Abstract
Background
While studies have suggested standard therapy for clinical T2N0 esophageal cancer should be primary surgery, we hypothesize there is a subgroup for whom induction therapy may result in improved overall survival (OS).
Methods
cT2N0 esophageal cancer patients receiving induction therapy or upfront esophagectomy (UE) were identified in the National Cancer Data Base (NCDB). UE patients were dichotomized as 1) pathologically upstaged or 2) same-or down-staged. Logistic regression models identified variables associated with upstaging and Kaplan-Meier analysis compared median OS.
Results
From 2006–2012, 932 (52.2%) cT2N0 patients received UE, while 853 (47.8%) received induction therapy first. 326/713 (45.7%) UE patients were upstaged. 87/326 (26.7%) patients had T upstaging, 98/326 (30.1%) had N upstaging, and 141/326 (43.3%) had both. Patients upstaged after UE had a higher tumor grade (35.1% versus 57.1% Grade 3), and a higher rate of lymphovascular invasion (LVI, 57.1% versus 17.7%), both p<0.001. Variables associated with upstaging included LVI (OR 6.0, 95% CI 2.9 – 12.5, p<0.001) and tumor grade 3 (OR 9.4, 1.8 – 48.4, p=0.007). Of upstaged UE patients, only 144 (44.2%) received adjuvant therapy. The median OS for cT2N0 patients upstaged after UE was 27.5 ± 2.5 months versus 43.9 ± 2.9 months for induction therapy patients (any resultant pathologic stage, p<0.001).
Conclusions
Half of all cT2N0 patients were pathologically upstaged after UE with worse survival compared to patients receiving induction therapy. Refining an upstaging model would help select patients for induction therapy and increase the rate of chemotherapy in patients at risk for systemic disease.
While national guidelines clearly delineate the role of endoscopic therapies for the earliest stage esophageal cancers (clinical Tis, T1aN0) and upfront surgery for T1bN0, a divergence in treatment options becomes evident when the clinician and patient encounter a clinical T2N0 diagnosis. [1] While in theory, this clinical stage should be amenable to upfront surgical resection, the likelihood of clinical T2N0 correlating to the same pathologic stage is low. Previous institutional reports have described that even with clinical staging performed by endoscopic ultrasound (EUS) and positron emission tomography (PET), undetected nodal disease was encountered with upfront esophagectomy in 39 – 55% of clinical T2N0 patients. [2,3] For operable patients with clinical evidence of nodal disease, induction therapy (either chemotherapy or chemoradiation therapy) is an acceptable treatment option prior to esophagectomy. [1,4,5] Conversely, it has also been described that 50% of cT2N0 patients are pathologically downstaged, and correlation with pathologic T2N0 occurs in less than 10%. [2]
Selecting the appropriate treatment for the operable cT2N0 patient presents a challenging scenario – for patients that are downstaged (or the few that remain pathologic T2N0), choosing induction therapy would be unnecessary and may come at the cost of patient deconditioning prior to surgery. For patients that are upstaged, not all of these patients may have the physiologic reserve to receive timely adjuvant therapy after esophagectomy. Recent reviews of national databases such as the Society of Thoracic Surgeons General Thoracic Surgery Database (STS GTDB) and the National Cancer Data Base (NCDB) had found that approximately 35 – 44% of cT2N0 patients are receiving induction therapy prior to esophagectomy. [6,7] However, a recent retrospective review of the NCDB found no survival benefit for cT2N0 patients receiving induction therapy, despite the fact that over 40% of patients were pathologically upstaged. [6]
Based on the likelihood of pathologic upstaging, there is possibly a subgroup of cT2N0 patients for whom induction therapy followed by esophagectomy would be recommended. In this study, we used the NCDB to identify and evaluate predictors of pathologic upstaging in cT2N0 patients. We hypothesize that by identifying this subgroup with an increased likelihood of pathologic upstaging, the use of induction therapy may be associated with an increase in overall survival that is not detected in the overall cohort of cT2N0 patients.
Patients and Methods
The NCDB Participant User File (PUF) for esophageal cancer was reviewed to identify all cT2N0 patients receiving esophagectomy. The NCDB is a joint program of the American College of Surgeons and the American Cancer Society, and includes patient, tumor, and treatment characteristics of approximately seventy percent of patients diagnosed at Commission on Cancer accredited cancer centers. The PUF contains deindentified patient and center information, and was exempt from institutional IRB review. As order of systemic therapy (neoadjuvant or adjuvant) was routinely coded beginning in 2006, the years of analysis in this study include 2006–2012, with at least two years of follow-up available for the most recent patients.
Patient characteristic variables abstracted included age, race (Caucasian or non-Caucasian), population (< or ≥ 250,000 individuals), income (average income in patient’s zip code is < or ≥ $38,000 per year), education level (percent of population in patient’s zip code without a high school education ≥21% or <21%), insurance status (uninsured, private, or government), cancer center type (academic versus community), and greatest circle distance of patient’s zip code to treatment center (in miles). As the overall number of Medicaid (n=83) and ‘other’ government insurance plans (military, federal workers, n=24) was small, this was combined into a government insurance category with Medicare patients (n=856). The Charlson/Deyo score was also abstracted as a measure of patient comorbidity, and is recorded as 0, 1, or ≥2, not including the patient’s known esophageal cancer malignancy. Patients recorded as having clinical M1 disease were excluded from analysis.
Descriptive statistics were expressed as mean ± standard deviation. Independent sample t tests were used to analyze normally distributed continuous data. χ2 tests were used to compare categorical data. Backwards stepwise multivariable logistic regression was performed to identify variables independently associated with being pathologically upstaged from cT2N0 esophageal cancer and receiving adjuvant therapy for upstaged upfront esophagectomy patients. Variables with a significant difference of p<0.05 were eligible for entry into the model. From the logistic regression model, the probability score for each patient in the model was analyzed using a receiver operating curve (ROC) with area under the curve (AUC) analysis. Overall survival of induction therapy patients versus upfront esophagectomy patients was then compared using Kaplan-Meier analysis and the log-rank test. P values of less than 0.05 were considered statistically significant.
Results
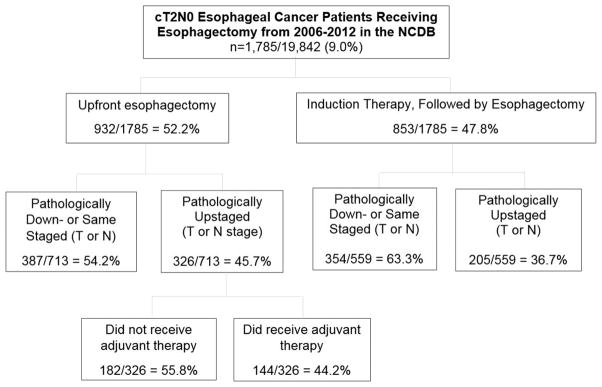
From 2006 – 2012, 1,785 cT2N0M0 patients that underwent esophagectomy were identified in the NCDB (Figure 1). This comprised 9.0% (1,785/19,842) of all esophagectomies in M0 patients during this period. Of the cT2N0 patients, 932 (52.2%) had upfront esophagectomy, while 853 (47.8%) received induction therapy (at least two cycles of either chemotherapy or chemoradiation therapy) prior to esophagectomy. Variables independently associated with an increasing likelihood of receiving induction therapy on multivariate analysis included higher education (<21% of population in patient’s zip code not having a high school diploma, OR 1.5, 95% CI 1.12 – 2.02, p=0.006), receiving care at a community cancer center (OR 1.7, 1.4 – 2.0, p<0.001) and more recent year of diagnosis (by year from 2006 – 2012, OR 1.12, 1.06 – 1.18, p<0.001). Increasing age was independently associated with a decreased likelihood of receiving induction therapy (by year, OR 0.96, 0.95 – 0.97, p<0.001).
Figure 1.
CONSORT diagram for cT2N0 esophageal cancer patients receiving esophagectomies from 2006–2012 as recorded in the NCDB.
1,272/1,785 (71.3%) of patients had complete pathologic T and N staging recorded. cT2N0 patients receiving upfront esophagectomy were significantly more likely to be pathologically upstaged versus induction therapy patients, 326/713 (45.7%) versus 205/559 (36.7%), p=0.001 and have positive surgical margins, 76/908 (8.4%) versus 40/824 (4.9%), p=0.003. Upfront esophagectomy patients were significantly more likely to receive pathologic T upstaging (228/713, 32.0% versus 137/559, 24.5%, p=0.003) and pathologic N upstaging (239/713, 33.5% versus 145/559, 25.9%, p=0.003).
While there was a significant difference in overall T and N pathologic upstaging between upfront esophagectomy patients and induction therapy patients, the patterns of disease in those patients that were upstaged demonstrated no difference (p=0.43). Specifically, of the 326 upstaged upfront esophagectomy patients with complete pathologic T and N staging, 87/326 (26.7%) had T upstaging, 98 /326 (30.1%) had N upstaging, and 141/326 (43.3%) had both T and N upstaging. Of the 205 induction therapy patients that were pathologically upstaged, 60/205 (29.3%) had T upstaging, 68/202 (33.2% had N upstaging), and 77/205 (37.6%) had both T and N upstaging.
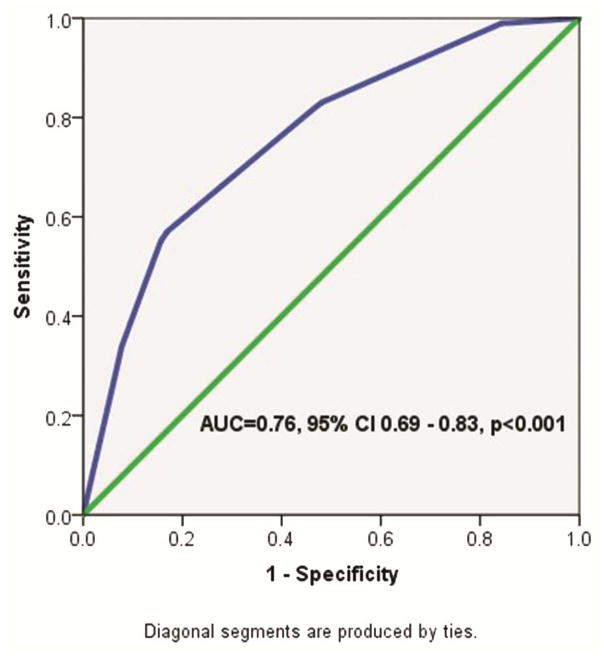
Of the 713/932 (76.5%) of upfront esophagectomy patients with complete pathologic staging data, upstaged patients were significantly more likely to have poorly-differentiated and undifferentiated tumor grades, presence of lymphovascular invasion, a more recent year of diagnosis, and have more lymph nodes examined (Table 1). On logistic regression, variables independently associated with increased likelihood of pathologic upstaging included lymphvascular invasion (OR 6.0, 95% CI 2.9 – 12.5, p<0.001) and poorly differentiated tumor grade (reference: well-differentiated, OR 9.4, 1.8 – 48.4, p=0.007). Moderately differentiated tumor grade approached significance (OR 5.2, 0.99 – 26.9, p=0.05). On ROC analysis, the AUC for tumor grade and lymphovascular invasion in predicting pathologic upstaging was 0.76 (95% CI 0.69 – 0.83), indicating a fair test above the null hypothesis (chance =0.5) (Figure 2).
Table 1.
Univariate comparison of patient and tumor characteristics of cT2N0 esophagectomy patients that were either pathologically down- or same-staged (n=387) or upstaged (n=326).
| Variable | Upfront Surgery Patients that Were Down or Same-Staged (n=387) | Upfront Surgery Patients that Were Upstaged (n=326) | P value |
|---|---|---|---|
|
| |||
| Age | 65.9 ± 10.6 | 65.2 ± 11.2 | 0.39 |
|
| |||
| Gender (male) | 310 (80.1%) | 277 (85.0%) | 0.09 |
|
| |||
| Race (Caucasian) | 350 (92.1%) | 298 (92.5%) | 0.83 |
|
| |||
| Distance from treatment center (miles) | 49.2 ± 145.4 | 49.0 ± 111.2 | 0.98 |
|
| |||
| Patient Location | |||
| <250,000 | 99 (27.5%) | 95 (31.1%) | 0.30 |
| ≥250,000 | 261 (72.5%) | 210 (68.9%) | |
|
| |||
| Education | |||
| ≥21% in zip code without high school diploma | 57 (15.3%) | 43 (13.7%) | 0.55 |
|
| |||
| Income (<$38,000) | 44 (11.8%) | 45 (14.3%) | 0.32 |
|
| |||
| Insurance | |||
| None | 7 (1.8%) | 8 (2.5%) | 0.83 |
| Private | 149 (38.8%) | 123 (38.2%) | |
| Government* | 228 (59.4%) | 191 (59.3%) | |
|
| |||
| Facility Type | |||
| Non-academic | 166 (42.9%) | 133 (40.8%) | 0.57 |
| Academic | 221 (57.1%) | 193 (59.2%) | |
|
| |||
| Charlson/Deyo Score | |||
| 0 | 265 (68.5%) | 213 (65.3%) | 0.64 |
| 1 | 93 (24.0%) | 84 (25.8%) | |
| ≥2 | 29 (7.5%) | 29 (8.9%) | |
|
| |||
| Year of Diagnosis | |||
| 2006 | 51 (13.2%) | 38 (11.7%) | 0.002 |
| 2007 | 56 (14.5%) | 40 (12.3%) | |
| 2008 | 93 (24.0%) | 72 (22.1%) | |
| 2009 | 97 (25.1%) | 53 (16.3%) | |
| 2010 | 37 (9.6%) | 47 (14.4%) | |
| 2011 | 32 (8.3%) | 41 (12.6%) | |
| 2012 | 21 (5.4%) | 35 (10.7%) | |
|
| |||
| Tumor Grade | |||
| Well differentiated | 44 (12.1%) | 7 (2.3%) | <0.001 |
| Moderately differentiated | 186 (51.0%) | 117 (37.7%) | |
| Poorly differentiated | 128 (35.1%) | 177 (57.1%) | |
| Undifferentiated | 7 (1.9%) | 9 (2.9%) | |
|
| |||
| Histology (adenocarcinoma) | 277 (75.9%) | 217 (78.1%) | 0.52 |
|
| |||
| Tumor size | 35.9 ± 74.9 | 42.4 ± 57.6 | 0.22 |
|
| |||
| Lymphovascular Invasion | 14 (17.7%) | 60 (57.1%) | <0.001 |
|
| |||
| Days from diagnosis to surgery | 56.3 ± 44.8 | 51.6 ± 41.4 | 0.15 |
|
| |||
| Number of regional lymph nodes examined | 14.3 ± 10.8 | 18.0 ± 11.8 | <0.001 |
|
| |||
| Number of positive lymph nodes | 0 | 2.7 ± 4.7 | <0.001 |
Government insurance includes Medicare, Medicaid, or other government insurance plans.
Figure 2.
ROC curve for predicting likelihood of pathologic upstaging in cT2N0 esophageal cancer patients receiving upfront esophagectomy. Input variables included tumor grade and presence or absence of lymphovascular invasion.
Of the 326 upstaged upfront esophagectomy patients, 144 (44.2%) went on to receive adjuvant therapy. Specifically, of those receiving adjuvant therapy, 53/142 (37.3%) received adjuvant chemotherapy, while 89/142 (62.7%) received adjuvant chemoradiation therapy. Characteristics of upstaged upfront esophagectomy patients receiving adjuvant therapy are listed in Table 2. Factors independently associated with increased likelihood of receiving adjuvant therapy for upstaged upfront esophagectomy patients on multivariate analysis included adenocarcinoma histology (OR 2.8, 95% CI 1.3 – 6.1, p=0.01), increasing pathologic nodal stage (reference: N0, N1: OR 4.7, 2.2 – 10.4, p<0.001, N2: OR 14.4, 3.4 – 60.0, p<0.001), and positive surgical margins (OR 2.6, 1.2 – 5.7, p=0.01), while those associated with a decreased likelihood of receiving adjuvant therapy included increasing age (by year, OR 0.94, 0.91 – 0.97, p<0.001) and increasing length of inpatient stay after esophagectomy (by day, OR 0.95, 0.92 – 0.98, p<0.001).
Table 2.
Univariate comparison of patient and tumor characteristics of cT2N0 upfront esophagectomy patients that were pathologically upstaged that did or did not receive adjuvant chemotherapy.
| Variable | Upstaged Upfront Esophagectomy, No Adjuvant (n=182) | Upstaged Upfront Esophagectomy, Received Adjuvant (n=144) | P value |
|---|---|---|---|
|
| |||
| Age (years) | 67.3 ± 11.2 | 62.6 ± 10.6 | <0.001 |
|
| |||
| Gender (male) | 150 (82.4%) | 127 (88.2%) | 0.15 |
|
| |||
| Race (Caucasian) | 160 (89.4%) | 138 (96.5%) | 0.02 |
|
| |||
| Population | |||
| <250,000 | 54 (31.4%) | 41 (30.8%) | 0.92 |
| ≥250,000 | 118 (68.6%) | 92 (69.2%) | |
|
| |||
| Greatest circle distance (miles) | 54.1 ± 81.6 | 42.6 ± 140.0 | 0.37 |
|
| |||
| Income | |||
| <$38,000 | 27 (15.3%) | 18 (13.0%) | 0.56 |
| ≥$38,000 | 149 (84.7%) | 120 (87.0%) | |
|
| |||
| Insurance | |||
| Uninsured | 5 (2.8%) | 3 (2.1%) | 0.001 |
| Private | 52 (29.1%) | 71 (49.7%) | |
| Government* | 122 (68.2%) | 69 (48.3%) | |
|
| |||
| Education | |||
| ≥21% no HS degree | 26 (14.8%) | 17 (12.2%) | 0.52 |
| <21% no HS degree | 150 (85.2%) | 122 (87.8%) | |
|
| |||
| Charlson/Deyo Score | |||
| 0 | 119 (65.4%) | 94 (65.3%) | 0.99 |
| 1 | 47 (25.8%) | 37 (25.7%) | |
| ≥2 | 16 (8.8%) | 13 (9.0%) | |
|
| |||
| Facility Type | |||
| Nonacademic | 75 (41.2%) | 58 (40.3%) | 0.87 |
| Academic | 107 (58.8%) | 86 (59.7%) | |
|
| |||
| Year of Diagnosis | |||
| 2006 | 23 (12.6%) | 15 (10.4%) | 0.40 |
| 2007 | 18 (9.9%) | 22 (15.3%) | |
| 2008 | 39 (21.4%) | 33 (22.9%) | |
| 2009 | 26 (14.3%) | 27 (18.8%) | |
| 2010 | 28 (15.4%) | 19 (13.2%) | |
| 2011 | 28 (15.4%) | 13 (9.0%) | |
| 2012 | 20 (11.0%) | 15 (10.4%) | |
|
| |||
| Histology | |||
| Squamous | 46 (29.3%) | 15 (12.4%) | 0.001 |
| Adenocarcinoma | 111 (70.7%) | 106 (87.6%) | |
|
| |||
| Tumor Grade | |||
| Well-differentiated | 4 (2.4%) | 3 (2.1%) | 0.18 |
| Moderately differentiated | 72 (42.6%) | 45 (31.9%) | |
| Poorly differentiated | 87 (51.5%) | 90 (63.8%) | |
| Undifferentiated | 6 (3.6%) | 3 (2.1%) | |
|
| |||
| Pathologic T Stage | |||
| 1 | 14 (7.7%) | 10 (6.9%) | 0.95 |
| 2 | 43 (23.6%) | 31 (21.5%) | |
| 3 | 122 (67.0%) | 101 (70.1%) | |
| 4 | 3 (1.6%) | 2 (1.4%) | |
|
| |||
| Pathologic N Stage | |||
| N0 | 66 (36.3%) | 21 (14.6%) | <0.001 |
| N1 | 102 (56.0%) | 101 (70.1%) | |
| N2 | 6 (3.3%) | 16 (11.1%) | |
| N3 | 8 (4.4%) | 6 (4.2%) | |
|
| |||
| Tumor size (mm) | 46.0 ± 74.9 | 37.8 ± 20.6 | 0.21 |
|
| |||
| Positive Surgical Margins | 22 (12.3%) | 35 (24.5%) | 0.004 |
|
| |||
| Number of Nodes Examined | 17.9 ± 12.4 | 18.2 ± 11.1 | 0.84 |
|
| |||
| Number of Nodes Positive | 2.1 ± 4.0 | 3.5 ± 5.3 | 0.007 |
|
| |||
| Length of Stay (days) | 18.6 ± 17.1 | 11.3 ± 8.3 | <0.001 |
|
| |||
| 30-day readmission | 20 (11.3%) | 10 (7.2%) | 0.22 |
Government insurance includes Medicare, Medicaid, or other government insurance plans.
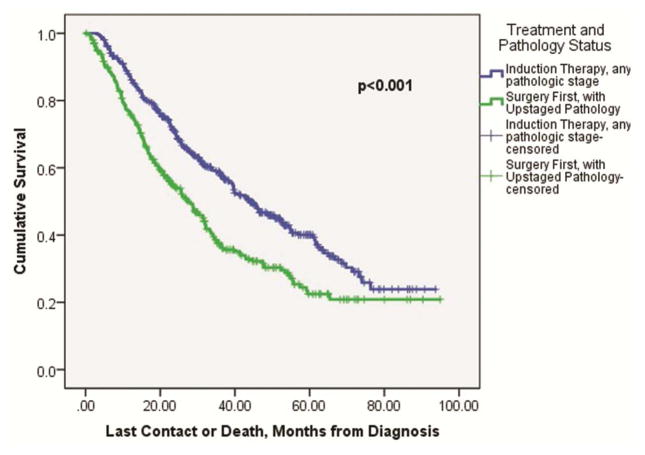
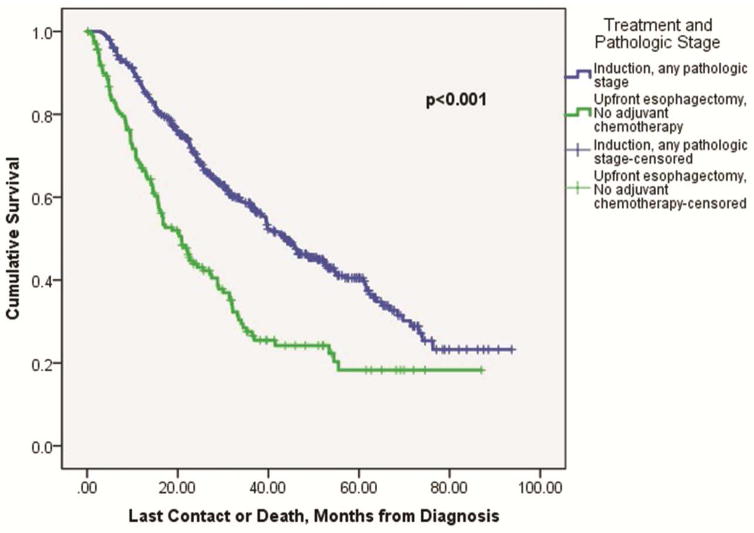
Patients that received induction therapy followed by esophagectomy with any resultant pathologic stage had improved overall median survival compared to upfront esophagectomy patients that were pathologically upstaged (43.9 ± 2.9 months versus 27.5 ± 2.5 months, p<0.001, Figure 3). The overall median survival difference between induction therapy patients (with any resultant pathologic stage) versus upstaged esophagectomy patients that went on to receive adjuvant therapy did not reach significance (43.8 ± 3.0 months versus 34.6 ± 4.2 months, p=0.14). However, the overall median survival difference between induction therapy patients (again, with any resultant pathologic stage) was significantly improved compared to the 182 (55.8%) upstaged upfront esophagectomy patients that did not go on to receive adjuvant therapy (43.8 ± 3.0 months versus 20.8 ± 2.3 months, p<0.001, Figure 4).
Figure 3.
Kaplan-Meier survival curve comparing overall survival among cT2N0 esophageal cancer patients that received upfront esophagectomy and were pathologically upstaged, versus induction therapy followed by esophagectomy patients, with any resultant pathologic stage.
Figure 4.
Kaplan-Meier curve comparing overall survival among cT2N0 esophageal cancer patients that were pathologically upstaged after upfront esophagectomy but did not receive adjuvant chemotherapy, versus induction therapy patients with any resultant pathologic stage after esophagectomy. In this series, 182/326 (55.8%) pathologically upstaged upfront esophagectomy patients did not receive adjuvant chemotherapy.
Comment
It is well known that a diagnosis of cT2N0 esophageal cancer presents a therapeutic dilemma in thoracic oncology due to inaccurate staging techniques at this time. The major findings of this study using the NCDB found that 1) almost half of cT2N0 upfront esophagectomy patients are pathologically upstaged and 2) while no significant difference in overall survival was detected between patients that received induction therapy (with any resultant pathologic stage) versus upstaged upfront esophagectomy patients that went on to receive adjuvant chemotherapy, more than half of upstaged upfront esophagectomy patients did not go on to receive adjuvant chemotherapy. Of this population that did not receive adjuvant chemotherapy after upstaging from upfront esophagectomy, overall survival was significantly worse than for those patients that received induction therapy. However, given the reticence to over-treat patients who may be down-or same staged with esophagectomy, we used the NCDB to identify preoperative predictors of upstaging, namely, histologic grade and presence of lymphovascular invasion, to identify a subgroup of cT2N0 patients that may be at particularly high risk for being upstaged, and would benefit from induction therapy.
Even when using both PET/CT and EUS staging modalities, the likelihood of cT2N0 esophageal cancer correlating with actual pathologic stage is exceedingly low. [2,3,6] Given the concern that such a significant proportion of these patients are upstaged, some practitioners opt for induction therapy prior to esophagectomy in cT2N0 patients. Both our review of the NCDB as well as a recent analysis by Duke University (evaluating cT2N0 patients from 1998–2011) found that almost half of all cT2N0 patients nationally are receiving induction therapy (either chemotherapy or chemoradiation therapy) [7]. To date, no randomized controlled trial has evaluated the role of induction therapy in cT2N0 esophageal cancer, likely due to the small numbers of patients that present with this clinical stage (in previous series, approximately 8–9% of esophageal cancer patients). [8,9] Previous institutional retrospective studies have failed to find a survival benefit among cT2N0 patients receiving either induction chemotherapy or chemoradiation therapy [3, 10]. One institution documented decreased survival with induction therapy compared to upfront esophagectomy for cT2N0 patients, however this was among a small sample size with only 8 patients receiving induction therapy. [9] As mentioned earlier, a recent review of the NCDB also found no overall survival benefit for cT2N0 patients receiving induction chemotherapy or chemoradiation therapy as on overall population analysis [7].
While these previous studies have not detected a survival benefit with induction therapy in cT2N0 patients, none to date have examined the outcomes between upstaged upfront esophagectomy patients that were able to receive adjuvant therapy versus those that were not. Both our NCDB review and the Duke study document that approximately half of upstaged upfront esophagectomy patients go on to receive adjuvant therapy. [7] An institutional study in the UK found that of upfront esophagectomy patients (T1-T4) that were determined to be pathologic N1, approximately 60% of patients were able to proceed with adjuvant chemoradiation therapy with a significant improvement in overall survival (47.5 months versus 14.1 months, p=0.001). [11] These findings from both retrospective institutional studies and national databases likely reflect selection biases that may hinder upstaged/node positive patients from receiving appropriate adjuvant therapy. In our study, patients of non-Caucasian race, non-private insurance, increasing age, and increased length of inpatient stay after esophagectomy were all found to receive adjuvant therapy less frequently, suggesting that both access to care and post-esophagectomy complication/frailty factors may be influencing uptake of adjuvant therapy when clinically indicated.
The finding that half of upstaged upfront esophagectomy patients did not obtain adjuvant therapy, with a resulting overall survival detriment, suggests that for this subgroup of patients induction therapy would be more appropriate. A retrospective review of upstaged cT2N0 esophageal cancer patients at our institution found that marked/intense uptake on staging PET was independently associated with pathologic upstaging in cT2N0 patients, while poorly differentiated tumor grade approached significance. [2] Another institutional study documented that an increasing length of time between staging EUS and esophagectomy decreased tumor staging accuracy from 90% when the two procedures were done within two weeks, but decreased to 40% when the interval was greater than one month. [12] Of note, this study did not find any significant differences in the accuracy of nodal staging over these time intervals. A previous review of the STS GTDB examining cT2N0 esophageal cancer patients found that male gender, increasing Zubrod score, and absence of prior thoracic surgical procedures were independently associated with pathologic upstaging. [6] Of note, histologic grade was not able to be considered in that analysis.
Of the preoperative variables examined in our model, increasing tumor grade and presence of lymphovascular invasion together produced a fair predictive value for pathologic upstaging, and could help identify patients at increased risk for pathologic upstaging. Currently, institutions may vary in the frequency with which lymphovascular invasion is reported from an endoscopic biopsy, as detection can be dependent on biopsy size and use of vascular and lymphatic specific staining techniques. Of note, two such stains used to identify lymphvascular invasion have been studied in relation to long-term outcomes. High levels of CD34 expression, a transmembrane protein that shows expression on vascular-associated tissue, have been shown to be associated with decreased survival in squamous cell esophageal cancer patients, but not in adenocarcinoma patients. [13] D2-40, a monoclonal antibody that specifically identifies lymphatic endothelium, has been shown to be independently associated with lymphovascular invasion and lymph node metastases in esophageal adenocarcinoma. [14] While a full review of pathologic markers in esophageal cancer and their prognostic ability is outside the scope of this manuscript, it is helpful to consider ways in which to glean additional information from esophageal cancer biopsy specimens, given the current clinical staging limitations and ‘blind spots’ of EUS and PET-CT in regards to cT2N0 status.
There are limitations to this large, retrospective analysis. While the NCDB is a robust database, it does not currently possess information on the modality of clinical staging used (PET/CT, EUS, or EUS with fine needle aspiration) to determine cT2N0 status. While previous institutional reviews have shown that even though a majority of their patients were receiving both EUS and PET/CT staging, pathologic upstaging after esophagectomy still persisted among 50–60% of cT2N0 patients. [2,3,15] For patients that may have only received one staging modality, the proportions of stage migration may be even higher. Another limitation is the inability to account for all selection biases and factors among patients that receive either induction therapy for cT2N0 esophageal cancer or adjuvant therapy for upstaged upfront cT2N0 esophagectomy patients. Additionally, we recognize that while a comparison between induction therapy patients and upfront esophagectomy patients is needed to compare rates of upstaging, a significant bias of this comparison is that it does not capture cT2N0 patients that began induction therapy and did not receive esophagectomy either due to progression of disease or physiologic deconditioning from the regimen that made the patient subsequently inoperable. However, the CROSS (Chemoradiotherapy for Oesophageal Cancer Followed by Surgery Study), a randomized controlled trial evaluating induction therapy versus upfront surgery in locally advanced esophageal cancer demonstrated that only 6% of induction therapy patients did not proceed to esophagectomy. [5] Either of these factors would select out patients from the induction group that had particularly aggressive disease or had some level of deconditioning that would have made them higher risk esophagectomy patients.
Despite these limitations, our conclusions from this analysis suggest that a predictive model could be helpful in identifying cT2N0 patients at increased risk of pathologic upstaging and assist in determining which patients may benefit from induction therapy. Additional prospective work is needed to validate this model, based primarily on tumor grade and presence of lymphvascular invasion, as well as to identify other factors associated with pathologic upstaging of cT2N0 esophageal cancer. Other variables to evaluate for a future model could include PET SUV and preoperative tumor length, which were unable to be captured in this analysis. An increasingly tailored approach in conjunction with routine staging practices such as PET/CT and EUS may help us better target appropriate patients for induction therapy and spare low-risk patients from unnecessary additional treatment.
Acknowledgments
Acknowledgements and Disclosures: Pamela Samson, MD, MPHS has grant support through NIH Cardiothoracic Surgery T32. Varun Puri, MD, MSCI has grant funding through NIH K07CA178120 and K12CA167540-02. Additionally, the NCDB is a joint effort of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. These organizations have not verified and are not responsible for the analytic or statistical methodology used in this study, and the conclusions drawn are solely those of the authors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Comprehensive Cancer Network (NCCN) [Accessed October 19, 2015];Guidelines: Esophageal and Esophagogastric Junction Cancers. Version 3.2015. Available at: http://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf.
- 2.Crabtree TD, Yacoub WN, Puri V, Azar R, et al. Endoscopic Ultrasound for Early Stage Esophageal Adenocarcinoma: Implications for Staging and Survival. Ann Thorac Surg. 2011;91:1509–16. doi: 10.1016/j.athoracsur.2011.01.063. [DOI] [PubMed] [Google Scholar]
- 3.Stiles BM, Mirza F, Coppolino A, Port JL, et al. Clinical T2-T3N0M0 Esophageal Cancer: The Risk of Node Positive Disease. Ann Thorac Surg. 2011;92:491–8. doi: 10.1016/j.athoracsur.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Kidane B, Coughlin S, Vogt K, Malthaner R. Preoperative chemotherapy for resectable thoracic esophageal cancer. Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD001556.pub3. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Hagen P, Hulshof MC, van Lanschott JJ, Steyerberg EW, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–84. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 6.Crabtree TD, Kosinski AS, Puri V, Burfeind W, et al. Evaluation of the Reliability of Clinical Staging of T2N0 Esophageal Cancer: A Review of the Society of Thoracic Surgeons Database. Ann Thorac Surg. 2013;96:382–90. doi: 10.1016/j.athoracsur.2013.03.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Speicher PJ, Ganapathi AM, Englum BR, Hartwig MG, et al. Induction Therapy Does Not Improve Survival for Clinical Stage T2N0 Esophageal Cancer. J Thorac Oncol. 2014;9:1195–1201. doi: 10.1097/JTO.0000000000000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardacker TJ, Ceppa D, Okereke I, Rieger KM, et al. Treatment of Clinical T2N0M0 Esophageal Cancer. Ann Surg Oncol. 2014;21:3739–3743. doi: 10.1245/s10434-014-3929-6. [DOI] [PubMed] [Google Scholar]
- 9.Rice TW, Mason DP, Murthy SC, Zuccaro G, et al. T2N0M0 esophageal cancer. J Thorac Cardiovasc Surg. 2007;133:317–24. doi: 10.1016/j.jtcvs.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 10.Zhang JQ, Hooker CM, Brock MV, Shin J, et al. Neoadjuvant Chemoradiation Therapy is Beneficial for Clinical Stage T2N0 Esophageal Cancer Patients Due to Inaccurate Preoperative Staging. Ann Thorac Surg. 2012;93:429–37. doi: 10.1016/j.athoracsur.2011.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bedard EL, Inculet RI, Malthaner RA, Brecevic E, et al. The Role of Surgery and Postoperative Chemoradiation Therapy in Patients with Lymph Node Positive Esophageal Carcinoma. Cancer. 2001;91(12):2423–30. [PubMed] [Google Scholar]
- 12.Fisher JM, Pohl H, Gordon SR, Gardner TB. The Impact of Time Elapsed Between Endoscopic Ultrasound and Esophagectomy on Concordance of Ultrasonographic and Pathologic Staging of Esophageal Malignancy. Dig Dis Sci. 2011;56:2987–2991. doi: 10.1007/s10620-011-1716-9. [DOI] [PubMed] [Google Scholar]
- 13.Goscinski MA, Nesland JM, Giercksky KE, Dhakai HP. Primary tumor vascularity in esophagus cancer. CD34 and HIF-α expression correlate with tumor progression. Histol Histopathol. 2013;28:1361–1368. doi: 10.14670/HH-28.1361. [DOI] [PubMed] [Google Scholar]
- 14.Saad RS, Lindner JL, Liu Y, Silverman JF. Lymphatic Vessel Density as Prognostic Marker in Esophageal Adenocarcinoma. [DOI] [PubMed] [Google Scholar]
- 15.Dolan JP, Kaur T, Diggs BS, Luna RA. Significant understaging is seen in clinically staged T2N0 esophageal cancer patients undergoing esophagectomy. Dis Esophagus. 2015 doi: 10.1111/dote.12334. Epub ahead of print. [DOI] [PubMed] [Google Scholar]