Abstract
In the period 2005–13 the US prescription drug market grew at an average annual pace of only 1.8 percent in real terms on an invoice price basis (that is, in constant dollars and before manufacturers’ rebates and discounts). But the growth rate increased dramatically in 2014, when the market expanded by 11.5 percent—which raised questions about future trends. We determined the impact of manufacturers’ rebates and discounts on prices and identified the underlying factors likely to influence prescription spending over the next decade. These include a strengthening of the innovation pipeline; consolidation among buyers such as wholesalers, pharmacy benefit managers, and health insurers; and reduced incidence of patent expirations, which means that fewer less costly generic drug substitutes will enter the market than in the recent past. While various forecasts indicate that pharmaceutical spending growth will moderate from its 2014 level, the business tension between buyers and sellers could play out in many different ways. This suggests that future spending trends remain highly uncertain.
Spending on retail prescription drugs in the United States increased by 12.2 percent in 2014, the largest annual increase since 2002.1 The total market for prescription drugs—including both retail and other channels, such as hospitals and clinics—increased by 11.5 percent in real invoice price terms (that is, prices adjusted for inflation and before manufacturers’ discounts and rebates) in 2014. In contrast, in the period 2005–13 the market increased only 1.8 percent annually, on average. Is this discontinuity a one-time event, or does it signal a permanent change in spending?
The pharmaceutical market landscape is evolving dramatically, and the impact on spending remains uncertain. Significant buyer consolidation has occurred in recent years through mergers and acquisitions of pharmacy benefit managers, insurers, and wholesalers. Meanwhile, manufacturers of brand-name drugs have become fragmented, with the top twenty manufacturers capturing a smaller share of market today than previously, and mergers and acquisitions have reduced the number of generic drug manufacturers.2
In recent years brand-name drug manufacturers have been raising the list prices of their products, but payers have been negotiating larger rebates for them. Under the Affordable Care Act (ACA), millions of people have obtained health insurance coverage, which has increased the demand for prescription drugs. At the same time, more people with health insurance are enrolling in plans that contain cost-sharing requirements for pharmaceutical products, including pharmacy deductibles and copayments.3
On the supply side, brand-name drug manufacturers cite research and development costs and high risk of failure to justify high pharmaceutical prices.4 On the demand side, however, efforts are focused on reducing spending. Understanding the roles and relative importance of these competing forces will help assess how they may play out in the ongoing “pharms race.”
Study Data And Methods
We analyzed US prescription drug spending trends using data from IMS Health’s National Sales Perspectives (NSP), which audits sales of pharmaceutical products from wholesalers to pharmacies and other outlets. These data differ from published National Health Expenditure Accounts1 compiled by the Centers for Medicare and Medicaid Services (CMS) in two major ways.
First, NSP calculates prescription drug spending through all channels: retail and mail-order direct sales to consumers and all sales to physicians, hospitals, nursing homes, and other institutions. In contrast, the prescription drug total in the National Health Expenditure Accounts consists only of sales through retail outlets, and purchases of many costly specialty medicines, such as the purchase of oncology drugs by institutions and other health care providers, are excluded. In 2014 these institutions and providers accounted for about 21.9 percent of total NSP prescription drug spending.
Second, the NSP is based on invoiced sales by wholesalers (and direct sales by manufacturers) to their customers, such as major national pharmacy chains, hospitals, clinics, group purchasing organizations, and other supply chain intermediaries. Although the invoiced data incorporate discounts for prompt payment, they typically do not capture off-invoice discounts and rebates frequently given by manufacturers to insurers, providers, and pharmacy benefit managers. The National Health Expenditure Accounts include an estimate of these discounts and rebates, as we do in this article. But because not all purchasers receive discounts and rebates, we also discuss sales before any discount or rebate is applied. We call these “invoice sales,” in contrast to “net sales.”
IMS Health has estimated average rebates by comparing NSP invoice sales with net sales data disclosed for some brand-name drugs in Securities and Exchange Commission (SEC) 10K filings by manufacturers. We computed the rebate share as the percentage difference between invoice and net sales, and we projected average rebate share by therapeutic class when SEC data were not available.5
Study Results
Overall Invoice Sales Trends And Their Decomposition
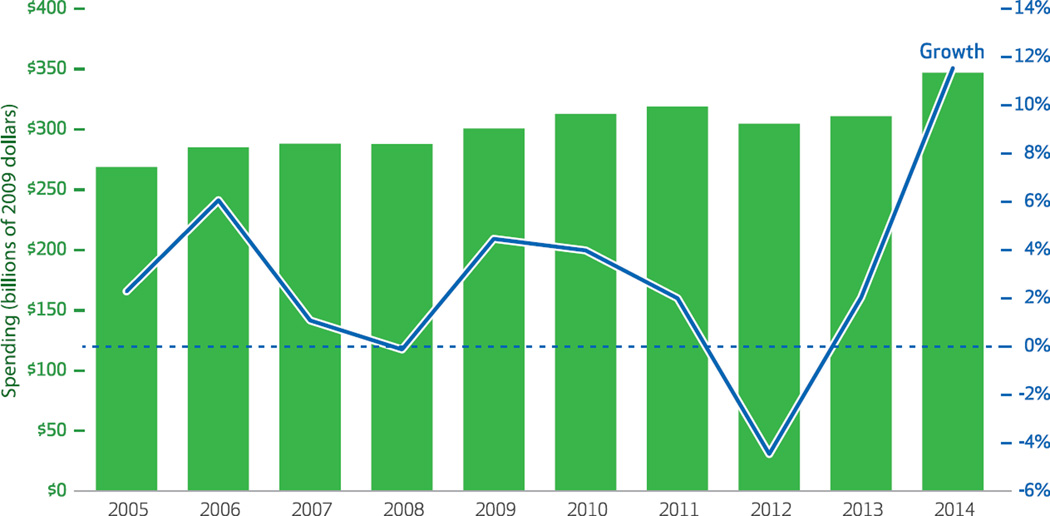
Annual changes in spending on prescription drugs fluctuated from a $14.2 billion decline from 2011 to 2012 to a $35.8 billion increase from 2013 to 2014 (Exhibit 1). Average spending in the period 2005–14 grew by $5.3 billion compounded annually, or 2.9 percent (data not shown).
Exhibit 1. Prescription drug spending based on invoice sales, and rate of spending growth.
SOURCE Authors’ analysis of data from the IMS National Sales Perspectives Dataset. NOTE Spending was adjusted to constant 2009 dollars using the St. Louis Federal Reserve quarterly gross domestic product price deflator series.
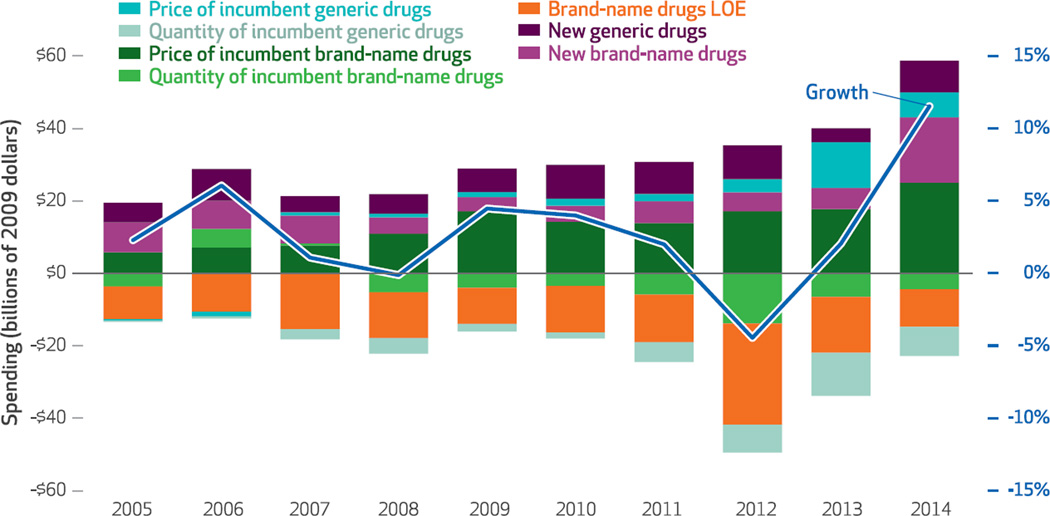
We decomposed this growth into its price and quantity components for brand-name and generic medications. Brand-name drugs were further segmented into new products introduced in the previous two years and drugs that lost market exclusivity because their patents expired (incumbent brand-name drugs) (Exhibit 2).6 Several striking patterns are apparent.
Exhibit 2. Price and quantity components of prescription drug spending based on invoice sales, and rate of spending growth.
SOURCE Authors’ analysis of data from the IMS National Sales Perspectives Dataset. NOTE Spending was adjusted to constant 2009 dollars using the St. Louis Federal Reserve quarterly gross domestic product price deflator series. Incumbent drugs are defined in the text. LOE is loss of exclusivity.
First, the dominant trend is that over much of the study period, growth in real pharmaceutical spending based on invoice sales decelerated, falling from a high of 6.1 percent in 2006 to a low of −4.5 percent in 2012. In each year since 2007, increased invoice prices for incumbent brand-name drugs accounted for the largest portion of overall spending growth; this became particularly prominent beginning in 2009.
Second, reductions in spending on brand-name drugs resulting from the loss of exclusivity of incumbent drugs accounted for most of the spending reductions during the study period. However, this component was smaller in 2014 than in previous years, which partially explains the 2014 discontinuity. Loss of exclusivity for incumbent brand-name drugs was particularly large in 2012, when a number of blockbuster brand-name drugs (those having annual sales of $1 billion or more)—including Lipitor, Plavix, Singulair, Actos, and Lexapro—faced generic competition for the first time. Altogether, brand-name products that lost exclusivity in 2012 accounted for $49 billion in real invoice spending in 2011 (data not shown). By 2013, spending on those products and their generic counterparts had declined almost 80 percent, to $10.5 billion.
Third, 2014 was the first time in the study period that spending increased significantly because of the introduction of new brand-name drugs, particularly the new generation of antiviral hepatitis C drugs (Sovaldi, Olysio, Viekira Pak, and Harvoni). Other newly launched drugs that treat cancers, multiple sclerosis, and diabetes also contributed to spending growth.
Fourth, while prices of incumbent generic drugs have been increasing slightly since 2007, in 2013 and 2014 these increases were markedly larger and contributed more significantly to overall spending increases, compared to earlier in the study period.
Gross And Net Sales And Prices
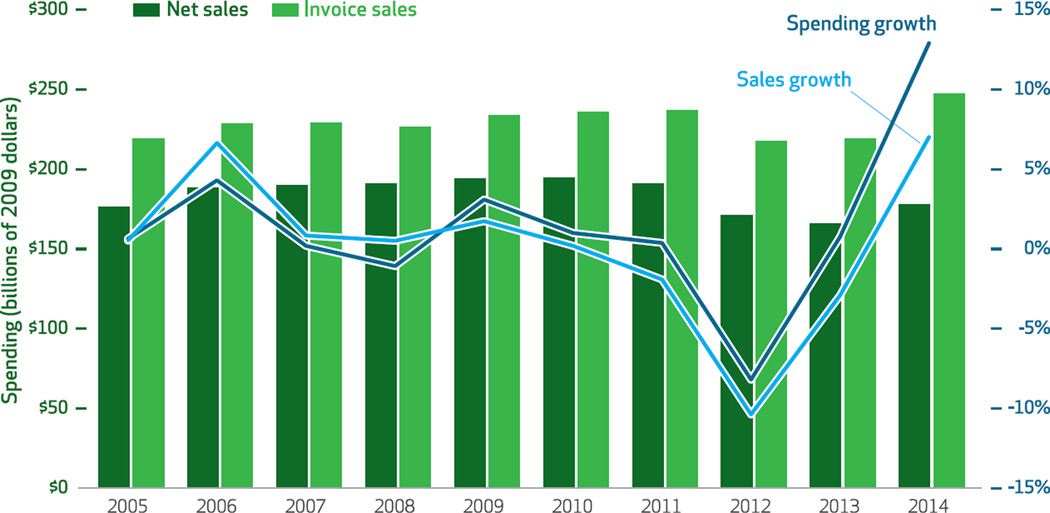
Between 2005 and 2012 manufacturers’ discounts and rebates reduced spending on brand-name drugs by approximately 18 percent each year (Exhibit 3). However, between 2010 and 2014 the discounts and rebates increased from about 18 percent to 28 percent of total spending on brand-name drugs (the difference between the net sales and invoice sales bars for each year). It is important to consider this increase when examining year-to-year changes.
Exhibit 3. Sales of brand-name drugs, before and after accounting for manufacturers’ discounts and rebates, and rates of sales and spending growth.
SOURCE Authors’ analysis of data from the IMS National Sales Perspectives Dataset. NOTES Sales and spending were adjusted to constant 2009 dollars using the St. Louis Federal Reserve quarterly gross domestic product price deflator series. Net sales take account of manufacturers’ off-invoice discounts and rebates; invoice sales do not.
To correctly identify the real growth rate of prices, we regressed price on fixed effects for product and time (results not shown). In particular, including product fixed effects eliminated the selection bias introduced by the fact that newer drugs tend to be more expensive than older ones. We estimated that the compounded annual real growth rate of invoice prices in the period 2005–14 was 6.4 percent, whereas the rate for net prices was only 5.4 percent—with most of the difference occurring after 2009.
The total value of discounts and rebates provided by manufacturers of brand-name drugs in the period 2010–14 was almost $260 billion (Exhibit 3)—the sum of the differences between invoice sales and net sales from 2010 to 2014. Because price increases for these drugs are the largest component in overall prescription drug spending growth, the difference between invoice and net prices has a significant impact on total spending: Incorporating rebates and discounts reduces the growth rate of real pharmaceutical spending overall from 2.0 percent to −0.3 percent in 2013 and from 11.5 percent to 7.4 percent in 2014 (data not shown).
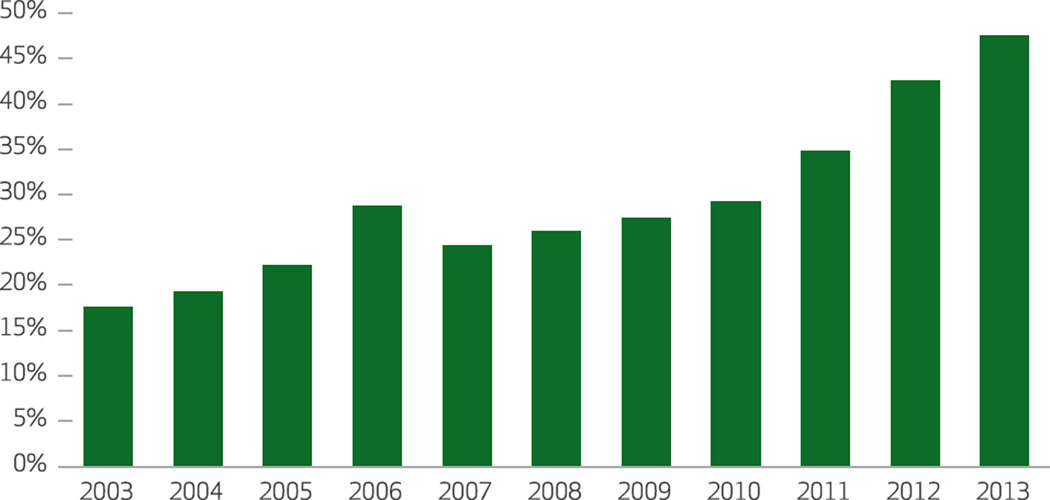
As a percentage of gross Medicaid drug expenditures, rebates increased dramatically from 17.6 percent in 2003 to 47.6 percent in 2013 (Exhibit 4). Most of the increase occurred between 2010 and 2013, as provisions of the ACA increased the mandated rebate rate. The growth in invoice prices for brand-name drugs in 2013–14 also triggered higher levels of Medicaid rebates as a result of automatic adjustments to rebates for drugs whose price growth rate exceeds the rate of general inflation.7
Exhibit 4. Manufacturers’ rebates to Medicaid as percentages of gross Medicaid drug expenditures.
SOURCE Authors’ analysis of data from the Medicaid Expenditure Reports, Centers for Medicare and Medicaid Services.
Rebates in the Medicare program have grown steadily, from 8.6 percent of total costs for brand-name and generic drugs (including the benefit portion of members’ premiums) in 2006 to 12.9 in 2013.8 In addition, the Medicare Coverage Gap Discount Program makes manufacturers’ discounts available to eligible Medicare beneficiaries who receive applicable drugs covered by Part D while in the coverage gap, which reduces net prices.9 Manufacturers offer additional discounts to federal, state, and local government purchasers through the 340B Drug Pricing Program.10
Manufacturers also provide rebates to private third-party payers, such as pharmacy benefit managers, hospitals, and insurers. Much less is known about these rebates than is known about public program rebates. However, information gathered by IMS Health suggests that these rebates tend to grow over the life cycle of drugs, peaking several years before patent expiration and generally ceasing after expiration and the entry into the market of generic drugs.
Considerable heterogeneity exists in the size of these rebates, both across firms and across products within firms.11 Products in crowded therapeutic areas tend to have larger rebates. Furthermore, discounts differ across buyers, with larger buyers able to extract greater price reductions, compared to smaller buyers—particularly if larger buyers can affect the market share of drugs through their formulary policies. In recent years, some pharmacy benefit managers have even negotiated inflation-protected provisions that incorporate automatic rebate increases whenever the manufacturer raises the list price, leaving the net price unchanged.
Discussion
Recent Trends In Drugs’ List Prices And Rebates
To some extent, increases in rebates are a consequence of ACA provisions, reflecting growth in brand-name drug prices in excess of the Consumer Price Index and the impact of increased rebate requirements for drugs provided through Medicaid. However, we also observed increases in invoice prices, which reflect manufacturer list prices. This raises the question of why manufacturers are increasing list prices.
High list prices combined with rebates that differ by payer and drug classes appear to reflect manufacturers’ ability to charge different net prices to various sets of consumers, a practice known as price discrimination. Pharmaceuticals—like movies, satellites, software, and many other products of high-technology industries—require very high up-front spending to develop and bring to market, but once launched, they have relatively small marginal costs of additional production. The manufacturers of such products can charge higher prices to relatively price-insensitive purchasers (those whose demand for a product is not affected by price) and much lower prices to more price-sensitive purchasers. For this strategy to be feasible, there must be sufficient numbers of purchasers willing to pay different prices, and purchasers who buy at a lower price must be prohibited from reselling at a higher one.
The following two market developments can explain the simultaneous increasing of list prices and rebates: the growth in importance of pharmacy benefit managers and the increase in the number of people who pay list prices for pharmaceuticals.
Pharmacy benefit managers have grown steadily in importance since the late 1980s, contracting with insurers or payers and provider networks, serving as intermediaries between manufacturers and insurers, and occasionally establishing their own mail-order pharmacy operations. The three largest benefit managers increased their share of the total commercial prescription volume from 42 percent in 2005 to 68 percent in 2015.12,13 This has increased their collective purchasing power and ability to extract rebates and discounts from manufacturers.
Simultaneously, the number of people without insurance coverage for drugs has been steadily falling since the introduction of the Medicare Part D benefit in 2006 and the ACA coverage expansions in 2014. However, the number of people enrolled in high-deductible health plans, particularly those with health savings accounts, has increased significantly. In 2015, 24 percent of covered employees were enrolled in a high-deductible health plan, up sixfold from 4 percent in 2006.14 As more people use these high-deductible accounts or enroll in plans with pharmacy deductibles, and as deductibles increase, drug companies have an incentive to increase their list prices for these relatively price-insensitive consumers.
Recent changes brought about by the ACA have also altered the landscape for people in the Medicare coverage gap. Before implementation of the ACA, those people had to bear the full cost of the drugs they purchased. One provision in the ACA mandated that manufacturers provide a 50 percent discount on the list price for Medicare enrollees in the coverage gap.14 Assuming that the price sensitivity of people in the coverage gap did not change, this reform—together with the increase in the Medicaid rebate mentioned above—creates incentives for manufacturers to raise their list prices. Commercial payers can afford to react modestly, because with a copay rate of 25 percent and a rebate of 50 percent off the list price, they bear only 25 percent of the list price.
Another recently expanded federal program is the 340B Drug Pricing Program, which requires manufacturers to provide outpatient drugs to eligible health care organizations or covered entities at significantly reduced prices. Established in 1992, the program has grown particularly rapidly in recent years.15,16 An indirect impact of the program is that it provides incentives for eligible entities such as hospitals to acquire physician practices, particularly those whose providers administer costly specialty drugs, since the practices can now purchase drugs less expensively than in the past. This phenomenon strengthens incentives in various ACA payment reform initiatives for hospitals to merge with one another and with physician practices.17 The resulting increase in the number of previously price-sensitive buyers who are now taking advantage of these discounts provides incentives for manufacturers to engage in price discrimination and raise list prices.
Finally, it should be noted that pharmacy benefit managers, wholesalers, and pharmacies have limited incentives to resist list price increases, since their profit “spreads” are often a percentage of list prices. Moreover, benefit managers frequently charge manufacturers a fixed portion of the list price to provide them with administrative services, which means that the managers’ revenues increase as list prices do. Of course, competition for business can cause benefit managers and pharmacies to reduce the amount they charge insurers and patients.
Loss Of Exclusivity On Brand-Name Drugs
In 2011 and 2012 numerous blockbuster drugs lost marketing exclusivity as their patents expired; as a result of generic drugs’ entering the market, spending on these brand-name drugs was drastically reduced. Cumulative spending reductions between 2011 and 2013 because of loss of exclusivity exceeded $56 billion (Exhibit 2). However, in 2014 no major drugs lost exclusivity, and as a result, related spending reductions were much smaller ($10.4 billion) than in previous years.
In the period 2016–20 several large-molecule (or biologic) blockbuster brand-name drugs—such as Rituxan, Novolog, Humira, and Avastin—are expected to lose patent protection. While drugs comparable to specific biologics, known as biosimilars, have been available in Europe since 2006, the Food and Drug Administration (FDA) did not approve the first biosimilar for sale in the United States until 2015. Thus far, only Zarxio (a biosimilar of filgrastim, marketed as Neupogen) and Inflectra (a biosimilar of infliximab, marketed as Remicade) have received FDA approval, although many more are expected to do so in the coming years.
The impact of the impending wave of biosimilar drugs is unclear, but there are several reasons to believe that they will not reduce spending on brand-name drugs as much as they will spending on small-molecule generic drugs. First, while most pharmacies, pharmacy benefit managers, and payers have established policies that allow them to switch to generic small-molecule drugs as soon as they become available, such measures would not apply equally to biosimilars. Standards for the interchangeability of biosimilars with their reference products have not yet been established or implemented.
The pharmaceutical market landscape is evolving dramatically, and the impact on spending remains uncertain.
Second, biosimilar manufacturing costs are generally considerably higher than those for small-molecule generic drugs, which constrains biosimilar entry into the market. However, some manufacturers’ prices for biosimilars in European markets suggest that there may be significant heterogeneity.18
Third, patients and their physicians might be more wary of switching from an original brand-name biologic to a biosimilar drug than they are about switching from an original brand-name small molecule to a generic product, in part because of concerns about immunogenicity—“the propensity of the therapeutic protein product to generate immune responses to itself and to related proteins or to induce immunologically related adverse clinical events.”19
Finally, the law is currently unsettled. Virtually every biosimilar product seeking FDA approval has been delayed by lawsuits filed by the holder of the patent on the original product. Until this litigation is resolved, the related uncertainty likely discourages potential entrants from even filing for approval.20
Generic Prices: From Steady Decreases To Sharp Increases
In 2014, 82 percent of all retail prescriptions (including those purchased by mail order and used in long-term care facilities) were dispensed as generic drugs, 6 percent as brand-name generics (those generics marketed with trade names), and 12 percent as brand-name drugs. By contrast, as a share of all retail prescription drug revenues, generics accounted for only 17 percent, brand-name generics 11 percent, and brand-name drugs 72 percent.21 Hence, despite their dominant share of dispensed prescriptions, generic drugs’ contributions to overall drug spending trends are relatively minor. This is partly because of the dramatically lower costs associated with generics, relative to brand-name drugs.22
We do not expect a détente in the pharms race.
Before 2013, price increases for incumbent generic drugs were negligible and contributed little to overall drug spending (Exhibit 2). However, in 2013 and 2014, changes in these drugs’ prices contributed significantly to increases in overall drug spending. What phenomena underlie this sharp change in the long-term trend of generic prices?
Many observers say that a principal driver of this change is the considerable merger and acquisition activity among generic drug manufacturers and between manufacturers of brand-name and generic drugs,2 although this activity and its impact have not been quantified. Additionally, small markets for specific molecules tend to attract fewer competitors than larger markets do,23 and there is an increased risk for extreme price increases in very small markets.
Rising prices generally create incentives for other firms to enter the market, which tends to increase supply and return prices to long-term levels. In the United States, however, competitive generic entry has been hindered by backlogs in the FDA’s handling of Abbreviated New Drug Applications (ANDAs), despite efforts to address this problem by the FDA, supported by the Generic Drug User Fee Amendments of 2012 (GDUFA).24
The FDA has committed to reducing the ANDA backlog and median review times. Moreover, user fee provisions of GDUFA are now under discussion by the FDA, manufacturers, and other stakeholders and expected to be renewed by October 2017. It is plausible to expect that to the extent that the FDA works diligently to reduce the ANDA backlog, which will result in additional competitors entering the market, upward pressures on price levels of generic drugs will be mitigated in the coming years. Recent evidence suggests that growth in generic drug prices has eased since 2014.25–27
What To Expect From The Innovation Pipeline?
A major reason behind the surge in spending on prescription drugs in 2014 was the introduction of several innovative treatments for diseases that affect large populations, such as hepatitis C, diabetes, and multiple sclerosis. Compared to previous treatments, the innovative ones are often more convenient (for example, they may be oral solids instead of injectables that need to be administered frequently) and tolerable (having fewer side effects), substantially more efficacious, or both.
The premier example of recent changes is the market for hepatitis C medicines. Before May 2011, standard therapy involved a combination of interferons and ribavirin, to be taken for up to forty-eight weeks. The treatment had severe side effects and a relatively low cure rate (about 50 percent). Beginning in May 2011, a series of new therapies, each improving on its predecessors, reached the US market, beginning with Merck’s Victrelis—which reduced treatment duration to twenty-four weeks and boasted a higher cure rate. Only a few days after approving Victrelis, the FDA approved Incivek, which reduced treatment to twelve weeks in some cases and decreased the number of daily tablets from twelve to six.28
Just two years later, a new generation of hepatitis C medications was launched, beginning in late 2013 with Johnson and Johnson’s Olysio and Gilead’s Sovaldi, followed by another Gilead product (Harvoni) and AbbVie’s Viekira Pak. These successive innovations boosted cure rates to more than 90 percent and simplified treatment, while reducing side effects by decreasing or eliminating the interferon and ribavirin treatment components. In 2014—as the number of patients treated reached about 141,000, compared to 17,000 in 2013—net real spending for new-generation hepatitis drugs increased by $7 billion, accounting for almost 40 percent of the net increase in overall US drug spending in 2014.21
The observed shift in the mix of newly launched drugs from those focused on small patient populations (including orphan drugs) to those with larger potential markets may continue in at least a few large therapeutic areas. For example, the FDA recently approved Praluent and Repatha, two members of a new family of cholesterol-lowering medicines that inhibit the enzyme proprotein convertase subtilisin/kexin type 9 (PCSK-9).While estimates of the potential market size differ and important long-term clinical studies have not yet been completed, some observers believe that the goal of treatment should be to eliminate all low-density lipoprotein (LDL) cholesterol, not just to reduce LDL levels to below 100 mg/dl, or even 70 mg/dl.29 If the clinical evidence continues to show cardiovascular and stroke benefits from driving LDL down to very low levels, the potential market for PCSK-9 inhibitors will likely be measured in billions of dollars.30
Another area that could experience a surge in innovation and spending is the PD-1 (programmed death) oncology drugs. These drugs are the result of a line of research that attempted to trigger immune responses in the presence of tumors. The first two molecules in this new class of drugs, Keytruda and Opdivo, were approved by the FDA in late 2014 and have the potential to be blockbusters. Looking at the current pipeline of drugs in clinical development, we conclude that the current innovation boom could be sustained for at least a few more years, particularly in the area of oncology—which means that we have likely not seen the last of innovation-spurred growth in spending.
Conclusion
Recent forecasts suggest a moderation in prescription drug spending growth after 2014, with the CMS Office of the Actuary projecting retail prescription drug spending growth to average 6.7 percent from 2016 through 2025.31 Other observers have reported moderation from 2014 growth levels in 2015.32,33The recent acceleration in FDA approvals of new drugs, innovative and increasingly patient-friendly formulations (from injectables that must be administered frequently to those that can be administered less frequently and to oral tablets and capsules that are taken once a day), and regulatory initiatives may all be contributing to new treatment options, improved outcomes, and faster drug spending growth. However, consolidation on the demand side by pharmacy benefit managers, insurers’ shift to increased patient copayments, and highly visible drug price increases by some manufacturers suggest that drug spending growth will likely be highly publicized and controversial. We do not expect a détente in the pharms race.
Acknowledgments
Ernst Berndt’s research on this project was supported in part by a grant from the National Institutes of Health (NIH) (Grant No. NIANIH/R01 AG043560) to the National Bureau of Economic Research (NBER). David Cutler’s research on this project was supported in part by a grant from the NIH (Grant No. P01AG005842). Any opinions and views expressed in this article and are not necessarily those of the NIH or the NBER.
Contributor Information
Murray Aitken, Email: MAitken@theimsinstitute.org, IMS Institute for Healthcare Informatics, in Parsippany, New Jersey.
Ernst R. Berndt, Louis B. Seley Professor of Applied Economics in the Alfred P. Sloan School of Management at the Massachusetts Institute of Technology, in Cambridge. He is a research associate at the National Bureau of Economics in Cambridge
David Cutler, Otto Eckstein Professor of Applied Economics in the Department of Economics at Harvard University, in Cambridge. He is a research associate at the National Bureau of Economics.
Michael Kleinrock, Research development at the IMS Institute for Healthcare Informatics, in Plymouth Meeting, Pennsylvania.
Luca Maini, Economics at Harvard University.
NOTES
- 1. CMS.gov; Baltimore (MD) Centers for Medicare and Medicaid Services; [cited 2016 Jul 13]. National Health Expenditures 2014 highlights [Internet] Available from: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/Downloads/highlights.pdf. [Google Scholar]
- 2.Understanding recent trends in generic drug prices [Internet] Washington (DC): ASPE; 2016. Jan 27, [cited 2016 Jul 13]. Department of Health and Human Services, Office of the Assistant Secretary for Planning and Evaluation. (ASPE Issue Brief). Available from: https://aspe.hhs.gov/sites/default/files/pdf/175071/GenericsDrugpaperr.pdf. [Google Scholar]
- 3.Emergence and impact of pharmacy deductibles: implications for patients in commercial health plans. Parsippany (NJ): The Institute; 2015. Sep, IMS Institute for Healthcare Informatics. [Google Scholar]
- 4.Prescription medicines: costs in context [Internet] Washington (DC): PhRMA; [cited 2016 Jul 19]. Pharmaceutical Research and Manufacturers of America. Available from: http://www.phrma.org/sites/default/files/pdf/prescription-medicines-costs-in-context-extended.pdf. [Google Scholar]
- 5.Branded medicine price increases and the impact of off-invoice discounts and rebates [Internet] Parsippany (NJ): The Institute; 2015. Nov, [cited 2016 Jul 13]. IMS Institute for Healthcare Informatics. Available from: https://www.imshealth.com/files/web/IMSH%20Institute/Healthcare%20Briefs/IIHI_Branded_Medicine_Price_Increases_Healthcare_Brief.pdf. [Google Scholar]
- 6.The difference in spending between two years can be thought of as the sum of four components: the change in volume sales, holding price constant: the change in price, holding volume sales constant; spending on new products—that is, products launched in the current calendar year; and reduced spending on brand-name drugs that have lost market exclusivity because of the expiration of patents or other forms of exclusivity. The product of change in volume sales and change in price is included in the volume component.
- 7. Medicaid.gov. Medicaid Drug Rebate Program [Internet] Baltimore (MD): Centers for Medicare and Medicaid Services; [cited 2016 Jul 13]. last updated 2016 Jun 16; Available from: https://www.medicaid.gov/Medicaid-CHIP-Program-Information/By-Topics/Benefits/Prescription-Drugs/Medicaid-Drug-Rebate-Program.html. [Google Scholar]
- 8.Boards of Trustees. 2015 annual report of the Boards of Trustees of the Federal Hospital Insurance and Federal Supplementary Medical Insurance Trust Funds [Internet] Baltimore (MD): Centers for Medicare and Medicaid Services; 2015. Jul 22, [cited 2106 Jul 13]. Available from: https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/reportstrustfunds/downloads/tr2015.pdf. [Google Scholar]
- 9. CMS.gov. Part D information for pharmaceutical manufacturers [Internet] Baltimore (MD): Centers for Medicare and Medicaid Services; [cited 2016 Jul 13]. last modified 2016 Jul 6; Available from: https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovGenIn/Pharma.html. [Google Scholar]
- 10.340B Drug Pricing Program [Internet] Rockville (MD): HRSA; [cited 2016 Jul 13]. Health Resources and Services Administration. Available from: http://www.hrsa.gov/opa/ [Google Scholar]
- 11.IMS Health. IMS Market Prognosis 2013–2017: North America—United States. London: IMS Health; 2013. [Google Scholar]
- 12.Unpublished research by IMS Institute for Healthcare Informatics based on IMS Health Rx Benefit Design.
- 13.2014 PBM market share [Internet] Plano (TX): The Institute; [cited 2016 Jul 13]. Pharmacy Benefit Management Institute. Available from: https://www.pbmi.com/2015/04/2014-pbm-market-share/ [Google Scholar]
- 14.Henry J. Employer health benefits: 2015 annual survey [Internet] Menlo Park (CA): KFF; 2015. Sep, [cited 2016 Jul 19]. Kaiser Family Foundation, Health Research and Educational Trust. Available from: http://kff.org/health-costs/report/2015-employer-health-benefits-survey/ [Google Scholar]
- 15.Conti RM, Bach PB. The 340B Drug Discount Program: hospitals generate profits by expanding reach to more affluent communities. Health Aff (Millwood) 2014;33(10):1786–1792. doi: 10.1377/hlthaff.2014.0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wynne B. The coming storm over the 340B Rx Drug Discount Program. [cited 2016 Jul 13];Health Affairs Blog [blog on the Internet] 2014 May 6; Available from: http://healthaffairs.org/blog/2014/05/06/the-coming-storm-over-the-340b-rx-drug-discount-program. [Google Scholar]
- 17.McCluskey PD. Rising costs, changing payments driving hospital mergers. Boston Globe. 2015 Sep 3; [Google Scholar]
- 18.Assessing biosimilar uptake and competition in European markets [Internet] Parsippany (NJ): The Institute; 2014. Oct, [cited 2016 Jul 13]. IMS Institute for Healthcare Informatics. Available from: www.theimsinstitute.org/assessingbiosimilarsineurope2014. [Google Scholar]
- 19.Guidance for industry: immunogenicity assessment for therapeutic protein products [Internet] Silver Spring (MD): FDA; 2014. Aug, [cited 2016 Jul 13]. Food and Drug Administration. Available from: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatory information/guidances/ucm338856.pdf. [Google Scholar]
- 20.For a discussion of the prospects for biosimilars and “biobetters,” see: Berndt ER, Trusheim MR. Biosimilar and biobetter scenarios for the US and Europe: what should we expect? In: Rosenberg A, Demeule B, editors. Biobetters: protein engineering to approach the curative. New York (NY): Springer-Verlag; 2015. pp. 315–360.
- 21.Medicine use and spending shifts: a review of the use of medicines in the U.S. in 2014 [Internet] Parsippany (NJ): The Institute; 2016. [cited 2016 Jul 29]. IMS Institute for Healthcare Informatics. c Available from: http://www.imshealth.com/en/thought-leadership/imsinstitute/reports/medicines-use-in-the-us-2014#ims-form. [Google Scholar]
- 22.Price declines after branded medicines lose exclusivity in the U.S. [Internet] Parsippany (NJ): The Institute; 2016. Jan, [cited 2016 Jul 13]. IMS Institute for Healthcare Informatics. Available from: http://www.theimsinstitute.org/brandedmedicinespricedecline. [Google Scholar]
- 23.Grabowski HG, Ridley DB, Schulman KA. Entry and competition in generic biologics. MDE Manage Decis Econ. 2007;28(4–5):439–51. [Google Scholar]
- 24.Gaugh DR. GDUFA past & present [Internet] Washington (DC): Generic Pharmaceutical Association; 2015. Jun 15, [cited 2016 Jul 13]. Available from: http://www.fda.gov/downloads/ForIndustry/UserFees/GenericDrugUserFees/UCM452611.pdf. [Google Scholar]
- 25.Fein AJ. In the third quarter, retail generic drug inflation kept on truckin’. [cited 2016 Jul 13];Drug Channels [serial on the Internet] 2014 Nov 18; Available from: http://www.drugchannels.net/2014/11/in-third-quarter-retail-generic-drug.html. [Google Scholar]
- 26.Fein AJ. Retail generic drug inflation eases, but the FDA keeps prices high. [cited 2016 Jul 13];Drug Channels [serial on the Internet] 2015 Apr 15; Available from: http://www.drugchannels.net/2015/04/retail-generic-drug-inflation-eases-but.html. [Google Scholar]
- 27.Fein AJ. The retail generic drug inflation slowdown: it’s real. [cited 2016 Jul 13];Drug Channels [serial on the Internet] 2015 Aug 25; Available from: http://www.drugchannels.net/2015/08/the-retail-generic-drug-inflation.html. [Google Scholar]
- 28.Pollack A. Second drug wins approval for treatment of hepatitis C. New York Times. 2011 May 23; [Google Scholar]
- 29.Shrank WH, Barlow JF, Brennan TA. New therapies in the treatment of high cholesterol: an argument to return to goal-based lipid guidelines. JAMA. 2015;314(14):1443–1444. doi: 10.1001/jama.2015.10017. [DOI] [PubMed] [Google Scholar]
- 30.Curfman G. PCSK9 inhibitors: a major advance in cholesterol-lowering drug therapy. [cited 2016 Jul 13];Harvard Health Blog [blog on the Internet] 2015 Mar 15; Available from: http://www.health.harvard.edu/blog/pcsk9-inhibitors-a-major-advance-in-cholesterol-low-eringdrug-therapy-201503157801. [Google Scholar]
- 31. CMS.gov. National Health Expenditure projections 2015–2025: forecast summary [Internet] Baltimore (MD): Centers for Medicare and Medicaid Services; [cited 2016 Jul 20]. Available from: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/Downloads/proj2015.pdf. [Google Scholar]
- 32.Medicines use and spending in the U.S.—a review of 2015 and outlook to 2020. Parsippany (NJ): The Institute; 2015. IMS Institute for Healthcare Informatics. [Google Scholar]
- 33.Express Scripts 2015 drug trend report [Internet] St. Louis (MO): The Lab; 2016. Mar, [cited 2016 Jul 13]. Express Scripts Lab. Available for download from: http://lab.express-scripts.com/lab/drug-trend-report. [Google Scholar]