Abstract
Background
Autosomal dominant hypophosphatemic rickets (ADHR) is a rare genetic disorder of phosphate homeostasis characterized, when severely expressed, by osteomalacia, suppressed levels of calcitriol, and renal phosphate wasting due to elevated levels of fibroblast growth factor 23 (FGF23). The disease is caused by heterozygous FGF23 mutations at the RXXR site that prevent cleavage of the intact hormone.
Objectives
An FGF23 mutation was identified in the proband an 85-year-old woman with elevated FGF23 levels, and her clinical course was characterized. Medical records revealed she was treated for rickets as an infant. She was then asymptomatic until soon after her 4th pregnancy, when she suffered incapacitating bone pain and weakness, age 37. Symptoms remitted with brief treatment.
Results
The proband and one son, but not other family members, were found to be heterozygous for the R176Q mutation in FGF23. Expression of this germ line mutation was strikingly different in both individuals in terms of skeletal health, FGF23 levels and disease activity.
Conclusions
The identified FGF23 mutation in two members of this family raises questions about molecular mechanisms that have led to intermittent increases in FGF23 synthesis and secretion, and disease expression.
Keywords: Autosomal dominant hypophosphatemic rickets, Osteomalacia, Fibroblast growth factor 23 (FGF23), Renal phosphate wasting, Metabolic bone disease
Introduction
Autosomal dominant hypophosphatemic rickets (ADHR, OMIM #193900) is a rare disorder of phosphate homeostasis resulting from heterozygous point mutations at amino acid residues 176 or 179 in fibroblast growth factor 23 (FGF23) [1]. These mutations disrupt enzymatic cleavage of the protein by a furin-like proprotein convertase, resulting in prolonged activity of this phosphate-regulating hormone [2–4]. In severe cases, ADHR is characterized by impaired mineralization of bone, low serum phosphate due to renal phosphate-wasting, and low or inappropriately normal 1,25-dihydroxyvitamin D3 (calcitriol) levels. These abnormalities are caused by enhanced FGF23 bioactivity, which reduces expression of NPT2a and NPT2c in the proximal renal tubules, and reduces calcitriol levels by diminishing the renal 1α-hydroxylase and increasing the 24-hydroxylase activity [5]. Incomplete penetrance and variable age of onset are described in ADHR, and fluctuations in FGF23 concentration in these patients have been demonstrated that correlate with disease severity. Remissions have been reported [6,7]. The factors responsible for these fluctuations that result in variable disease expression remain unknown.
We herein report a family with ADHR in which the proband, now an 85 year old woman, was described in hospital records to have had rickets as a child, from which she was apparently cured. At age 37, she developed severe osteomalacia due to ‘phosphate diabetes,’ a condition then defined by excessive urinary phosphate due to a defect in renal tubular reabsorption of phosphate. She was extensively studied at the Massachusetts General Hospital (MGH) by Drs. Nagant de Deuxchaisnes and Krane, and their findings were published in a case series in which she is case 2 [8]. At age 84, she was again evaluated at MGH and a heterozygous FGF23 mutation (R176Q) was identified in her and one of her children, who was said to be ‘rachitic’ as an infant. This child, now in his 57th year, reached normal adult height with no evidence of disease throughout childhood, adolescence or adulthood. He shows no clinical or laboratory abnormalities in recent testing. This is the longest follow-up reported on an individual affected by ADHR, which gives novel insights into the natural history of this rare disease.
Material and methods
Interviews with the family, clinical history and physical examination of the patient and genetic analysis of the family for the FGF23 mutation were performed at the MGH. The patient's medical records from 1965 were reviewed in detail, and pertinent clinical and laboratory findings described in the original report were extracted. FGF23 levels were measured using an immunometric enzyme assay performed at Mayo Medical Laboratories (Rochester, Minnesota) using methods previously described [9]. Mutational analysis of the FGF23 gene was performed at GeneDx (Gaithersburg, Maryland) for the proband. The authors confirmed this mutation and performed subsequent genetic testing for the identified R176Q mutation in FGF23 in every living family member using described methods [1,10]. Informed consent was obtained from the proband and all participating family members using a protocol approved by Partners Internal Review Board (Boston, Massachusetts).
Results
The proband was born in France in 1926, the 3rd child of non-consanguineous parents. By age 1 1/2 years, her limbs began to bow with weight-bearing, and she was brought to a hospital near Strasbourg for corrective orthopedic surgery by age 4. As detailed in the medical records, she underwent bilateral osteotomies and was casted for 6 months, then wore orthopedic molds on her lower extremities over the next year. Her father's letters describe that throughout her childhood, she was on “a healthy diet, fortifying to the bones,” and that “both medications and ultraviolet rays were prescribed.” The patient remembers neither illness nor progressive deformity in her youth. In 1945, the patient married an American soldier and immigrated to the United States.
Two years after the birth of her 4th and last child, she began to suffer physical impairments (age 35). She described progressive weakness in her arms and legs, rib pain and difficulty walking. She had been diagnosed with goiter and hypothyroidism a year earlier. At age 37, she was admitted to the MGH. She was taking no medications. She had normal thyroid function, and the following laboratory findings: her hemoglobin was 12.4 mg/dL (reference 12.0–16.0 g/dL); serial serum calcium levels were 9.0, 9.4, and 9.1 mg/dL (reference 8.5–10.5 mg/dL), with serum phosphorus levels of 1.3, 2.1, and 2.4 mg/dL (reference 2.6–4.5 mg/dL), respectively. It is unknown whether these were fasting levels. Tubular reabsorption of phosphate was estimated to be only 85%, when serum phosphorus measured 1.3 mg/dL. Serum iron on admission was 84 µg/dL (reference 50–150 µg/dL).
The patient suffered no significant loss of height between the ages of 37 and 84 years (Table 1). She had the onset of menarche at age 13, and menopause at age 39. At the MGH, she was determined to have severe osteomalacia by bone biopsy, and was diagnosed as suffering from adult phosphate diabetes. There was no evidence of Fanconi's syndrome to account for renal phosphate wasting, no glycosuria or proteinuria, and no evidence for a urinary concentrating defect. Malabsorption was excluded as a cause of hypophosphatemia. Measured Vitamin A and carotene in serum were normal. There was no evidence by history of heavy metal poisoning.
Table 1.
Adult height, year of birth, and birth weight of patient's children.
| Family member | Adult height |
Year of birth |
Birth weight and height |
|---|---|---|---|
| Father | 5′ 7″ | 1892 | |
| Mother | 5′ 6″ | 1901 | |
| Brother | 6′ 0″ | 1923 | |
| Sister | 5′ 4″ | 1925 | |
| Patient I-1 (age 19) | 5′ 1″ | 1926 | |
| (Age 37) | 4′ 11″ | ||
| (Age 84) | 4′ 11″ | ||
| Patient's eldest daughter (II-4)a | 1948 | 6 lb 9 oz, 21″ | |
| Patient's eldest son (II-3) | 5′ 9″ | 1950 | 6 lb 3 oz, 20.5″ |
| Patient's son FGF23+ (II-2) | 5′ 7″ | 1955 | 7 lb 10.5 oz, 20″ |
| Patient's youngest daughter (II-1) | 5′ 3″ | 1960 | 6 lb 13 oz, 19″ |
Daughter II-4 died age 62; her adult height is unknown.
Fasting blood chemistries taken from the patient and her 4 children in 1964 showed no abnormalities in the children's studies. The patient's fasting calcium was 8.8 mg/dL (reference 8.5–10.5 mg/dL) with the serum phosphorus 1.5 (reference 3.0–4.5 mg/dL), and serum alkaline phosphatase 4.9 Bodansky units (reference range: 2.0–6.0 Bodansky units). The patient's extensive laboratory studies during this admission were previously reported [8]. These studies revealed a serum phosphorus that became even lower with dietary restriction of phosphate (measured tubular re-absorption of phosphate 68%); and that was refractory to simple phosphate repletion. It was not until both calcium and neutral sodium phosphate (MGH pharmacy) were given in combination with 50,000 IU ergocalciferol daily that the serum and urinary calcium levels normalized, she entered a positive calcium balance and the serum phosphorus began to rise [8]. Apart from this inappropriate loss of phosphate in the urine, her renal function was normal throughout. We do not have radiographic images any longer, just the written reports from the medical records, which describe pseudofractures in the ribs, pelvis and proximal femurs, with deformity of the pelvis and left femur. The radiograph of the skull was normal.
The patient was discharged on a prolonged trial of oral neutral sodium phosphate, prescribed as 6 capsules (0.3 g) every 3 h. Ingestion of milk and other dairy products were encouraged. The bone pain diminished gradually in her thighs, ribs and shoulders, resolving completely by week 6 of treatment as her muscular strength and gait normalized. The serum phosphorus level at 6 weeks was 3.8 mg/dL 3 h after the last dose of oral phosphorus, and the serum calcium was 9.8 mg/dL. A repeat iliac crest biopsy performed 7 months later showed “a marked reduction in the amount of osteoid seams” [8]. There was radiographic healing of pseudofractures. She chose to cease gradually her phosphate supplements after 3 years. Milk and other dairy products were substituted according to the patient.
There are many insights that were drawn from the 1967 paper (case 2), [8] but two shall be mentioned here. One is the observation that “Her rickets healed in the epiphyses of the extremities that were immobilized in plaster following the osteotomies performed at age four” [8] (healing of immobilized epiphyses in refractory rickets has been described by Tobler et al [11]). The second was the recommendation that phosphorus be dosed every 4 h; this was derived from the observation that this regimen resulted in transient improvements in serum phosphorus that lasted for several hours.
In 2010, she returned to the MGH Rheumatology clinic; she was 84 years old and recovering from a gastrointestinal illness. She was taking calcium carbonate 500 mg with Vitamin D 200 IU 3 times a day with meals. Recent past medical history was notable for an ankle fracture (2009), adult onset diabetes mellitus, hypertension and a myocardial infarction in 2005. The patient's height was 4′ 11″ and she weighed 128 lb (BMI 26.1). Physical examination showed a long torso to lower extremity ratio, with mild kyphoscoliosis. She had a slightly rocking gait due to a leg length discrepancy, and some internal rotation of the right lower extremity, particularly evident at the right knee. The left hip showed no internal or external rotation. Her muscle strength was normal. Early Bouchard's nodes were noted in her hands. There was no bone tenderness.
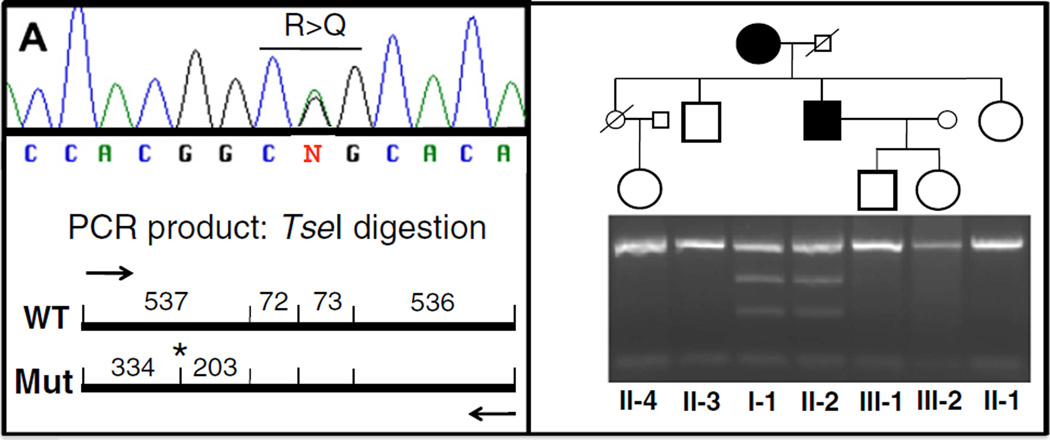
In 2011, she was found to be heterozygous for a guanine (G) to adenine (A) exchange at codon 176 replacing arginine with glutamine (R176Q), as described in ADHR. [2,4] No DMP1 mutation was identified. A PHEX mutation was not explored. One of her sons (II-2) is also a carrier of the same FGF23 mutation (Fig. 1). We suspect that he had clinical manifestations of rickets as an infant when his mother described him as having a “pigeon chest” and “knock-knees” as a toddler. With cod liver oil, sunlight and adequate nutrition, he remained asymptomatic and his final adult height (5′ 7″) was usual for the family (Table 1). He is currently healthy with normal serum phosphate and FGF23 levels, and he has two healthy children, who are not carriers of the FGF23 mutation. His two living siblings as well as the only daughter of his deceased sister are also healthy and do not carry the FGF23 mutation. Recent laboratory data are shown in Table 2.
Fig. 1.
A portion of the FGF23 gene was amplified from genomic DNA obtained from the proband and available family members as described in Material and methods section, using the forward primer 5′-CTCAACGCCCTAAGAACTG-3′ and the reverse primer 5′-CCTCATTTCAGCAAGCATCA-3′. The G-to-A nucleotide change, which causes the R176Q mutation, introduces a site for the restriction endonuclease TseI (*), leading to the generation of two additional fragments that are 334 bp and 203 bp in length.
Table 2.
Current laboratory values of patient I-1 and affected son (II-2).
| Test (reference ranges) | Patient | Son | |||
|---|---|---|---|---|---|
| 9/2010 | 4/2011 Fasting |
7/2011 Fasting |
4/2011 Fasting |
7/2011 Fasting |
|
| Ca (8.5–10.5 mg/dL) | 10.1 | 10.0 | 10.5 | 9.8 | 9.5 |
| Phosphorus (2.6–4.5 mg/dL) | 1.9 | 3.5 | 2.9 | 2.9 | 3.2 |
| Magnesium (1.4–2.0 meq/L) | 1.7 | 1.7 | 1.7 | 1.7 | 1.8 |
| Vitamin D (> 32 ng/mL) | 34 | 22 | 39 | 21 | |
| 1,25 Vitamin D (18–78 pg/mL) | 10 | 14 | 43.5 | 39 | |
| Albumin (3.3–5.0 g/dL) | 4.3 | 4.2 | 4.4 | 4.8 | |
| Alkaline phosphatase (30–100 U/L) | 108 | 78 | 79 | 103 | |
| Parathyroid hormone (10–60 pg/mL) |
30 | 30 | 21 | 42 | 47 |
| FGF23 (< 180 RU/mL) | 785 | 414 | 70 | ||
| Creatinine (0.60–1.50 mg/dL) | 1.14 | 1.0 | 0.99 | 0.8 | 0.82 |
| Glucose (70–110 mg/dL) | 143 | ||||
| Tubular reabsorption of phosphorus (> 80%, calculated based on creatinine) |
47.4% | 89.7% | 84.2%. | 89.0% | 89.8% |
| T1-TSH (0.40–5.00 uU/mL) | 3.66 | 5.03 | 2.30 | ||
| Hemoglobin (12.0–16.0 g/dL) | 12.7 | 13.0 | 13.9 | ||
| Fe (30–160 µg/dL) | 118 | 80 | |||
| TIBC (230–404 µg/dL) | 365 | 303 | |||
| Ferritin (10–200 ng/mL) | 29 | 71 | |||
Although the medical records document that both Dr. Nagant de Deuxchaisnes, Dr. Krane and subsequent physicians recommended some form of Vitamin D supplementation each time they saw her in the years that followed, it is unclear from her history what she took, and for how long. There is no record of her taking cod liver oil after her childhood. Her renal function remained stable, her serum creatinine 0.8–1.0 mg/dL during these years, increasing only slightly over the last few years. The most recent measurement was 1.13 mg/dL in 2011. The serum phosphorus level (reference range 2.6–4.5 mg/dL) generally remained in the low normal range, as did the tubular reabsorption of phosphorus the times this was measured (reference range > 80%). Review of laboratory studies of random serum phosphorus from the years from 1992 to 2011 are as follows: 3.2 (1992), 2.6 (1994), 2.8 (1995), 2.8 (1995), 2.7 (1997), 2.7 (1998), 2.3 (1999), 2.8 (2003), 2.7 (2005), 2.9 (2006), 2.6 (2007), 2.0 (2008), 1.9 (2010), and 2.9 (2011). Her serum calcium levels and complete blood cell counts were unremarkable throughout the 20 years of clinic visits. There was no evidence of chronic inflammation or iron deficiency. A recent bone mineral density of her lumbar spine measured 0.62 g/cm2 (T score −2.6, Z score −0.4); femoral neck measured 0.56 g/cm2 (T score −2.6, Z score −0.1).
Discussion
ADHR is a rare disorder of phosphate homeostasis that is caused by heterozygous FGF23 mutations at the RXXR cleavage site that prevent cleavage of the hormone thereby prolonging its biological activity [1,4,12]. In this case study, we describe a woman with the onset of ADHR disease in infancy that then remits until her 4th decade, when she suffered profound muscle weakness and bone pain, and was treated for 3 years with neutral sodium phosphate for ‘phosphate diabetes.’ Since that time, she has remained in sustained clinical remission, despite elevations in her serum FGF23 levels measured over the last couple of years.
Winters and his colleagues [13] are credited with the first description of ADHR when their early studies indicated that hypophosphatemic rickets can occur in a pattern of male-to-male transmission, thus excluding X-linked hypophosphatemia. Other case reports of familial hypophosphatemic rickets consistent with ADHR were published, one describing hypophosphatemia affecting a father, his son and two daughters in 1966; [14] and a second describing a family with an autosomal dominant pattern of inheritance in 1971 [15]. The disease remains rare worldwide, [16] although its variable age of onset and incomplete clinical expression may lead to underestimating its prevalence.
In comparison to XLH, ADHR is typically more variable in disease expression, including delayed onset and spontaneous remission. However, Winter et al. already reported XLH patients with affected children, who revealed no evidence of clinically or radiologically detectable bone disease [13]. Variable penetrance can thus occur in both autosomal dominant and X-linked hypophosphatemic disorders.
Econs and McEnery were the first to describe two patterns of onset in a large kindred with ADHR, some presenting in childhood and some with onset in adulthood [7]. Of those presenting in childhood, two infant boys ‘out grew’ the phosphate-wasting, with normalization of serum phosphate and renal handling of phosphate. The disease course in these boys may well describe our patient's son (II-2), who in his infancy was described as suffering from mild rickets, but who grew to normal adult height and currently shows no evidence for hypophosphatemia, has normal FGF23 level, and no increase in urinary phosphate excretion. In contrast to the disease course in these male children, three previously described women, all carriers of the R176Q mutation, showed delayed disease onset, with no evidence of disease during infancy [7]. Our female patient presented with bowed legs during infancy, required osteotomies during childhood, and then seemed to enter a sustained clinical remission until 2 years after the birth of her 4th child. She was treated at age 37, and again entered a clinical remission.
Recent studies revealed that iron could be one of the factors contributing to the regulation of FGF23 expression [17,18]. Low iron levels were shown to correlate negatively with FGF23 levels in ADHR patients as determined by an assay measuring either the intact hormone alone (iFGF23 assay) and by an assay measuring intact as well as C-terminal FGF23 (cFGF23 assay); in healthy controls only cFGF23 levels correlated with iron deficiency [19]. Mouse models of ADHR fed a low iron diet corroborated these findings, as did wild type animals [20]. Durham and his colleagues had previously reported that there is an inverse correlation between low serum ferritin levels and cFGF23 levels, a correlation that was not present when iFGF23 was measured [21]. Taken together these findings indicate that cleavage of the biologically active hormone occurs normally in the absence a mutation at the RXXR cleavage site, thereby preventing hypophosphatemia during iron deficiency.
Our patient was never shown to be overtly iron deficient, but her pregnancies may have led to some degree of iron deficiency sufficient to increase FGF23 production. It remains unknown whether her history of hypothyroidism and early menopause contributed to the disease course. During 85 years of ADHR marked by variable periods of hypophosphatemia, our patient never developed renal dysfunction or nephrocalcinosis, which is different from patients with XLH. The XLH patients frequently acquire this complication of oral phosphate therapy, and 1,25(OH)2 Vitamin D prescribed to prevent or limit secondary hyperparathyroidism.
Estrogen deficiency and the end of obligate iron losses through menses after menopause may have improved our patient's phosphate homeostasis. In rat models, estrogens regulate the expression of sodium-dependent phosphate co-transporter IIb (NaPi-IIb) in the gut, enhancing gastrointestinal absorption of phosphate [22]. In ovariectomized rats, estrogen was also shown to enhance urinary phosphate excretion by reducing NaPi-IIa expression [23]. Evidence for interplay between estrogen and iron deficiency was previously suggested when examining regulation of transferrin expression [24]. Future studies are needed to unravel the role of iron, gastrointestinal phosphate transport, energy metabolism, [25] and other hormones on the expression of ADHR.
Conclusion
This elderly woman with ADHR followed from infancy, through her childbearing years to her current age of 85, has provided intriguing observations into the consequences of FGF23 on skeletal health. In her, serum levels of FGF23 have not consistently correlated with low serum phosphorus, nor are they correlated with early cardiovascular or renal disease. We know the levels of FGF23 in ADHR do not approximate those in chronic kidney disease [26]. While aortic calcification is now visible on plain films, she did not suffer a myocardial infarction until she was in her late 70s. By her long life and history, this woman reminds us of how little we yet understand of the metabolic consequences of FGF23 in skeletal health, and the determinants of disease expression.
Acknowledgments
The authors would like to thank Dr. Stephen M. Krane for his insights and critique of this paper, and his scholarship that made this story possible. We would also like to thank M. Reyes for expert technical help in the search for the FGF23 mutation in the available members of this family.
Contributor Information
Margaret Seton, Email: mseton@partners.org.
Harald Jüppner, Email: jueppner@helix.mgh.harvard.edu.
References
- 1.White KE, Evans WE, O'Riordan JLH, et al. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000 Nov;26(3):345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 2.White KE, Carn G, Lorenz-Depiereux B, Benet-Pages A, Strom TM, Econs MJ. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int. 2001;60(6):2079–2086. doi: 10.1046/j.1523-1755.2001.00064.x. [DOI] [PubMed] [Google Scholar]
- 3.Shimada T, Muto T, Urakawa I, et al. Mutant FGF-23 responsible for autosomal dominant hypophosphatemic rickets is resistant to proteolytic cleavage and causes hypophosphatemia in vivo. Endocrinology. 2002;143(8):3179–3182. doi: 10.1210/endo.143.8.8795. [DOI] [PubMed] [Google Scholar]
- 4.Bai XY, Miao D, Goltzman D, Karaplis AC. The autosomal dominant hypophosphatemic rickets R176Q mutation in fibroblast growth factor 23 resists proteolytic cleavage and enhances in vivo biological potency. J Biol Chem. 2003;278(11):9843–9849. doi: 10.1074/jbc.M210490200. [DOI] [PubMed] [Google Scholar]
- 5.Tenenhouse HS, Martel J, Gauthier C, Segawa H, Miyamoto K. Differential effects of Npt2a gene ablation and X-linked Hyp mutation on renal expression of Npt2c. Am J Physiol Renal Physiol. 2003 Dec;285(6):F1271–F1278. doi: 10.1152/ajprenal.00252.2003. [DOI] [PubMed] [Google Scholar]
- 6.Imel EA, Hui SL, Econs MJ. FGF23 concentrations vary with disease status in autosomal dominant hypophosphatemic rickets. J Bone Miner Res. 2007;22(4):520–526. doi: 10.1359/jbmr.070107. [DOI] [PubMed] [Google Scholar]
- 7.Econs MJ, McEnery PT. Autosomal dominant hypophosphatemic rickets/osteomalacia: clinical characterization of a novel renal phosphate-wasting disorder. J Clin Endocrinol Metab. 1997;82(2):674–681. doi: 10.1210/jcem.82.2.3765. [DOI] [PubMed] [Google Scholar]
- 8.Nagant de Deuxchaisnes C, Krane SM. The treatment of adult phosphate diabetes and Fanconi syndrome with neutral sodium phosphate. Am J Med. 1967 Oct;43(4):508–543. doi: 10.1016/0002-9343(67)90177-5. [DOI] [PubMed] [Google Scholar]
- 9.Jonsson KB, Zahradnik R, Larsson T, et al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003;348(17):1656–1663. doi: 10.1056/NEJMoa020881. [DOI] [PubMed] [Google Scholar]
- 10.Bergwitz C, Banerjee S, Abu-Zahra H, et al. Defective O-glycosylation due to a novel homozygous S129P mutation is associated with lack of fibroblast growth factor 23 secretion and tumoral calcinosis. J Clin Endocrinol Metab. 2009 Nov;94(11):4267–4274. doi: 10.1210/jc.2009-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prader A, Taillard W, Tobler R. Primary familial vitamin D-resistant rickets (phosphate diabetes) Helv Paediatr Acta. 1956 Sep;11(3):209–255. [PubMed] [Google Scholar]
- 12.Econs MJ, McEnery PT, Lennon F, Speer MC. Autosomal dominant hypophosphatemic rickets is linked to chromosome 12p13. J Clin Invest. 1997;100(11):2653–2657. doi: 10.1172/JCI119809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winters RW, Graham JB, Williams TF, Mc FV, Burnett CH. A genetic study of familial hypophosphatemia and vitamin D resistant rickets with a review of the literature. Medicine (Baltimore) 1958 May;37(2):97–142. doi: 10.1097/00005792-195805000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Harrison HE, Harrison HC, Lifshitz F, Johnson AD. Growth disturbance in hereditary hypophosphatemia. Am J Dis Child. 1966 Oct;112(4):290–297. doi: 10.1001/archpedi.1966.02090130064005. [DOI] [PubMed] [Google Scholar]
- 15.Bianchine JW, Stambler AA, Harrison HE. Familial hypophosphatemic rickets showing autosomal dominant inheritance. Birth Defects Orig Artic Ser. 1971 May;7(6):287–295. [PubMed] [Google Scholar]
- 16.Sun Y, Wang O, Xia W, et al. FGF23 analysis of a Chinese family with autosomal dominant hypophosphatemic rickets. J Bone Miner Metab. 2012 Jan;30(1):78–84. doi: 10.1007/s00774-011-0285-5. [DOI] [PubMed] [Google Scholar]
- 17.Gattineni J, Baum M. Genetic disorders of phosphate regulation. Pediatr Nephrol. 2012 Feb;14 doi: 10.1007/s00467-012-2103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saito H, Kusano K, Kinosaki M, et al. Human fibroblast growth factor-23 mutants suppress Na+-dependent phosphate co-transport activity and 1alpha,25-dihydroxyvitamin D3 production. J Biol Chem. 2003;278(4):2206–2211. doi: 10.1074/jbc.M207872200. [DOI] [PubMed] [Google Scholar]
- 19.Imel EA, Peacock M, Gray AK, Padgett LR, Hui SL, Econs MJ. Iron modifies plasma FGF23 differently in autosomal dominant hypophosphatemic rickets and healthy humans. J Clin Endocrinol Metab. 2011;96(11):3541–3549. doi: 10.1210/jc.2011-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farrow EG, Yu X, Summers LJ, et al. Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (FGF23) knock-in mice. Proc Natl Acad Sci U S A. 2011;108(46):E1146–E11455. doi: 10.1073/pnas.1110905108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Durham BH, Joseph F, Bailey LM, Fraser WD. The association of circulating ferritin with serum concentrations of fibroblast growth factor-23 measured by three commercial assays. Ann Clin Biochem. 2007 Sep;44(Pt 5):463–466. doi: 10.1258/000456307781646102. [DOI] [PubMed] [Google Scholar]
- 22.Xu H, Uno JK, Inouye M, et al. Regulation of intestinal NaPi-IIb cotransporter gene expression by estrogen. Am J Physiol Gastrointest Liver Physiol. 2003 Dec;285(6):G1317–G1324. doi: 10.1152/ajpgi.00172.2003. [DOI] [PubMed] [Google Scholar]
- 23.Guttmann-Rubinstein L, Lichtstein D, Ilani A, Gal-Moscovici A, Scherzer P, Rubinger D. Evidence of a parathyroid hormone-independent chronic effect of estrogen on renal phosphate handling and sodium-dependent phosphate cotransporter type IIa expression. Horm Metab Res. 2010 Apr;42(4):230–236. doi: 10.1055/s-0029-1246182. [DOI] [PubMed] [Google Scholar]
- 24.McKnight GS, Lee DC, Palmiter RD. Transferrin gene expression. Regulation of mRNA transcription in chick liver by steroid hormones and iron deficiency. J Biol Chem. 1980;255(1):148–153. [PubMed] [Google Scholar]
- 25.Ishiguro M, Yamamoto H, Masuda M, et al. Thyroid hormones regulate phosphate homoeostasis through transcriptional control of the renal type IIa sodium-dependent phosphate co-transporter (Npt2a) gene. Biochem J. 2010;427(1):161–169. doi: 10.1042/BJ20090671. [DOI] [PubMed] [Google Scholar]
- 26.Juppner H. Phosphate and FGF-23. Kidney Int Suppl. 2011 Apr;121:S24–S27. doi: 10.1038/ki.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]