Abstract
Background
Controversy exists about the incremental utility of nontraditional lipid biomarkers [e.g., apolipoprotein (apo) B, apo A-I, and non-HDL-C] in improving cardiovascular disease (CVD) risk prediction when added to a conventional model of traditional risk factors (e.g., total cholesterol, LDL cholesterol, HDL cholesterol, sex, age, smoking status, and blood pressure). Here we present a systematic review that was conducted to assess the use of nontraditional lipid biomarkers including apo B, apo A-I, apo B/A-I ratio, and non-HDL-C in improving CVD risk prediction after controlling for the traditional risk factors in populations at risk for cardiovascular events.
Content
This systematic review used the Laboratory Medicine Best Practices (LMBP™) A-6 methods. A total of 9 relevant studies published before and including July 2015 comprised the evidence base for this review. Results from this systematic review indicated that after the adjustment for standard nonlipid and lipid CVD risk factors, nontraditional apolipoprotein biomarkers apo B (overall effect = relative risk: 1.31; 95% CI, 1.22–1.40; 4 studies) and apo B/apo A-I ratio (overall effect = relative risk: 1.31; 95% CI, 1.11–1.38; 7 studies) resulted in significant improvement in long-term CVD risk assessment.
Summary
Available evidence showed that nontraditional lipid biomarkers apo B and apo B/apo I ratio can improve the risk prediction for cardiovascular events after controlling for the traditional risk factors for the populations at risk. However, because of insufficient evidence, no conclusions could be made for the effectiveness of apo A-I and non-HDL-C lipid markers to predict the CVD events, indicating a need for more research in this field.
Cardiovascular disease (CVD)8 remains the leading cause of death and inpatient hospital care in the United States (1–4). Research has shown that approximately 50% of population-attributable risk of developing CVD is associated with abnormalities in lipid biomarker profile (5). Most of the existing guidelines have used traditional lipid levels and other risk factors, namely total cholesterol (TC), LDL cholesterol (LDL-C), HDL cholesterol (HDL-C), triglycerides (TG), and nonlipid risk factors, e.g., high blood pressure, cigarette smoking, diabetes, age, sex, diet, and obesity, as risk factor scores for CVD risk prediction (6, 7). Based on recent studies, there is a growing interest to investigate whether nontraditional lipid-related markers add incremental value to standard prognostic models containing information on TC, HDL-C, and other conventional risk factors and can improve the CVD risk prediction (5). The utility of nontraditional markers in risk assessment is best examined by combining them with a model that includes traditional risk factors (8, 9). Non-HDL cholesterol (non-HDL-C), apolipoprotein (apo) B, and apo A-I are among the most investigated nontraditional lipid biomarkers and hence were the focus of this study (10–12).
QUALITY GAP: LIPID BIOMARKERS AND CVD RISK
Traditional risk factors provide estimates of plasma pool sizes and do not necessarily relate to the flux of cholesterol between lipoproteins and tissues, which may be more relevant to the process of atherosclerosis. Controversy exists about the effectiveness of traditional lipid tests to accurately predict risk of cardiovascular events, causing potential for missed opportunities for prevention and leading to suboptimal clinical management because these do not (a) account for the variability in cholesterol subfraction content, (b) measure lipoprotein particle size and number, or (c) provide information suggestive of changes associated with insulin resistance progression. Two major trends may be further compromising the ability of LDL-C to serve as the best surrogate for atherogenic lipo-proteins to target CVD risk reduction. First, the prevalence of obesity in the US remains high (13), leading to a higher prevalence of mixed dyslipidemia and more discrepancies between LDL-C and other lipoproteins such as apo B, LDL particle number, and non-HDL-C (14–16). Second, evidence suggests that even when LDL-C is within the normal range, significant residual cardiovascular risk remains (17). Because a majority of patients on conventional lipid-lowering treatment have either diabetes or some component of the metabolic syndrome, there is an increasing need to identify the degree of residual cardiovascular risk (14–16, 18–20).
The objective of this review was to evaluate the available evidence to compare the incremental utility of apolipoprotein and non-HDL lipid bio-markers to the traditional lipid measures (e.g., TC, TG, HDL-C) and other nonlipid standard risk factors (e.g., smoking status, high blood pressure, type 2 diabetes) for risk prediction of CVD events. For the purposes of this review, cardiovascular events of interest included ischemic heart disease, congestive heart failure, stable angina, unstable angina, myocardial infarction, and CVD death.
DESCRIPTION OF EVALUATED PRACTICES
This review evaluated the effectiveness of the following 4 biomarkers (practices) to improve the prediction of CVD events when added to traditional lipid biomarkers (e.g., TC, HDL-C): (i) apo B; (ii) apo A-I; (iii) apo B/apo A-I ratio; and (iv) non-HDL cholesterol (non-HDL-C).
Apo B
Apo B is the primary apolipoprotein of chylomicrons, VLDL, intermediate-density lipoprotein, and LDL particles (14, 21, 22). Importantly, there is 1 apo B-100 molecule per hepatic-derived lipoprotein; hence, measurement of apo B can quantify the number of lipoprotein particles by noting the total apo B-100 concentration in the circulation (more specific to LDL particle concentration) (23). Furthermore, high levels of apo B are indicative of a higher risk even when LDL-C or non-HDL-C levels commonly stay low in highly atherogenic states such as the metabolic syndrome and type 2 diabetes (24, 25). Prospective studies suggest that concentrations of apo B are superior indicators of vascular/heart disease and CVD risk prediction than standard lipid profile, e.g., TC and LDL-C (22, 26–31).
Apo A-I
Apo A-I is the major apolipoprotein in the HDL particles (32), accounting for 70% of all HDL-associated proteins (16) and mediates many of the anti-atherogenic functions of HDL (33). HDL-C levels are inversely correlated with risk for CVD, but HDL-C is heterogeneous in composition and size and varies widely across patients; thus, apo A-I is potentially more accurate than HDL-C in reflecting the “atheroprotective” potential of lipid metabolism (16, 18, 32).
Apo B/A-I ratio
The apolipoprotein B/A-I ratio is used as a measure of the proatherogenic to anti-atherogenic cholesterol (34). It was found to be strongly associated with CVD risk, (16, 29, 35, 36) and in some cases more than that of other cholesterol ratios (34, 36). Furthermore, compared to other lipid ratios, apolipoprotein B/A-I ratio may be more accurate in risk prediction, particularly among high-risk individuals (37).
Non-HDL-C
Non-HDL-C is the difference between the TC concentration and the HDL-C concentration, providing an estimate of cholesterol in the atherogenic particles including intermediate-density lipoprotein, VLDL, lipoprotein(a), and LDL (31, 38). Although the CVD risk prediction is based on the increased concentrations of TC (39, 40), mostly from increased LDL-C, research has shown the utility of non-HDL-C in the prevention of CVD (41) and varying CVD risk prediction across several studies (11, 18, 25, 28, 31, 36, 42). The latest guidelines for both European and American Cardiological Societies emphasize the importance of this parameter for assessing the risk of atherosclerosis and coronary heart disease.
METHODS
This systematic evidence review was conducted using the Laboratory Medicine Best Practices (LMBP) Initiative's “A-6” systematic review methods, which is reported in detail elsewhere (43) (LMBP™ website). In brief, the process includes formation of a review team that includes a review coordinator, data abstractors, CDC liaison, and subject matter experts (expert panel team) with the expertise in the area of cardiovascular medicine, laboratory management, and evidence review methods. The team worked under the oversight of the LMBP Workgroup. Supplemental Appendix A lists the members of the expert panel team for this review; see the Data Supplement that accompanies the online version of this article at http://www.jalm.org/content/vol1/issue2. The results of the evidence-based best practice are presented to and approved by the LMBP Work-group team (Supplemental Appendix B lists the LMBP Workgroup members; see the online Data Supplement).
Ask (A-1): review question and analytic framework
Review question
What practices are effective at improving the risk prediction (or risk estimation) for CVD events among the populations at risk, specifically ischemic heart disease, congestive heart failure, angina, myocardial infarction, and CVD death, when supplemented to the traditional lipid (e.g., TC, LDL, TG, and HDL) and nonlipid (e.g., age, sex, smoking status, and blood pressure) risk factors?
This review question is addressed in the context of an analytic framework as depicted in Fig. 1.
Fig. 1.
Analytic framework.
The following were the relevant Population, Intervention/Practice, Comparator, and Outcome (PICO) elements considered for this review.
Population
Men (>35 years)
Women (>45 years)
Younger adults (≥20 years) with multiple cardiovascular risk factors for CVD
No previously diagnosed CVD or diabetes at baseline
In ambulatory (including primary, specialty care) and inpatient settings
Interventions
Practices using nonstandard lipo-protein measurements in addition to the existing traditional risk factors (e.g., TC, TG, LDL-C, HDL-C, age, sex, smoking status, and blood pressure) for calculating cardiovascular risk assessment. The following lipid biomarkers were considered for this review:
Apo B
Apo A-I
Apo B/apo A-I ratio
Non-HDL-C
Comparison
Practices using traditional risk factors (e.g., TC and HDL-C, age, sex, smoking status, and blood pressure) alone to calculate CVD risk prediction.
Outcome
Improvement in the 10-year risk prediction of CVD events (e.g., myocardial infarction, ischemic heart disease, and CVD death) upon adding the nonstandard biomarker. Studies with follow-up period <10 years were still included in the review but were penalized in the study quality rating because of type 1 censoring of the findings (44).
Inclusion/exclusion criteria for studies to be included in this review
Inclusion criteria
To meet the eligibility criteria for this review, a study had to (a) address one or more of the proposed practices of interest in the context of CVD outcomes; (b) target populations in the studies who met the population criteria—that is, at-risk populations described above, with no previously diagnosed CVD or diabetes; (c) report the outcome(s) of interest—that is, improvement in the 10-year risk prediction of CVD events (e.g., myocardial infarction, CVD death) due to the addition of 1 of the 4 practices; and (d) provide comparison data to calculate the effectiveness of practices of interest (e.g., pre- and post-intervention data, concurrent comparison data).
In addition, interventions were considered to be included in this review if the biomarker of interest was added to a model or algorithm of the traditional lipid profile (e.g., TC, TG, HDL-C) and other risk factor (e.g., high blood pressure, cigarette smoking, diabetes, family history of premature heart disease, age, sex, diet, obesity, and physical inactivity) for predicting CVD risk. The practices were considered individually (e.g., the combination of apo B and non-HDL-C simultaneously added into a model was not considered a practice of interest), unless the results for the effectiveness of each practice was reported separately. A practice was considered effective if the fit of a model containing all traditional risk factors was significantly improved through the addition of a practice.
Exclusion criteria
The exclusion criteria were as follows: (a) previously diagnosed CVD or symptomatic coronary artery disease at baseline and (b) previously diagnosed diabetes at baseline.
Acquire (A-2): search for evidence
A comprehensive electronic literature search was conducted to retrieve the relevant evidence published before and including July 2015. Three databases were used for a formal literature search: PubMed, CINAHL, and EMBASE (focusing on international biomedical literature). Details of the formal literature search strategy can be found in Supplemental Appendix C (see the online Data Supplement). In addition, the systematic review team used other sources to locate relevant studies including hand searches (e.g., the citations from retrieved studies, Google scholar) and referrals from the experts in the field (e.g., expert panel team). To collect relevant unpublished data, researchers in the field, laboratories, and institutions were invited through personal requests and the LMBP website, but the review team did not receive any relevant unpublished data to be included in this review.
Appraise (A-3): screening, data abstraction, and quality scoring of individual studies
During the initial screening process, studies were excluded if they did not satisfy the inclusion criteria for this review as described in a previous section. Each eligible study was abstracted and assessed for quality of execution by 2 independent reviewers. Data abstraction was conducted by using the standardized LMBP abstraction methods and abstraction form. All differences were resolved through consensus. After the full abstraction, each study was evaluated for quality scoring to minimize any issue related to internal and external validity using LMBP quality assessment methods (43).
Details on the rating process of individual studies can be found elsewhere (43). Each study was classified into 1 of 3 quality ratings: good (8–10 score), fair (5–7 score), and poor (≤4 score). Studies with poor quality ratings were excluded from the effect size metaanalyses and the overall practice evidence base. See Supplemental Appendix D (in the online Data Supplement) for the Evidence Summary Tables containing quality ratings for each study.
Analyze (A-4): summarization of results and strength of the effect magnitude
Results from all included studies were variously reported risk ratios: odds ratios, relative risks, or hazard ratios. For the analyses purposes, these ratios were assumed to approximate the same measure of relative risk. Metaanalysis was performed to calculate the overall grand mean effect recommended by Borenstein et al. (45). A random-effects model was used for these statistics to perform meta-analysis because (a) not all the studies compared the same mixture of nontraditional lipid biomarkers to the traditional risk factors to improve the CVD risk prediction and (b) the long-term CVD risk prediction was based on different clinical CVD events in individual studies. To evaluate the effectiveness of these interventions, pooled point estimates across studies were expressed as an overall grand mean with CIs. When possible, all metaanalysis results were presented as forest plots, where the vertical line labeled “1” equals “no/minimal” difference between practices, and estimates to the right of the line favor the tested practice, i.e., improved risk prediction of CVD events due to the assessed practice. However, as apo A-I levels inversely correlate with risk for CVD (i.e., high apo A-I levels are protective against future cardiovascular events), the effect estimates less than 1 were considered favorable for development of CVD risk prediction.
For the effectiveness strength rating, the point estimate from each study between ≤1 and ≤2.0 was considered as a “moderate” magnitude of effectiveness; and any point estimate >2.0 was considered a “substantial” magnitude of effect. Final conclusions and recommendations for the overall effectiveness were based on the criteria including number of studies, quality of available evidence, consistency of results, and magnitude of effect estimates. Criteria for these ratings are described in greater detail elsewhere (43).
RESULTS
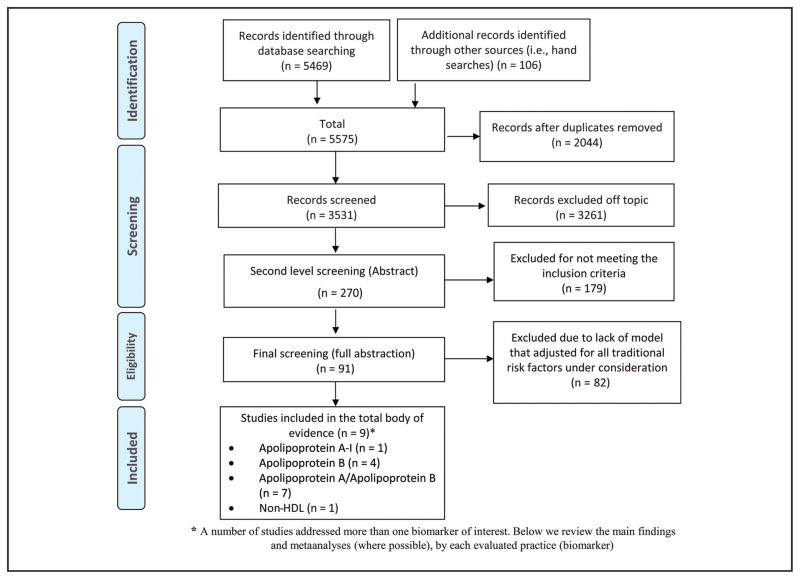
A total of 5575 bibliographic records were retrieved from the literature search, of which 106 were identified from other sources (e.g., hand searches, referrals). A total of 5305 studies were excluded (2044 duplicates and 3261 were not relevant to the topic). The remaining 270 published studies were screened further, and 179 were excluded. The remaining 91 studies were subjected to full abstraction and quality assessment; finally, 9 independent published studies (34, 46–53) met the inclusion criteria and comprised the total body of evidence (Fig. 2).
Fig. 2.
Biomarkers and risk of CVD review search flow diagram.
Apo B
Four eligible studies (48, 49, 51, 52) examined the associations between the plasma apo B levels when added to other traditional lipids with CVD risk prediction. The combined evidence from all included studies indicated that the risk assessment to develop long-term CVD events was significantly improved by adding apo B marker to the traditional risk factors (overall effect = relative risk: 1.31, 95% CI, 1.22–1.40, Fig. 3). The total evidence showed consistently favorable association of apo B lipid marker with the better long-term CVD risk prediction. The effect estimates for the CVD risk prediction were statistically significant from all included studies (48, 49, 52) but 1 study (51). The overall evidence was derived from 3 good quality studies (49, 51, 52) and 1 “fair” quality study (48) (Fig. 3).
Fig. 3.
Risk prediction for CVD events due to apo B vs other traditional risk factors.
Conclusions
Applying the LMBP criteria (43), the overall strength of evidence is considered moderate to conclude that the addition of apo B bio-marker to the traditional risk factors can improve the risk prediction for cardiovascular events for populations at risk (Table 1).
Table 1.
Body of evidence LMBP ratings for apo B.
| Study, year | Study quality rating | Effect size rating |
|---|---|---|
| Steffen et al., 2015 | Good | Moderate |
| Kappelle et al., 2011 | Fair | Moderate |
| St-Pierre et al., 2006 | Good | Moderate |
| Lamarche et al., 1996 | Good | Moderate |
| Body of evidence ratings | 1 Fair/moderate | |
| 3 Good/moderate | ||
| Consistency | Yes | |
| Overall strength | Moderate | |
LMBP working group (WG) recommendation (apo B)
Based on the moderate evidence of effectiveness, lipoprotein apo B measure is recommended to improve the risk prediction for cardiovascular events when added to other traditional risk factors for the populations at risk (e.g., men >35 years, women >45 years, and younger adults ≥20 years old with multiple risk factors for CVD, in ambulatory and inpatient settings). This recommendation is based on consistently favorable results from 3 “good” quality and 1 fair quality studies.
Apo A-I
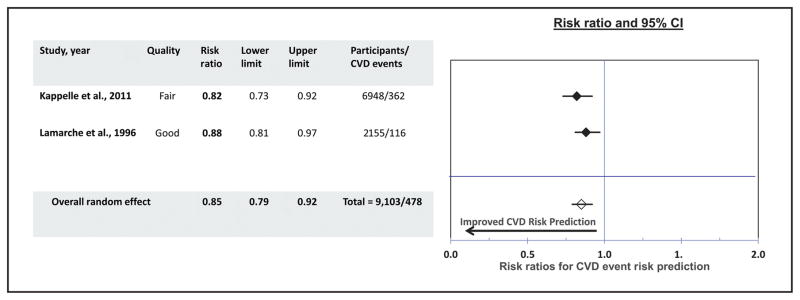
Two studies (48, 49) qualified to be included in the evidence that reported the impact of the measures of apo A-I in CVD risk prediction. One study was rated good quality of execution (49) and the other study was of fair quality (48). Combined results from both studies showed the favorable association of apo A-I marker with the CVD risk prediction—that is, the participants with the higher apo A-I levels tended to have lower risk of developing CVD events (overall random effect: 0.85; 95% CI, 0.79–0.92) (Fig. 4).
Fig. 4.
Risk prediction for CVD events due to apo A-I vs other traditional risk factors.
Conclusions
Applying the LMBP criteria, due to the limited available evidence, the overall strength of evidence is considered “insufficient” to conclude that the addition of apo A-I to the traditional risk factors can improve the risk prediction for cardiovascular events for populations at risk.
LMBP WG recommendation (apo A-I)
Because of the insufficient available evidence, no recommendations could be made for or against the effectiveness of apo A-I practices to predict the CVD events.
Apo B/apo A-I
Seven studies provided evidence for the effectiveness of apo B/A-I ratio. Results from 4 included studies (46, 47, 50, 51) were presented as hazard ratios, 2 as relative risk (34, 48), and 1 as odds ratio (53); the results from these studies were combined to calculate overall grand mean estimate of effectiveness. The combined results from the total evidence showed a consistent and positive association of apo B/A-I ratio marker to the development of long-term CVD events [overall grand mean estimate: 1.31 (95% CI, 1.11–1.38)] (Fig. 5). The results were statistically significant from all but 2 studies (47, 51). Of 7 studies, 4 studies (34, 47, 50, 51) had a quality rating of good, and 3 (46, 48, 53) were of fair quality. One study (50) showed a substantial effect estimate but had a large CI, indicating a less precise estimate. Six studies (34, 46–48, 51, 53) showed moderate effect for the CVD risk prediction (Fig. 5).
Fig. 5.
Risk prediction for CVD events due to apo B/apo A-I ratio vs other traditional risk factors.
Conclusions
Applying the LMBP criteria, the overall strength of evidence is considered moderate to conclude that the addition of apo B/apo I ratio to the traditional risk factors can improve the risk prediction for cardiovascular events for populations at risk (Table 2).
Table 2.
Body of evidence LMBP ratings for apo B/A-I.
| Study, year | Study quality rating | Effect size rating |
|---|---|---|
| Steffen et al., 2015 | Good | Moderate |
| Kappelle et al., 2011 | Fair | Moderate |
| Sierra-Johnson et al., 2009 | Good | Substantial |
| Ingelsson et al., 2007 | Good | Moderate |
| van der Steeg et al., 2007 | Fair | Moderate |
| Ingelsson et al., 2005 | Fair | Moderate |
| Walldius et al., 2004 | Good | Moderate |
| Body of evidence ratings | 1 Good/substantial | |
| 3 Good; 3 fair/moderate | ||
| Consistency | Yes | |
| Overall strength | Moderate | |
LMBP WG recommendation (apo B/apo A-I ratio)
According to the LMBP methods, based on the moderate evidence of effectiveness the lipo-protein apo B/apo A-I ratio is recommended to improve the risk prediction for cardiovascular events when added to other traditional risk factors for the populations at risk (e.g., men >35 years, women >45 years, younger adults ≥20 years old with multiple risk factors for CVD, in ambulatory and inpatient settings). This recommendation is developed based on evidence from 4 good and 3 fair quality studies (Table 2).
Non-HDL-C
Only 1 fair quality study (48) was identified investigating the association of non-HDL-C with CVD outcomes after controlling for traditional risk factors. The results from this prospective cohort study showed that non-HDL-C lipid marker when adjusted for nonlipid (e.g., age, sex) and lipid (e.g., triglycerides) risk factors were associated with better CVD risk prediction at the 7.9-year follow-up period (hazard ratio: 1.25; 95% CI, 1.11–1.41).
Conclusions
Applying the LMBP criteria (43), the overall strength of evidence is considered insufficient to conclude at the time that the measures of non-HDL-C can improve the risk prediction for cardiovascular events for populations at risk.
LMBP WG recommendation (non-HDL-C)
Because of the insufficient available evidence, no recommendations could be made for or against the effectiveness of non-HDL-C practices to predict the CVD events (Table 3).
Table 3.
Body of evidence LMBP ratings for non-HDL-C.
| Study, year | Study quality rating | Effect size rating |
|---|---|---|
| Kappelle, 2011 | Fair | Moderate |
| Body of evidence ratings | 1 Fair/moderate | |
| Consistency | Not applicable | |
| Overall strength | Insufficient | |
DISCUSSION
Best practices recommendations
Based on the findings from this systematic review, below are the LMBP workgroup recommendations for 4 evaluated practices in this review.
Lipoprotein apo B and apo B/apo A-I ratio measures are recommended to improve the risk pre-diction for cardiovascular events when added to other traditional risk factors for the populations at risk (i.e., men >35 years, women >45 years, and younger adults ≥20 years old with multiple risk factors for CVD, in ambulatory and inpatient settings).
Because of the insufficient evidence, no recommendations could be made for or against their effectiveness of apo A-I and non-HDL-C practices to improve the prediction of CVD events. This result does not discount the utility of these lipid markers in this context, but points to the lack of sufficient and consistent evidence in the literature. This is partly driven by the fact that many studies had to be excluded from the analyses because they did not include or adjust for all of the traditional risk factors included in this review.
This review examined 4 nontraditional lipid bio-markers, namely apo B, apo A-I, apo B/A-I ratio, and non-HDL-C, for improving CVD risk assessment. In contrast to previous guidelines, to date, some published reviews focus on 1 biomarker (22, 54) whereas others examine several biomarkers (3, 31, 55). The intent of the current review was not to determine how well the lipid biomarkers predict the risk of CVD in comparison to or as a replacement to traditional lipid and nonlipid risk factors, but rather, how do these emerging lipoproteins improve the risk prediction for CVD when added to traditional cardiovascular risk factors.
The lipid biomarkers examined in this review have great potential utility in the field of cardiovascular health and have been investigated for at least 2 decades. Undoubtedly, the literature is available to answer different types of review questions in this field. Based on the existing evidence, major Canadian guideline groups have concluded that specific apolipoproteins should be included into CVD screening biomarkers as an alternative to the traditional cholesterol indices to estimate risk and to guide therapy (56, 57). Yet, limited evidence was available to answer our review question, “Did the lipid biomarker provide additional benefit beyond traditional risk factors?” Our findings are consistent with current national guidelines in the use of apo B (55). We extend and add to those findings by investigating the use of other lipid biomarkers (apo A-I, apo B/A-I ratio, and non-HDL-C), which, to the best of our knowledge, have not been assessed in the same review and subjected to the same rigorous review criteria. It is important to acknowledge that the practices and outcomes used in this systematic review agree with the general principles of, but do not necessarily mimic, the current (ATP [Adult Treatment Panel] IV) (55) and past (ATP III) (58) guidelines for CVD risk prevention in the general population. The approach for this review was intended to be independent and stand alone and was thoroughly evaluated with guidance from the expert panel. This review was initiated while ATP III guidelines were enacted; the ATP IV guidelines were published towards the end. The traditional risk factors used in this review are in common with those assessed in both guidelines and are considered standard practice. For the applicability of the review, substantial and moderate findings were reported from Sweden (50), the US (46, 47, 50, 51), Canada (49, 52), the Netherlands (48, 53), and Denmark (58). In contrast, ATP IV guidelines (55) report risk equations from a pooled population of non-Hispanic African Americans and whites. Several studies reviewed herein provided promising results.
Considerations for implementation
Apo B is measured mainly by immunonephelometric or immunoturbidimetric assays. Efforts to improve variability among these assays have been made by harmonizing measurements using a thoroughly characterized immunoassay. Because apo B is a well-characterized analyte, it has the potential of being standardized and linked to the International System of Units (SI system), which is not possible with LDL-C, which is often only indirectly measured and harmonized to a thoroughly characterized ultracentrifugation method (16, 33, 60, 61). Like apo B, apo A-I is mainly measured with immunonephelometric or immunoturbidimetric assays. These assays are being harmonized to a thoroughly characterized immunoassay, while HDL-C (2) assays are standardized to a thoroughly characterized ultracentrifugation method (23, 33, 61). Non-HDL-C is simply calculated as the difference between total plasma cholesterol and HDL-C. Since it can be calculated directly from routine lipid tests, it does not incur additional cost, making it more readily available (54, 62). Since non-HDL-C does not depend on triglycerides, it can be calculated from nonfasting samples.
Economic evaluation
No eligible economic evaluations were identified for analysis of cost-effectiveness.
Potential harms
The use of additional lipid biomarkers could require an additional venipuncture. All venipuncture procedures pose a minimal risk to clinical staff of needle stick injury and exposure to infectious or other harmful agents. In addition, patients identified at intermediate risk to develop CVD events may become candidates for unnecessary additional testing to better stratify risk and for aggressive medical therapy (e.g., lipid lowering, blood pressure control) for secondary CVD prevention (63).
Study limitations
The scope and clinical relevance of this review is confined to CVD events and excludes stroke, which has been included in the outcome for CVD risk assessment in the recent national guidelines for assessment of cardiovascular risk (55). Most of the evidence for this review is from prospective studies with populations having a single race/ethnicity, thus limiting generalizability. However, across the studies, there was a variety of findings supporting the need to develop population-specific risk prediction systems. In some cases, the follow-up period was <10 years, which may introduce bias into the case ascertainment process. However, this concern was compensated during the quality scoring of these studies. Differences in inherent or baseline risk status may arise from the type of population used in the studies (community based vs clinic based), which may affect study results. In addition, the restriction to English language studies may also introduce bias.
Several studies summarized in this review did not control for variations in measurement method and interindividual variation—for example, this review assumed that all biomarker tests used in individual studies performed at the same level of analytical quality. The impact of these differences on the outcome of this review is not known.
Future research needs
There was sufficient evidence to make recommendations for 2 risk biomarkers based on the moderate strength of evidence. However, more research is needed to strengthen the evidence rating and also to make recommendations for the other 2 biomarkers (e.g., apo A-I and non-HDL-C). Examination of the literature revealed several deficits that need to be highlighted as best practices for high-quality studies examining the added benefit of nontraditional lipid biomarkers to predicting CVD events. Several studies did not adjust for all the traditional risk factors and provided incomplete information about the demographics of the population. Model descriptions varied, and in some cases, it was difficult to understand the model selection criteria, model specifications, and what factors were included in the model. Several studies lacked sufficient raw data eliminating the ability to replicate findings. Finally, many studies used different analytical methods to calculate the effectiveness of evaluated practices; thus, we were not able to combine the result from those studies to perform metaanalysis to produce an overall grand mean of effectiveness. It is desirable for future studies to use common standardized analytical methods.
The aim of this study was to assess the additive benefits obtained by adding the lipid biomarkers to a panel of traditional risk factors. Thus, the potential benefits of replacing the traditional risk factors with the new biomarkers, especially in patients with conditions known to have highly altered lipid particle profiles, was not assessed and requires further investigation.
Supplementary Material
IMPACT STATEMENT.
The findings from this study are beneficial for the population at risk of developing adverse cardiovascular events, including men (>35 years), women (>45 years), and younger adults (≥20 years) with multiple cardiovascular risk factors for cardiovascular disease (CVD). Existing guidelines mainly used traditional lipid levels and other risk factors—namely total cholesterol, LDL, HDL, triglycerides, high blood pressure, smoking, diabetes, age, and sex—as risk factor scores for CVD risk prediction. This study evaluated the incremental utility of nontraditional lipid biomarkers (e.g., apo B, apo A-I, and non-HDL-C) in improving CVD risk prediction to a conventional model of traditional risk factors.
Acknowledgments
This work was funded by the CDC under contract number SP0700-00-D-3180, Delivery Order 0723, “Laboratory Medicine Preparedness: Best Practices.” Names and affiliations of LMBP Workgroup can be found at http://wwwn.cdc.gov/futurelabmedicine/default.aspx. The authors recognize Melissa Gustafson (Battelle Librarian), LMBP CVD Biomarkers Expert Panel, LMBP Workgroup members, and Joanna Taliano, Reference Librarian (CDC).
Footnotes
Nonstandard abbreviations: CVD, cardiovascular disease; TC, total cholesterol; LDL-C, LDL cholesterol; HDL-C, HDL cholesterol; TG, triglycerides; apo, apolipoprotein; LMBP, Laboratory Medicine Best Practices; ATP, Adult Treatment Panel.
Disclaimer: The findings and conclusions of this article are those of the authors and do not necessarily represent the views of the CDC.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 4 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; (c) final approval of the published article; and (d) agreement to be accountable for all aspects of the article thus ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved.
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form. Employment or Leadership: None declared. Consultant or Advisory Role: None declared. Stock Ownership: None declared. Honoraria: None declared. Research Funding: R. Christenson, CDC. Expert Testimony: None declared. Patents: None declared.
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, or preparation or approval of manuscript.
References
- 1.World Health Organization. [Accessed July 2016];Fact sheet: Cardiovascular diseases (CVDs) 2014 http://www.who.int/mediacentre/factsheets/fs317/en/
- 2.Force USPST. The Guide to Clinical Preventive Services 2010–2011: Recommendations of the U.S. Preventive Services Task Force. Rockville (MD): Agency for Healthcare Research and Quality (US); 2010. US Preventive Services Task Force Guides to Clinical Preventive Services. [PubMed] [Google Scholar]
- 3.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:e50–103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Laslett LJ, Alagona PBL, Jr, Clark BABL, 3rd, Drozda JPBL, Jr, Saldivar F, Wilson SR, et al. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. J Am Coll Cardiol. 2012;60(25 Suppl):S1– 49. doi: 10.1016/j.jacc.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Arsenault BJ, Despres JP, Stroes ES, Wareham NJ, Kastelein JJ, Khaw KT, et al. Lipid assessment, metabolic syndrome and coronary heart disease risk. Eur J Clin Invest. 2010;40:1081–93. doi: 10.1111/j.1365-2362.2010.02357.x. [DOI] [PubMed] [Google Scholar]
- 6.Parish S, Offer A, Clarke R, Hopewell JC, Hill MR, Otvos JD, et al. Lipids and lipoproteins and risk of different vascular events in the MRC/BHF Heart Protection Study. Circulation. 2012;125:2469–78. doi: 10.1161/CIRCULATIONAHA.111.073684. [DOI] [PubMed] [Google Scholar]
- 7.Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012): The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts) Eur Heart J. 2012 doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 8.Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MS, et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119:2408–16. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moons KG, Kengne AP, Woodward M, Royston P, Vergouwe Y, Altman DG, et al. Risk prediction models: I. Development, internal validation, and assessing the incremental value of a new (bio)marker. Heart. 2012;98:683–90. doi: 10.1136/heartjnl-2011-301246. [DOI] [PubMed] [Google Scholar]
- 10.Clarke R, Emberson JR, Parish S, Palmer A, Shipley M, Linksted P, et al. Cholesterol fractions and apolipoproteins as risk factors for heart disease mortality in older men. Arch Intern Med. 2007;167:1373– 8. doi: 10.1001/archinte.167.13.1373. [DOI] [PubMed] [Google Scholar]
- 11.Goliasch G, Oravec S, Blessberger H, Dostal E, Hoke M, Wojta J, et al. Relative importance of different lipid risk factors for the development of myocardial infarction at a very young age (≤40 years of age) Eur J Clin Invest. 2012;42:631–6. doi: 10.1111/j.1365-2362.2011.02629.x. [DOI] [PubMed] [Google Scholar]
- 12.Langlois MR. Laboratory approaches for predicting and managing the risk of cardiovascular disease: postanalytical opportunities of lipid and lipoprotein testing. Clin Chem Lab Med. 2012;50:1169–81. doi: 10.1515/cclm-2011-0636. [DOI] [PubMed] [Google Scholar]
- 13.Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of Obesity Among Adults and Youth: United States, 2011–2014. NCHS Data Brief. 2015;(219):1–8. [PubMed] [Google Scholar]
- 14.Davidson MH, Ballantyne CM, Jacobson TA, Bittner VA, Braun LT, Brown AS, et al. Clinical utility of inflammatory markers and advanced lipoprotein testing: advice from an expert panel of lipid specialists. J Clin Lipidol. 2011;5:338–67. doi: 10.1016/j.jacl.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Sniderman AD, De Graaf J, Couture P. Low-density lipoprotein-lowering strategies: target versus maximalist versus population percentile. Curr Opin Cardiol. 2012;27:405–11. doi: 10.1097/HCO.0b013e328353fed5. [DOI] [PubMed] [Google Scholar]
- 16.Davidson MH. Apolipoprotein measurements: is more widespread use clinically indicated? Clin Cardiol. 2009;32:482–6. doi: 10.1002/clc.20559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cziraky MJ, Watson KE, Talbert RL. Targeting low HDL-cholesterol to decrease residual cardiovascular risk in the managed care setting. J Manag Care Pharm. 2008;14(8 Suppl):S3–28. quiz S30–1. [PubMed] [Google Scholar]
- 18.Dallmeier D, Koenig W. Strategies for vascular disease prevention: the role of lipids and related markers including apolipoproteins, low-density lipoproteins (LDL)-particle size, high sensitivity C-reactive protein (hs-CRP), lipoprotein-associated phospholipase A2 (Lp-PLA(2)) and lipoprotein(a) (Lp(a)) Best Pract Res Clin Endocrinol Metab. 2014;28:281–94. doi: 10.1016/j.beem.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Brindle P, Beswick A, Fahey T, Ebrahim S. Accuracy and impact of risk assessment in the primary prevention of cardiovascular disease: a systematic review. Heart. 2006;92:1752–9. doi: 10.1136/hrt.2006.087932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson R, Kerr A, Wells S. Vascular risk calculators: essential but flawed clinical tools? Circulation. 2013;127:1929–31. doi: 10.1161/CIRCULATIONAHA.113.002650. [DOI] [PubMed] [Google Scholar]
- 21.Benn M, Stene MC, Nordestgaard BG, Jensen GB, Steffensen R, Tybjaerg-Hansen A. Common and rare alleles in apolipoprotein B contribute to plasma levels of low-density lipoprotein cholesterol in the general population. J Clin Endocrinol Metab. 2008;93:1038–45. doi: 10.1210/jc.2007-1365. [DOI] [PubMed] [Google Scholar]
- 22.Contois JH, McConnell JP, Sethi AA, Csako G, Devaraj S, Hoefner DM, et al. Apolipoprotein B and cardiovascular disease risk: Position statement from the AACC Lipoproteins and Vascular Diseases Division Working Group on Best Practices. Clin Chem. 2009;55:407–19. doi: 10.1373/clinchem.2008.118356. [DOI] [PubMed] [Google Scholar]
- 23.Walldius G, Jungner I. The apoB/apoA-I ratio: a strong, new risk factor for cardiovascular disease and a target for lipid-lowering therapy–A review of the evidence. J Intern Med. 2006;259:493–519. doi: 10.1111/j.1365-2796.2006.01643.x. [DOI] [PubMed] [Google Scholar]
- 24.Mora S, Buring JE, Ridker PM. Discordance of low-density lipoprotein (LDL) cholesterol with alternative LDL-related measures and future coronary events. Circulation. 2014;129:553–61. doi: 10.1161/CIRCULATIONAHA.113.005873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sniderman AD, Islam S, Yusuf S, McQueen MJ. Discordance analysis of apolipoprotein B and non-high density lipoprotein cholesterol as markers of cardiovascular risk in the INTERHEART study. Atherosclerosis. 2012;225:444–9. doi: 10.1016/j.atherosclerosis.2012.08.039. [DOI] [PubMed] [Google Scholar]
- 26.Di Angelantonio E, Gao P, Pennells L, Kaptoge S, Caslake M, Thompson A, et al. Lipid-related markers and cardiovascular disease prediction. JAMA. 2012;307:2499–506. doi: 10.1001/jama.2012.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gigante B, Leander K, Vikstrom M, Frumento P, Carlsson AC, Bottai M, et al. Elevated ApoB serum levels strongly predict early cardiovascular events. Heart. 2012;98:1242–5. doi: 10.1136/heartjnl-2012-301852. [DOI] [PubMed] [Google Scholar]
- 28.Holme I, Aastveit AH, Jungner I, Walldius G. Relationships between lipoprotein components and risk of myocardial infarction: age, gender and short versus longer follow-up periods in the Apolipoprotein MOrtality RISk study (AMORIS) J Intern Med. 2008;264:30–8. doi: 10.1111/j.1365-2796.2008.01925.x. [DOI] [PubMed] [Google Scholar]
- 29.Meisinger C, Loewel H, Mraz W, Koenig W. Prognostic value of apolipoprotein B and A-I in the prediction of myocardial infarction in middle-aged men and women: results from the MONICA/KORA Augsburg cohort study. Eur Heart J. 2005;26:271–8. doi: 10.1093/eurheartj/ehi003. [DOI] [PubMed] [Google Scholar]
- 30.Shai I, Rimm EB, Hankinson SE, Curhan G, Manson JE, Rifai N, et al. Multivariate assessment of lipid parameters as predictors of coronary heart disease among postmenopausal women: potential implications for clinical guidelines. Circulation. 2004;110:2824–30. doi: 10.1161/01.CIR.0000146339.57154.9B. [DOI] [PubMed] [Google Scholar]
- 31.Sniderman AD, Williams K, Contois JH, Monroe HM, McQueen MJ, de Graaf J, et al. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes. 2011;4:337–45. doi: 10.1161/CIRCOUTCOMES.110.959247. [DOI] [PubMed] [Google Scholar]
- 32.Walldius G, Jungner I, Holme I, Aastveit AH, Kolar W, Steiner E. High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): A prospective study. Lancet. 2001;358:2026–33. doi: 10.1016/S0140-6736(01)07098-2. [DOI] [PubMed] [Google Scholar]
- 33.Marcovina S, Packard CJ. Measurement and meaning of apolipoprotein AI and apolipoprotein B plasma levels. J Intern Med. 2006;259:437–46. doi: 10.1111/j.1365-2796.2006.01648.x. [DOI] [PubMed] [Google Scholar]
- 34.Walldius G, Jungner I, Aastveit AH, Holme I, Furberg CD, Sniderman AD. The apoB/apoA-I ratio is better than the cholesterol ratios to estimate the balance between plasma proatherogenic and antiatherogenic lipoproteins and to predict coronary risk. Clin Chem Lab Med. 2004;42:1355–63. doi: 10.1515/CCLM.2004.254. [DOI] [PubMed] [Google Scholar]
- 35.Lind L, Vessby B, Sundstrom J. The apolipoprotein B/AI ratio and the metabolic syndrome independently predict risk for myocardial infarction in middle-aged men. Arterioscler Thromb Vasc Biol. 2006;26:406–10. doi: 10.1161/01.ATV.0000197827.12431.d0. [DOI] [PubMed] [Google Scholar]
- 36.McQueen MJ, Hawken S, Wang X, Ounpuu S, Sniderman A, Probstfield J, et al. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case-control study. Lancet. 2008;372:224–33. doi: 10.1016/S0140-6736(08)61076-4. [DOI] [PubMed] [Google Scholar]
- 37.Sniderman AD, Jungner I, Holme I, Aastveit A, Walldius G. Errors that result from using the TC/HDL C ratio rather than the apoB/apoA-I ratio to identify the lipoprotein-related risk of vascular disease. J Intern Med. 2006;259:455–61. doi: 10.1111/j.1365-2796.2006.01649.x. [DOI] [PubMed] [Google Scholar]
- 38.Bittner V, Hardison R, Kelsey SF, Weiner BH, Jacobs AK, Sopko G. Non-high-density lipoprotein cholesterol levels predict five-year outcome in the Bypass Angioplasty Revascularization Investigation (BARI) Circulation. 2002;106:2537–42. doi: 10.1161/01.cir.0000038496.57570.06. [DOI] [PubMed] [Google Scholar]
- 39.Ghosh J, Mishra TK, Rao YN, Aggarwal SK. Oxidised LDL, HDL cholesterol, LDL cholesterol levels in patients of coronary artery disease Indian. J Clin Biochem. 2006;21:181–4. doi: 10.1007/BF02913092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar A, Nagtilak S, Sivakanesan R, Gunasekera S. Cardiovascular risk factors in elderly normolipidemic acute myocardial infarct patients–A case controlled study from India. Southeast Asian J Trop Med Public Health. 2009;40:581–92. [PubMed] [Google Scholar]
- 41.Liu J, Sempos C, Donahue RP, Dorn J, Trevisan M, Grundy SM. Joint distribution of non-HDL and LDL cholesterol and coronary heart disease risk prediction among individuals with and without diabetes. Diabetes Care. 2005;28:1916–21. doi: 10.2337/diacare.28.8.1916. [DOI] [PubMed] [Google Scholar]
- 42.Pischon T, Girman CJ, Sacks FM, Rifai N, Stampfer MJ, Rimm EB. Non-high-density lipoprotein cholesterol and apolipoprotein B in the prediction of coronary heart disease in men. Circulation. 2005;112:3375–83. doi: 10.1161/CIRCULATIONAHA.104.532499. [DOI] [PubMed] [Google Scholar]
- 43.Christenson RH, Snyder SR, Shaw CS, Derzon JH, Black RS, Mass D, et al. Laboratory medicine best practices: systematic evidence review and evaluation methods for quality improvement. Clin Chem. 2011;57:816–25. doi: 10.1373/clinchem.2010.157131. [DOI] [PubMed] [Google Scholar]
- 44.Prinja S, Gupta N, Verma R. Censoring in clinical trials: review of survival analysis techniques. Indian J Community Med. 2010;35:217–21. doi: 10.4103/0970-0218.66859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borenstein MHL, Higgins JPT, Rothstein HR. Introduction to meta-analysis. Chichester (UK): Wiley; 2009. [Google Scholar]
- 46.Ingelsson E, Arnlov J, Sundstrom J, Zethelius B, Vessby B, Lind L. Novel metabolic risk factors for heart failure. J Am Coll Cardiol. 2005;46:2054–60. doi: 10.1016/j.jacc.2005.07.059. [DOI] [PubMed] [Google Scholar]
- 47.Ingelsson E, Schaefer EJ, Contois JH, McNamara JR, Sullivan L, Keyes MJ, et al. Clinical utility of different lipid measures for prediction of coronary heart disease in men and women. JAMA. 2007;298:776–85. doi: 10.1001/jama.298.7.776. [DOI] [PubMed] [Google Scholar]
- 48.Kappelle PJ, Gansevoort RT, Hillege JL, Wolffenbuttel BH, Dullaart RP. Apolipoprotein B/A-I and total cholesterol/high-density lipoprotein cholesterol ratios both predict cardiovascular events in the general population independently of nonlipid risk factors, albuminuria and C-reactive protein. J Intern Med. 2011;269:232–42. doi: 10.1111/j.1365-2796.2010.02323.x. [DOI] [PubMed] [Google Scholar]
- 49.Lamarche B, Moorjani S, Lupien PJ, Cantin B, Bernard PM, Dagenais GR, et al. Apolipoprotein A-I and B levels and the risk of ischemic heart disease during a five-year follow-up of men in the Quebec cardiovascular study. Circulation. 1996;94:273–8. doi: 10.1161/01.cir.94.3.273. [DOI] [PubMed] [Google Scholar]
- 50.Sierra-Johnson J, Fisher RM, Romero-Corral A, Somers VK, Lopez-Jimenez F, Ohrvik J, et al. Concentration of apolipoprotein B is comparable with the apolipoprotein B/apolipoprotein A-I ratio and better than routine clinical lipid measurements in predicting coronary heart disease mortality: Findings from a multi-ethnic US population. Eur Heart J. 2009;30:710–7. doi: 10.1093/eurheartj/ehn347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steffen BT, Guan W, Remaley AT, Paramsothy P, Heckbert SR, McClelland RL, et al. Use of lipoprotein particle measures for assessing coronary heart disease risk post-American Heart Association/American College of Cardiology guidelines: The Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:448–54. doi: 10.1161/ATVBAHA.114.304349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.St-Pierre AC, Cantin B, Dagenais GR, Despres JP, Lamarche B. Apolipoprotein-B, low-density lipoprotein cholesterol, and the long-term risk of coronary heart disease in men. Am J Cardiol. 2006;97:997–1001. doi: 10.1016/j.amjcard.2005.10.060. [DOI] [PubMed] [Google Scholar]
- 53.van der Steeg WA, Boekholdt SM, Stein EA, El-Harchaoui K, Stroes ES, Sandhu MS, et al. Role of the apolipoprotein B-apolipoprotein A-I ratio in cardiovascular risk assessment: A case-control analysis in EPIC-Norfolk. Ann Intern Med. 2007;146:640–8. doi: 10.7326/0003-4819-146-9-200705010-00007. [DOI] [PubMed] [Google Scholar]
- 54.Blaha MJ, Blumenthal RS, Brinton EA, Jacobson TA. The importance of non-HDL cholesterol reporting in lipid management. J Clin Lipidol. 2008;2:267–73. doi: 10.1016/j.jacl.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 55.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Sr, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935–59. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anderson TJ, Gregoire J, Hegele RA, Couture P, Mancini GB, McPherson R, et al. 2012 update of the Canadian Cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2013;29:151–67. doi: 10.1016/j.cjca.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 57.Genest J, Frohlich J, Fodor G, McPherson R. Recommendations for the management of dyslipidemia and the prevention of cardiovascular disease: summary of the 2003 update. CMAJ. 2003;169:921–4. [PMC free article] [PubMed] [Google Scholar]
- 58.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 59.Benn M, Nordestgaard BG, Jensen GB, Tybjaerg-Hansen A. Improving prediction of ischemic cardiovascular disease in the general population using apolipoprotein B: The Copenhagen City Heart Study. Arterioscler Thromb Vasc Biol. 2007;27:661–70. doi: 10.1161/01.ATV.0000255580.73689.8e. [DOI] [PubMed] [Google Scholar]
- 60.Miller WG, Myers GL, Sakurabayashi I, Bachmann LM, Caudill SP, Dziekonski A, et al. Seven direct methods for measuring HDL and LDL cholesterol compared with ultracentrifugation reference measurement procedures. Clin Chem. 2010;56:977–86. doi: 10.1373/clinchem.2009.142810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rifai NWG, Dominiczak MH. Handbook of lipoprotein testing. Washington (DC): AACC Press; 1997. [Google Scholar]
- 62.Ramjee V, Sperling LS, Jacobson TA. Non-high-density lipoprotein cholesterol versus apolipoprotein B in cardiovascular risk stratification: do the math. J Am Coll Cardiol. 2011;58:457–63. doi: 10.1016/j.jacc.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 63.Smith SC, Jr, Amsterdam E, Balady GJ, Bonow RO, Fletcher GF, Froelicher V, et al. Prevention Conference V: Beyond secondary prevention: identifying the high-risk patient for primary prevention: tests for silent and inducible ischemia: Writing Group II. Circulation. 2000;101:E12–6. doi: 10.1161/01.cir.101.1.e12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.