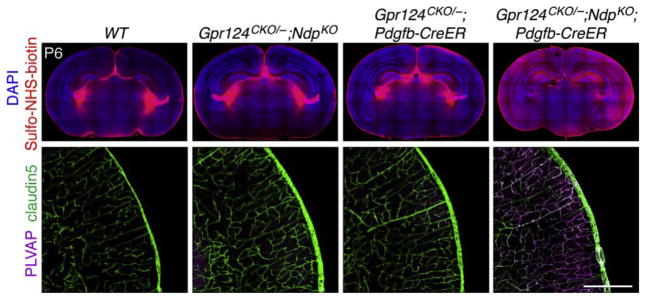
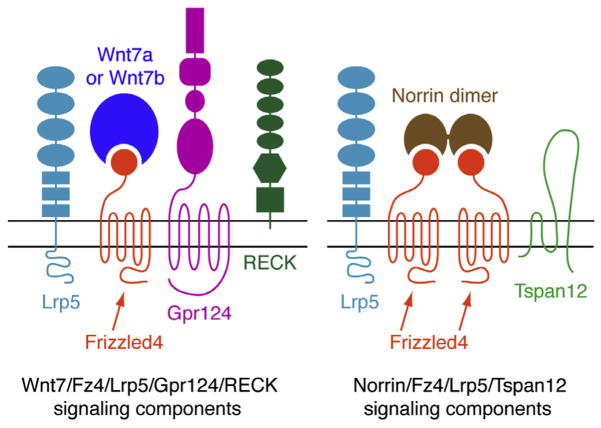
Recent work on Fz4 signaling has led to the discovery of two additional proteins, Gpr124 and Reck, that function in ECs as Wnt7a- and Wnt7b-specific coactivators of canonical Wnt signaling. Based on its predicted trans-membrane topography, Gpr124 had been categorized as a member of the adhesion class of orphan G-protein-coupled receptors. The first link between Gpr124 and vascular biology came with the discovery that GPR124 transcripts are abundant in human tumor vasculature but rare in quiescent adult vasculature (Carson-Walter et al., 2001; St Croix et al., 2000). The second link came with the discovery that Gpr124−/− embryos exhibit anatomically localized defects in CNS angiogenesis that are virtually identical to the defects observed in Wnt7a and Wnt7b double mutant embryos (Anderson et al., 2011; Cullen et al., 2011; Kuhnert et al., 2010; Stenman et al., 2008). This second observation led Zhou and Nathans (2014) and Posokhova et al. (2015) to test whether Gpr124 promotes signaling by Wnt7a and Wnt7b. Remarkably, in transfected cells Gpr124 stimulated signaling by these two Wnts but had little or no effect on signaling by Norrin or any of the other 17 mammalian Wnts. In vivo, various double mutant combinations show that the Wnt7a/Wnt7b/Gpr124/Fz system (which likely signals through Fz4 as well as other EC-expressed Frizzleds) and the Norrin/Tspan12/Fz4 system function redundantly to promote angiogenesis in the embryonic spinal cord and hindbrain and to maintain BBB integrity in many brain regions, including the cerebral cortex (Fig. 6).
Figure 6.
Redundant actions of Gpr124 and Norrin in maintaining BBB integrity. Coronal sections through the cortex and thalamus of postnatal day (P)6 mice with the indicated genotypes. Pdgfb-CreER recombines specifically in ECs. 4-Hydroxytamoxifen (4HT) was delivered by intraperitoneal injection at P3–P4. Intravascular Sulfo-NHS-biotin leaks from the choroid plexus in the lateral ventricles in all brain samples (upper panels), but it leaks extensively into the brain parenchyma only in the sample that is deleted for both Gpr124 and Ndp (upper right panel). Intracerebral capillaries in the WT brain (lower left) and in the brains from mice with either one functional copy of Gpr124 or one functional copy ofNdp (lower middletwo panels) expressclaudin5(a hallmark ofCNS vasculature) and suppress plasmalemma vesicle-associated protein (PLVAP; a hallmark of peripheral vasculature). Deletion of both Gpr124 and Ndp reverses this expression pattern (lower right panel). Scale bar, 200 μm. From Zhou and Nathans (2014).
The second coactivator, Reck, is a GPI-anchored protein that synergizes with Gpr124 to specifically stimulate Wnt7a/Wnt7b-mediated canonical Wnt signaling in transfected cells (Vanhollebeke et al., 2015). In zebrafish, Reck morphants phenocopy Gpr124 mutants and morphants, and eliminating either Reck or Gpr124 blocks CNS angiogenesis and eliminates EC-specific expression of a reporter for canonical Wnt signaling.
These experiments reveal a general role for canonical Wnt signaling in ECs during embryonic and retinal angiogenesis and BBB development and maintenance. They also reveal the existence of auxiliary receptor proteins that enhance ligand specificity (Fig. 7). The high level of GPR124 expression in tumor ECs suggests that canonical Wnt signaling in ECs may play a role in tumor angiogenesis.
Figure 7.
Schematic of ligand and receptor components involved in canonical Wnt signaling in CNS ECs. Horizontal black lines represent the plasma membrane; extracellular space is above and cytoplasm is below. Additional components, such as Lrp6, other Frizzleds, and other Wnts, are also involved, but their roles are not yet well defined. Modified from Zhou and Nathans (2014).
6. FRIZZLED3 AND FRIZZLED6
Among mammalian Frizzled genes, Fz6 is the family member that most closely resembles Drosophila fz, as determined by the macroscopic appearance of its loss-of-function phenotype (Guo, Hawkins, & Nathans, 2004).
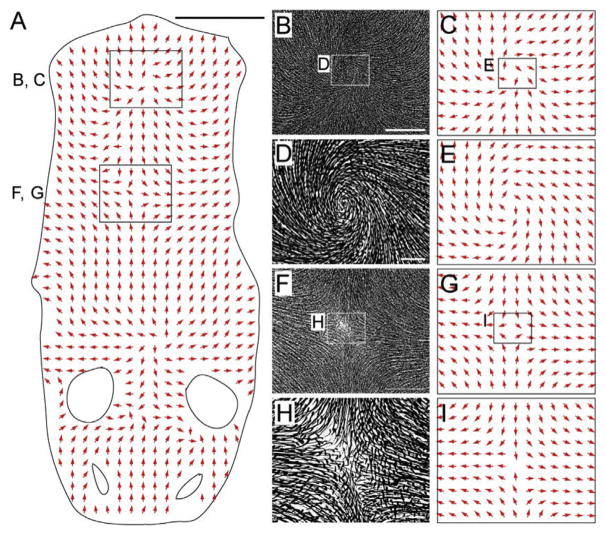
Fz6 is expressed in the epidermis and in hair follicles starting at ~E13. Fz6−/− mice are healthy and fertile, with skin and hair follicles that appear microscopically normal. However, at the earliest stages of their development, the orientations of Fz6−/− hair follicles are aberrant and in many regions nearly randomized. By contrast, in WT mice, immature follicle orientations generally differ by less than 30° from their final orientations, which are precisely coordinated with the body and limb axes. During the first postnatal week, Fz6−/− follicles gradually reorient to create progressively expanding zones of local order, such as whorls or cruciforms (Fig. 8; Wang, Badea, & Nathans, 2006; Wang et al., 2010). As the number of follicles encompassed by the individual patterns increases and the follicle orientations conform more perfectly to the local geometry of each pattern, the number of patterning centers decreases, a progression that appears to represent a competition between neighboring patterns. In WT mice, a more subtle shift in follicle orientations occurs during the same time period, leading to the nearly parallel alignment that characterizes the mature coat.
Figure 8.
Hair patterns in a Fz6−/−;Vangl2Lp/+ back skin at P8. (A) Vector map showing hair follicle orientations over the head and back in a flat-mount skin from a P8 Fz6−/−; Vangl2Lp/+ mouse. Anterior is down; posterior is up. The small ovals are the eye positions; the larger ovals are the ear positions. Four patterning centers are present along the midline. (B–I) The central regions of the two most posterior patterning centers are enlarged in panels B, C, F, and G, and further enlarged in panels D, E, H, and I, as indicated by the rectangles in panels A, B, C, F, and G. Spatial order among follicles is evident at long range (~1 cm) and short range (e.g., between neighboring follicles; tens of microns). Scale bars: A, 1 cm; B and F, 2 mm; D and H, 300 μm. From Wang, Chang, and Nathans (2010).
These and other observations indicate that hair follicle orientation is determined by two fundamentally distinct systems. An embryonic system that requires Fz6 sets up the global pattern of hair follicles orientations, and a postnatal system that is independent of Fz6 refines the initial orientations to minimize differences between neighboring follicles. The refinement system can be modeled with a two-dimensional Ising lattice and a local consensus algorithm of the type that is commonly used to study the behavior of interacting vectors in condensed matter physics (Wang, Badea, et al., 2006). These models are agnostic with regard to molecular and cellular mechanisms, but they capture the global behavior of the system and its responses to various perturbations, such as the juxtaposition of WT and mutant tissue. It is interesting that the wing hairs of Drosophila PCP mutants also exhibit large-scale patterns, such as whorls, that are characterized by local order (Taylor, Abramova, Charlton, & Adler, 1998). The similarity in the large-scale patterns formed by PCP-deficient mammalian and insect epidermal structures is especially striking in light of the very different cellular structures involved: a mammalian hair follicle is an elongated cylinder formed by dozens to hundreds of epidermal cells, whereas a Drosophila wing hair is an actin-based protrusion that emerges from one side of an epithelial cell (Wong & Adler, 1993).
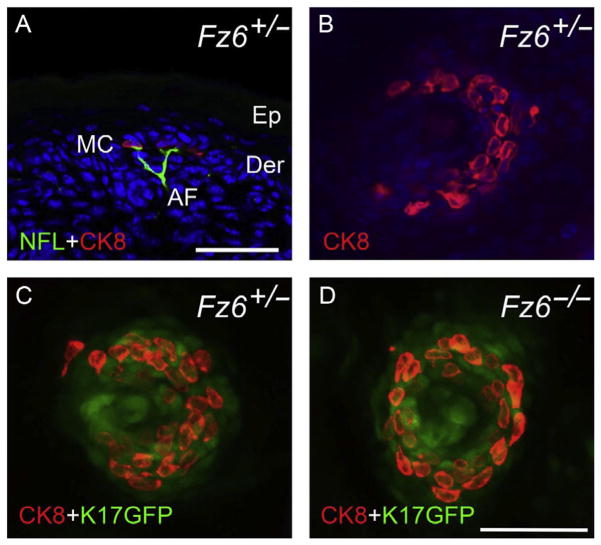
Hair follicles are associated with auxiliary structures that are polarized in a direction that matches the central follicle: a pair of follicle-derived sebaceous glands, arrector pili muscles, sensory nerve endings, and Merkel cell clusters (epithelial derivatives that are involved in mechanosensation). The largest follicles, which give rise to guard or tylotrich hairs, have all of these structures; the smaller follicles lack Merkel cells. The role of the central follicle in directing the polarized assembly of these different structures was analyzed in Fz6−/− mice (Chang & Nathans, 2013). Sebaceous glands, arrector pili muscles, and sensory nerve endings were observed to track the orientation of their associated follicle. Merkel cell clusters on the back skin are normally arranged in a semicircle around the base of the guard hair follicles with the opening of the semicircle pointing toward the anterior. In Fz6−/− mice, the Merkel cell clusters are arranged in a closed circle (Fig. 9). These data are consistent with the idea that Fz6−/− skin is missing global polarity information and that the circularly symmetric Merkel cell arrangement represents a default response to a nonpolarized environment.
Figure 9.
Loss of polarity in the arrangement of Merkel cells in Fz6−/− mice. (A) Vertical section of P1 back skin from a phenotypically WT Fz6+/− mouse. Merkel cells (MC) are visualized with anticytokeratin8 (CK8) immunostaining and afferent sensory nerve fibers (AF) are visualized with antineurofilament (NFL) immunostaining. Ep, epidermis; Der, dermis. (B–D) Flat mounts of P1 skin in which Merkel cells are visualized with CK8 and the central guard hair follicle is visualized with a Keratin (K)17-GFP transgene. Anterior is to the left. The phenotypically WT Fz6+/− Merkel cells form a semicircle that opens toward the anterior. The Fz6−/− Merkel cells form a closed circle. Scale bar, 50 μm. From Chang and Nathans (2013).
In humans, FZD6 loss-of-function mutations have been identified in several families with autosomal recessive nail dysplasia (Frojmark et al., 2011; Naz et al., 2012; Wilson et al., 2013). A follow-up histologic and molecular analysis of claw development in Fz6−/− mice shows dysplastic growth and downregulation of multiple claw-specific keratin genes in addition to Wnt, bone morphogenetic protein, and Hedgehog genes (Cui et al., 2013). The data imply that Fz6 affects epidermal derivatives in a manner that extends beyond spatial patterning: the simplest explanation is that Fz6 couples directly to the canonical Wnt pathway in nail bed epithelial cells.
Like Fz6, Fz3 appears to signal through the PCP/tissue polarity system, but the Fz3−/− phenotype is limited to the nervous system where it controls axon growth and guidance in the forebrain, in cranial and spinal motor neurons, in spinal sensory neurons, and in the sympathetic nervous system (Armstrong, Ryu, Chieco, & Kuruvilla, 2011; Fenstermaker et al., 2010; Hua, Jeon, Caterina, & Nathans, 2014; Hua, Smallwood, & Nathans, 2013; Lyuksyutova et al., 2003; Wang, Thekdi, Smallwood, Macke, & Nathans, 2002). In the Fz3−/− forebrain, thalamocortical axons grow extensively but never enter the internal capsule, instead projecting inferiorly to innervate the contralateral thalamus (Fig. 10). Similarly, nigrostriatal dopaminergic neurons and brainstem serotonergic axons show extensive misrouting, with many axons growing caudally instead of rostrally. In the WT spinal cord, sensory axons that originate in the dorsal horn cross the midline before turning rostrally toward the brain. In the Fz3−/− spinal cord, these sensory axons cross the midline appropriately but appear to lack rostrocaudal cues once their growth cones arrive on the contralateral side. Fz3−/− spinal sensory axons appear to be incapable of finding their appropriate targets in the brainstem and thalamus as determined by behavioral testing of mice that lack Fz3 in all tissues caudal to the neck (Fz3CKO/−;Cdx1-Cre). As adults, these mice show no evidence that thermal or mechanical information from the feet can be transmitted to the brain (Hua, Jeon, et al., 2014). Finally, Fz3−/− motor neurons in the dorsal fore- and hindlimbs exhibit a distinctive stalling phenotype in the nerve plexus at the base of the limbs, suggesting that Fz3-dependent signals are required to sense or respond to molecular cues from intermediate targets (Hua et al., 2013).
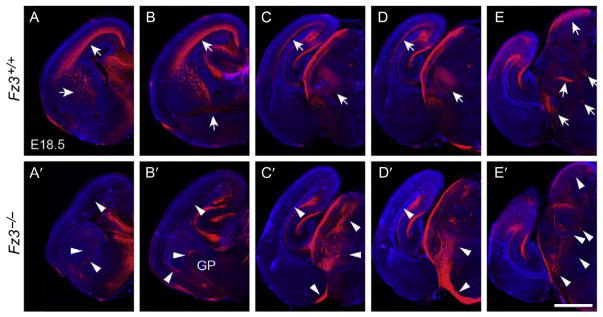
Figure 10.
Thalamocortical axon guidance defects in the Fz3−/− forebrain at E18.5. Serial coronal sections through the forebrains of E18.5 Fz3−/− and Fz3+/+ fetuses stained with anti-neurofilament antibodies. The midline of each hemibrain image is at the right border of each panel. The sections are arranged from rostral (A and A′) to caudal (E and E′). In the Fz3+/+ brain, arrows in (A–D) trace the thalamocortical projections originating in the thalamus (D) and projecting via the internal capsule (A–C) to the cortex (A–D). In the Fz3−/− brain, thalamic axons track inferiorly with most crossing the midline to the contralateral thalamus (D′) and a minority projecting to the inferior border of the cortex (C′). Additional axon guidance defects are indicated by arrowheads in (A′–E′); see Hua, Jeon, et al. (2014) for details. GP, globus pallidus. Scale bar, 1 mm. From Hua, Jeon, et al. (2014).
The developmental roles of Fz3 and Fz6 extend substantially beyond the processes revealed by single gene mutations. Fz3−/−;Fz6−/− embryos reveal redundant functions in neural tube closure and in rotationally orientating the kinocilium/stereociliary bundle complex on the apical face of inner ear sensory hair cells (Wang, Guo, & Nathans, 2006). Additionally, epidermis-specific double knockout of Fz3 and Fz6 (in K14-Cre; Fz3CKO/CKO;Fz6−/− mice) reveals a redundant role for these genes in orienting the filiform papillae on the dorsal surface of the tongue (Hua, Chang, et al., 2014). Stimulated by the partial redundancy of Fz3 and Fz6, Hua, Chang, et al. (2014) asked whether this redundancy can be extended to other tissues by enlarging the domains of Fz3 and Fz6 gene expression. These experiments demonstrated that ubiquitous expression of Fz3 fully rescues the Fz6−/− hair follicle orientation phenotype and ubiquitous expression of Fz6 partially rescues the Fz3−/− axon guidance phenotype. Thus, the many diverse contexts in which Fz3 and Fz6 act are likely based on a conserved signaling role for these two proteins.
Mutations of Celsr1, Celsr2, and Celsr3, mammalian homologues of the Drosophila core PCP gene Flamingo/Starry night (Fmi/Stan), produce neural tube closure, axon guidance, neuronal migration, inner ear sensory hair cell, and hair follicle phenotypes that closely resemble the phenotypes in Fz3−/−, Fz6−/−, and Fz3−/−;Fz6−/− mice (Curtin et al., 2003; Qu et al., 2010; Ravni, Qu, Goffinet, & Tissir, 2009; Tissir, Bar, Jossin, De Backer, & Goffinet, 2005; Zhou et al., 2008). Similarly, Vangl2Lp/Lp fetuses exhibit defects in inner ear sensory hair cell orientation and neural tube closure that closely resemble the defects observed in Fz3−/−;Fz6−/− fetuses (Kibar et al., 2001; Montcouquiol et al., 2003; Murdoch, Doudney, Paternotte, Copp, & Stanier, 2001; Strong & Hollander, 1949). Vangl2Lp/Lp fetuses also exhibit a hair follicle orientation defect, but, interestingly, the Vangl2Lp/Lp follicles are initially oriented perpendicular to the plane of the skin whereas WT and Fz6−/− follicles are oriented obliquely (Devenport & Fuchs, 2008; Wang et al., 2010). Despite such minor differences, the overall similarities in affected tissues and in polarity defects among Celsr, Vangl, and Fz3/Fz6 mutants support the idea that the diverse Fz3−/−, Fz6−/−, and Fz3−/−; Fz6−/− phenotypes reflect the activity of a mammalian version of Drosophila PCP.
7. CONCLUSION
The past three decades represent the first phase of exploration of the Frizzled family. At least some of the functions of most mammalian Frizzled family members are now known at a descriptive level, and for three family members the signaling pathways in which they participate have been identified. Despite the enormous progress made during this time, our current level of understanding remains rudimentary. To cite three examples: (1) the determinants of Wnt–Frizzled specificity are still unknown, and there is a strong suspicion that additional modulatory/specificity proteins remain to be discovered, (2) the regulation of Frizzled expression, trafficking, and stability is just starting to be explored, with the discovery of R-spondin regulation of Frizzled degradation via the ubiquitin ligase Znrf3 representing a tantalizing appetizer (Hao et al., 2012; Koo et al., 2012), and (3) the role of Frizzled receptors in human disease—both inherited and acquired—is still in its infancy.
Perhaps the greatest challenge is to define the biochemical role of Frizzled proteins in polarity signaling. Tissue polarity has been recognized as an essential attribute of animal development for more than 75 years, with the early microsurgical investigations of polarity in the insect cuticle preparing the intellectual foundations for today’s molecular genetic experiments (Lawrence, 1966; Locke, 1959; Wigglesworth, 1940). Tissue polarity signaling also has a connection to the larger world of complex systems, of which biological pattern formation represents one part (Ball, 1999). The local nature of cell–cell signaling suggests that tissue polarity uses conceptually simple “rules” of information transfer that can be modified in various ways to create anatomic diversity. In keeping with this idea, the study of cellular automata demonstrates that large-scale iterative applications of simple rules can generate remarkably complex patterns (Wolfram, 2002). As new mechanistic insights emerge in the field of tissue polarity signaling, it will be of great interest to try to capture the fundamental characteristics of this system in mathematical models, an effort that is already underway (Amonlirdviman et al., 2005; Axelrod & Tomlin, 2011; Wang, Badea, et al., 2006).
Acknowledgments
Supported by the Howard Hughes Medical Institute, the Ellison Medical Foundation, and the National Eye institute (NIH).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- Albers J, Schulze J, Beil FT, Gebauer M, Baranowsky A, Keller J, et al. Control of bone formation by the serpentine receptor Frizzled-9. The Journal of Cell Biology. 2011;192(6):1057–1072. doi: 10.1083/jcb.201008012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allache R, De Marco P, Merello E, Capra V, Kibar Z. Role of the planar cell polarity gene CELSR1 in neural tube defects and caudal agenesis. Birth Defects Research. Part A, Clinical and Molecular Teratology. 2012;94(3):176–181. doi: 10.1002/bdra.23002. [DOI] [PubMed] [Google Scholar]
- Amonlirdviman K, Khare NA, Tree DR, Chen WS, Axelrod JD, Tomlin CJ. Mathematical modeling of planar cell polarity to understand domineering non-autonomy. Science. 2005;307(5708):423–426. doi: 10.1126/science.1105471. [DOI] [PubMed] [Google Scholar]
- Anderson KD, Pan L, Yang XM, Hughes VC, Walls JR, Dominguez MG, et al. Angiogenic sprouting into neural tissue requires Gpr124, an orphan G protein-coupled receptor. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(7):2807–2812. doi: 10.1073/pnas.1019761108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong A, Ryu YK, Chieco D, Kuruvilla R. Frizzled3 is required for neurogenesis and target innervation during sympathetic nervous system development. The Journal of Neuroscience. 2011;31(7):2371–2381. doi: 10.1523/JNEUROSCI.4243-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au KS, Ashley-Koch A, Northrup H. Epidemiologic and genetic aspects of spina bifida and other neural tube defects. Developmental Disabilities Research Reviews. 2010;16(1):6–15. doi: 10.1002/ddrr.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod JD, Tomlin CJ. Modeling the control of planar cell polarity. Wiley Interdisciplinary Reviews Systems Biology and Medicine. 2011;3(5):588–605. doi: 10.1002/wsbm.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball P. The self-made tapestry: Pattern formation in nature. Oxford: Oxford University Press; 1999. p. 287. [Google Scholar]
- Baron R, Kneissel M. Wnt signaling in bone homeostasis and disease: From human mutations to treatments. Nature Medicine. 2013;19(2):179–192. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- Berger W, Meindl A, van de Pol TJ, Cremers FP, Ropers HH, Doerner C, et al. Isolation of a candidate gene for Norrie disease by positional cloning. Nature Genetics. 1992;1(3):199–203. doi: 10.1038/ng0692-199. [DOI] [PubMed] [Google Scholar]
- Berger W, Ropers H. Norrie disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. New York, NY, USA: McGraw-Hill; 2001. pp. 5977–5985. [Google Scholar]
- Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, et al. A new member of the frizzled family from drosophila functions as a wingless receptor. Nature. 1996;382(6588):225–230. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- Bhanot P, Fish M, Jemison JA, Nusse R, Nathans J, Cadigan KM. Frizzled and frizzled-2 function as redundant receptors for wingless during drosophila embryonic development. Development. 1999;126(18):4175–4186. doi: 10.1242/dev.126.18.4175. [DOI] [PubMed] [Google Scholar]
- Bhat KM. Frizzled and frizzled 2 play a partially redundant role in wingless signaling and have similar requirements to wingless in neurogenesis. Cell. 1998;95(7):1027–1036. doi: 10.1016/s0092-8674(00)81726-2. [DOI] [PubMed] [Google Scholar]
- Biggs LC, Goudy SL, Dunnwald M. Palatogenesis and cutaneous repair: A two-headed coin. Developmental Dynamics. 2015;244(3):289–310. doi: 10.1002/dvdy.24224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson-Walter EB, Watkins DN, Nanda A, Vogelstein B, Kinzler KW, St Croix B. Cell surface tumor endothelial markers are conserved in mice and humans. Cancer Research. 2001;61(18):6649–6655. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Temporal trends in the incidence of birth defects—United States. MMWR. Morbidity and Mortality Weekly Report. 1997;46(49):1171–1176. [PubMed] [Google Scholar]
- Chan SD, Karpf DB, Fowlkes ME, Hooks M, Bradley MS, Vuong V, et al. Two homologs of the drosophila polarity gene frizzled (fz) are widely expressed in mammalian tissues. The Journal of Biological Chemistry. 1992;267(35):25202–25207. [PubMed] [Google Scholar]
- Chang TH, Hsieh FL, Zebisch M, Harlos K, Elegheert J, Jones EY. Structure and functional properties of Norrin mimic Wnt for signalling with Frizzled4, Lrp5/6, and proteoglycan. eLife. 2015:4. doi: 10.7554/eLife.06554. http://dx.doi.org/10.7554/eLife.06554. [DOI] [PMC free article] [PubMed]
- Chang H, Nathans J. Responses of hair follicle-associated structures to loss of planar cell polarity signaling. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(10):E908–E917. doi: 10.1073/pnas.1301430110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Hendriks RW, Jobling MA, Powell JF, Breakefield XO, Sims KB, et al. Isolation and characterization of a candidate gene for Norrie disease. Nature Genetics. 1992;1(3):204–208. doi: 10.1038/ng0692-204. [DOI] [PubMed] [Google Scholar]
- Chi L, Zhang S, Lin Y, Prunskaite-Hyyrylainen R, Vuolteenaho R, Itaranta P, et al. Sprouty proteins regulate ureteric branching by coordinating reciprocal epithelial Wnt11, mesenchymal Gdnf and stromal Fgf7 signalling during kidney development. Development. 2004;131(14):3345–3356. doi: 10.1242/dev.01200. [DOI] [PubMed] [Google Scholar]
- Cui CY, Klar J, Georgii-Heming P, Frojmark AS, Baig SM, Schlessinger D, et al. Frizzled6 deficiency disrupts the differentiation process of nail development. The Journal of Investigative Dermatology. 2013;133(8):1990–1997. doi: 10.1038/jid.2013.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen M, Elzarrad MK, Seaman S, Zudaire E, Stevens J, Yang MY, et al. GPR124, an orphan G protein-coupled receptor, is required for CNS-specific vascularization and establishment of the blood–brain barrier. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(14):5759–5764. doi: 10.1073/pnas.1017192108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin JA, Quint E, Tsipouri V, Arkell RM, Cattanach B, Copp AJ, et al. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Current Biology. 2003;13(13):1129–1133. doi: 10.1016/s0960-9822(03)00374-9. [DOI] [PubMed] [Google Scholar]
- Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(2):641–646. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Prat A. The blood–brain barrier. Cold Spring Harbor Perspectives in Biology. 2015;7(1):a020412. doi: 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marco P, Merello E, Rossi A, Piatelli G, Cama A, Kibar Z, et al. FZD6 is a novel gene for human neural tube defects. Human Mutation. 2012;33(2):384–390. doi: 10.1002/humu.21643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenport D, Fuchs E. Planar polarization in embryonic epidermis orchestrates global asymmetric morphogenesis of hair follicles. Nature Cell Biology. 2008;10(11):1257–1268. doi: 10.1038/ncb1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolk H, Loane M, Garne E. The prevalence of congenital anomalies in Europe. Advances in Experimental Medicine and Biology. 2010;686:349–364. doi: 10.1007/978-90-481-9485-8_20. [DOI] [PubMed] [Google Scholar]
- Duke-Elder S. System of ophthalmology: Normal and abnormal development. St. Louis: C.V. Mosby; 1964. pp. 764–770. [Google Scholar]
- Fenstermaker AG, Prasad AA, Bechara A, Adolfs Y, Tissir F, Goffinet A, et al. Wnt/planar cell polarity signaling controls the anterior-posterior organization of monoaminergic axons in the brainstem. The Journal of Neuroscience. 2010;30(47):16053–16064. doi: 10.1523/JNEUROSCI.4508-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frojmark AS, Schuster J, Sobol M, Entesarian M, Kilander MB, Gabrikova D, et al. Mutations in frizzled 6 cause isolated autosomal-recessive nail dysplasia. American Journal of Human Genetics. 2011;88(6):852–860. doi: 10.1016/j.ajhg.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruttiger M. Development of the retinal vasculature. Angiogenesis. 2007;10(2):77–88. doi: 10.1007/s10456-007-9065-1. [DOI] [PubMed] [Google Scholar]
- Goldberg MF. Persistent fetal vasculature (PFV): An integrated interpretation of signs and symptoms associated with persistent hyperplastic primary vitreous (PHPV). LIV Edward Jackson Memorial Lecture. American Journal of Ophthalmology. 1997;124(5):587–626. doi: 10.1016/s0002-9394(14)70899-2. [DOI] [PubMed] [Google Scholar]
- Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development. 2011;138(10):1877–1892. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubb D, Garcia-Bellido A. A genetic analysis of the determination of cuticular polarity during development in Drosophila melanogaster. Journal of Embryology and Experimental Morphology. 1982;68:37–57. [PubMed] [Google Scholar]
- Guo N, Hawkins C, Nathans J. Frizzled6 controls hair patterning in mice. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(25):9277–9281. doi: 10.1073/pnas.0402802101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao HX, Xie Y, Zhang Y, Charlat O, Oster E, Avello M, et al. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature. 2012;485(7397):195–200. doi: 10.1038/nature11019. [DOI] [PubMed] [Google Scholar]
- Heilmann A, Schinke T, Bindl R, Wehner T, Rapp A, Haffner-Luntzer M, et al. The Wnt serpentine receptor frizzled-9 regulates new bone formation in fracture healing. PloS One. 2013;8(12):e84232. doi: 10.1371/journal.pone.0084232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh JC, Rattner A, Smallwood PM, Nathans J. Biochemical characterization of Wnt-frizzled interactions using a soluble, biologically active vertebrate Wnt protein. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(7):3546–3551. doi: 10.1073/pnas.96.7.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua ZL, Chang H, Wang Y, Smallwood PM, Nathans J. Partial interchangeability of Fz3 and Fz6 in tissue polarity signaling for epithelial orientation and axon growth and guidance. Development. 2014;141(20):3944–3954. doi: 10.1242/dev.110189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua ZL, Jeon S, Caterina MJ, Nathans J. Frizzled3 is required for the development of multiple axon tracts in the mouse central nervous system. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(29):E3005–E3014. doi: 10.1073/pnas.1406399111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua ZL, Smallwood PM, Nathans J. Frizzled3 controls axonal development in distinct populations of cranial and spinal motor neurons. eLife. 2013;2:e01482. doi: 10.7554/eLife.01482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Tamai Y, Zorn AM, Yoshida H, Seldin MF, Nishikawa S, et al. Mouse Wnt receptor gene Fzd5 is essential for yolk sac and placental angio-genesis. Development. 2001;128(1):25–33. doi: 10.1242/dev.128.1.25. [DOI] [PubMed] [Google Scholar]
- Janda CY, Waghray D, Levin AM, Thomas C, Garcia KC. Structural basis of Wnt recognition by Frizzled. Science. 2012;337(6090):59–64. doi: 10.1126/science.1222879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joeng KS, Schumacher CA, Zylstra-Diegel CR, Long F, Williams BO. Lrp5 and Lrp6 redundantly control skeletal development in the mouse embryo. Developmental Biology. 2011;359(2):222–229. doi: 10.1016/j.ydbio.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junge HJ, Yang S, Burton JB, Paes K, Shu X, French DM, et al. TSPAN12 regulates retinal vascular development by promoting Norrin- but not Wnt-induced FZD4/beta-catenin signaling. Cell. 2009;139(2):299–311. doi: 10.1016/j.cell.2009.07.048. [DOI] [PubMed] [Google Scholar]
- Ke J, Harikumar KG, Erice C, Chen C, Gu X, Wang L, et al. Structure and function of norrin in assembly and activation of a frizzled 4-Lrp5/6 complex. Genes & Development. 2013;27(21):2305–2319. doi: 10.1101/gad.228544.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibar Z, Bosoi CM, Kooistra M, Salem S, Finnell RH, De Marco P, et al. Novel mutations in VANGL1 in neural tube defects. Human Mutation. 2009;30(7):E706–E715. doi: 10.1002/humu.21026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibar Z, Salem S, Bosoi CM, Pauwels E, De Marco P, Merello E, et al. Contribution of VANGL2 mutations to isolated neural tube defects. Clinical Genetics. 2011;80(1):76–82. doi: 10.1111/j.1399-0004.2010.01515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibar Z, Torban E, McDearmid JR, Reynolds A, Berghout J, Mathieu M, et al. Mutations in VANGL1 associated with neural-tube defects. The New England Journal of Medicine. 2007;356(14):1432–1437. doi: 10.1056/NEJMoa060651. [DOI] [PubMed] [Google Scholar]
- Kibar Z, Vogan KJ, Groulx N, Justice MJ, Underhill DA, Gros P. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant loop-tail. Nature Genetics. 2001;28(3):251–255. doi: 10.1038/90081. [DOI] [PubMed] [Google Scholar]
- Koike J, Takagi A, Miwa T, Hirai M, Terada M, Katoh M. Molecular cloning of Frizzled-10, a novel member of the Frizzled gene family. Biochemical and Biophysical Research Communications. 1999;262(1):39–43. doi: 10.1006/bbrc.1999.1161. [DOI] [PubMed] [Google Scholar]
- Koo BK, Spit M, Jordens I, Low TY, Stange DE, van de Wetering M, et al. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature. 2012;488(7413):665–669. doi: 10.1038/nature11308. [DOI] [PubMed] [Google Scholar]
- Kuhnert F, Mancuso MR, Shamloo A, Wang HT, Choksi V, Florek M, et al. Essential regulation of CNS angiogenesis by the orphan G protein-coupled receptor GPR124. Science. 2010;330(6006):985–989. doi: 10.1126/science.1196554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence PA. Gradients in the insect segment: The orientation of hairs in the milkweed bug Oncopeltus fasciatus. The Journal of Experimental Biology. 1966;44:607–620. doi: 10.1242/jeb.44.3.507. [DOI] [PubMed] [Google Scholar]
- Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, et al. Wnt/beta-catenin signaling controls development of the blood–brain barrier. The Journal of Cell Biology. 2008;183(3):409–417. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Bafico A, Harris VK, Aaronson SA. A novel mechanism for Wnt activation of canonical signaling through the LRP6 receptor. Molecular and Cellular Biology. 2003;23(16):5825–5835. doi: 10.1128/MCB.23.16.5825-5835.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Bakeri H, Li T, Swaroop A. Regulation of retinal progenitor expansion by frizzled receptors: Implications for microphthalmia and retinal coloboma. Human Molecular Genetics. 2012;21(8):1848–1860. doi: 10.1093/hmg/ddr616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Nathans J. An essential role for frizzled 5 in mammalian ocular development. Development. 2008;135(21):3567–3576. doi: 10.1242/dev.028076. [DOI] [PubMed] [Google Scholar]
- Liu C, Wang Y, Smallwood PM, Nathans J. An essential role for Frizzled5 in neuronal survival in the parafascicular nucleus of the thalamus. The Journal of Neuroscience. 2008;28(22):5641–5653. doi: 10.1523/JNEUROSCI.1056-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke M. The cuticular pattern in an insect Rhodnius prolixus Stal. The Journal of Experimental Biology. 1959;36:459–477. [Google Scholar]
- Luhmann UF, Lin J, Acar N, Lammel S, Feil S, Grimm C, et al. Role of the Norrie disease pseudoglioma gene in sprouting angiogenesis during development of the retinal vasculature. Investigative Ophthalmology & Visual Science. 2005;46(9):3372–3382. doi: 10.1167/iovs.05-0174. [DOI] [PubMed] [Google Scholar]
- Lyuksyutova AI, Lu CC, Milanesio N, King LA, Guo N, Wang Y, et al. Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science. 2003;302(5652):1984–1988. doi: 10.1126/science.1089610. [DOI] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Developmental Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar A, Vainio S, Kispert A, McMahon J, McMahon AP. Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development. 2003;130(14):3175–3185. doi: 10.1242/dev.00520. [DOI] [PubMed] [Google Scholar]
- Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature. 2003;423(6936):173–177. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- Muller HA, Samanta R, Wieschaus E. Wingless signaling in the Drosophila embryo: Zygotic requirements and the role of the frizzled genes. Development. 1999;126(3):577–586. doi: 10.1242/dev.126.3.577. [DOI] [PubMed] [Google Scholar]
- Murdoch JN, Doudney K, Paternotte C, Copp AJ, Stanier P. Severe neural tube defects in the loop-tail mouse result from mutation of Lpp1, a novel gene involved in floor plate specification. Human Molecular Genetics. 2001;10(22):2593–2601. doi: 10.1093/hmg/10.22.2593. [DOI] [PubMed] [Google Scholar]
- Naz G, Pasternack SM, Perrin C, Mattheisen M, Refke M, Khan S, et al. FZD6 encoding the Wnt receptor frizzled 6 is mutated in autosomal-recessive nail dysplasia. The British Journal of Dermatology. 2012;166(5):1088–1094. doi: 10.1111/j.1365-2133.2011.10800.x. [DOI] [PubMed] [Google Scholar]
- Nikopoulos K, Gilissen C, Hoischen A, van Nouhuys CE, Boonstra FN, Blokland EA, et al. Next-generation sequencing of a 40 Mb linkage interval reveals TSPAN12 mutations in patients with familial exudative vitreoretinopathy. American Journal of Human Genetics. 2010;86(2):240–247. doi: 10.1016/j.ajhg.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikopoulos K, Venselaar H, Collin RW, Riveiro-Alvarez R, Boonstra FN, Hooymans JM, et al. Overview of the mutation spectrum in familial exudative vitreoretinopathy and norrie disease with identification of 21 novel variants in FZD4, LRP5, and NDP. Human Mutation. 2010;31(6):656–666. doi: 10.1002/humu.21250. [DOI] [PubMed] [Google Scholar]
- Nusse R. Wnt signaling. Cold Spring Harbor Perspectives in Biology. 2012;4(5) doi: 10.1101/cshperspect.a011163. http://dx.doi.org/10.1101/cshperspect.a011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posokhova E, Shukla A, Seaman S, Volate S, Hilton MB, Wu B, et al. GPR124 functions as a Wnt7-specific coactivator of canonical beta-catenin signaling. Cell Reports. 2015;10(2):123–130. doi: 10.1016/j.celrep.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter JA, Ali M, Gilmour DF, Rice A, Kondo H, Hayashi K, et al. Mutations in TSPAN12 cause autosomal-dominant familial exudative vitreoretinopathy. American Journal of Human Genetics. 2010;86(2):248–253. doi: 10.1016/j.ajhg.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter JA, Davidson AE, Ali M, Gilmour DF, Parry DA, Mintz-Hittner HA, et al. Recessive mutations in TSPAN12 cause retinal dysplasia and severe familial exudative vitreoretinopathy (FEVR) Investigative Ophthalmology & Visual Science. 2012;53(6):2873–2879. doi: 10.1167/iovs.11-8629. [DOI] [PubMed] [Google Scholar]
- Qin M, Hayashi H, Oshima K, Tahira T, Hayashi K, Kondo H. Complexity of the genotype-phenotype correlation in familial exudative vitreoretinopathy with mutations in the LRP5 and/or FZD4 genes. Human Mutation. 2005;26(2):104–112. doi: 10.1002/humu.20191. [DOI] [PubMed] [Google Scholar]
- Qu Y, Glasco DM, Zhou L, Sawant A, Ravni A, Fritzsch B, et al. Atypical cadherins Celsr1-3 differentially regulate migration of facial branchiomotor neurons in mice. The Journal of Neuroscience. 2010;30(28):9392–9401. doi: 10.1523/JNEUROSCI.0124-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranheim EA, Kwan HC, Reya T, Wang YK, Weissman IL, Francke U. Frizzled 9 knock-out mice have abnormal B-cell development. Blood. 2005;105(6):2487–2494. doi: 10.1182/blood-2004-06-2334. [DOI] [PubMed] [Google Scholar]
- Ravni A, Qu Y, Goffinet AM, Tissir F. Planar cell polarity cadherin Celsr1 regulates skin hair patterning in the mouse. The Journal of Investigative Dermatology. 2009;129(10):2507–2509. doi: 10.1038/jid.2009.84. [DOI] [PubMed] [Google Scholar]
- Rehm HL, Zhang DS, Brown MC, Burgess B, Halpin C, Berger W, et al. Vascular defects and sensorineural deafness in a mouse model of Norrie disease. The Journal of Neuroscience. 2002;22(11):4286–4292. doi: 10.1523/JNEUROSCI.22-11-04286.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille J, MacDonald ML, Kaykas A, Sheldahl LC, Zeisler J, Dube MP, et al. Mutant frizzled-4 disrupts retinal angiogenesis in familial exudative vitreoretinopathy. Nature Genetics. 2002;32(2):326–330. doi: 10.1038/ng957. [DOI] [PubMed] [Google Scholar]
- Robitaille JM, Zheng B, Wallace K, Beis MJ, Tatlidil C, Yang J, et al. The role of Frizzled-4 mutations in familial exudative vitreoretinopathy and Coats disease. The British Journal of Ophthalmology. 2011;95(4):574–579. doi: 10.1136/bjo.2010.190116. [DOI] [PubMed] [Google Scholar]
- Salvo J, Lyubasyuk V, Xu M, Wang H, Wang F, Nguyen D, et al. Next-generation sequencing and novel variant determination in a cohort of 92 familial exudative vitreoretinopathy patients. Investigative Ophthalmology & Visual Science. 2015;56(3):1937–1946. doi: 10.1167/iovs.14-16065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkelaars Q, Fierro-Constain L, Renard E, Hill AL, Borchiellini C. Insights into Frizzled evolution and new perspectives. Evolution & Development. 2015;17(2):160–169. doi: 10.1111/ede.12115. [DOI] [PubMed] [Google Scholar]
- Shah SM, Kang YJ, Christensen BL, Feng AS, Kollmar R. Expression of Wnt receptors in adult spiral ganglion neurons: Frizzled 9 localization at growth cones of regenerating neurites. Neuroscience. 2009;164(2):478–487. doi: 10.1016/j.neuroscience.2009.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood PM, Williams J, Xu Q, Leahy DJ, Nathans J. Mutational analysis of Norrin-Frizzled4 recognition. The Journal of Biological Chemistry. 2007;282(6):4057–4068. doi: 10.1074/jbc.M609618200. [DOI] [PubMed] [Google Scholar]
- St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, et al. Genes expressed in human tumor endothelium. Science. 2000;289(5482):1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322(5905):1247–1250. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- Strong LC, Hollander WF. Hereditary loop-tail in the house mouse accompanied by imperforate vagina and with lethal craniorachischisis when homozygous. The Journal of Heredity. 1949;40:329–334. [Google Scholar]
- Taylor J, Abramova N, Charlton J, Adler PN. Van Gogh: A new Drosophila tissue polarity gene. Genetics. 1998;150(1):199–210. doi: 10.1093/genetics/150.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissir F, Bar I, Jossin Y, De Backer O, Goffinet AM. Protocadherin Celsr3 is crucial in axonal tract development. Nature Neuroscience. 2005;8(4):451–457. doi: 10.1038/nn1428. [DOI] [PubMed] [Google Scholar]
- Toomes C, Bottomley HM, Scott S, Mackey DA, Craig JE, Appukuttan B, et al. Spectrum and frequency of FZD4 mutations in familial exudative vitreoretinopathy. Investigative Ophthalmology & Visual Science. 2004;45(7):2083–2090. doi: 10.1167/iovs.03-1044. [DOI] [PubMed] [Google Scholar]
- Vanhollebeke B, Stone OA, Bostaille N, Cho C, Zhou Y, Maquet E, et al. Tip cell-specific requirement for an atypical Gpr124- and Reck-dependent Wnt/beta-catenin pathway during brain angiogenesis. eLife. 2015;4 doi: 10.7554/eLife.06489. http://dx.doi.org/10.7554/eLife.06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson CR, Conover S, Adler PN. A Drosophila tissue polarity locus encodes a protein containing seven potential transmembrane domains. Nature. 1989;338(6212):263–264. doi: 10.1038/338263a0. [DOI] [PubMed] [Google Scholar]
- Wang Y, Badea T, Nathans J. Order from disorder: Self-organization in mammalian hair patterning. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(52):19800–19805. doi: 10.1073/pnas.0609712104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chang H, Nathans J. When whorls collide: The development of hair patterns in frizzled 6 mutant mice. Development. 2010;137(23):4091–4099. doi: 10.1242/dev.057455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Guo N, Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. The Journal of Neuroscience. 2006;26(8):2147–2156. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Huso D, Cahill H, Ryugo D, Nathans J. Progressive cerebellar, auditory, and esophageal dysfunction caused by targeted disruption of the frizzled-4 gene. The Journal of Neuroscience. 2001;21(13):4761–4771. doi: 10.1523/JNEUROSCI.21-13-04761.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Macke JP, Abella BS, Andreasson K, Worley P, Gilbert DJ, et al. A large family of putative transmembrane receptors homologous to the product of the Drosophila tissue polarity gene frizzled. The Journal of Biological Chemistry. 1996;271(8):4468–4476. doi: 10.1074/jbc.271.8.4468. [DOI] [PubMed] [Google Scholar]
- Wang HY, Malbon CC. Wnt signaling, Ca2+, and cyclic GMP: Visualizing frizzled functions. Science. 2003;300(5625):1529–1530. doi: 10.1126/science.1085259. [DOI] [PubMed] [Google Scholar]
- Wang Y, Rattner A, Zhou Y, Williams J, Smallwood PM, Nathans J. Norrin/Frizzled4 signaling in retinal vascular development and blood brain barrier plasticity. Cell. 2012;151(6):1332–1344. doi: 10.1016/j.cell.2012.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YK, Samos CH, Peoples R, Perez-Jurado LA, Nusse R, Francke U. A novel human homologue of the Drosophila frizzled wnt receptor gene binds wingless protein and is in the Williams syndrome deletion at 7q11.23. Human Molecular Genetics. 1997;6:465–472. doi: 10.1093/hmg/6.3.465. [DOI] [PubMed] [Google Scholar]
- Wang Y, Thekdi N, Smallwood PM, Macke JP, Nathans J. Frizzled-3 is required for the development of major fiber tracts in the rostral CNS. The Journal of Neuroscience. 2002;22(19):8563–8573. doi: 10.1523/JNEUROSCI.22-19-08563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigglesworth VB. Local and general factors in the development of ‘pattern’ in Rhodnius prolixus. The Journal of Experimental Biology. 1940;17:180–200. [Google Scholar]
- Wilson NJ, Hansen CD, Azkur D, Kocabas CN, Metin A, Coskun Z, et al. Recessive mutations in the gene encoding frizzled 6 cause twenty nail dystrophy—Expanding the differential diagnosis for pachyonychia congenita. Journal of Dermatological Science. 2013;70(1):58–60. doi: 10.1016/j.jdermsci.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Wolfram S. A new kind of science. Champagne, IL: Wolfram Media, Inc; 2002. p. 1197. [Google Scholar]
- Wong LL, Adler PN. Tissue polarity genes of Drosophila regulate the sub-cellular location for prehair initiation in pupal wing cells. The Journal of Cell Biology. 1993;123(1):209–221. doi: 10.1083/jcb.123.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Wang Y, Dabdoub A, Smallwood PM, Williams J, Woods C, et al. Vascular development in the retina and inner ear: Control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116(6):883–895. doi: 10.1016/s0092-8674(04)00216-8. [DOI] [PubMed] [Google Scholar]
- Ye X, Smallwood P, Nathans J. Expression of the Norrie disease gene (Ndp) in developing and adult mouse eye, ear, and brain. Gene Expression Patterns. 2011;11(1–2):151–155. doi: 10.1016/j.gep.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Wang Y, Cahill H, Yu M, Badea TC, Smallwood PM, et al. Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell. 2009;139(2):285–298. doi: 10.1016/j.cell.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Wang Y, Rattner A, Nathans J. Genetic mosaic analysis reveals a major role for frizzled 4 and frizzled 8 in controlling ureteric growth in the developing kidney. Development. 2011;138(6):1161–1172. doi: 10.1242/dev.057620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Smallwood PM, Wang Y, Vidaltamayo R, Reed R, Nathans J. Frizzled 1 and frizzled 2 genes function in palate, ventricular septum and neural tube closure: General implications for tissue fusion processes. Development. 2010;137(21):3707–3717. doi: 10.1242/dev.052001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Ye X, Guo N, Nathans J. Frizzled 2 and frizzled 7 function redundantly in convergent extension and closure of the ventricular septum and palate: Evidence for a network of interacting genes. Development. 2012;139(23):4383–4394. doi: 10.1242/dev.083352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Fuhrmann S, Vetter ML. A nonautonomous role for retinal frizzled-5 in regulating hyaloid vitreous vasculature development. Investigative Ophthalmology & Visual Science. 2008;49(12):5561–5567. doi: 10.1167/iovs.08-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Aviles C, Abel RA, Almli CR, McQuillen P, Pleasure SJ. Hippocampal and visuospatial learning defects in mice with a deletion of frizzled 9, a gene in the Williams syndrome deletion interval. Development. 2005;132(12):2917–2927. doi: 10.1242/dev.01871. [DOI] [PubMed] [Google Scholar]
- Zhao C, Guan W, Pleasure SJ. A transgenic marker mouse line labels Cajal-Retzius cells from the cortical hem and thalamocortical axons. Brain Research. 2006;1077(1):48–53. doi: 10.1016/j.brainres.2006.01.042. [DOI] [PubMed] [Google Scholar]
- Zhou L, Bar I, Achouri Y, Campbell K, De Backer O, Hebert JM, et al. Early forebrain wiring: Genetic dissection using conditional Celsr3 mutant mice. Science. 2008;320(5878):946–949. doi: 10.1126/science.1155244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Nathans J. Gpr124 controls CNS angiogenesis and blood–brain barrier integrity by promoting ligand-specific canonical Wnt signaling. Developmental Cell. 2014;31(2):248–256. doi: 10.1016/j.devcel.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Wang Y, Tischfield M, Williams J, Smallwood PM, Rattner A, et al. Canonical Wnt signaling components in vascular development and barrier formation. The Journal of Clinical Investigation. 2014;124(9):3825–3846. doi: 10.1172/JCI76431. [DOI] [PMC free article] [PubMed] [Google Scholar]