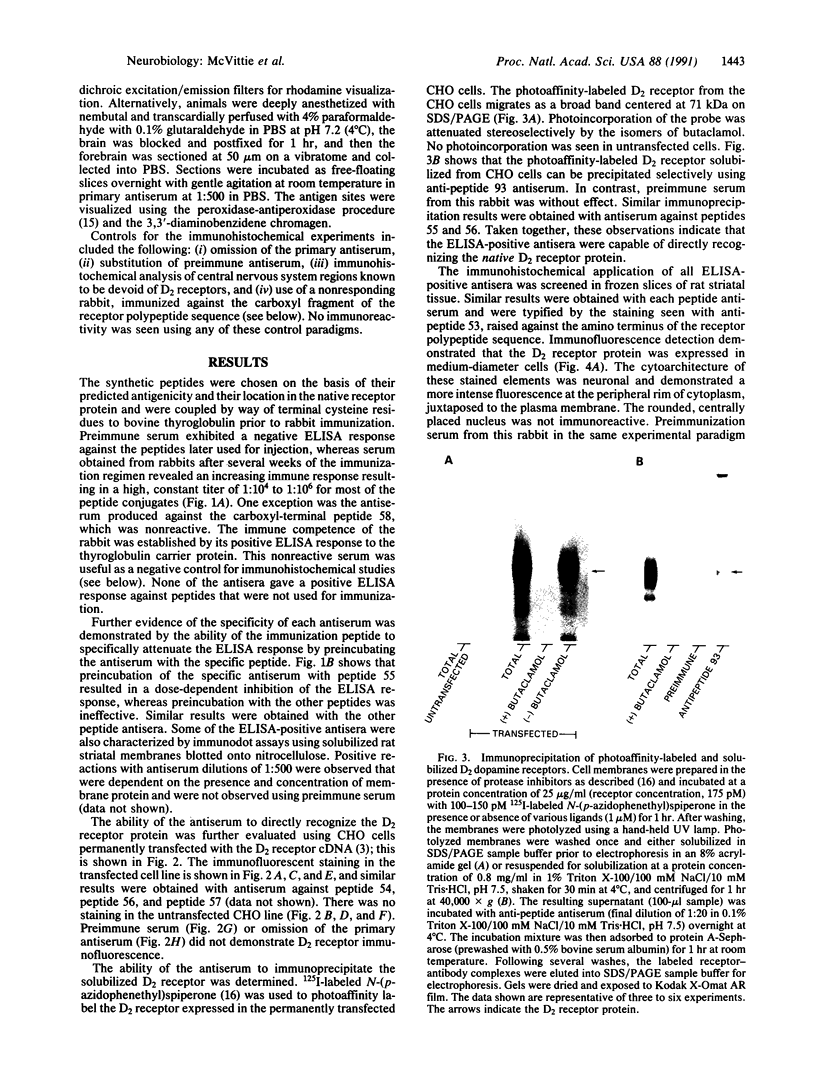
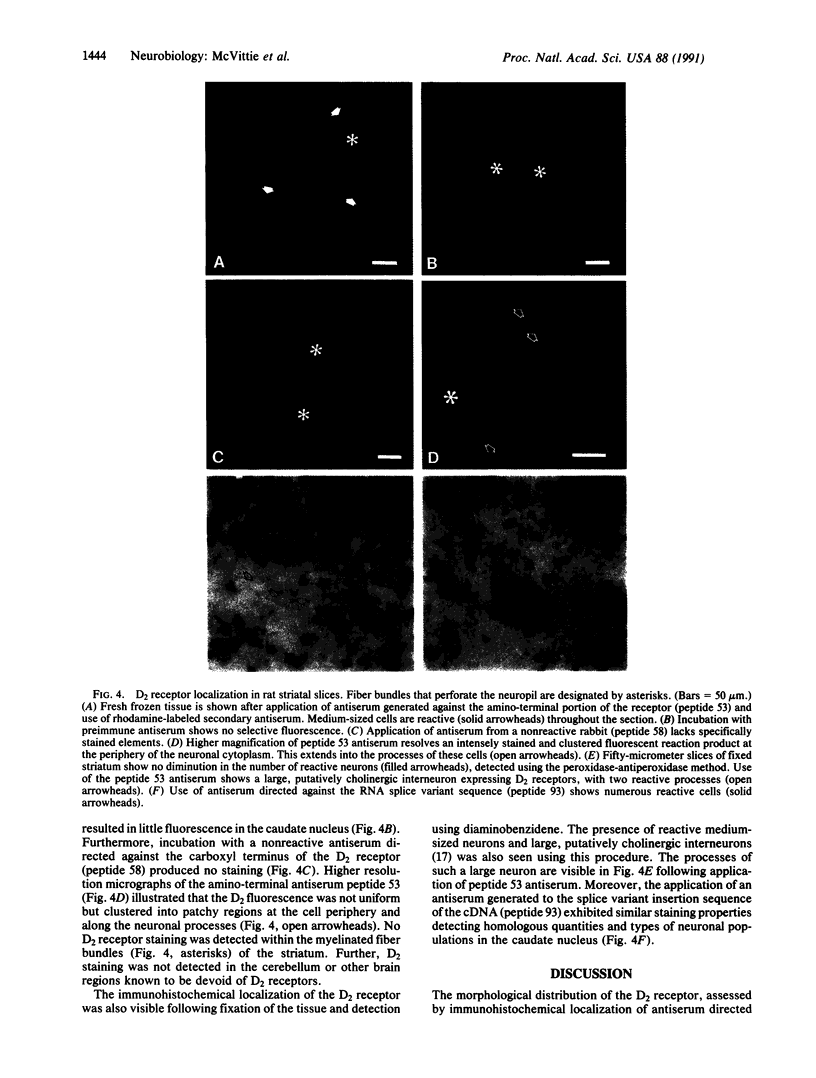
Abstract
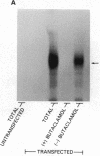
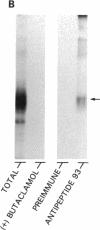
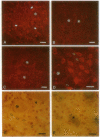
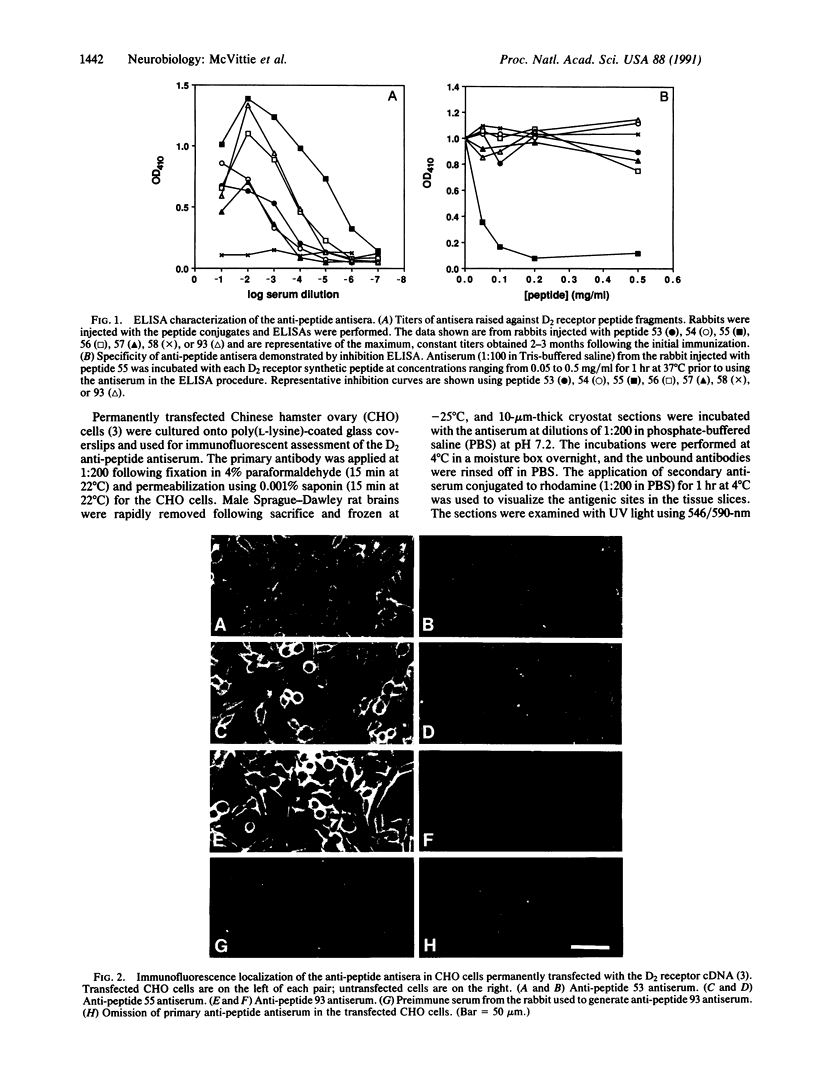
Seven different peptides of 14-23 residues in length based on the predicted amino acid sequence of the cloned rat D2 receptor cDNA were used as immunogens to develop antibodies in rabbits. Two of these peptides were derived from the amino terminus and four were from the third cytoplasmic loop, including one to the splice variant insertion sequence and one to the carboxyl terminus of the receptor protein. These peptides were conjugated to bovine thyroglobulin prior to rabbit immunization. Antibody production was monitored by a solid-phase ELISA. With the exception of the carboxyl-terminal peptide, all of the peptide immunogens produced antiserum of high titer ranging from 1:10(4) to 1:10(6) on ELISA. Specificity of the reaction was demonstrated by the absence of a response in the preimmune serum and by the absence of cross-reactivity between the various antisera and the nonimmunization peptides. Moreover, preincubation of the antiserum with the immunization peptide, but not other peptides, blocked the subsequent ELISA reactions. Some of the antisera were additionally characterized by immunodot assays using solubilized rat striatal membranes blotted onto nitrocellulose. Positive reactions with antiserum dilutions of 1:500 were observed that were dependent on the presence and concentration of membrane protein and were not observed using preimmune serum. Additionally, immunofluorescent staining by the D2 receptor antiserum was observed on cells that had been transfected with the D2 receptor cDNA but not on untransfected cells. Immunoprecipitation of the photoaffinity-labelled and solubilized D2 receptor also suggested that the antisera were able to directly recognize the native receptor protein. Immunohistochemical localization of the D2 receptor in slices of fresh frozen and perfusion-fixed rat brain was performed using these antisera. Within the striatum, about 50% of the medium-sized neurons were labeled as well as large, putatively cholinergic interneurons.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert P. R., Neve K. A., Bunzow J. R., Civelli O. Coupling of a cloned rat dopamine-D2 receptor to inhibition of adenylyl cyclase and prolactin secretion. J Biol Chem. 1990 Feb 5;265(4):2098–2104. [PubMed] [Google Scholar]
- Altar C. A., Marien M. R. Picomolar affinity of 125I-SCH 23982 for D1 receptors in brain demonstrated with digital subtraction autoradiography. J Neurosci. 1987 Jan;7(1):213–222. doi: 10.1523/JNEUROSCI.07-01-00213.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amlaiky N., Caron M. G. Identification of the D2-dopamine receptor binding subunit in several mammalian tissues and species by photoaffinity labeling. J Neurochem. 1986 Jul;47(1):196–204. doi: 10.1111/j.1471-4159.1986.tb02850.x. [DOI] [PubMed] [Google Scholar]
- Ariano M. A., Monsma F. J., Jr, Barton A. C., Kang H. C., Haugland R. P., Sibley D. R. Direct visualization and cellular localization of D1 and D2 dopamine receptors in rat forebrain by use of fluorescent ligands. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8570–8574. doi: 10.1073/pnas.86.21.8570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariano M. A. Striatal D1 dopamine receptor distribution following chemical lesion of the nigrostriatal pathway. Brain Res. 1988 Mar 8;443(1-2):204–214. doi: 10.1016/0006-8993(88)91614-9. [DOI] [PubMed] [Google Scholar]
- Boyson S. J., McGonigle P., Molinoff P. B. Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. J Neurosci. 1986 Nov;6(11):3177–3188. doi: 10.1523/JNEUROSCI.06-11-03177.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Toso R., Sommer B., Ewert M., Herb A., Pritchett D. B., Bach A., Shivers B. D., Seeburg P. H. The dopamine D2 receptor: two molecular forms generated by alternative splicing. EMBO J. 1989 Dec 20;8(13):4025–4034. doi: 10.1002/j.1460-2075.1989.tb08585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson T. M., Barone P., Sidhu A., Wamsley J. K., Chase T. N. Quantitative autoradiographic localization of D-1 dopamine receptors in the rat brain: use of the iodinated ligand [125I]SCH 23982. Neurosci Lett. 1986 Aug 4;68(3):261–266. doi: 10.1016/0304-3940(86)90499-4. [DOI] [PubMed] [Google Scholar]
- Giros B., Sokoloff P., Martres M. P., Riou J. F., Emorine L. J., Schwartz J. C. Alternative splicing directs the expression of two D2 dopamine receptor isoforms. Nature. 1989 Dec 21;342(6252):923–926. doi: 10.1038/342923a0. [DOI] [PubMed] [Google Scholar]
- Grandy D. K., Marchionni M. A., Makam H., Stofko R. E., Alfano M., Frothingham L., Fischer J. B., Burke-Howie K. J., Bunzow J. R., Server A. C. Cloning of the cDNA and gene for a human D2 dopamine receptor. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9762–9766. doi: 10.1073/pnas.86.24.9762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebabian J. W., Calne D. B. Multiple receptors for dopamine. Nature. 1979 Jan 11;277(5692):93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff J. H., Mansour A., Bunzow J. R., Van Tol H. H., Watson S. J., Jr, Civelli O. Distribution of D2 dopamine receptor mRNA in rat brain. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7625–7628. doi: 10.1073/pnas.86.19.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengod G., Martinez-Mir M. I., Vilaró M. T., Palacios J. M. Localization of the mRNA for the dopamine D2 receptor in the rat brain by in situ hybridization histochemistry. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8560–8564. doi: 10.1073/pnas.86.21.8560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsma F. J., Jr, McVittie L. D., Gerfen C. R., Mahan L. C., Sibley D. R. Multiple D2 dopamine receptors produced by alternative RNA splicing. Nature. 1989 Dec 21;342(6252):926–929. doi: 10.1038/342926a0. [DOI] [PubMed] [Google Scholar]
- Phelps P. E., Houser C. R., Vaughn J. E. Immunocytochemical localization of choline acetyltransferase within the rat neostriatum: a correlated light and electron microscopic study of cholinergic neurons and synapses. J Comp Neurol. 1985 Aug 15;238(3):286–307. doi: 10.1002/cne.902380305. [DOI] [PubMed] [Google Scholar]
- Stoof J. C., De Boer T., Sminia P., Mulder A. H. Stimulation of D2-dopamine receptors in rat neostriatum inhibits the release of acetylcholine and dopamine but does not affect the release of gamma-aminobutyric acid, glutamate or serotonin. Eur J Pharmacol. 1982 Oct 22;84(3-4):211–214. doi: 10.1016/0014-2999(82)90204-7. [DOI] [PubMed] [Google Scholar]
- Weick B. G., Walters J. R. Effects of D1 and D2 dopamine receptor stimulation on the activity of substantia nigra pars reticulata neurons in 6-hydroxydopamine lesioned rats: D1/D2 coactivation induces potentiated responses. Brain Res. 1987 Mar 10;405(2):234–246. doi: 10.1016/0006-8993(87)90293-9. [DOI] [PubMed] [Google Scholar]