Abstract
Background
The aim was to assess the influence of long-term treatment with tumor necrosis factor alpha (TNF-α) inhibitors on total cholesterol (TC), triglycerides (TG), low-density lipoprotein (LDL), high-density lipoprotein (HDL), and atherogenic index (AI) in rheumatoid arthritis (RA), psoriatic arthritis (PsA), and ankylosing spondylitis (AS) patients.
Methods
A retrospective cohort study was conducted on RA, PsA, and AS patients treated with TNF-α inhibitors for at least 270 days between 2001 and 2011. Levels of TC, TG, LDL, and HDL and the AI were compared with baseline values at 0–6, 6–12, 12–18, and 18–24 months. Patients were further subdivided into three groups according to their HMG CoA reductase inhibitor (statin) treatment status in order to assess their effect on the results.
Results
The records of 311 patients (152 RA, 90 PsA, and 69 AS) were reviewed. TC and TG increased following treatment with TNF-α inhibitors, from 180.85 ± 2.12 mg/dl and 116.00 ± 3.55 mg/dl at baseline to 188.12 ± 2.35 mg/dl (p = 0.02) and 132.02 ± 4.63 mg/dl at 0–6 months (p < 0.01), respectively, and to 184.88 ± 2.09 mg/dl (p = 0.02) and 129.36 ± 4.32 mg/dl at 18–24 months (p < 0.01), respectively. AI increased following treatment with TNF-α inhibitors, from –0.032 ± 0.017 at baseline to 0.004 ± 0.019 at 18–24 months (p < 0.01). LDL decreased significantly in patients who were treated with statins before and during the entire study period, from 119.97 ± 2.86 mg/dl at baseline to 104.02 ± 3.57 mg/dl at 18–24 months (p < 0.01), in contrast to an increase in LDL values in patients who did not receive statins during the study.
Conclusions
TNF-α inhibitor treatment was associated with a significant increase in TC and TG levels and the AI. Adding statins to the treatment was associated with a significant decrease in LDL levels.
Keywords: TNF-α inhibitors, Lipid profile, Rheumatoid arthritis, Ankylosing spondylitis, Psoriatic arthritis
Background
Atherosclerosis is a major cause of morbidity and mortality in systemic autoimmune diseases. Inflammation is central to the pathogenesis of atherosclerosis and is an important risk factor for vascular disease [1]. An increase in the atherogenic index (AI) is associated with an increase in the atherosclerosis process [2]. It is therefore important to treat cardiovascular risk factors, including dyslipidemia, in these patients.
The influence of tumor necrosis factor alpha (TNF-α) inhibitors on lipid profile [3–17] and AI [3, 5, 7–9, 11, 13, 14, 17] in patients with rheumatologic diseases has been examined in several studies (Table 1), with inconsistent results. In some studies TNF-α inhibitors promoted an atherogenic lipid profile, while in others there was no such effect. None of the studies assessed the time-dependent impact of TNF-α inhibitors on the lipid profile of ankylosing spondylitis (AS), psoriatic arthritis (PsA), and rheumatoid arthritis (RA) patients as a group.
Table 1.
Literature on the influence of TNF-α inhibitors on lipid profile
| Author | Publication year | Place | Follow up (weeks) | Patient number | Disease | Drug | Research type | TCa | TGa | LDLa | HDLa | AIa |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Popa et al. [4] | 2005 | The Netherlands | 2 | 33 | RA | ADA | Prospective | ↑ | ↔ | ↔ | ↑ | |
| Vis et al. [6] | 2005 | The Netherlands | 6 | 69 | RA | INF | Prospective | ↑ | ↑ | |||
| Seriolo et al. [8] | 2006 | Italy | 24 | 34 | RA | ETA, ADA, INF | Prospective | ↑ | ↔ | ↑ | ↔ | |
| Allanore et al. [10] | 2006 | France | 30 | 56 | RA | INF | Prospective | ↑ | ↑ | ↑ | ||
| Popa et al. [9] | 2007 | The Netherlands | 52 | 55 | RA | INF | Prospective | ↑ | ↑ | ↔ | ↔ | ↑ |
| Tam et al. [11] | 2007 | China | 14 | 19 | RA | INF | Prospective | ↑ | ↑ | ↑ | ↑ | ↔ |
| Peters et al. [13] | 2007 | The Netherlands | 48 | 80 | RA | INF | Prospective | ↔ | ↔ | ↔ | ↔ | |
| Wijbrandts et al. [16] | 2009 | The Netherlands | 52 | 50 | RA | ADA | Prospective | ↔ | ↔ | ↔ | ↔ | |
| Mathieu et al. [7] | 2010 | France | 14 | 34 | AS | ETA, ADA, INF | Prospective | ↑ | ↔ | ↔ | ↑ | ↔ |
| Castro et al. [12] | 2011 | Brazil | 3 | 15 | PA | INF | Prospective | ↔ | ↑ | ↔ | ↔ | |
| Daïen et al. [3] | 2012 | 2–52 | 13 articles | RA | ETA, ADA, INF | Systematic review, meta-analysis | ↑ | ↑ | ↔ | ↑ | ↔ | |
| Curtis et al. [5] | 2012 | United States | Median > 2 years | 289 | RA | TNFi | Retrospective | ↑ | ↑ | ↑ | ↑ | ↔ |
| Di Minno et al. [14] | 2014 | 2–52 | 1707 | RA | TNFi | Meta-analysis | ↑ | ↑ | ↔ | ↑ | ↔ | |
| 30 articles | PsA | |||||||||||
| AS | ||||||||||||
| Souto et al. [15] | 2015 | 12–24 | 25 articles | RA | ADA, INF | Systematic review, meta-analysis | ↔ | ↔ | ↔ | ↔ | ||
| Chen et al. [25] | 2015 | Taiwan | 24 | 48 | RA | ETA, ADA | Prospective | ↔ | ↔ | ↔ | ↑ | ↔ |
| Present study | 2016 | Israel | 104 | 311 | RA | TNFi | Retrospective | ↑ | ↑ | ↔ | ↔ | ↑ |
| PsA | ||||||||||||
| AS |
aOnly significant changes (p ≤ 0.05) at the end of the follow-up period were included
TNF-α tumor necrosis factor alpha, TC total cholesterol, TG triglycerides, LDL low-density lipoprotein, HDL high-density lipoprotein, AI, atherogenic index, RA rheumatoid arthritis, AS ankylosing spondylitis, PsA psoriatic arthritis, ADA adalimumab, INF infliximab, ETA etanercept, TNFi TNF inhibitors
↑ Increased, ↔ Unchanged
Our objective was to assess the influence of TNF-α inhibitors treatment on the lipid profile and the AI of patients with AS, PsA, and RA at various time points up to 2 years of treatment.
Methods
Study population
A retrospective cohort analysis was conducted on the database of Clalit Health Services (CHS) in Haifa and Western Galilee districts in northern Israel. CHS is the biggest healthcare provider in Israel, with over 1 million members in this area (approximately 50 % of the total population of the region). CHS maintains a comprehensive computerized database with continuous input from pharmaceutical, medical, laboratory, and administrative computerized operators. The CHS database and our study cohort were described in a previous study [18]. Briefly, the database for biological agents included in the Israeli ‘health basket’ contains diagnoses of specific rheumatic diseases as determined by a rheumatologist. The data are linked through a unique national identification number to the pharmaceutical, medical, and laboratory databases.
Medical charts of patients who met the following criteria were reviewed: minimum 18 years old; diagnosis under one of the codes—‘rheumatoid arthritis’, ‘psoriatic arthritis’, and ‘ankylosing spondylitis’—and approval for biologic treatment included in the Israeli health basket; treated with TNF-α inhibitors between 2001 and 2011; began TNF-α inhibitors during the study period and were treated for at least 270 consecutive days; and had baseline lipid levels measured before starting treatment with TNF-α inhibitors and at least three lipid profile tests during the four time periods (0–6 months, 6–12 months, 12–18 months, and 18–24 months) (Fig. 1).
Fig. 1.
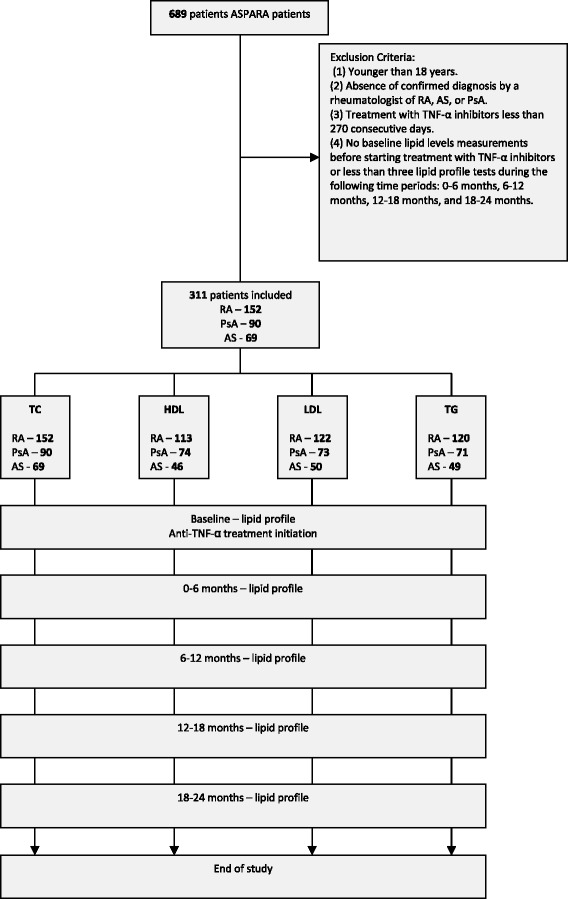
Study flow. TNF-α tumor necrosis factor alpha, TC total cholesterol, TG triglycerides, LDL low-density lipoprotein, HDL high-density lipoprotein, RA rheumatoid arthritis, AS ankylosing spondylitis, PsA psoriatic arthritis
The following data were collected: demographics (age, gender); rheumatologist diagnosis (RA, PsA, AS); comorbidities (diabetes, hypertension, hyperlipidemia, ischemic heart disease); type and dates of pharmacy-dispensed medication (TNF-α inhibitors, steroids, disease-modifying anti-rheumatic drugs (DMARDs), HMG CoA reductase inhibitors (statins), fibrates); diabetes treatment; and laboratory tests results—lipid profile that included total cholesterol (TC), triglycerides (TG), low-density lipoprotein (LDL), and high-density lipoprotein (HDL). AI was calculated by the following formula: AI = log (TG/HDL), with TG and HDL expressed in molar concentrations [2].
The patients were subdivided into three groups according to statin treatment:
Patients not treated with statins.
Patients who started statin therapy during the study period, after the initiation of treatment with TNF-α inhibitors.
Patients who were treated with statins prior to and during the entire study period.
Patients from Groups 2 and 3 were included only if the type and dose of statin did not change during the study period. Patients treated with fibrates, which are known to reduce TG levels [19], were excluded from the analysis in the TG group.
Statistical methods
Descriptive statistics are presented with continuous variables expressed as mean or median and standard deviation or standard error and categorical variables as frequencies and proportions. Comparisons of continuous patient characteristics among the three diagnostic groups (RA, PsA, AS) were performed by analysis of variance (ANOVA) or Kruskal–Wallis test, according to data distribution. Categorical variables were compared using the chi-square test.
The effect of TNF-α inhibitors therapy on lipid profile was assessed by comparing the levels of lipid particles at each time point with the baseline prior to treatment. Each lipid particle was analyzed in univariable and multivariable models, adjusted for the following study parameters: hyperlipidemia, statin treatment, steroid treatment, rheumatic diseases (RA, PsA, AS), obesity, smoking, diabetes, hypertension, ischemic heart disease, gender, and age.
Comparisons at various times were performed by repeated-measures mixed-model ANOVA. This procedure takes into account the intracorrelation of repeated measurements carried out on the same subject and does not exclude subjects with incomplete data at follow-up. Stratification analysis by statin use was employed in a multivariable model in patients treated concomitantly with a TNF-α inhibitor and statins after adjustment for hyperlipidemia, hypertension, obesity, gender, and age.
The analyses were conducted with the PASW (SPSS) 19 statistical package (PASW Software Statistic, SPSS, Chicago, IL, USA). All statistical tests were two-sided and statistical significance was considered p ≤ 0.05.
Results
Study population
Of the 689 patients treated with TNF-α inhibitors between 2001 and 2011, 311 met the inclusion criteria: 152 RA patients, 90 PsA patients, and 69 AS patients. The mean age of RA and PsA patients was higher (47.6 ± 19.3 and 50.2 ± 13.4 years, respectively) than that of AS patients (39.7 ± 15.2 years, p <0. 01). As expected, there were more females in the RA group (74.3 %) than in the AS group (36.0 %, p < 0.01). Corticosteroids were used more often in the RA group (58.7 %) than in the PsA and AS groups (29.2 % and 26.6 %, respectively, p < 0.01). Diabetes, hyperlipidemia, and hypertension were more prevalent in the PsA group (19.0 %, p = 0.03; 52.2 %, p = 0.02; and 40.0 %, p = 0.02, respectively) than in the RA group (11.2 %, 37.5 %, and 36.2 %, respectively) and the AS group (1.4 %, 32.0 %, and 20.3 %, respectively). The incidence of ischemic heart disease did not differ significantly between the groups. Smoking was more prevalent in the AS group (45.0 %, p = 0.02) than in the RA and PsA groups (26.3 % and 30.0 %, respectively). Baseline values of TC and LDL did not differ significantly between the groups. HDL and TG levels were significantly lower in patients with AS. Higher TG levels were found in PsA patients. There was no difference at baseline between the three groups in the percentage of patients treated with statins (Table 2). We excluded nine patients treated with fibrates from the TG group. These nine patients were treated with a constant dose of fibrates throughout the study and exhibited no significant changes in HDL and LDL values when TNF-α inhibitor was introduced; they were included in the TC, HDL, and LDL analysis.
Table 2.
Baseline characteristics of patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis
| Rheumatoid arthritis | Psoriatic arthritis | Ankylosing spondylitis | p value | ||
|---|---|---|---|---|---|
| (n = 152) | (n = 90) | (n = 69) | |||
| Age (years) | 47.6 ± 19.3 | 50.2 ± 13.4 | 39.7 ± 15.2 | <0.0001 | |
| 50.0 | 52.0 | 38 | |||
| Baseline (mg/dl) | TCa,b | 180.6 ± 37.6 | 186.4 ± 35.2 | 174.3 ± 38.9 | NS |
| 183.0 | 185.0 | 172.0 | |||
| HDLa,c | 53.92 ± 15.67 | 51.23 ± 14.51 | 46.40 ± 13.31 | 0.003 | |
| 52.08 | 48.25 | 43.38 | |||
| LDLa,d | 108.77 ± 25.63 | 114.43 ± 22.31 | 111.67 ± 34.09 | NS | |
| 110.70 | 117.14 | 109.39 | |||
| TGa,e | 111.35 ± 53.44 | 128.36 ± 57.32 | 109.49 ± 53.87 | 0.04 | |
| 96.46 | 119.00 | 106.00 | |||
| Gender | Male | 39 (25.7) | 26 (28.9) | 44 (64.0) | <0.0001 |
| Female | 113 (74.3) | 64 (71.1) | 25 (36.0) | ||
| Statin treatment | Treated before and during treatment with TNF-α inhibitors | 25 (18.4) | 22 (24.4) | 9 (13.0) | NS |
| Began treatment after beginning TNF-α inhibitors treatment | 18 (11.8) | 11 (12.2) | 8 (11.6) | ||
| Medication | Classical DMARDsf | 93 (62.0) | 35 (39.3) | 20 (31.3) | <0.0001 |
| Steroids | 88 (58.7) | 26 (29.2) | 17 (26.6) | <0.0001 | |
| Smoking | Current or past | 40 (26.3) | 27 (30.0) | 31 (44.9) | 0.02 |
| Obesity | 27 (17.8) | 22 (24.4) | 6 (8.7) | 0.04 | |
| Hyperlipidemia | 57 (37.5) | 47 (52.2) | 22 (32.0) | 0.02 | |
| Diabetes | 17 (11.2) | 17 (18.9) | 1 (1.4) | 0.003 | |
| Hypertension | 55 (36.2) | 36 (40.0) | 14 (20.3) | 0.02 | |
| Ischemic heart disease | 21 (13.8) | 7 (7.8) | 4 (5.8) | NS | |
Data presented as standard deviation with median below, or as n (%)
aBecause of missing values of lipid profile at various time points, the number of patients for each lipid profile test differed
bTC: 152 RA, 90 PsA, and 69 AS patients
cHDL: 113 RA, 74 PsA, and 46 AS patients
dLDL: 122RA, 73 PsA, and 50 AS patients
eTG: 120 RA, 71 PsA, and 49 AS patients
fClassical DMARDs: leflunomide, azathioprine, cyclosporin, mercaptopurine, methotrexate, hydroxychloroquine, sulfasalazine
TC total cholesterol, HDL high-density lipoprotein, LDL low-density lipoprotein, TG, triglycerides, TNF-α tumor necrosis factor alpha, DMARD disease-modifying anti-rheumatic drug, RA rheumatoid arthritis, AS ankylosing spondylitis, PsA psoriatic arthritis, NS not significant
Lipid profile
TC and TG levels increased following TNF-α inhibitor treatment, mainly during the first 6 months of treatment, and decreased thereafter but remained significantly higher than baseline at last follow-up (Table 3). LDL and HDL levels did not change significantly following introduction of TNF-α inhibitors.
Table 3.
Effects of TNF-α inhibitor treatment on lipid profile
| Time | ||||||
|---|---|---|---|---|---|---|
| Baseline value before treatment | 0–6 months | 6–12 months | 12–18 months | 18–24 months | ||
| Total cholesterol (mg/dl) | Mean ± standard error | 180.85 ± 2.12 | 188.12 ± 2.35 | 183.80 ± 2.14 | 184.48 ± 2.08 | 184.88 ± 2.09 |
| p value | <0.0001 | NS | 0.030 | 0.015 | ||
| Multivariate modela
p value |
0.018 | NS | 0.041 | 0.018 | ||
| HDL (mg/dl) | Mean ± standard error | 51.8 ± 1.07 | 53.55 ± 1.21 | 51.06 ± 1.13 | 52.04 ± 1.18 | 52.05 ± 1.18 |
| p value | NS | NS | NS | NS | ||
| Multivariate modela
p value |
NS | NS | NS | NS | ||
| LDL (mg/dl) | Mean ± standard error | 108.70 ± 1.55 | 109.00 ± 1.92 | 108.42 ± 1.76 | 108.54 ± 1.75 | 108.67 ± 1.70 |
| p value | NS | NS | NS | NS | ||
| Multivariate modela
p value |
NS | NS | NS | NS | ||
| Triglycerides (mg/dl) | Mean ± standard error | 116.00 ± 3.55 | 132.02 ± 4.63 | 124.47 ± 3.95 | 128.72 ± 4.17 | 129.36 ± 4.32 |
| p value | <0.0001 | 0.005 | <0.0001 | <0.0001 | ||
| Multivariate modela
p value |
<0.0001 | 0.004 | <0.0001 | <0.0001 | ||
| Atherogenic index (LogTG/HDL) | Mean ± standard error | –0.032 ± 0.017 | –0.001 ± 0.02 | –0.01 ± 0.019 | 0.009 ± 0.018 | 0.004 ± 0.019 |
| p value | 0.005 | 0.043 | 0.001 | 0.003 | ||
| Multivariate modela
p value |
0.007 | 0.04 | 0.001 | 0.004 | ||
aAdjusted for hyperlipidemia, statin treatment, steroid treatment, rheumatic diseases (RA, AS, PsA), obesity, smoking, diabetes, hypertension, ischemic heart disease, gender, and age
TNF-α tumor necrosis factor alpha, HDL high-density lipoprotein, LDL low-density lipoprotein, TG, triglycerides, NS not significant, RA rheumatoid arthritis, AS ankylosing spondylitis, PsA psoriatic arthritis
The same pattern was found in the multivariate model after adjustment for hyperlipidemia, statin treatment, steroid treatment, rheumatic diseases (RA, PsA, AS), obesity, smoking, diabetes, hypertension, ischemic heart disease, gender, and age.
Atherogenic index
The AI increased significantly following TNF-α inhibitor treatment (Table 3). The same increase was found in the multivariate model after adjustment for hyperlipidemia, statin treatment, steroid treatment, rheumatic diseases (RA, PsA, AS), obesity, smoking, diabetes, hypertension, ischemic heart disease, gender, and age.
Co-administration of TNF-α inhibitors and statins
The effect of the interaction between TNF-α inhibitors and statins on the various lipid parameters after adjustment for hyperlipidemia, hypertension, obesity, gender, and age reached statistical significance for LDL (p < 0.01), TC (p = 0.02), and TG (p < 0.01) values (Table 4). No effect of the interaction was found in HDL values and therefore data were not analyzed further.
Table 4.
Effects of TNF-α inhibitor treatment on lipid profile: stratification analysis by statin use
| No statins | Statin treatment begun after initiating TNF-α inhibitor treatment | Statin treatment begun before and continued during TNF-α inhibitor treatment | |||||
|---|---|---|---|---|---|---|---|
| Time period | Mean ± standard error | p value | Mean ± standard error | p value | Mean ± standard error | p value | |
| Total cholesterola (mg/dl) | Reference | 183.73 ± 3.26 | 188.96 ± 8.92 | 182.04 ± 5.65 | |||
| 0–6 months | 188.63 ± 3.42 | 0.007 | 208.22 ± 9.02 | 0.005 | 188.78 ± 5.73 | NS | |
| 6–12 months | 185.20 ± 3.29 | NS | 197.92 ± 8.74 | NS | 184.05 ± 5.60 | NS | |
| 12–18 months | 187.77 ± 3.22 | 0.03 | 198.55 ± 9.15 | 0.04 | 180.42 ± 5.48 | NS | |
| 18–24 months | 188.62 ± 3.34 | NS | 199.38 ± 8.46 | 0.01 | 178.89 ± 5.14 | NS | |
| LDLa (mg/dl) | Reference | 103.13 ± 3.68 | 112.10 ± 5.62 | 119.97 ± 2.86 | |||
| 0–6 months | 106.01 ± 3.83 | 0.057 | 123.42 ± 7.62 | 0.05 | 107.84 ± 3.96 | <0.001 | |
| 6–12 months | 105.63 ± 3.80 | NS | 115.35 ± 7.05 | NS | 108.26 ± 4.07 | <0.001 | |
| 12–18 months | 106.85 ± 3.80 | 0.02 | 119.67 ± 6.38 | 0.03 | 101.85 ± 3.57 | <0.001 | |
| 18–24 months | 107.70 ± 3.76 | 0.001 | 115.89 ± 6.81 | NS | 104.02 ± 3.57 | <0.001 | |
| Triglyceridesa (mg/dl) | Reference | 119.41 ± 9.75 | 179.31 ± 19.96 | 142.14 ± 9.89 | |||
| 0–6 months | 134.33 ± 10.24 | <0.001 | 223.91 ± 24.84 | 0.002 | 147.33 ± 10.67 | NS | |
| 6–12 months | 128.80 ± 10.05 | 0.02 | 186.09 ± 20.47 | NS | 145.81 ± 10.15 | NS | |
| 12–18 months | 135.89 ± 10.19 | 0.001 | 181.31 ± 20.98 | NS | 149.60 ± 10.16 | NS | |
| 18–24 months | 135.99 ± 10.20 | <0.001 | 194.68 ± 23.42 | NS | 146.72 ± 10.82 | NS | |
aAdjusted for hyperlipidemia, hypertension, obesity, gender, and age
TNF-α tumor necrosis factor alpha, LDL low-density lipoprotein, NS not significant
LDL levels rose higher than baseline values (103.13 ± 3.68 mg/dl) in patients treated with TNF-α inhibitors but not with statins, reaching statistical significance at 12–18 months (106.85 ± 3.80 mg/dl, p = 0.024) and 18–24 months (107.70 ± 3.76 mg/dl, p = 0.001). LDL in patients who began statins after the initiation of TNF-α inhibitors reached peak value at 6 months and 12–18 months. In contrast, LDL decreased significantly in patients who were treated with statins before starting TNF-α inhibitors and continued throughout the study: from 119.97 ± 2.86 mg/dl at baseline to 107.84 ± 3.96 mg/dl at 0–6 months (p < 0.01), 108.26 ± 4.07 mg/dl at 6–12 months (p < 0.01), 101.85 ± 3.57 mg/dl at 12–18 months (p < 0.01), and 104.02 ± 3.57 mg/dl at 18–24 months (p < 0.01) (Table 4).
TC increased in patients not treated with TNF-α inhibitors or statins, from 183.73 ± 3.26 mg/dl at baseline to 188.63 ± 3.42 mg/dl at 0–6 months (p < 0.01) and 187.77 ± 3.22 mg/dl at 12–18 months (p = 0.03). In patients who began statins after the initiation of TNF-α inhibitors, TC increased from 188.96 ± 8.92 mg/dl at baseline to 208.22 ± 9.02 mg/dl at 0–6 months (p < 0.01), 198.55 ± 9.15 mg/dl at 12–18 months (p = 0.04), and 199.38 ± 8.46 mg/dl at 18–24 months (p = 0.01). TC values in patients treated with statins before initiation of TNF-α inhibitors did not change significantly.
The same pattern was observed in TG values, which increased significantly in patients treated with TNF-α inhibitors but not statins, and in patients who began statins after the initiation of TNF-α inhibitors, but only in the first 6 months. TG values increased, but not significantly, in patients treated with statins before TNF-α inhibitor initiation and during the whole study period (Table 4).
Discussion
The demographic and clinical characteristics of our cohort are consistent with those described in the literature. AS patients were young and most were males, while RA patients were older, needed more treatment with corticosteroids, and most were females [20, 21]. A large meta-analysis by Armstrong et al. in 2013 [22] showed an increased risk of metabolic syndrome in patients with psoriasis (odds ratio 2.26). According to our data, diabetes, hyperlipidemia, and hypertension were more prevalent in PsA patients than in RA and AS patients (Table 2).
TNF-α inhibitors revolutionized the treatment of inflammatory arthritic conditions [23, 24]. In our study, TNF-α inhibitors significantly increased the AI, TC, and TG levels without significantly changing LDL and HDL levels. They also significantly increased LDL, TC, and TG levels in patients who were not treated with statins during the study. This was in contrast to the decrease in LDL levels in patients who were treated with statins prior to initiation of TNF-α inhibitors and continued treatment throughout the study.
A comparison of our results with those reported in the literature must take into account the different study designs. The 15 studies that assessed the effects of TNF-α inhibitors on lipid profile (Table 1) [3–17] were of shorter duration, ranging from 2 to 30 weeks in nine studies [4, 6–8, 10–12, 15, 17] and up to 52 weeks in six other studies [3, 5, 9, 13, 14, 16]. Follow-up in our study was 104 weeks. We conducted our analysis on pooled data of rheumatic patients in the ASPARA cohort while most of the published studies analyzed a single disease: 12 studies of RA, one study of PsA, and one study of AS. Sample size also varied: 15–80 patients in published studies [4, 6–13, 16, 17] compared with 311 patients in our cohort. Six of the studies were performed in patients treated with infliximab (INF) alone, and the rest included data on different types of TNF-α inhibitors such as INF, etanercept (ETA), and/or adalimumab (ADA) (11 of 15 studies) [3, 4, 6–13, 15, 17]. Our study was performed in patients treated with INF, ETA, or ADA.
Our finding of a significant increase in TC levels following TNF-α inhibitor treatment (Table 3) is in line with the results of 10 of the 15 studies mentioned [3–11, 14]. Six of the 15 studies also reported an increase in TG levels following TNF-α inhibitors [3, 5, 9, 11, 12, 14], similar to our findings, while the other nine showed no significant changes in TG levels. Nine of the 15 studies studied the effect of TNF-α inhibitors on the AI [3, 5, 7–9, 11, 13, 14, 17]. Only one of them showed an increase in the AI [9], similar to our results, while the others did not demonstrate significant changes of the AI. Concomitant treatment with statins, known to reduce TG, TC, LDL, and TNF-α levels [25, 26], could account for the lack of increase in TG levels and consequently the AI in those studies. Three studies that showed an increase in TG values included patients on drugs that affect the lipid profile, such as statins [5, 9, 12]; the studies that did not show significant changes in TG levels did not include them in their calculations [4, 6–8, 10, 13, 15, 16].
Our results showed no significant changes in the HDL and LDL values following TNF-α inhibitor treatment. The 10 studies from the literature [3–8, 10, 11, 14, 17] (Table 1) that did report increased levels of HDL could be explained by the longer follow-up period in our study. Wijbrandts et al. [16] reported a significant increase in HDL levels after 16 weeks of ADA treatment, although the level returned to baseline by the end of the 52-week study. Similarly, HDL levels in our study increased during the first 24 weeks and returned to near baseline levels later, although none of these changes was statistically significant (Table 3). Like in our cohort, nine of the 15 studies that assessed the effects of TNF-α inhibitors on lipid profile [3, 4, 7, 9, 12, 14–17] showed no significant change in LDL level following TNF-α inhibitor treatment. Because this could be due to concomitant treatment with statins, which can lower LDL values [25, 26], we tested whether there was an interaction between TNF-α inhibitor and statin treatment that affected the lipid profile.
LDL, TG, and TC levels increased significantly in patients treated with TNF-α inhibitors but not with statins. LDL and TC levels also increased significantly in patients who began statin therapy during the study, while TG increased significantly only during the first 6 months in this group. LDL values in patients treated with statins before and during the entire study period decreased significantly throughout the study. Thus, concomitant treatment with TNF-α inhibitor and statins may protect against the expected increase in LDL, helping reduce the cardiovascular risk in rheumatic patients in addition to treating the inflammatory component.
To our knowledge, no other study has demonstrated the benefit to LDL levels of adding statin treatment to TNF-α inhibitors. A meta-analysis of 31 observational studies showed that the TNF-α gene is involved in the pathogenesis of the metabolic syndrome (the 308A variant of this gene has been associated with elevated levels of insulin and blood pressure) [27]. Another study reported an association between polymorphisms of TNF-α (TNF-αG-238A) and LDL level [28]. These investigators also found that TNF-α-C-857T—a polymorphism of the TNF-α gene promoter that increases transcription—is associated with increased LDL values and the formation of atherosclerotic carotid plaques in type 2 diabetes patients. Carriers of TNF-α-C-857T had significantly increased LDL values compared with noncarriers. There was no difference in LDL values in patients who were not treated with statins regardless of the carrier state of TNF-α-C-857T. In contrast, carriers of TNF-α-C-857T who were treated with statins had increased LDL levels compared with noncarriers [29]. In other words, there is a link between statin therapy, TNF-α activity level, and LDL. These findings, together with our results, call for further research into the impact on LDL levels of adding statin treatment in patients with increased activity levels of TNF-α (as in carriers who harbor the TNF-α-C-857T polymorphism) compared with patients with low activity levels of TNF-α (such as those receiving TNF-α inhibitors).
There are some limitations in our study. In addition to being a retrospective and observational study without a control group, the study was conducted on a relatively small cohort of patients which included those who were taking various medications, including steroids and fibrates, and had illnesses such as diabetes that could affect lipid metabolism. We dealt with this limitation by constructing statistical models for each lipid profile parameter adjusted for possible confounders (Tables 3 and 4). Being a retrospective study, follow-up data were not complete on all subjects; nevertheless, in order to include them in the analyses, we used repeated measures with mixed models that allow for unbalanced data and calculated the intracorrelation of repeated measurements carried out on the same subject. We estimated the drug intake according to pharma dispensed prescriptions. It would be interesting to evaluate the effects of long-term anti-TNF therapy on carotid artery US findings and its association with lipid profiles changes in further studies.
Conclusions
The study documents the real-life, long-term follow-up and treatment of RA, PsA, and AS patients being treated with TNF-α inhibitors. We found that this treatment promotes an atherogenic lipid profile most pronounced in patients not treated by statins, evidenced by a significant increase in the AI, LDL, TC, and TG levels. Statin therapy before and during treatment with TNF-α inhibitors may protect against the expected rise in LDL values. While there were negative changes in the lipid profile under TNF-α inhibitor treatment, they were relatively small and not statistically significant, and their clinical significance is unclear.
Coronary disease is the main cause of death in rheumatic diseases and the importance of reducing cardiovascular risk factors, including dyslipidemia, in these patients cannot be overemphasized. Prospective studies on the long-term effects of TNF-α inhibitors on lipid profile, with and without statins, are required to assess the cardiovascular risk in these patients.
Acknowledgements
Not applicable
Funding
Not applicable
Availability of supporting data
Not applicable
Authors’ contributions
SH participated in the study design and coordination, obtained data, participated in the statistical analysis, and wrote the manuscript. UM obtained data, participated in the study design, and drafted the manuscript. JF participated in analysis and interpretation of data and drafted the manuscript. LE participated in the design of the study and drafted the manuscript. IL participated in the design of the study, performed the statistical analysis, and drafted the manuscript. SC participated in the study design, and helped draft the manuscript. DZ conceived the study, participated in its design and coordination, and in analysis and interpretation of data, and drafted the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable
Ethical approval and consent to participate
The study was approved by the ethics committee at Carmel Medical Center. Approval number CMC 06014722. The approval included exemption from individual consent.
Source of support
None declared
Abbreviations
- ADA
Adalimumab
- AI
Atherogenic index
- AS
Ankylosing spondylitis
- DMARD
Disease-modifying anti-rheumatic drug
- ETA
Etanercept
- HDL
High-density lipoprotein
- INF
Infliximab
- LDL
Low-density lipoprotein
- PsA
Psoriatic arthritis
- RA
Rheumatoid arthritis
- TC
Total cholesterol
- TG
Triglycerides
- TNF-α
Tumor necrosis factor alpha
Contributor Information
Shadi Hassan, Email: shadihasan@gmail.com.
Uzi Milman, Email: uzimy@netvision.net.il.
Joy Feld, Email: joyfeld@gmail.com.
Lihi Eder, Email: benlihi@gmail.com.
Idit Lavi, Email: idit_lavi@clalit.org.il.
Shai Cohen, Email: ShaiCo@clalit.org.il.
Devy Zisman, Phone: 972-48250486, Email: devyzisman@gmail.com.
References
- 1.Abou-Raya A, Abou-Raya S. Inflammation: a pivotal link between autoimmune diseases and atherosclerosis. Autoimmun Rev. 2006;5:331–337. doi: 10.1016/j.autrev.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Shen S, Lu Y, Qi H, Li F, Shen Z, Wu L, Yang C, Wang L, Shui K, Wang Y, Qiang D, Yun J, Weng X. Association between ideal cardiovascular health and the atherogenic index of plasma. Med (Baltimore) 2016;95:e3866. doi: 10.1097/MD.0000000000003866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daïen CI, Duny Y, Barnetche T, Daurès JP, Combe B, Morel J. Effect of TNF inhibitors on lipid profile in rheumatoid arthritis: a systematic review with meta-analysis. Ann Rheum Dis. 2012;71:862–868. doi: 10.1136/annrheumdis-2011-201148. [DOI] [PubMed] [Google Scholar]
- 4.Popa C, Netea MG, Radstake T, Van der Meer JW, Stalenhoef AF, van Riel PL, Barerra P. Influence of anti-tumour necrosis factor therapy on cardiovascular risk factors in patients with active rheumatoid arthritis. Ann Rheum Dis. 2005;64:303–305. doi: 10.1136/ard.2004.023119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curtis JR, John A, Baser O. Dyslipidemia and changes in lipid profiles associated with rheumatoid arthritis and initiation of anti-tumor necrosis factor therapy. Arthritis Care Res (Hoboken) 2012;64:1282–1291. doi: 10.1002/acr.21693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vis M, Nurmohamed MT, Wolbink G, Voskuyl AE, de Koning M, van de Stadt R, Twisk JW, Dijkmans BA, Lems WF. Short term effects of infliximab on the lipid profile in patients with rheumatoid arthritis. J Rheumatol. 2005;32:252–255. [PubMed] [Google Scholar]
- 7.Mathieu S, Dubost JJ, Tournadre A, Malochet-Guinamand S, Ristori JM, Soubrier M. Effects of 14 weeks of TNF alpha blockade treatment on lipid profile in ankylosing spondylitis. Joint Bone Spine. 2010;77:50–52. doi: 10.1016/j.jbspin.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Seriolo B, Paolino S, Sulli A, Fasciolo D, Cutolo M. Effects of anti-TNF-alpha treatment on lipid profile in patients with active rheumatoid arthritis. Ann N Y Acad Sci. 2006;1069:414–419. doi: 10.1196/annals.1351.039. [DOI] [PubMed] [Google Scholar]
- 9.Popa C, van den Hoogen FH, Radstake TR, Netea MG, Eijsbouts AE, den Heijer M, van der Meer JW, van Riel PL, Stalenhoef AF, Barrera P. Modulation of lipoprotein plasma concentrations during long-term anti-TNF therapy in patients with active rheumatoid arthritis. Ann Rheum Dis. 2007;66:1503–1507. doi: 10.1136/ard.2006.066191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allanore Y, Kahan A, Sellam J, Ekindjian OG, Borderie D. Effects of repeated infliximab therapy on serum lipid profile in patients with refractory rheumatoid arthritis. Clin Chim Acta. 2006;365:143–148. doi: 10.1016/j.cca.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Tam LS, Tomlinson B, Chu TT, Li TK, Li EK. Impact of TNF inhibition on insulin resistance and lipids levels in patients with rheumatoid arthritis. Clin Rheumatol. 2007;26:1495–1498. doi: 10.1007/s10067-007-0539-8. [DOI] [PubMed] [Google Scholar]
- 12.Castro KR, Aikawa NE, Saad CG, Moraes JC, Medeiros AC, Mota LM, Silva CA, Bonfá E, Carvalho JF. Infliximab induces increase in triglyceride levels in psoriatic arthritis patients. Clin Dev Immunol. 2011;2011:352686. doi: 10.1155/2011/352686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peters MJ, Vis M, van Halm VP, Wolbink GJ, Voskuyl AE, Lems WF, Dijkmans BA, Twisk JW, de Koning MH, van de Stadt RJ, Nurmohamed MT. Changes in lipid profile during infliximab and corticosteroid treatment in rheumatoid arthritis. Ann Rheum Dis. 2007;66:958–961. doi: 10.1136/ard.2006.059691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Minno MN, Ambrosino P, Peluso R, Di Minno A, Lupoli R, Dentali F, CaRRDs Study Group Lipid profile changes in patients with rheumatic diseases receiving a treatment with TNF-α blockers: a meta-analysis of prospective studies. Ann Med. 2014;46:73–83. doi: 10.3109/07853890.2013.874661. [DOI] [PubMed] [Google Scholar]
- 15.Souto A, Salgado E, Maneiro JR, Mera A, Carmona L, Gómez-Reino JJ. Lipid profile changes in patients with chronic inflammatory arthritis treated with biologic agents and tofacitinib in randomized clinical trials: a systematic review and meta-analysis. Arthritis Rheumatol. 2015;67:117–127. doi: 10.1002/art.38894. [DOI] [PubMed] [Google Scholar]
- 16.Wijbrandts CA, van Leuven SI, Boom HD, Gerlag DM, Stroes EG, Kastelein JJ, Tak PP. Sustained changes in lipid profile and macrophage migration inhibitory factor levels after anti-tumour necrosis factor therapy in rheumatoid arthritis. Ann Rheum Dis. 2009;68:1316–1321. doi: 10.1136/ard.2007.086728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen DY, Chen YM, Hsieh TY, Hsieh CW, Lin CC, Lan JL. Significant effects of biologic therapy on lipid profiles and insulin resistance in patients with rheumatoid arthritis. Arthritis Res Ther. 2015;17:52. doi: 10.1186/s13075-015-0559-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zisman D, Haddad A, Hashoul S, Laor A, Bitterman H, Rosner I, Eder L, Balbir-Gurman A, Mader R, Milman U. Hospitalizations of patients treated with anti-tumor necrosis factor-α agents—a retrospective cohort analysis. J Rheumatol. 2013;40:16–22. doi: 10.3899/jrheum.111516. [DOI] [PubMed] [Google Scholar]
- 19.Koh KK, Quon MJ, Shin KC, Lim S, Lee Y, Sakuma I, Lee K, Han SH, Shin EK. Significant differential effects of omega-3 fatty acids and fenofibrate in patients with hypertriglyceridemia. Atherosclerosis. 2012;220:537–544. doi: 10.1016/j.atherosclerosis.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 20.Liao KP, Karlson EW. Classification and epidemiology of rheumatoid arthritis. In: Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, editors. Rheumatology. 6. St. Louis: Mosby (Elsevier) Publishing; 2015. pp. 691–697. [Google Scholar]
- 21.Rudwaleit M. Classification and epidemiology of spondyloarthritis. In: Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, editors. Rheumatology. 6. St. Louis: Mosby (Elsevier) Publishing; 2015. pp. 941–945. [Google Scholar]
- 22.Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and metabolic syndrome: a systematic review and meta-analysis of observational studies. J Am Acad Dermatol. 2013;68:654–662. doi: 10.1016/j.jaad.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 23.Dixon WG, Watson KD, Lunt M, Hyrich KL, British Society for Rheumatology Biologics Register Control Centre Consortium. British Society for Rheumatology Biologics Register. Silman AJ, Symmons DP. Reduction in the incidence of myocardial infarction in patients with rheumatoid arthritis who respond to anti-tumor necrosis factor alpha therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2007;56:2905–2912. doi: 10.1002/art.22809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobsson LT, Turesson C, Nilsson JA, Petersson IF, Lindqvist E, Saxne T, Geborek P. Treatment with TNF blockers and mortality risk in patients with rheumatoid arthritis. Ann Rheum Dis. 2007;66:670–675. doi: 10.1136/ard.2006.062497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen X, Xun K, Chen L, Wang Y. TNF-alpha, a potent lipid metabolism regulator. Cell Biochem Funct. 2009;27:407–416. doi: 10.1002/cbf.1596. [DOI] [PubMed] [Google Scholar]
- 26.Palmer SC, Navaneethan SD, Craig JC, Johnson DW, Perkovic V, Hegbrant J, Strippoli GF. HMG CoA reductase inhibitors (statins) for kidney transplant recipients. Cochrane Database Syst Rev. 2014;31:5. doi: 10.1002/14651858.CD005019.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sookoian SC, Gonzalez C, Pirola CJ. Meta-analysis on the G-308A tumor necrosis factor alpha gene variant and phenotypes associated with the metabolic syndrome. Obes Res. 2005;13:2122–2131. doi: 10.1038/oby.2005.263. [DOI] [PubMed] [Google Scholar]
- 28.Shiau MY, Wu CY, Huang CN, Hu SW, Lin SJ, Chang YH. TNF-alpha polymorphisms and type 2 diabetes mellitus in Taiwanese patients. Tissue Antigens. 2003;61:393–397. doi: 10.1034/j.1399-0039.2003.00059.x. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi T, Takahashi K, Yamashina M, Maesawa C, Kajiwara T, Taneichi H, Takebe N, Kaneko Y, Masuda T, Satoh J. Association of the TNF-{alpha}-C-857T polymorphism with resistance to the cholesterol-lowering effect of HMG-CoA reductase inhibitors in type 2 diabetic subjects. Diabetes Care. 2010;33:463–466. doi: 10.2337/dc09-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]


