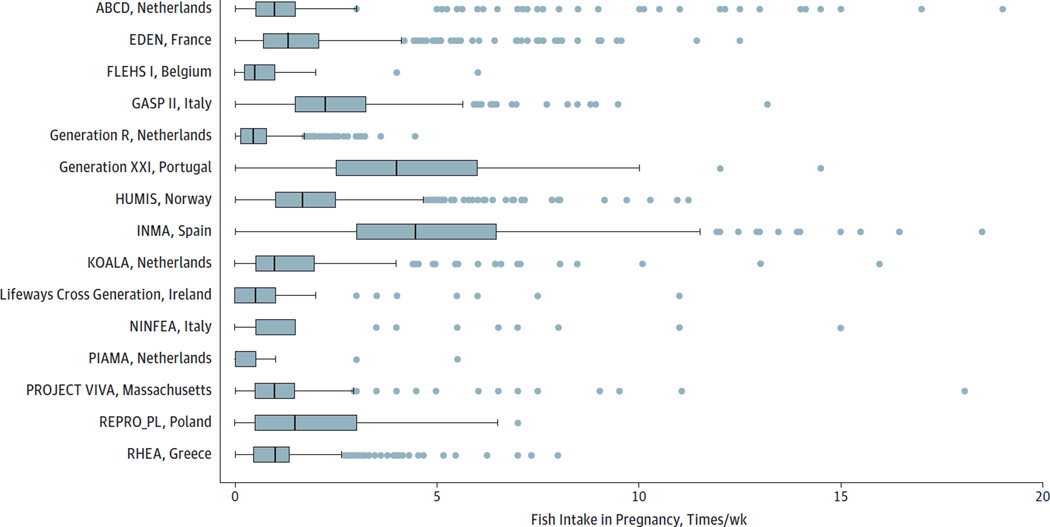
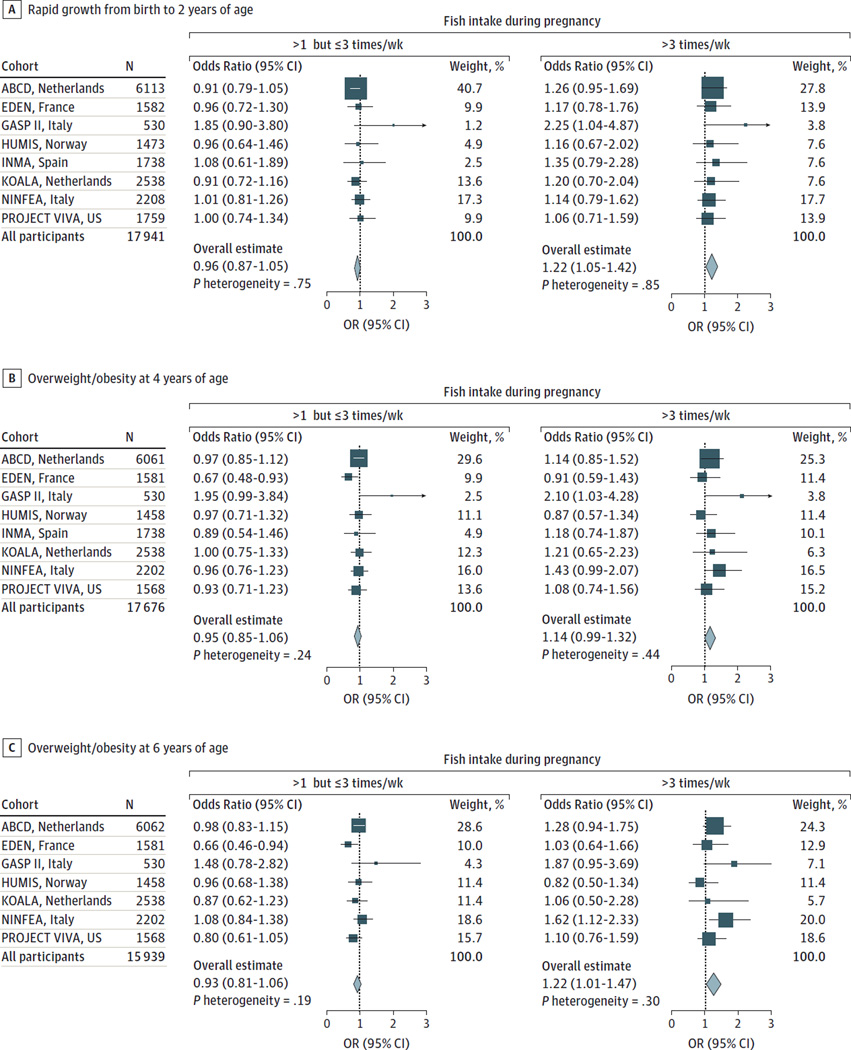
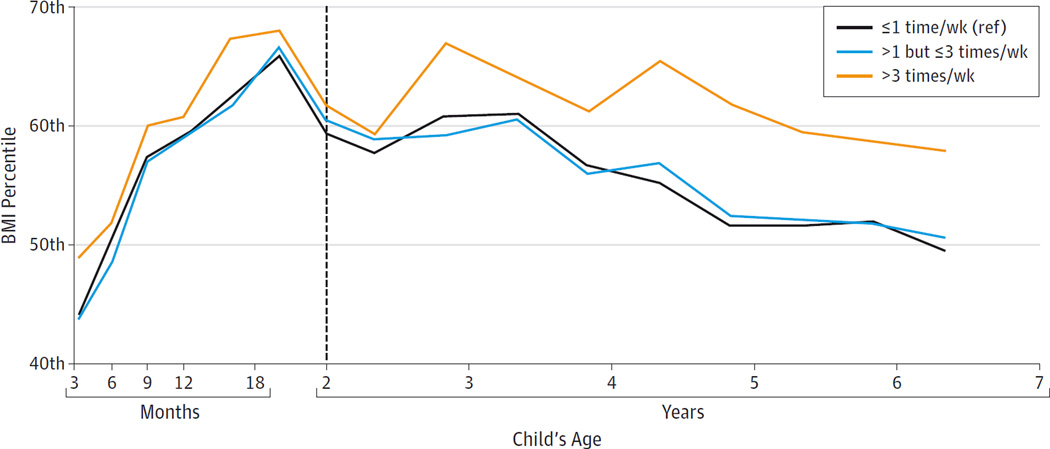
Nikos Stratakis
Nikos Stratakis, MSc
1Department of Social Medicine, Faculty of Medicine, University of Crete, Heraklion, Greece (Stratakis, Roumeliotaki, Chatzi); Section of Complex Genetics, Department of Genetics and Cell Biology, NUTRIM School of Nutrition and Translational Research in Metabolism, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Stratakis, Zeegers); Obesity Prevention Program, Harvard Pilgrim Health Care Institute, Department of Population Medicine, Harvard Medical School, Boston, Massachusetts (Oken, Rifas-Shiman); Department of Clinical Epidemiology, Predictive Medicine and Public Health, University of Porto Medical School, Porto, Portugal (Barros); Epidemology Research Unit, Institute of Public Health, University of Porto, Porto, Portugal (Barros); Public Health Division of Gipuzkoa, Basque Government; Health Research Institute, Biodonostia, San Sebastián, Spain (Basterrechea, Oliveira); Centros de Investigación Biomédica en Red Epidemiología y Salud Pública, Spain (Basterrechea, Murcia, Oliveira, Sunyer, Vrijheid, Kogevinas); Centre for Research in Epidemiology and Biostatistics Paris Sorbonne Cité, Institut National de la Santé et de la Recherche Medicale, Early Origin of the Child Development and Health Team, Villejuif, France (Charles, Heude); Université Paris Descartes, Villejuif, France (Charles, Heude); Norwegian Institute of Public Health, Oslo, Norway (Eggesbø, Iszatt); Department of Epidemiology, Lazio Regional Health System, Rome, Italy (Forastiere); Generation R Study Group, Department of Epidemiology, Erasmus University Medical Centre, Rotterdam, Netherlands (Gaillard, Jaddoe); Institute for Risk Assessment Sciences, Utrecht University, Utrecht, Netherlands (Gehring, Porta); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Govarts); Department of Environmental Epidemiology, Nofer Institute of Occupational Medicine, Lodz, Poland (Hanke); School of Public Health, Physiotherapy, and Population Science, University College Dublin, Dublin, Ireland (Kelleher, Viljoen); Department of Epidemiology, CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Mommers, Polańska, Thijs); Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana–Universitat Jaume I, Universitat de València Joint Research Unit of Epidemiology and Environmental Health, Valencia, Spain (Murcia); Cancer Epidemiology Unit, Department of Medical Sciences, University of Turin and Reference Centre for Epidemiology and Cancer Prevention in Piemonte, Turin, Italy (Pizzi, Richiardi); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Schoeters); University of Antwerp, Antwerp, Belgium; University of Southern Denmark, Odense, Denmark (Schoeters); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Sunyer, Vrijheid); Pompeu Fabra University, Barcelona, Spain (Sunyer); Department of Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain (Vrijheid); Department of Public Health, Academic Medical Centre, University of Amsterdam, Amsterdam, Netherlands (Vrijkotte); Centre for Nutrition, Prevention and Health Services, National Institute for Public Health and the Environment, Bilthoven, Netherlands (Wijga); CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Zeegers); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Kogevinas); Institut Hospital del Mar d'Investigacions Mèdiques, Barcelona, Spain (Kogevinas); National School of Public Health, Athens, Greece (Kogevinas)
1,
Theano Roumeliotaki
Theano Roumeliotaki, MPH
1Department of Social Medicine, Faculty of Medicine, University of Crete, Heraklion, Greece (Stratakis, Roumeliotaki, Chatzi); Section of Complex Genetics, Department of Genetics and Cell Biology, NUTRIM School of Nutrition and Translational Research in Metabolism, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Stratakis, Zeegers); Obesity Prevention Program, Harvard Pilgrim Health Care Institute, Department of Population Medicine, Harvard Medical School, Boston, Massachusetts (Oken, Rifas-Shiman); Department of Clinical Epidemiology, Predictive Medicine and Public Health, University of Porto Medical School, Porto, Portugal (Barros); Epidemology Research Unit, Institute of Public Health, University of Porto, Porto, Portugal (Barros); Public Health Division of Gipuzkoa, Basque Government; Health Research Institute, Biodonostia, San Sebastián, Spain (Basterrechea, Oliveira); Centros de Investigación Biomédica en Red Epidemiología y Salud Pública, Spain (Basterrechea, Murcia, Oliveira, Sunyer, Vrijheid, Kogevinas); Centre for Research in Epidemiology and Biostatistics Paris Sorbonne Cité, Institut National de la Santé et de la Recherche Medicale, Early Origin of the Child Development and Health Team, Villejuif, France (Charles, Heude); Université Paris Descartes, Villejuif, France (Charles, Heude); Norwegian Institute of Public Health, Oslo, Norway (Eggesbø, Iszatt); Department of Epidemiology, Lazio Regional Health System, Rome, Italy (Forastiere); Generation R Study Group, Department of Epidemiology, Erasmus University Medical Centre, Rotterdam, Netherlands (Gaillard, Jaddoe); Institute for Risk Assessment Sciences, Utrecht University, Utrecht, Netherlands (Gehring, Porta); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Govarts); Department of Environmental Epidemiology, Nofer Institute of Occupational Medicine, Lodz, Poland (Hanke); School of Public Health, Physiotherapy, and Population Science, University College Dublin, Dublin, Ireland (Kelleher, Viljoen); Department of Epidemiology, CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Mommers, Polańska, Thijs); Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana–Universitat Jaume I, Universitat de València Joint Research Unit of Epidemiology and Environmental Health, Valencia, Spain (Murcia); Cancer Epidemiology Unit, Department of Medical Sciences, University of Turin and Reference Centre for Epidemiology and Cancer Prevention in Piemonte, Turin, Italy (Pizzi, Richiardi); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Schoeters); University of Antwerp, Antwerp, Belgium; University of Southern Denmark, Odense, Denmark (Schoeters); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Sunyer, Vrijheid); Pompeu Fabra University, Barcelona, Spain (Sunyer); Department of Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain (Vrijheid); Department of Public Health, Academic Medical Centre, University of Amsterdam, Amsterdam, Netherlands (Vrijkotte); Centre for Nutrition, Prevention and Health Services, National Institute for Public Health and the Environment, Bilthoven, Netherlands (Wijga); CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Zeegers); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Kogevinas); Institut Hospital del Mar d'Investigacions Mèdiques, Barcelona, Spain (Kogevinas); National School of Public Health, Athens, Greece (Kogevinas)
1,
Emily Oken
Emily Oken, MD
1Department of Social Medicine, Faculty of Medicine, University of Crete, Heraklion, Greece (Stratakis, Roumeliotaki, Chatzi); Section of Complex Genetics, Department of Genetics and Cell Biology, NUTRIM School of Nutrition and Translational Research in Metabolism, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Stratakis, Zeegers); Obesity Prevention Program, Harvard Pilgrim Health Care Institute, Department of Population Medicine, Harvard Medical School, Boston, Massachusetts (Oken, Rifas-Shiman); Department of Clinical Epidemiology, Predictive Medicine and Public Health, University of Porto Medical School, Porto, Portugal (Barros); Epidemology Research Unit, Institute of Public Health, University of Porto, Porto, Portugal (Barros); Public Health Division of Gipuzkoa, Basque Government; Health Research Institute, Biodonostia, San Sebastián, Spain (Basterrechea, Oliveira); Centros de Investigación Biomédica en Red Epidemiología y Salud Pública, Spain (Basterrechea, Murcia, Oliveira, Sunyer, Vrijheid, Kogevinas); Centre for Research in Epidemiology and Biostatistics Paris Sorbonne Cité, Institut National de la Santé et de la Recherche Medicale, Early Origin of the Child Development and Health Team, Villejuif, France (Charles, Heude); Université Paris Descartes, Villejuif, France (Charles, Heude); Norwegian Institute of Public Health, Oslo, Norway (Eggesbø, Iszatt); Department of Epidemiology, Lazio Regional Health System, Rome, Italy (Forastiere); Generation R Study Group, Department of Epidemiology, Erasmus University Medical Centre, Rotterdam, Netherlands (Gaillard, Jaddoe); Institute for Risk Assessment Sciences, Utrecht University, Utrecht, Netherlands (Gehring, Porta); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Govarts); Department of Environmental Epidemiology, Nofer Institute of Occupational Medicine, Lodz, Poland (Hanke); School of Public Health, Physiotherapy, and Population Science, University College Dublin, Dublin, Ireland (Kelleher, Viljoen); Department of Epidemiology, CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Mommers, Polańska, Thijs); Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana–Universitat Jaume I, Universitat de València Joint Research Unit of Epidemiology and Environmental Health, Valencia, Spain (Murcia); Cancer Epidemiology Unit, Department of Medical Sciences, University of Turin and Reference Centre for Epidemiology and Cancer Prevention in Piemonte, Turin, Italy (Pizzi, Richiardi); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Schoeters); University of Antwerp, Antwerp, Belgium; University of Southern Denmark, Odense, Denmark (Schoeters); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Sunyer, Vrijheid); Pompeu Fabra University, Barcelona, Spain (Sunyer); Department of Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain (Vrijheid); Department of Public Health, Academic Medical Centre, University of Amsterdam, Amsterdam, Netherlands (Vrijkotte); Centre for Nutrition, Prevention and Health Services, National Institute for Public Health and the Environment, Bilthoven, Netherlands (Wijga); CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Zeegers); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Kogevinas); Institut Hospital del Mar d'Investigacions Mèdiques, Barcelona, Spain (Kogevinas); National School of Public Health, Athens, Greece (Kogevinas)
1,
Henrique Barros
Henrique Barros, PhD
1Department of Social Medicine, Faculty of Medicine, University of Crete, Heraklion, Greece (Stratakis, Roumeliotaki, Chatzi); Section of Complex Genetics, Department of Genetics and Cell Biology, NUTRIM School of Nutrition and Translational Research in Metabolism, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Stratakis, Zeegers); Obesity Prevention Program, Harvard Pilgrim Health Care Institute, Department of Population Medicine, Harvard Medical School, Boston, Massachusetts (Oken, Rifas-Shiman); Department of Clinical Epidemiology, Predictive Medicine and Public Health, University of Porto Medical School, Porto, Portugal (Barros); Epidemology Research Unit, Institute of Public Health, University of Porto, Porto, Portugal (Barros); Public Health Division of Gipuzkoa, Basque Government; Health Research Institute, Biodonostia, San Sebastián, Spain (Basterrechea, Oliveira); Centros de Investigación Biomédica en Red Epidemiología y Salud Pública, Spain (Basterrechea, Murcia, Oliveira, Sunyer, Vrijheid, Kogevinas); Centre for Research in Epidemiology and Biostatistics Paris Sorbonne Cité, Institut National de la Santé et de la Recherche Medicale, Early Origin of the Child Development and Health Team, Villejuif, France (Charles, Heude); Université Paris Descartes, Villejuif, France (Charles, Heude); Norwegian Institute of Public Health, Oslo, Norway (Eggesbø, Iszatt); Department of Epidemiology, Lazio Regional Health System, Rome, Italy (Forastiere); Generation R Study Group, Department of Epidemiology, Erasmus University Medical Centre, Rotterdam, Netherlands (Gaillard, Jaddoe); Institute for Risk Assessment Sciences, Utrecht University, Utrecht, Netherlands (Gehring, Porta); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Govarts); Department of Environmental Epidemiology, Nofer Institute of Occupational Medicine, Lodz, Poland (Hanke); School of Public Health, Physiotherapy, and Population Science, University College Dublin, Dublin, Ireland (Kelleher, Viljoen); Department of Epidemiology, CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Mommers, Polańska, Thijs); Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana–Universitat Jaume I, Universitat de València Joint Research Unit of Epidemiology and Environmental Health, Valencia, Spain (Murcia); Cancer Epidemiology Unit, Department of Medical Sciences, University of Turin and Reference Centre for Epidemiology and Cancer Prevention in Piemonte, Turin, Italy (Pizzi, Richiardi); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Schoeters); University of Antwerp, Antwerp, Belgium; University of Southern Denmark, Odense, Denmark (Schoeters); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Sunyer, Vrijheid); Pompeu Fabra University, Barcelona, Spain (Sunyer); Department of Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain (Vrijheid); Department of Public Health, Academic Medical Centre, University of Amsterdam, Amsterdam, Netherlands (Vrijkotte); Centre for Nutrition, Prevention and Health Services, National Institute for Public Health and the Environment, Bilthoven, Netherlands (Wijga); CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Zeegers); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Kogevinas); Institut Hospital del Mar d'Investigacions Mèdiques, Barcelona, Spain (Kogevinas); National School of Public Health, Athens, Greece (Kogevinas)
1,
Mikel Basterrechea
Mikel Basterrechea
1Department of Social Medicine, Faculty of Medicine, University of Crete, Heraklion, Greece (Stratakis, Roumeliotaki, Chatzi); Section of Complex Genetics, Department of Genetics and Cell Biology, NUTRIM School of Nutrition and Translational Research in Metabolism, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Stratakis, Zeegers); Obesity Prevention Program, Harvard Pilgrim Health Care Institute, Department of Population Medicine, Harvard Medical School, Boston, Massachusetts (Oken, Rifas-Shiman); Department of Clinical Epidemiology, Predictive Medicine and Public Health, University of Porto Medical School, Porto, Portugal (Barros); Epidemology Research Unit, Institute of Public Health, University of Porto, Porto, Portugal (Barros); Public Health Division of Gipuzkoa, Basque Government; Health Research Institute, Biodonostia, San Sebastián, Spain (Basterrechea, Oliveira); Centros de Investigación Biomédica en Red Epidemiología y Salud Pública, Spain (Basterrechea, Murcia, Oliveira, Sunyer, Vrijheid, Kogevinas); Centre for Research in Epidemiology and Biostatistics Paris Sorbonne Cité, Institut National de la Santé et de la Recherche Medicale, Early Origin of the Child Development and Health Team, Villejuif, France (Charles, Heude); Université Paris Descartes, Villejuif, France (Charles, Heude); Norwegian Institute of Public Health, Oslo, Norway (Eggesbø, Iszatt); Department of Epidemiology, Lazio Regional Health System, Rome, Italy (Forastiere); Generation R Study Group, Department of Epidemiology, Erasmus University Medical Centre, Rotterdam, Netherlands (Gaillard, Jaddoe); Institute for Risk Assessment Sciences, Utrecht University, Utrecht, Netherlands (Gehring, Porta); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Govarts); Department of Environmental Epidemiology, Nofer Institute of Occupational Medicine, Lodz, Poland (Hanke); School of Public Health, Physiotherapy, and Population Science, University College Dublin, Dublin, Ireland (Kelleher, Viljoen); Department of Epidemiology, CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Mommers, Polańska, Thijs); Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana–Universitat Jaume I, Universitat de València Joint Research Unit of Epidemiology and Environmental Health, Valencia, Spain (Murcia); Cancer Epidemiology Unit, Department of Medical Sciences, University of Turin and Reference Centre for Epidemiology and Cancer Prevention in Piemonte, Turin, Italy (Pizzi, Richiardi); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Schoeters); University of Antwerp, Antwerp, Belgium; University of Southern Denmark, Odense, Denmark (Schoeters); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Sunyer, Vrijheid); Pompeu Fabra University, Barcelona, Spain (Sunyer); Department of Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain (Vrijheid); Department of Public Health, Academic Medical Centre, University of Amsterdam, Amsterdam, Netherlands (Vrijkotte); Centre for Nutrition, Prevention and Health Services, National Institute for Public Health and the Environment, Bilthoven, Netherlands (Wijga); CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Zeegers); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Kogevinas); Institut Hospital del Mar d'Investigacions Mèdiques, Barcelona, Spain (Kogevinas); National School of Public Health, Athens, Greece (Kogevinas)
1,
Marie-Aline Charles
Marie-Aline Charles, MD
1Department of Social Medicine, Faculty of Medicine, University of Crete, Heraklion, Greece (Stratakis, Roumeliotaki, Chatzi); Section of Complex Genetics, Department of Genetics and Cell Biology, NUTRIM School of Nutrition and Translational Research in Metabolism, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Stratakis, Zeegers); Obesity Prevention Program, Harvard Pilgrim Health Care Institute, Department of Population Medicine, Harvard Medical School, Boston, Massachusetts (Oken, Rifas-Shiman); Department of Clinical Epidemiology, Predictive Medicine and Public Health, University of Porto Medical School, Porto, Portugal (Barros); Epidemology Research Unit, Institute of Public Health, University of Porto, Porto, Portugal (Barros); Public Health Division of Gipuzkoa, Basque Government; Health Research Institute, Biodonostia, San Sebastián, Spain (Basterrechea, Oliveira); Centros de Investigación Biomédica en Red Epidemiología y Salud Pública, Spain (Basterrechea, Murcia, Oliveira, Sunyer, Vrijheid, Kogevinas); Centre for Research in Epidemiology and Biostatistics Paris Sorbonne Cité, Institut National de la Santé et de la Recherche Medicale, Early Origin of the Child Development and Health Team, Villejuif, France (Charles, Heude); Université Paris Descartes, Villejuif, France (Charles, Heude); Norwegian Institute of Public Health, Oslo, Norway (Eggesbø, Iszatt); Department of Epidemiology, Lazio Regional Health System, Rome, Italy (Forastiere); Generation R Study Group, Department of Epidemiology, Erasmus University Medical Centre, Rotterdam, Netherlands (Gaillard, Jaddoe); Institute for Risk Assessment Sciences, Utrecht University, Utrecht, Netherlands (Gehring, Porta); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Govarts); Department of Environmental Epidemiology, Nofer Institute of Occupational Medicine, Lodz, Poland (Hanke); School of Public Health, Physiotherapy, and Population Science, University College Dublin, Dublin, Ireland (Kelleher, Viljoen); Department of Epidemiology, CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Mommers, Polańska, Thijs); Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana–Universitat Jaume I, Universitat de València Joint Research Unit of Epidemiology and Environmental Health, Valencia, Spain (Murcia); Cancer Epidemiology Unit, Department of Medical Sciences, University of Turin and Reference Centre for Epidemiology and Cancer Prevention in Piemonte, Turin, Italy (Pizzi, Richiardi); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Schoeters); University of Antwerp, Antwerp, Belgium; University of Southern Denmark, Odense, Denmark (Schoeters); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Sunyer, Vrijheid); Pompeu Fabra University, Barcelona, Spain (Sunyer); Department of Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain (Vrijheid); Department of Public Health, Academic Medical Centre, University of Amsterdam, Amsterdam, Netherlands (Vrijkotte); Centre for Nutrition, Prevention and Health Services, National Institute for Public Health and the Environment, Bilthoven, Netherlands (Wijga); CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Zeegers); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Kogevinas); Institut Hospital del Mar d'Investigacions Mèdiques, Barcelona, Spain (Kogevinas); National School of Public Health, Athens, Greece (Kogevinas)
1,
Merete Eggesbø
Merete Eggesbø, PhD
1Department of Social Medicine, Faculty of Medicine, University of Crete, Heraklion, Greece (Stratakis, Roumeliotaki, Chatzi); Section of Complex Genetics, Department of Genetics and Cell Biology, NUTRIM School of Nutrition and Translational Research in Metabolism, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Stratakis, Zeegers); Obesity Prevention Program, Harvard Pilgrim Health Care Institute, Department of Population Medicine, Harvard Medical School, Boston, Massachusetts (Oken, Rifas-Shiman); Department of Clinical Epidemiology, Predictive Medicine and Public Health, University of Porto Medical School, Porto, Portugal (Barros); Epidemology Research Unit, Institute of Public Health, University of Porto, Porto, Portugal (Barros); Public Health Division of Gipuzkoa, Basque Government; Health Research Institute, Biodonostia, San Sebastián, Spain (Basterrechea, Oliveira); Centros de Investigación Biomédica en Red Epidemiología y Salud Pública, Spain (Basterrechea, Murcia, Oliveira, Sunyer, Vrijheid, Kogevinas); Centre for Research in Epidemiology and Biostatistics Paris Sorbonne Cité, Institut National de la Santé et de la Recherche Medicale, Early Origin of the Child Development and Health Team, Villejuif, France (Charles, Heude); Université Paris Descartes, Villejuif, France (Charles, Heude); Norwegian Institute of Public Health, Oslo, Norway (Eggesbø, Iszatt); Department of Epidemiology, Lazio Regional Health System, Rome, Italy (Forastiere); Generation R Study Group, Department of Epidemiology, Erasmus University Medical Centre, Rotterdam, Netherlands (Gaillard, Jaddoe); Institute for Risk Assessment Sciences, Utrecht University, Utrecht, Netherlands (Gehring, Porta); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Govarts); Department of Environmental Epidemiology, Nofer Institute of Occupational Medicine, Lodz, Poland (Hanke); School of Public Health, Physiotherapy, and Population Science, University College Dublin, Dublin, Ireland (Kelleher, Viljoen); Department of Epidemiology, CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Mommers, Polańska, Thijs); Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana–Universitat Jaume I, Universitat de València Joint Research Unit of Epidemiology and Environmental Health, Valencia, Spain (Murcia); Cancer Epidemiology Unit, Department of Medical Sciences, University of Turin and Reference Centre for Epidemiology and Cancer Prevention in Piemonte, Turin, Italy (Pizzi, Richiardi); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Schoeters); University of Antwerp, Antwerp, Belgium; University of Southern Denmark, Odense, Denmark (Schoeters); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Sunyer, Vrijheid); Pompeu Fabra University, Barcelona, Spain (Sunyer); Department of Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain (Vrijheid); Department of Public Health, Academic Medical Centre, University of Amsterdam, Amsterdam, Netherlands (Vrijkotte); Centre for Nutrition, Prevention and Health Services, National Institute for Public Health and the Environment, Bilthoven, Netherlands (Wijga); CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Zeegers); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Kogevinas); Institut Hospital del Mar d'Investigacions Mèdiques, Barcelona, Spain (Kogevinas); National School of Public Health, Athens, Greece (Kogevinas)
1,
Francesco Forastiere
Francesco Forastiere, PhD
1Department of Social Medicine, Faculty of Medicine, University of Crete, Heraklion, Greece (Stratakis, Roumeliotaki, Chatzi); Section of Complex Genetics, Department of Genetics and Cell Biology, NUTRIM School of Nutrition and Translational Research in Metabolism, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Stratakis, Zeegers); Obesity Prevention Program, Harvard Pilgrim Health Care Institute, Department of Population Medicine, Harvard Medical School, Boston, Massachusetts (Oken, Rifas-Shiman); Department of Clinical Epidemiology, Predictive Medicine and Public Health, University of Porto Medical School, Porto, Portugal (Barros); Epidemology Research Unit, Institute of Public Health, University of Porto, Porto, Portugal (Barros); Public Health Division of Gipuzkoa, Basque Government; Health Research Institute, Biodonostia, San Sebastián, Spain (Basterrechea, Oliveira); Centros de Investigación Biomédica en Red Epidemiología y Salud Pública, Spain (Basterrechea, Murcia, Oliveira, Sunyer, Vrijheid, Kogevinas); Centre for Research in Epidemiology and Biostatistics Paris Sorbonne Cité, Institut National de la Santé et de la Recherche Medicale, Early Origin of the Child Development and Health Team, Villejuif, France (Charles, Heude); Université Paris Descartes, Villejuif, France (Charles, Heude); Norwegian Institute of Public Health, Oslo, Norway (Eggesbø, Iszatt); Department of Epidemiology, Lazio Regional Health System, Rome, Italy (Forastiere); Generation R Study Group, Department of Epidemiology, Erasmus University Medical Centre, Rotterdam, Netherlands (Gaillard, Jaddoe); Institute for Risk Assessment Sciences, Utrecht University, Utrecht, Netherlands (Gehring, Porta); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Govarts); Department of Environmental Epidemiology, Nofer Institute of Occupational Medicine, Lodz, Poland (Hanke); School of Public Health, Physiotherapy, and Population Science, University College Dublin, Dublin, Ireland (Kelleher, Viljoen); Department of Epidemiology, CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Mommers, Polańska, Thijs); Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana–Universitat Jaume I, Universitat de València Joint Research Unit of Epidemiology and Environmental Health, Valencia, Spain (Murcia); Cancer Epidemiology Unit, Department of Medical Sciences, University of Turin and Reference Centre for Epidemiology and Cancer Prevention in Piemonte, Turin, Italy (Pizzi, Richiardi); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Schoeters); University of Antwerp, Antwerp, Belgium; University of Southern Denmark, Odense, Denmark (Schoeters); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Sunyer, Vrijheid); Pompeu Fabra University, Barcelona, Spain (Sunyer); Department of Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain (Vrijheid); Department of Public Health, Academic Medical Centre, University of Amsterdam, Amsterdam, Netherlands (Vrijkotte); Centre for Nutrition, Prevention and Health Services, National Institute for Public Health and the Environment, Bilthoven, Netherlands (Wijga); CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Zeegers); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Kogevinas); Institut Hospital del Mar d'Investigacions Mèdiques, Barcelona, Spain (Kogevinas); National School of Public Health, Athens, Greece (Kogevinas)
1,
Romy Gaillard
Romy Gaillard, PhD
1Department of Social Medicine, Faculty of Medicine, University of Crete, Heraklion, Greece (Stratakis, Roumeliotaki, Chatzi); Section of Complex Genetics, Department of Genetics and Cell Biology, NUTRIM School of Nutrition and Translational Research in Metabolism, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Stratakis, Zeegers); Obesity Prevention Program, Harvard Pilgrim Health Care Institute, Department of Population Medicine, Harvard Medical School, Boston, Massachusetts (Oken, Rifas-Shiman); Department of Clinical Epidemiology, Predictive Medicine and Public Health, University of Porto Medical School, Porto, Portugal (Barros); Epidemology Research Unit, Institute of Public Health, University of Porto, Porto, Portugal (Barros); Public Health Division of Gipuzkoa, Basque Government; Health Research Institute, Biodonostia, San Sebastián, Spain (Basterrechea, Oliveira); Centros de Investigación Biomédica en Red Epidemiología y Salud Pública, Spain (Basterrechea, Murcia, Oliveira, Sunyer, Vrijheid, Kogevinas); Centre for Research in Epidemiology and Biostatistics Paris Sorbonne Cité, Institut National de la Santé et de la Recherche Medicale, Early Origin of the Child Development and Health Team, Villejuif, France (Charles, Heude); Université Paris Descartes, Villejuif, France (Charles, Heude); Norwegian Institute of Public Health, Oslo, Norway (Eggesbø, Iszatt); Department of Epidemiology, Lazio Regional Health System, Rome, Italy (Forastiere); Generation R Study Group, Department of Epidemiology, Erasmus University Medical Centre, Rotterdam, Netherlands (Gaillard, Jaddoe); Institute for Risk Assessment Sciences, Utrecht University, Utrecht, Netherlands (Gehring, Porta); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Govarts); Department of Environmental Epidemiology, Nofer Institute of Occupational Medicine, Lodz, Poland (Hanke); School of Public Health, Physiotherapy, and Population Science, University College Dublin, Dublin, Ireland (Kelleher, Viljoen); Department of Epidemiology, CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Mommers, Polańska, Thijs); Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana–Universitat Jaume I, Universitat de València Joint Research Unit of Epidemiology and Environmental Health, Valencia, Spain (Murcia); Cancer Epidemiology Unit, Department of Medical Sciences, University of Turin and Reference Centre for Epidemiology and Cancer Prevention in Piemonte, Turin, Italy (Pizzi, Richiardi); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Schoeters); University of Antwerp, Antwerp, Belgium; University of Southern Denmark, Odense, Denmark (Schoeters); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Sunyer, Vrijheid); Pompeu Fabra University, Barcelona, Spain (Sunyer); Department of Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain (Vrijheid); Department of Public Health, Academic Medical Centre, University of Amsterdam, Amsterdam, Netherlands (Vrijkotte); Centre for Nutrition, Prevention and Health Services, National Institute for Public Health and the Environment, Bilthoven, Netherlands (Wijga); CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Zeegers); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Kogevinas); Institut Hospital del Mar d'Investigacions Mèdiques, Barcelona, Spain (Kogevinas); National School of Public Health, Athens, Greece (Kogevinas)
1,
Ulrike Gehring
Ulrike Gehring, PhD
1Department of Social Medicine, Faculty of Medicine, University of Crete, Heraklion, Greece (Stratakis, Roumeliotaki, Chatzi); Section of Complex Genetics, Department of Genetics and Cell Biology, NUTRIM School of Nutrition and Translational Research in Metabolism, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Stratakis, Zeegers); Obesity Prevention Program, Harvard Pilgrim Health Care Institute, Department of Population Medicine, Harvard Medical School, Boston, Massachusetts (Oken, Rifas-Shiman); Department of Clinical Epidemiology, Predictive Medicine and Public Health, University of Porto Medical School, Porto, Portugal (Barros); Epidemology Research Unit, Institute of Public Health, University of Porto, Porto, Portugal (Barros); Public Health Division of Gipuzkoa, Basque Government; Health Research Institute, Biodonostia, San Sebastián, Spain (Basterrechea, Oliveira); Centros de Investigación Biomédica en Red Epidemiología y Salud Pública, Spain (Basterrechea, Murcia, Oliveira, Sunyer, Vrijheid, Kogevinas); Centre for Research in Epidemiology and Biostatistics Paris Sorbonne Cité, Institut National de la Santé et de la Recherche Medicale, Early Origin of the Child Development and Health Team, Villejuif, France (Charles, Heude); Université Paris Descartes, Villejuif, France (Charles, Heude); Norwegian Institute of Public Health, Oslo, Norway (Eggesbø, Iszatt); Department of Epidemiology, Lazio Regional Health System, Rome, Italy (Forastiere); Generation R Study Group, Department of Epidemiology, Erasmus University Medical Centre, Rotterdam, Netherlands (Gaillard, Jaddoe); Institute for Risk Assessment Sciences, Utrecht University, Utrecht, Netherlands (Gehring, Porta); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Govarts); Department of Environmental Epidemiology, Nofer Institute of Occupational Medicine, Lodz, Poland (Hanke); School of Public Health, Physiotherapy, and Population Science, University College Dublin, Dublin, Ireland (Kelleher, Viljoen); Department of Epidemiology, CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Mommers, Polańska, Thijs); Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana–Universitat Jaume I, Universitat de València Joint Research Unit of Epidemiology and Environmental Health, Valencia, Spain (Murcia); Cancer Epidemiology Unit, Department of Medical Sciences, University of Turin and Reference Centre for Epidemiology and Cancer Prevention in Piemonte, Turin, Italy (Pizzi, Richiardi); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Schoeters); University of Antwerp, Antwerp, Belgium; University of Southern Denmark, Odense, Denmark (Schoeters); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Sunyer, Vrijheid); Pompeu Fabra University, Barcelona, Spain (Sunyer); Department of Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain (Vrijheid); Department of Public Health, Academic Medical Centre, University of Amsterdam, Amsterdam, Netherlands (Vrijkotte); Centre for Nutrition, Prevention and Health Services, National Institute for Public Health and the Environment, Bilthoven, Netherlands (Wijga); CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Zeegers); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Kogevinas); Institut Hospital del Mar d'Investigacions Mèdiques, Barcelona, Spain (Kogevinas); National School of Public Health, Athens, Greece (Kogevinas)
1,
Eva Govarts
Eva Govarts, MSc
1Department of Social Medicine, Faculty of Medicine, University of Crete, Heraklion, Greece (Stratakis, Roumeliotaki, Chatzi); Section of Complex Genetics, Department of Genetics and Cell Biology, NUTRIM School of Nutrition and Translational Research in Metabolism, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Stratakis, Zeegers); Obesity Prevention Program, Harvard Pilgrim Health Care Institute, Department of Population Medicine, Harvard Medical School, Boston, Massachusetts (Oken, Rifas-Shiman); Department of Clinical Epidemiology, Predictive Medicine and Public Health, University of Porto Medical School, Porto, Portugal (Barros); Epidemology Research Unit, Institute of Public Health, University of Porto, Porto, Portugal (Barros); Public Health Division of Gipuzkoa, Basque Government; Health Research Institute, Biodonostia, San Sebastián, Spain (Basterrechea, Oliveira); Centros de Investigación Biomédica en Red Epidemiología y Salud Pública, Spain (Basterrechea, Murcia, Oliveira, Sunyer, Vrijheid, Kogevinas); Centre for Research in Epidemiology and Biostatistics Paris Sorbonne Cité, Institut National de la Santé et de la Recherche Medicale, Early Origin of the Child Development and Health Team, Villejuif, France (Charles, Heude); Université Paris Descartes, Villejuif, France (Charles, Heude); Norwegian Institute of Public Health, Oslo, Norway (Eggesbø, Iszatt); Department of Epidemiology, Lazio Regional Health System, Rome, Italy (Forastiere); Generation R Study Group, Department of Epidemiology, Erasmus University Medical Centre, Rotterdam, Netherlands (Gaillard, Jaddoe); Institute for Risk Assessment Sciences, Utrecht University, Utrecht, Netherlands (Gehring, Porta); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Govarts); Department of Environmental Epidemiology, Nofer Institute of Occupational Medicine, Lodz, Poland (Hanke); School of Public Health, Physiotherapy, and Population Science, University College Dublin, Dublin, Ireland (Kelleher, Viljoen); Department of Epidemiology, CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Mommers, Polańska, Thijs); Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana–Universitat Jaume I, Universitat de València Joint Research Unit of Epidemiology and Environmental Health, Valencia, Spain (Murcia); Cancer Epidemiology Unit, Department of Medical Sciences, University of Turin and Reference Centre for Epidemiology and Cancer Prevention in Piemonte, Turin, Italy (Pizzi, Richiardi); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Schoeters); University of Antwerp, Antwerp, Belgium; University of Southern Denmark, Odense, Denmark (Schoeters); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Sunyer, Vrijheid); Pompeu Fabra University, Barcelona, Spain (Sunyer); Department of Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain (Vrijheid); Department of Public Health, Academic Medical Centre, University of Amsterdam, Amsterdam, Netherlands (Vrijkotte); Centre for Nutrition, Prevention and Health Services, National Institute for Public Health and the Environment, Bilthoven, Netherlands (Wijga); CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Zeegers); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Kogevinas); Institut Hospital del Mar d'Investigacions Mèdiques, Barcelona, Spain (Kogevinas); National School of Public Health, Athens, Greece (Kogevinas)
1,
Wojciech Hanke
Wojciech Hanke, PhD
1Department of Social Medicine, Faculty of Medicine, University of Crete, Heraklion, Greece (Stratakis, Roumeliotaki, Chatzi); Section of Complex Genetics, Department of Genetics and Cell Biology, NUTRIM School of Nutrition and Translational Research in Metabolism, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Stratakis, Zeegers); Obesity Prevention Program, Harvard Pilgrim Health Care Institute, Department of Population Medicine, Harvard Medical School, Boston, Massachusetts (Oken, Rifas-Shiman); Department of Clinical Epidemiology, Predictive Medicine and Public Health, University of Porto Medical School, Porto, Portugal (Barros); Epidemology Research Unit, Institute of Public Health, University of Porto, Porto, Portugal (Barros); Public Health Division of Gipuzkoa, Basque Government; Health Research Institute, Biodonostia, San Sebastián, Spain (Basterrechea, Oliveira); Centros de Investigación Biomédica en Red Epidemiología y Salud Pública, Spain (Basterrechea, Murcia, Oliveira, Sunyer, Vrijheid, Kogevinas); Centre for Research in Epidemiology and Biostatistics Paris Sorbonne Cité, Institut National de la Santé et de la Recherche Medicale, Early Origin of the Child Development and Health Team, Villejuif, France (Charles, Heude); Université Paris Descartes, Villejuif, France (Charles, Heude); Norwegian Institute of Public Health, Oslo, Norway (Eggesbø, Iszatt); Department of Epidemiology, Lazio Regional Health System, Rome, Italy (Forastiere); Generation R Study Group, Department of Epidemiology, Erasmus University Medical Centre, Rotterdam, Netherlands (Gaillard, Jaddoe); Institute for Risk Assessment Sciences, Utrecht University, Utrecht, Netherlands (Gehring, Porta); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Govarts); Department of Environmental Epidemiology, Nofer Institute of Occupational Medicine, Lodz, Poland (Hanke); School of Public Health, Physiotherapy, and Population Science, University College Dublin, Dublin, Ireland (Kelleher, Viljoen); Department of Epidemiology, CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Mommers, Polańska, Thijs); Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana–Universitat Jaume I, Universitat de València Joint Research Unit of Epidemiology and Environmental Health, Valencia, Spain (Murcia); Cancer Epidemiology Unit, Department of Medical Sciences, University of Turin and Reference Centre for Epidemiology and Cancer Prevention in Piemonte, Turin, Italy (Pizzi, Richiardi); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Schoeters); University of Antwerp, Antwerp, Belgium; University of Southern Denmark, Odense, Denmark (Schoeters); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Sunyer, Vrijheid); Pompeu Fabra University, Barcelona, Spain (Sunyer); Department of Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain (Vrijheid); Department of Public Health, Academic Medical Centre, University of Amsterdam, Amsterdam, Netherlands (Vrijkotte); Centre for Nutrition, Prevention and Health Services, National Institute for Public Health and the Environment, Bilthoven, Netherlands (Wijga); CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Zeegers); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Kogevinas); Institut Hospital del Mar d'Investigacions Mèdiques, Barcelona, Spain (Kogevinas); National School of Public Health, Athens, Greece (Kogevinas)
1,
Barbara Heude
Barbara Heude, PhD
1Department of Social Medicine, Faculty of Medicine, University of Crete, Heraklion, Greece (Stratakis, Roumeliotaki, Chatzi); Section of Complex Genetics, Department of Genetics and Cell Biology, NUTRIM School of Nutrition and Translational Research in Metabolism, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Stratakis, Zeegers); Obesity Prevention Program, Harvard Pilgrim Health Care Institute, Department of Population Medicine, Harvard Medical School, Boston, Massachusetts (Oken, Rifas-Shiman); Department of Clinical Epidemiology, Predictive Medicine and Public Health, University of Porto Medical School, Porto, Portugal (Barros); Epidemology Research Unit, Institute of Public Health, University of Porto, Porto, Portugal (Barros); Public Health Division of Gipuzkoa, Basque Government; Health Research Institute, Biodonostia, San Sebastián, Spain (Basterrechea, Oliveira); Centros de Investigación Biomédica en Red Epidemiología y Salud Pública, Spain (Basterrechea, Murcia, Oliveira, Sunyer, Vrijheid, Kogevinas); Centre for Research in Epidemiology and Biostatistics Paris Sorbonne Cité, Institut National de la Santé et de la Recherche Medicale, Early Origin of the Child Development and Health Team, Villejuif, France (Charles, Heude); Université Paris Descartes, Villejuif, France (Charles, Heude); Norwegian Institute of Public Health, Oslo, Norway (Eggesbø, Iszatt); Department of Epidemiology, Lazio Regional Health System, Rome, Italy (Forastiere); Generation R Study Group, Department of Epidemiology, Erasmus University Medical Centre, Rotterdam, Netherlands (Gaillard, Jaddoe); Institute for Risk Assessment Sciences, Utrecht University, Utrecht, Netherlands (Gehring, Porta); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Govarts); Department of Environmental Epidemiology, Nofer Institute of Occupational Medicine, Lodz, Poland (Hanke); School of Public Health, Physiotherapy, and Population Science, University College Dublin, Dublin, Ireland (Kelleher, Viljoen); Department of Epidemiology, CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Mommers, Polańska, Thijs); Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana–Universitat Jaume I, Universitat de València Joint Research Unit of Epidemiology and Environmental Health, Valencia, Spain (Murcia); Cancer Epidemiology Unit, Department of Medical Sciences, University of Turin and Reference Centre for Epidemiology and Cancer Prevention in Piemonte, Turin, Italy (Pizzi, Richiardi); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Schoeters); University of Antwerp, Antwerp, Belgium; University of Southern Denmark, Odense, Denmark (Schoeters); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Sunyer, Vrijheid); Pompeu Fabra University, Barcelona, Spain (Sunyer); Department of Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain (Vrijheid); Department of Public Health, Academic Medical Centre, University of Amsterdam, Amsterdam, Netherlands (Vrijkotte); Centre for Nutrition, Prevention and Health Services, National Institute for Public Health and the Environment, Bilthoven, Netherlands (Wijga); CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Zeegers); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Kogevinas); Institut Hospital del Mar d'Investigacions Mèdiques, Barcelona, Spain (Kogevinas); National School of Public Health, Athens, Greece (Kogevinas)
1,
Nina Iszatt
Nina Iszatt, PhD
1Department of Social Medicine, Faculty of Medicine, University of Crete, Heraklion, Greece (Stratakis, Roumeliotaki, Chatzi); Section of Complex Genetics, Department of Genetics and Cell Biology, NUTRIM School of Nutrition and Translational Research in Metabolism, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Stratakis, Zeegers); Obesity Prevention Program, Harvard Pilgrim Health Care Institute, Department of Population Medicine, Harvard Medical School, Boston, Massachusetts (Oken, Rifas-Shiman); Department of Clinical Epidemiology, Predictive Medicine and Public Health, University of Porto Medical School, Porto, Portugal (Barros); Epidemology Research Unit, Institute of Public Health, University of Porto, Porto, Portugal (Barros); Public Health Division of Gipuzkoa, Basque Government; Health Research Institute, Biodonostia, San Sebastián, Spain (Basterrechea, Oliveira); Centros de Investigación Biomédica en Red Epidemiología y Salud Pública, Spain (Basterrechea, Murcia, Oliveira, Sunyer, Vrijheid, Kogevinas); Centre for Research in Epidemiology and Biostatistics Paris Sorbonne Cité, Institut National de la Santé et de la Recherche Medicale, Early Origin of the Child Development and Health Team, Villejuif, France (Charles, Heude); Université Paris Descartes, Villejuif, France (Charles, Heude); Norwegian Institute of Public Health, Oslo, Norway (Eggesbø, Iszatt); Department of Epidemiology, Lazio Regional Health System, Rome, Italy (Forastiere); Generation R Study Group, Department of Epidemiology, Erasmus University Medical Centre, Rotterdam, Netherlands (Gaillard, Jaddoe); Institute for Risk Assessment Sciences, Utrecht University, Utrecht, Netherlands (Gehring, Porta); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Govarts); Department of Environmental Epidemiology, Nofer Institute of Occupational Medicine, Lodz, Poland (Hanke); School of Public Health, Physiotherapy, and Population Science, University College Dublin, Dublin, Ireland (Kelleher, Viljoen); Department of Epidemiology, CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Mommers, Polańska, Thijs); Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana–Universitat Jaume I, Universitat de València Joint Research Unit of Epidemiology and Environmental Health, Valencia, Spain (Murcia); Cancer Epidemiology Unit, Department of Medical Sciences, University of Turin and Reference Centre for Epidemiology and Cancer Prevention in Piemonte, Turin, Italy (Pizzi, Richiardi); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Schoeters); University of Antwerp, Antwerp, Belgium; University of Southern Denmark, Odense, Denmark (Schoeters); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Sunyer, Vrijheid); Pompeu Fabra University, Barcelona, Spain (Sunyer); Department of Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain (Vrijheid); Department of Public Health, Academic Medical Centre, University of Amsterdam, Amsterdam, Netherlands (Vrijkotte); Centre for Nutrition, Prevention and Health Services, National Institute for Public Health and the Environment, Bilthoven, Netherlands (Wijga); CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Zeegers); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Kogevinas); Institut Hospital del Mar d'Investigacions Mèdiques, Barcelona, Spain (Kogevinas); National School of Public Health, Athens, Greece (Kogevinas)
1,
Vincent W Jaddoe
Vincent W Jaddoe, PhD
1Department of Social Medicine, Faculty of Medicine, University of Crete, Heraklion, Greece (Stratakis, Roumeliotaki, Chatzi); Section of Complex Genetics, Department of Genetics and Cell Biology, NUTRIM School of Nutrition and Translational Research in Metabolism, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Stratakis, Zeegers); Obesity Prevention Program, Harvard Pilgrim Health Care Institute, Department of Population Medicine, Harvard Medical School, Boston, Massachusetts (Oken, Rifas-Shiman); Department of Clinical Epidemiology, Predictive Medicine and Public Health, University of Porto Medical School, Porto, Portugal (Barros); Epidemology Research Unit, Institute of Public Health, University of Porto, Porto, Portugal (Barros); Public Health Division of Gipuzkoa, Basque Government; Health Research Institute, Biodonostia, San Sebastián, Spain (Basterrechea, Oliveira); Centros de Investigación Biomédica en Red Epidemiología y Salud Pública, Spain (Basterrechea, Murcia, Oliveira, Sunyer, Vrijheid, Kogevinas); Centre for Research in Epidemiology and Biostatistics Paris Sorbonne Cité, Institut National de la Santé et de la Recherche Medicale, Early Origin of the Child Development and Health Team, Villejuif, France (Charles, Heude); Université Paris Descartes, Villejuif, France (Charles, Heude); Norwegian Institute of Public Health, Oslo, Norway (Eggesbø, Iszatt); Department of Epidemiology, Lazio Regional Health System, Rome, Italy (Forastiere); Generation R Study Group, Department of Epidemiology, Erasmus University Medical Centre, Rotterdam, Netherlands (Gaillard, Jaddoe); Institute for Risk Assessment Sciences, Utrecht University, Utrecht, Netherlands (Gehring, Porta); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Govarts); Department of Environmental Epidemiology, Nofer Institute of Occupational Medicine, Lodz, Poland (Hanke); School of Public Health, Physiotherapy, and Population Science, University College Dublin, Dublin, Ireland (Kelleher, Viljoen); Department of Epidemiology, CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Mommers, Polańska, Thijs); Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana–Universitat Jaume I, Universitat de València Joint Research Unit of Epidemiology and Environmental Health, Valencia, Spain (Murcia); Cancer Epidemiology Unit, Department of Medical Sciences, University of Turin and Reference Centre for Epidemiology and Cancer Prevention in Piemonte, Turin, Italy (Pizzi, Richiardi); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Schoeters); University of Antwerp, Antwerp, Belgium; University of Southern Denmark, Odense, Denmark (Schoeters); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Sunyer, Vrijheid); Pompeu Fabra University, Barcelona, Spain (Sunyer); Department of Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain (Vrijheid); Department of Public Health, Academic Medical Centre, University of Amsterdam, Amsterdam, Netherlands (Vrijkotte); Centre for Nutrition, Prevention and Health Services, National Institute for Public Health and the Environment, Bilthoven, Netherlands (Wijga); CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Zeegers); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Kogevinas); Institut Hospital del Mar d'Investigacions Mèdiques, Barcelona, Spain (Kogevinas); National School of Public Health, Athens, Greece (Kogevinas)
1,
Cecily Kelleher
Cecily Kelleher, DMed
1Department of Social Medicine, Faculty of Medicine, University of Crete, Heraklion, Greece (Stratakis, Roumeliotaki, Chatzi); Section of Complex Genetics, Department of Genetics and Cell Biology, NUTRIM School of Nutrition and Translational Research in Metabolism, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Stratakis, Zeegers); Obesity Prevention Program, Harvard Pilgrim Health Care Institute, Department of Population Medicine, Harvard Medical School, Boston, Massachusetts (Oken, Rifas-Shiman); Department of Clinical Epidemiology, Predictive Medicine and Public Health, University of Porto Medical School, Porto, Portugal (Barros); Epidemology Research Unit, Institute of Public Health, University of Porto, Porto, Portugal (Barros); Public Health Division of Gipuzkoa, Basque Government; Health Research Institute, Biodonostia, San Sebastián, Spain (Basterrechea, Oliveira); Centros de Investigación Biomédica en Red Epidemiología y Salud Pública, Spain (Basterrechea, Murcia, Oliveira, Sunyer, Vrijheid, Kogevinas); Centre for Research in Epidemiology and Biostatistics Paris Sorbonne Cité, Institut National de la Santé et de la Recherche Medicale, Early Origin of the Child Development and Health Team, Villejuif, France (Charles, Heude); Université Paris Descartes, Villejuif, France (Charles, Heude); Norwegian Institute of Public Health, Oslo, Norway (Eggesbø, Iszatt); Department of Epidemiology, Lazio Regional Health System, Rome, Italy (Forastiere); Generation R Study Group, Department of Epidemiology, Erasmus University Medical Centre, Rotterdam, Netherlands (Gaillard, Jaddoe); Institute for Risk Assessment Sciences, Utrecht University, Utrecht, Netherlands (Gehring, Porta); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Govarts); Department of Environmental Epidemiology, Nofer Institute of Occupational Medicine, Lodz, Poland (Hanke); School of Public Health, Physiotherapy, and Population Science, University College Dublin, Dublin, Ireland (Kelleher, Viljoen); Department of Epidemiology, CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Mommers, Polańska, Thijs); Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana–Universitat Jaume I, Universitat de València Joint Research Unit of Epidemiology and Environmental Health, Valencia, Spain (Murcia); Cancer Epidemiology Unit, Department of Medical Sciences, University of Turin and Reference Centre for Epidemiology and Cancer Prevention in Piemonte, Turin, Italy (Pizzi, Richiardi); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Schoeters); University of Antwerp, Antwerp, Belgium; University of Southern Denmark, Odense, Denmark (Schoeters); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Sunyer, Vrijheid); Pompeu Fabra University, Barcelona, Spain (Sunyer); Department of Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain (Vrijheid); Department of Public Health, Academic Medical Centre, University of Amsterdam, Amsterdam, Netherlands (Vrijkotte); Centre for Nutrition, Prevention and Health Services, National Institute for Public Health and the Environment, Bilthoven, Netherlands (Wijga); CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Zeegers); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Kogevinas); Institut Hospital del Mar d'Investigacions Mèdiques, Barcelona, Spain (Kogevinas); National School of Public Health, Athens, Greece (Kogevinas)
1,
Monique Mommers
Monique Mommers, PhD
1Department of Social Medicine, Faculty of Medicine, University of Crete, Heraklion, Greece (Stratakis, Roumeliotaki, Chatzi); Section of Complex Genetics, Department of Genetics and Cell Biology, NUTRIM School of Nutrition and Translational Research in Metabolism, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Stratakis, Zeegers); Obesity Prevention Program, Harvard Pilgrim Health Care Institute, Department of Population Medicine, Harvard Medical School, Boston, Massachusetts (Oken, Rifas-Shiman); Department of Clinical Epidemiology, Predictive Medicine and Public Health, University of Porto Medical School, Porto, Portugal (Barros); Epidemology Research Unit, Institute of Public Health, University of Porto, Porto, Portugal (Barros); Public Health Division of Gipuzkoa, Basque Government; Health Research Institute, Biodonostia, San Sebastián, Spain (Basterrechea, Oliveira); Centros de Investigación Biomédica en Red Epidemiología y Salud Pública, Spain (Basterrechea, Murcia, Oliveira, Sunyer, Vrijheid, Kogevinas); Centre for Research in Epidemiology and Biostatistics Paris Sorbonne Cité, Institut National de la Santé et de la Recherche Medicale, Early Origin of the Child Development and Health Team, Villejuif, France (Charles, Heude); Université Paris Descartes, Villejuif, France (Charles, Heude); Norwegian Institute of Public Health, Oslo, Norway (Eggesbø, Iszatt); Department of Epidemiology, Lazio Regional Health System, Rome, Italy (Forastiere); Generation R Study Group, Department of Epidemiology, Erasmus University Medical Centre, Rotterdam, Netherlands (Gaillard, Jaddoe); Institute for Risk Assessment Sciences, Utrecht University, Utrecht, Netherlands (Gehring, Porta); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Govarts); Department of Environmental Epidemiology, Nofer Institute of Occupational Medicine, Lodz, Poland (Hanke); School of Public Health, Physiotherapy, and Population Science, University College Dublin, Dublin, Ireland (Kelleher, Viljoen); Department of Epidemiology, CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Mommers, Polańska, Thijs); Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana–Universitat Jaume I, Universitat de València Joint Research Unit of Epidemiology and Environmental Health, Valencia, Spain (Murcia); Cancer Epidemiology Unit, Department of Medical Sciences, University of Turin and Reference Centre for Epidemiology and Cancer Prevention in Piemonte, Turin, Italy (Pizzi, Richiardi); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Schoeters); University of Antwerp, Antwerp, Belgium; University of Southern Denmark, Odense, Denmark (Schoeters); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Sunyer, Vrijheid); Pompeu Fabra University, Barcelona, Spain (Sunyer); Department of Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain (Vrijheid); Department of Public Health, Academic Medical Centre, University of Amsterdam, Amsterdam, Netherlands (Vrijkotte); Centre for Nutrition, Prevention and Health Services, National Institute for Public Health and the Environment, Bilthoven, Netherlands (Wijga); CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Zeegers); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Kogevinas); Institut Hospital del Mar d'Investigacions Mèdiques, Barcelona, Spain (Kogevinas); National School of Public Health, Athens, Greece (Kogevinas)
1,
Mario Murcia
Mario Murcia, MSc
1Department of Social Medicine, Faculty of Medicine, University of Crete, Heraklion, Greece (Stratakis, Roumeliotaki, Chatzi); Section of Complex Genetics, Department of Genetics and Cell Biology, NUTRIM School of Nutrition and Translational Research in Metabolism, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Stratakis, Zeegers); Obesity Prevention Program, Harvard Pilgrim Health Care Institute, Department of Population Medicine, Harvard Medical School, Boston, Massachusetts (Oken, Rifas-Shiman); Department of Clinical Epidemiology, Predictive Medicine and Public Health, University of Porto Medical School, Porto, Portugal (Barros); Epidemology Research Unit, Institute of Public Health, University of Porto, Porto, Portugal (Barros); Public Health Division of Gipuzkoa, Basque Government; Health Research Institute, Biodonostia, San Sebastián, Spain (Basterrechea, Oliveira); Centros de Investigación Biomédica en Red Epidemiología y Salud Pública, Spain (Basterrechea, Murcia, Oliveira, Sunyer, Vrijheid, Kogevinas); Centre for Research in Epidemiology and Biostatistics Paris Sorbonne Cité, Institut National de la Santé et de la Recherche Medicale, Early Origin of the Child Development and Health Team, Villejuif, France (Charles, Heude); Université Paris Descartes, Villejuif, France (Charles, Heude); Norwegian Institute of Public Health, Oslo, Norway (Eggesbø, Iszatt); Department of Epidemiology, Lazio Regional Health System, Rome, Italy (Forastiere); Generation R Study Group, Department of Epidemiology, Erasmus University Medical Centre, Rotterdam, Netherlands (Gaillard, Jaddoe); Institute for Risk Assessment Sciences, Utrecht University, Utrecht, Netherlands (Gehring, Porta); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Govarts); Department of Environmental Epidemiology, Nofer Institute of Occupational Medicine, Lodz, Poland (Hanke); School of Public Health, Physiotherapy, and Population Science, University College Dublin, Dublin, Ireland (Kelleher, Viljoen); Department of Epidemiology, CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Mommers, Polańska, Thijs); Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana–Universitat Jaume I, Universitat de València Joint Research Unit of Epidemiology and Environmental Health, Valencia, Spain (Murcia); Cancer Epidemiology Unit, Department of Medical Sciences, University of Turin and Reference Centre for Epidemiology and Cancer Prevention in Piemonte, Turin, Italy (Pizzi, Richiardi); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Schoeters); University of Antwerp, Antwerp, Belgium; University of Southern Denmark, Odense, Denmark (Schoeters); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Sunyer, Vrijheid); Pompeu Fabra University, Barcelona, Spain (Sunyer); Department of Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain (Vrijheid); Department of Public Health, Academic Medical Centre, University of Amsterdam, Amsterdam, Netherlands (Vrijkotte); Centre for Nutrition, Prevention and Health Services, National Institute for Public Health and the Environment, Bilthoven, Netherlands (Wijga); CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Zeegers); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Kogevinas); Institut Hospital del Mar d'Investigacions Mèdiques, Barcelona, Spain (Kogevinas); National School of Public Health, Athens, Greece (Kogevinas)
1,
Andreia Oliveira
Andreia Oliveira, PhD
1Department of Social Medicine, Faculty of Medicine, University of Crete, Heraklion, Greece (Stratakis, Roumeliotaki, Chatzi); Section of Complex Genetics, Department of Genetics and Cell Biology, NUTRIM School of Nutrition and Translational Research in Metabolism, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Stratakis, Zeegers); Obesity Prevention Program, Harvard Pilgrim Health Care Institute, Department of Population Medicine, Harvard Medical School, Boston, Massachusetts (Oken, Rifas-Shiman); Department of Clinical Epidemiology, Predictive Medicine and Public Health, University of Porto Medical School, Porto, Portugal (Barros); Epidemology Research Unit, Institute of Public Health, University of Porto, Porto, Portugal (Barros); Public Health Division of Gipuzkoa, Basque Government; Health Research Institute, Biodonostia, San Sebastián, Spain (Basterrechea, Oliveira); Centros de Investigación Biomédica en Red Epidemiología y Salud Pública, Spain (Basterrechea, Murcia, Oliveira, Sunyer, Vrijheid, Kogevinas); Centre for Research in Epidemiology and Biostatistics Paris Sorbonne Cité, Institut National de la Santé et de la Recherche Medicale, Early Origin of the Child Development and Health Team, Villejuif, France (Charles, Heude); Université Paris Descartes, Villejuif, France (Charles, Heude); Norwegian Institute of Public Health, Oslo, Norway (Eggesbø, Iszatt); Department of Epidemiology, Lazio Regional Health System, Rome, Italy (Forastiere); Generation R Study Group, Department of Epidemiology, Erasmus University Medical Centre, Rotterdam, Netherlands (Gaillard, Jaddoe); Institute for Risk Assessment Sciences, Utrecht University, Utrecht, Netherlands (Gehring, Porta); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Govarts); Department of Environmental Epidemiology, Nofer Institute of Occupational Medicine, Lodz, Poland (Hanke); School of Public Health, Physiotherapy, and Population Science, University College Dublin, Dublin, Ireland (Kelleher, Viljoen); Department of Epidemiology, CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Mommers, Polańska, Thijs); Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana–Universitat Jaume I, Universitat de València Joint Research Unit of Epidemiology and Environmental Health, Valencia, Spain (Murcia); Cancer Epidemiology Unit, Department of Medical Sciences, University of Turin and Reference Centre for Epidemiology and Cancer Prevention in Piemonte, Turin, Italy (Pizzi, Richiardi); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Schoeters); University of Antwerp, Antwerp, Belgium; University of Southern Denmark, Odense, Denmark (Schoeters); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Sunyer, Vrijheid); Pompeu Fabra University, Barcelona, Spain (Sunyer); Department of Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain (Vrijheid); Department of Public Health, Academic Medical Centre, University of Amsterdam, Amsterdam, Netherlands (Vrijkotte); Centre for Nutrition, Prevention and Health Services, National Institute for Public Health and the Environment, Bilthoven, Netherlands (Wijga); CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Zeegers); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Kogevinas); Institut Hospital del Mar d'Investigacions Mèdiques, Barcelona, Spain (Kogevinas); National School of Public Health, Athens, Greece (Kogevinas)
1,
Costanza Pizzi
Costanza Pizzi, PhD
1Department of Social Medicine, Faculty of Medicine, University of Crete, Heraklion, Greece (Stratakis, Roumeliotaki, Chatzi); Section of Complex Genetics, Department of Genetics and Cell Biology, NUTRIM School of Nutrition and Translational Research in Metabolism, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Stratakis, Zeegers); Obesity Prevention Program, Harvard Pilgrim Health Care Institute, Department of Population Medicine, Harvard Medical School, Boston, Massachusetts (Oken, Rifas-Shiman); Department of Clinical Epidemiology, Predictive Medicine and Public Health, University of Porto Medical School, Porto, Portugal (Barros); Epidemology Research Unit, Institute of Public Health, University of Porto, Porto, Portugal (Barros); Public Health Division of Gipuzkoa, Basque Government; Health Research Institute, Biodonostia, San Sebastián, Spain (Basterrechea, Oliveira); Centros de Investigación Biomédica en Red Epidemiología y Salud Pública, Spain (Basterrechea, Murcia, Oliveira, Sunyer, Vrijheid, Kogevinas); Centre for Research in Epidemiology and Biostatistics Paris Sorbonne Cité, Institut National de la Santé et de la Recherche Medicale, Early Origin of the Child Development and Health Team, Villejuif, France (Charles, Heude); Université Paris Descartes, Villejuif, France (Charles, Heude); Norwegian Institute of Public Health, Oslo, Norway (Eggesbø, Iszatt); Department of Epidemiology, Lazio Regional Health System, Rome, Italy (Forastiere); Generation R Study Group, Department of Epidemiology, Erasmus University Medical Centre, Rotterdam, Netherlands (Gaillard, Jaddoe); Institute for Risk Assessment Sciences, Utrecht University, Utrecht, Netherlands (Gehring, Porta); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Govarts); Department of Environmental Epidemiology, Nofer Institute of Occupational Medicine, Lodz, Poland (Hanke); School of Public Health, Physiotherapy, and Population Science, University College Dublin, Dublin, Ireland (Kelleher, Viljoen); Department of Epidemiology, CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Mommers, Polańska, Thijs); Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana–Universitat Jaume I, Universitat de València Joint Research Unit of Epidemiology and Environmental Health, Valencia, Spain (Murcia); Cancer Epidemiology Unit, Department of Medical Sciences, University of Turin and Reference Centre for Epidemiology and Cancer Prevention in Piemonte, Turin, Italy (Pizzi, Richiardi); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Schoeters); University of Antwerp, Antwerp, Belgium; University of Southern Denmark, Odense, Denmark (Schoeters); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Sunyer, Vrijheid); Pompeu Fabra University, Barcelona, Spain (Sunyer); Department of Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain (Vrijheid); Department of Public Health, Academic Medical Centre, University of Amsterdam, Amsterdam, Netherlands (Vrijkotte); Centre for Nutrition, Prevention and Health Services, National Institute for Public Health and the Environment, Bilthoven, Netherlands (Wijga); CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Zeegers); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Kogevinas); Institut Hospital del Mar d'Investigacions Mèdiques, Barcelona, Spain (Kogevinas); National School of Public Health, Athens, Greece (Kogevinas)
1,
Kinga Polańska
Kinga Polańska, PhD
1Department of Social Medicine, Faculty of Medicine, University of Crete, Heraklion, Greece (Stratakis, Roumeliotaki, Chatzi); Section of Complex Genetics, Department of Genetics and Cell Biology, NUTRIM School of Nutrition and Translational Research in Metabolism, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Stratakis, Zeegers); Obesity Prevention Program, Harvard Pilgrim Health Care Institute, Department of Population Medicine, Harvard Medical School, Boston, Massachusetts (Oken, Rifas-Shiman); Department of Clinical Epidemiology, Predictive Medicine and Public Health, University of Porto Medical School, Porto, Portugal (Barros); Epidemology Research Unit, Institute of Public Health, University of Porto, Porto, Portugal (Barros); Public Health Division of Gipuzkoa, Basque Government; Health Research Institute, Biodonostia, San Sebastián, Spain (Basterrechea, Oliveira); Centros de Investigación Biomédica en Red Epidemiología y Salud Pública, Spain (Basterrechea, Murcia, Oliveira, Sunyer, Vrijheid, Kogevinas); Centre for Research in Epidemiology and Biostatistics Paris Sorbonne Cité, Institut National de la Santé et de la Recherche Medicale, Early Origin of the Child Development and Health Team, Villejuif, France (Charles, Heude); Université Paris Descartes, Villejuif, France (Charles, Heude); Norwegian Institute of Public Health, Oslo, Norway (Eggesbø, Iszatt); Department of Epidemiology, Lazio Regional Health System, Rome, Italy (Forastiere); Generation R Study Group, Department of Epidemiology, Erasmus University Medical Centre, Rotterdam, Netherlands (Gaillard, Jaddoe); Institute for Risk Assessment Sciences, Utrecht University, Utrecht, Netherlands (Gehring, Porta); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Govarts); Department of Environmental Epidemiology, Nofer Institute of Occupational Medicine, Lodz, Poland (Hanke); School of Public Health, Physiotherapy, and Population Science, University College Dublin, Dublin, Ireland (Kelleher, Viljoen); Department of Epidemiology, CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Mommers, Polańska, Thijs); Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana–Universitat Jaume I, Universitat de València Joint Research Unit of Epidemiology and Environmental Health, Valencia, Spain (Murcia); Cancer Epidemiology Unit, Department of Medical Sciences, University of Turin and Reference Centre for Epidemiology and Cancer Prevention in Piemonte, Turin, Italy (Pizzi, Richiardi); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Schoeters); University of Antwerp, Antwerp, Belgium; University of Southern Denmark, Odense, Denmark (Schoeters); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Sunyer, Vrijheid); Pompeu Fabra University, Barcelona, Spain (Sunyer); Department of Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain (Vrijheid); Department of Public Health, Academic Medical Centre, University of Amsterdam, Amsterdam, Netherlands (Vrijkotte); Centre for Nutrition, Prevention and Health Services, National Institute for Public Health and the Environment, Bilthoven, Netherlands (Wijga); CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Zeegers); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Kogevinas); Institut Hospital del Mar d'Investigacions Mèdiques, Barcelona, Spain (Kogevinas); National School of Public Health, Athens, Greece (Kogevinas)
1,
Daniela Porta
Daniela Porta, MSc
1Department of Social Medicine, Faculty of Medicine, University of Crete, Heraklion, Greece (Stratakis, Roumeliotaki, Chatzi); Section of Complex Genetics, Department of Genetics and Cell Biology, NUTRIM School of Nutrition and Translational Research in Metabolism, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Stratakis, Zeegers); Obesity Prevention Program, Harvard Pilgrim Health Care Institute, Department of Population Medicine, Harvard Medical School, Boston, Massachusetts (Oken, Rifas-Shiman); Department of Clinical Epidemiology, Predictive Medicine and Public Health, University of Porto Medical School, Porto, Portugal (Barros); Epidemology Research Unit, Institute of Public Health, University of Porto, Porto, Portugal (Barros); Public Health Division of Gipuzkoa, Basque Government; Health Research Institute, Biodonostia, San Sebastián, Spain (Basterrechea, Oliveira); Centros de Investigación Biomédica en Red Epidemiología y Salud Pública, Spain (Basterrechea, Murcia, Oliveira, Sunyer, Vrijheid, Kogevinas); Centre for Research in Epidemiology and Biostatistics Paris Sorbonne Cité, Institut National de la Santé et de la Recherche Medicale, Early Origin of the Child Development and Health Team, Villejuif, France (Charles, Heude); Université Paris Descartes, Villejuif, France (Charles, Heude); Norwegian Institute of Public Health, Oslo, Norway (Eggesbø, Iszatt); Department of Epidemiology, Lazio Regional Health System, Rome, Italy (Forastiere); Generation R Study Group, Department of Epidemiology, Erasmus University Medical Centre, Rotterdam, Netherlands (Gaillard, Jaddoe); Institute for Risk Assessment Sciences, Utrecht University, Utrecht, Netherlands (Gehring, Porta); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Govarts); Department of Environmental Epidemiology, Nofer Institute of Occupational Medicine, Lodz, Poland (Hanke); School of Public Health, Physiotherapy, and Population Science, University College Dublin, Dublin, Ireland (Kelleher, Viljoen); Department of Epidemiology, CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Mommers, Polańska, Thijs); Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana–Universitat Jaume I, Universitat de València Joint Research Unit of Epidemiology and Environmental Health, Valencia, Spain (Murcia); Cancer Epidemiology Unit, Department of Medical Sciences, University of Turin and Reference Centre for Epidemiology and Cancer Prevention in Piemonte, Turin, Italy (Pizzi, Richiardi); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Schoeters); University of Antwerp, Antwerp, Belgium; University of Southern Denmark, Odense, Denmark (Schoeters); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Sunyer, Vrijheid); Pompeu Fabra University, Barcelona, Spain (Sunyer); Department of Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain (Vrijheid); Department of Public Health, Academic Medical Centre, University of Amsterdam, Amsterdam, Netherlands (Vrijkotte); Centre for Nutrition, Prevention and Health Services, National Institute for Public Health and the Environment, Bilthoven, Netherlands (Wijga); CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Zeegers); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Kogevinas); Institut Hospital del Mar d'Investigacions Mèdiques, Barcelona, Spain (Kogevinas); National School of Public Health, Athens, Greece (Kogevinas)
1,
Lorenzo Richiardi
Lorenzo Richiardi, PhD
1Department of Social Medicine, Faculty of Medicine, University of Crete, Heraklion, Greece (Stratakis, Roumeliotaki, Chatzi); Section of Complex Genetics, Department of Genetics and Cell Biology, NUTRIM School of Nutrition and Translational Research in Metabolism, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Stratakis, Zeegers); Obesity Prevention Program, Harvard Pilgrim Health Care Institute, Department of Population Medicine, Harvard Medical School, Boston, Massachusetts (Oken, Rifas-Shiman); Department of Clinical Epidemiology, Predictive Medicine and Public Health, University of Porto Medical School, Porto, Portugal (Barros); Epidemology Research Unit, Institute of Public Health, University of Porto, Porto, Portugal (Barros); Public Health Division of Gipuzkoa, Basque Government; Health Research Institute, Biodonostia, San Sebastián, Spain (Basterrechea, Oliveira); Centros de Investigación Biomédica en Red Epidemiología y Salud Pública, Spain (Basterrechea, Murcia, Oliveira, Sunyer, Vrijheid, Kogevinas); Centre for Research in Epidemiology and Biostatistics Paris Sorbonne Cité, Institut National de la Santé et de la Recherche Medicale, Early Origin of the Child Development and Health Team, Villejuif, France (Charles, Heude); Université Paris Descartes, Villejuif, France (Charles, Heude); Norwegian Institute of Public Health, Oslo, Norway (Eggesbø, Iszatt); Department of Epidemiology, Lazio Regional Health System, Rome, Italy (Forastiere); Generation R Study Group, Department of Epidemiology, Erasmus University Medical Centre, Rotterdam, Netherlands (Gaillard, Jaddoe); Institute for Risk Assessment Sciences, Utrecht University, Utrecht, Netherlands (Gehring, Porta); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Govarts); Department of Environmental Epidemiology, Nofer Institute of Occupational Medicine, Lodz, Poland (Hanke); School of Public Health, Physiotherapy, and Population Science, University College Dublin, Dublin, Ireland (Kelleher, Viljoen); Department of Epidemiology, CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Mommers, Polańska, Thijs); Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana–Universitat Jaume I, Universitat de València Joint Research Unit of Epidemiology and Environmental Health, Valencia, Spain (Murcia); Cancer Epidemiology Unit, Department of Medical Sciences, University of Turin and Reference Centre for Epidemiology and Cancer Prevention in Piemonte, Turin, Italy (Pizzi, Richiardi); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Schoeters); University of Antwerp, Antwerp, Belgium; University of Southern Denmark, Odense, Denmark (Schoeters); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Sunyer, Vrijheid); Pompeu Fabra University, Barcelona, Spain (Sunyer); Department of Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain (Vrijheid); Department of Public Health, Academic Medical Centre, University of Amsterdam, Amsterdam, Netherlands (Vrijkotte); Centre for Nutrition, Prevention and Health Services, National Institute for Public Health and the Environment, Bilthoven, Netherlands (Wijga); CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Zeegers); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Kogevinas); Institut Hospital del Mar d'Investigacions Mèdiques, Barcelona, Spain (Kogevinas); National School of Public Health, Athens, Greece (Kogevinas)
1,
Sheryl L Rifas-Shiman
Sheryl L Rifas-Shiman, MPH
1Department of Social Medicine, Faculty of Medicine, University of Crete, Heraklion, Greece (Stratakis, Roumeliotaki, Chatzi); Section of Complex Genetics, Department of Genetics and Cell Biology, NUTRIM School of Nutrition and Translational Research in Metabolism, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Stratakis, Zeegers); Obesity Prevention Program, Harvard Pilgrim Health Care Institute, Department of Population Medicine, Harvard Medical School, Boston, Massachusetts (Oken, Rifas-Shiman); Department of Clinical Epidemiology, Predictive Medicine and Public Health, University of Porto Medical School, Porto, Portugal (Barros); Epidemology Research Unit, Institute of Public Health, University of Porto, Porto, Portugal (Barros); Public Health Division of Gipuzkoa, Basque Government; Health Research Institute, Biodonostia, San Sebastián, Spain (Basterrechea, Oliveira); Centros de Investigación Biomédica en Red Epidemiología y Salud Pública, Spain (Basterrechea, Murcia, Oliveira, Sunyer, Vrijheid, Kogevinas); Centre for Research in Epidemiology and Biostatistics Paris Sorbonne Cité, Institut National de la Santé et de la Recherche Medicale, Early Origin of the Child Development and Health Team, Villejuif, France (Charles, Heude); Université Paris Descartes, Villejuif, France (Charles, Heude); Norwegian Institute of Public Health, Oslo, Norway (Eggesbø, Iszatt); Department of Epidemiology, Lazio Regional Health System, Rome, Italy (Forastiere); Generation R Study Group, Department of Epidemiology, Erasmus University Medical Centre, Rotterdam, Netherlands (Gaillard, Jaddoe); Institute for Risk Assessment Sciences, Utrecht University, Utrecht, Netherlands (Gehring, Porta); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Govarts); Department of Environmental Epidemiology, Nofer Institute of Occupational Medicine, Lodz, Poland (Hanke); School of Public Health, Physiotherapy, and Population Science, University College Dublin, Dublin, Ireland (Kelleher, Viljoen); Department of Epidemiology, CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Mommers, Polańska, Thijs); Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana–Universitat Jaume I, Universitat de València Joint Research Unit of Epidemiology and Environmental Health, Valencia, Spain (Murcia); Cancer Epidemiology Unit, Department of Medical Sciences, University of Turin and Reference Centre for Epidemiology and Cancer Prevention in Piemonte, Turin, Italy (Pizzi, Richiardi); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Schoeters); University of Antwerp, Antwerp, Belgium; University of Southern Denmark, Odense, Denmark (Schoeters); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Sunyer, Vrijheid); Pompeu Fabra University, Barcelona, Spain (Sunyer); Department of Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain (Vrijheid); Department of Public Health, Academic Medical Centre, University of Amsterdam, Amsterdam, Netherlands (Vrijkotte); Centre for Nutrition, Prevention and Health Services, National Institute for Public Health and the Environment, Bilthoven, Netherlands (Wijga); CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Zeegers); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Kogevinas); Institut Hospital del Mar d'Investigacions Mèdiques, Barcelona, Spain (Kogevinas); National School of Public Health, Athens, Greece (Kogevinas)
1,
Greet Schoeters
Greet Schoeters, PhD
1Department of Social Medicine, Faculty of Medicine, University of Crete, Heraklion, Greece (Stratakis, Roumeliotaki, Chatzi); Section of Complex Genetics, Department of Genetics and Cell Biology, NUTRIM School of Nutrition and Translational Research in Metabolism, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Stratakis, Zeegers); Obesity Prevention Program, Harvard Pilgrim Health Care Institute, Department of Population Medicine, Harvard Medical School, Boston, Massachusetts (Oken, Rifas-Shiman); Department of Clinical Epidemiology, Predictive Medicine and Public Health, University of Porto Medical School, Porto, Portugal (Barros); Epidemology Research Unit, Institute of Public Health, University of Porto, Porto, Portugal (Barros); Public Health Division of Gipuzkoa, Basque Government; Health Research Institute, Biodonostia, San Sebastián, Spain (Basterrechea, Oliveira); Centros de Investigación Biomédica en Red Epidemiología y Salud Pública, Spain (Basterrechea, Murcia, Oliveira, Sunyer, Vrijheid, Kogevinas); Centre for Research in Epidemiology and Biostatistics Paris Sorbonne Cité, Institut National de la Santé et de la Recherche Medicale, Early Origin of the Child Development and Health Team, Villejuif, France (Charles, Heude); Université Paris Descartes, Villejuif, France (Charles, Heude); Norwegian Institute of Public Health, Oslo, Norway (Eggesbø, Iszatt); Department of Epidemiology, Lazio Regional Health System, Rome, Italy (Forastiere); Generation R Study Group, Department of Epidemiology, Erasmus University Medical Centre, Rotterdam, Netherlands (Gaillard, Jaddoe); Institute for Risk Assessment Sciences, Utrecht University, Utrecht, Netherlands (Gehring, Porta); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Govarts); Department of Environmental Epidemiology, Nofer Institute of Occupational Medicine, Lodz, Poland (Hanke); School of Public Health, Physiotherapy, and Population Science, University College Dublin, Dublin, Ireland (Kelleher, Viljoen); Department of Epidemiology, CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Mommers, Polańska, Thijs); Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana–Universitat Jaume I, Universitat de València Joint Research Unit of Epidemiology and Environmental Health, Valencia, Spain (Murcia); Cancer Epidemiology Unit, Department of Medical Sciences, University of Turin and Reference Centre for Epidemiology and Cancer Prevention in Piemonte, Turin, Italy (Pizzi, Richiardi); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Schoeters); University of Antwerp, Antwerp, Belgium; University of Southern Denmark, Odense, Denmark (Schoeters); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Sunyer, Vrijheid); Pompeu Fabra University, Barcelona, Spain (Sunyer); Department of Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain (Vrijheid); Department of Public Health, Academic Medical Centre, University of Amsterdam, Amsterdam, Netherlands (Vrijkotte); Centre for Nutrition, Prevention and Health Services, National Institute for Public Health and the Environment, Bilthoven, Netherlands (Wijga); CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Zeegers); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Kogevinas); Institut Hospital del Mar d'Investigacions Mèdiques, Barcelona, Spain (Kogevinas); National School of Public Health, Athens, Greece (Kogevinas)
1,
Jordi Sunyer
Jordi Sunyer, PhD
1Department of Social Medicine, Faculty of Medicine, University of Crete, Heraklion, Greece (Stratakis, Roumeliotaki, Chatzi); Section of Complex Genetics, Department of Genetics and Cell Biology, NUTRIM School of Nutrition and Translational Research in Metabolism, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Stratakis, Zeegers); Obesity Prevention Program, Harvard Pilgrim Health Care Institute, Department of Population Medicine, Harvard Medical School, Boston, Massachusetts (Oken, Rifas-Shiman); Department of Clinical Epidemiology, Predictive Medicine and Public Health, University of Porto Medical School, Porto, Portugal (Barros); Epidemology Research Unit, Institute of Public Health, University of Porto, Porto, Portugal (Barros); Public Health Division of Gipuzkoa, Basque Government; Health Research Institute, Biodonostia, San Sebastián, Spain (Basterrechea, Oliveira); Centros de Investigación Biomédica en Red Epidemiología y Salud Pública, Spain (Basterrechea, Murcia, Oliveira, Sunyer, Vrijheid, Kogevinas); Centre for Research in Epidemiology and Biostatistics Paris Sorbonne Cité, Institut National de la Santé et de la Recherche Medicale, Early Origin of the Child Development and Health Team, Villejuif, France (Charles, Heude); Université Paris Descartes, Villejuif, France (Charles, Heude); Norwegian Institute of Public Health, Oslo, Norway (Eggesbø, Iszatt); Department of Epidemiology, Lazio Regional Health System, Rome, Italy (Forastiere); Generation R Study Group, Department of Epidemiology, Erasmus University Medical Centre, Rotterdam, Netherlands (Gaillard, Jaddoe); Institute for Risk Assessment Sciences, Utrecht University, Utrecht, Netherlands (Gehring, Porta); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Govarts); Department of Environmental Epidemiology, Nofer Institute of Occupational Medicine, Lodz, Poland (Hanke); School of Public Health, Physiotherapy, and Population Science, University College Dublin, Dublin, Ireland (Kelleher, Viljoen); Department of Epidemiology, CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Mommers, Polańska, Thijs); Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana–Universitat Jaume I, Universitat de València Joint Research Unit of Epidemiology and Environmental Health, Valencia, Spain (Murcia); Cancer Epidemiology Unit, Department of Medical Sciences, University of Turin and Reference Centre for Epidemiology and Cancer Prevention in Piemonte, Turin, Italy (Pizzi, Richiardi); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Schoeters); University of Antwerp, Antwerp, Belgium; University of Southern Denmark, Odense, Denmark (Schoeters); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Sunyer, Vrijheid); Pompeu Fabra University, Barcelona, Spain (Sunyer); Department of Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain (Vrijheid); Department of Public Health, Academic Medical Centre, University of Amsterdam, Amsterdam, Netherlands (Vrijkotte); Centre for Nutrition, Prevention and Health Services, National Institute for Public Health and the Environment, Bilthoven, Netherlands (Wijga); CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Zeegers); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Kogevinas); Institut Hospital del Mar d'Investigacions Mèdiques, Barcelona, Spain (Kogevinas); National School of Public Health, Athens, Greece (Kogevinas)
1,
Carel Thijs
Carel Thijs, PhD
1Department of Social Medicine, Faculty of Medicine, University of Crete, Heraklion, Greece (Stratakis, Roumeliotaki, Chatzi); Section of Complex Genetics, Department of Genetics and Cell Biology, NUTRIM School of Nutrition and Translational Research in Metabolism, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Stratakis, Zeegers); Obesity Prevention Program, Harvard Pilgrim Health Care Institute, Department of Population Medicine, Harvard Medical School, Boston, Massachusetts (Oken, Rifas-Shiman); Department of Clinical Epidemiology, Predictive Medicine and Public Health, University of Porto Medical School, Porto, Portugal (Barros); Epidemology Research Unit, Institute of Public Health, University of Porto, Porto, Portugal (Barros); Public Health Division of Gipuzkoa, Basque Government; Health Research Institute, Biodonostia, San Sebastián, Spain (Basterrechea, Oliveira); Centros de Investigación Biomédica en Red Epidemiología y Salud Pública, Spain (Basterrechea, Murcia, Oliveira, Sunyer, Vrijheid, Kogevinas); Centre for Research in Epidemiology and Biostatistics Paris Sorbonne Cité, Institut National de la Santé et de la Recherche Medicale, Early Origin of the Child Development and Health Team, Villejuif, France (Charles, Heude); Université Paris Descartes, Villejuif, France (Charles, Heude); Norwegian Institute of Public Health, Oslo, Norway (Eggesbø, Iszatt); Department of Epidemiology, Lazio Regional Health System, Rome, Italy (Forastiere); Generation R Study Group, Department of Epidemiology, Erasmus University Medical Centre, Rotterdam, Netherlands (Gaillard, Jaddoe); Institute for Risk Assessment Sciences, Utrecht University, Utrecht, Netherlands (Gehring, Porta); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Govarts); Department of Environmental Epidemiology, Nofer Institute of Occupational Medicine, Lodz, Poland (Hanke); School of Public Health, Physiotherapy, and Population Science, University College Dublin, Dublin, Ireland (Kelleher, Viljoen); Department of Epidemiology, CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Mommers, Polańska, Thijs); Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana–Universitat Jaume I, Universitat de València Joint Research Unit of Epidemiology and Environmental Health, Valencia, Spain (Murcia); Cancer Epidemiology Unit, Department of Medical Sciences, University of Turin and Reference Centre for Epidemiology and Cancer Prevention in Piemonte, Turin, Italy (Pizzi, Richiardi); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Schoeters); University of Antwerp, Antwerp, Belgium; University of Southern Denmark, Odense, Denmark (Schoeters); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Sunyer, Vrijheid); Pompeu Fabra University, Barcelona, Spain (Sunyer); Department of Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain (Vrijheid); Department of Public Health, Academic Medical Centre, University of Amsterdam, Amsterdam, Netherlands (Vrijkotte); Centre for Nutrition, Prevention and Health Services, National Institute for Public Health and the Environment, Bilthoven, Netherlands (Wijga); CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Zeegers); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Kogevinas); Institut Hospital del Mar d'Investigacions Mèdiques, Barcelona, Spain (Kogevinas); National School of Public Health, Athens, Greece (Kogevinas)
1,
Karien Viljoen
Karien Viljoen, PhD
1Department of Social Medicine, Faculty of Medicine, University of Crete, Heraklion, Greece (Stratakis, Roumeliotaki, Chatzi); Section of Complex Genetics, Department of Genetics and Cell Biology, NUTRIM School of Nutrition and Translational Research in Metabolism, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Stratakis, Zeegers); Obesity Prevention Program, Harvard Pilgrim Health Care Institute, Department of Population Medicine, Harvard Medical School, Boston, Massachusetts (Oken, Rifas-Shiman); Department of Clinical Epidemiology, Predictive Medicine and Public Health, University of Porto Medical School, Porto, Portugal (Barros); Epidemology Research Unit, Institute of Public Health, University of Porto, Porto, Portugal (Barros); Public Health Division of Gipuzkoa, Basque Government; Health Research Institute, Biodonostia, San Sebastián, Spain (Basterrechea, Oliveira); Centros de Investigación Biomédica en Red Epidemiología y Salud Pública, Spain (Basterrechea, Murcia, Oliveira, Sunyer, Vrijheid, Kogevinas); Centre for Research in Epidemiology and Biostatistics Paris Sorbonne Cité, Institut National de la Santé et de la Recherche Medicale, Early Origin of the Child Development and Health Team, Villejuif, France (Charles, Heude); Université Paris Descartes, Villejuif, France (Charles, Heude); Norwegian Institute of Public Health, Oslo, Norway (Eggesbø, Iszatt); Department of Epidemiology, Lazio Regional Health System, Rome, Italy (Forastiere); Generation R Study Group, Department of Epidemiology, Erasmus University Medical Centre, Rotterdam, Netherlands (Gaillard, Jaddoe); Institute for Risk Assessment Sciences, Utrecht University, Utrecht, Netherlands (Gehring, Porta); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Govarts); Department of Environmental Epidemiology, Nofer Institute of Occupational Medicine, Lodz, Poland (Hanke); School of Public Health, Physiotherapy, and Population Science, University College Dublin, Dublin, Ireland (Kelleher, Viljoen); Department of Epidemiology, CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Mommers, Polańska, Thijs); Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana–Universitat Jaume I, Universitat de València Joint Research Unit of Epidemiology and Environmental Health, Valencia, Spain (Murcia); Cancer Epidemiology Unit, Department of Medical Sciences, University of Turin and Reference Centre for Epidemiology and Cancer Prevention in Piemonte, Turin, Italy (Pizzi, Richiardi); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Schoeters); University of Antwerp, Antwerp, Belgium; University of Southern Denmark, Odense, Denmark (Schoeters); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Sunyer, Vrijheid); Pompeu Fabra University, Barcelona, Spain (Sunyer); Department of Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain (Vrijheid); Department of Public Health, Academic Medical Centre, University of Amsterdam, Amsterdam, Netherlands (Vrijkotte); Centre for Nutrition, Prevention and Health Services, National Institute for Public Health and the Environment, Bilthoven, Netherlands (Wijga); CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Zeegers); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Kogevinas); Institut Hospital del Mar d'Investigacions Mèdiques, Barcelona, Spain (Kogevinas); National School of Public Health, Athens, Greece (Kogevinas)
1,
Martine Vrijheid
Martine Vrijheid, PhD
1Department of Social Medicine, Faculty of Medicine, University of Crete, Heraklion, Greece (Stratakis, Roumeliotaki, Chatzi); Section of Complex Genetics, Department of Genetics and Cell Biology, NUTRIM School of Nutrition and Translational Research in Metabolism, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Stratakis, Zeegers); Obesity Prevention Program, Harvard Pilgrim Health Care Institute, Department of Population Medicine, Harvard Medical School, Boston, Massachusetts (Oken, Rifas-Shiman); Department of Clinical Epidemiology, Predictive Medicine and Public Health, University of Porto Medical School, Porto, Portugal (Barros); Epidemology Research Unit, Institute of Public Health, University of Porto, Porto, Portugal (Barros); Public Health Division of Gipuzkoa, Basque Government; Health Research Institute, Biodonostia, San Sebastián, Spain (Basterrechea, Oliveira); Centros de Investigación Biomédica en Red Epidemiología y Salud Pública, Spain (Basterrechea, Murcia, Oliveira, Sunyer, Vrijheid, Kogevinas); Centre for Research in Epidemiology and Biostatistics Paris Sorbonne Cité, Institut National de la Santé et de la Recherche Medicale, Early Origin of the Child Development and Health Team, Villejuif, France (Charles, Heude); Université Paris Descartes, Villejuif, France (Charles, Heude); Norwegian Institute of Public Health, Oslo, Norway (Eggesbø, Iszatt); Department of Epidemiology, Lazio Regional Health System, Rome, Italy (Forastiere); Generation R Study Group, Department of Epidemiology, Erasmus University Medical Centre, Rotterdam, Netherlands (Gaillard, Jaddoe); Institute for Risk Assessment Sciences, Utrecht University, Utrecht, Netherlands (Gehring, Porta); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Govarts); Department of Environmental Epidemiology, Nofer Institute of Occupational Medicine, Lodz, Poland (Hanke); School of Public Health, Physiotherapy, and Population Science, University College Dublin, Dublin, Ireland (Kelleher, Viljoen); Department of Epidemiology, CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Mommers, Polańska, Thijs); Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana–Universitat Jaume I, Universitat de València Joint Research Unit of Epidemiology and Environmental Health, Valencia, Spain (Murcia); Cancer Epidemiology Unit, Department of Medical Sciences, University of Turin and Reference Centre for Epidemiology and Cancer Prevention in Piemonte, Turin, Italy (Pizzi, Richiardi); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Schoeters); University of Antwerp, Antwerp, Belgium; University of Southern Denmark, Odense, Denmark (Schoeters); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Sunyer, Vrijheid); Pompeu Fabra University, Barcelona, Spain (Sunyer); Department of Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain (Vrijheid); Department of Public Health, Academic Medical Centre, University of Amsterdam, Amsterdam, Netherlands (Vrijkotte); Centre for Nutrition, Prevention and Health Services, National Institute for Public Health and the Environment, Bilthoven, Netherlands (Wijga); CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Zeegers); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Kogevinas); Institut Hospital del Mar d'Investigacions Mèdiques, Barcelona, Spain (Kogevinas); National School of Public Health, Athens, Greece (Kogevinas)
1,
Tanja G M Vrijkotte
Tanja G M Vrijkotte, PhD
1Department of Social Medicine, Faculty of Medicine, University of Crete, Heraklion, Greece (Stratakis, Roumeliotaki, Chatzi); Section of Complex Genetics, Department of Genetics and Cell Biology, NUTRIM School of Nutrition and Translational Research in Metabolism, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Stratakis, Zeegers); Obesity Prevention Program, Harvard Pilgrim Health Care Institute, Department of Population Medicine, Harvard Medical School, Boston, Massachusetts (Oken, Rifas-Shiman); Department of Clinical Epidemiology, Predictive Medicine and Public Health, University of Porto Medical School, Porto, Portugal (Barros); Epidemology Research Unit, Institute of Public Health, University of Porto, Porto, Portugal (Barros); Public Health Division of Gipuzkoa, Basque Government; Health Research Institute, Biodonostia, San Sebastián, Spain (Basterrechea, Oliveira); Centros de Investigación Biomédica en Red Epidemiología y Salud Pública, Spain (Basterrechea, Murcia, Oliveira, Sunyer, Vrijheid, Kogevinas); Centre for Research in Epidemiology and Biostatistics Paris Sorbonne Cité, Institut National de la Santé et de la Recherche Medicale, Early Origin of the Child Development and Health Team, Villejuif, France (Charles, Heude); Université Paris Descartes, Villejuif, France (Charles, Heude); Norwegian Institute of Public Health, Oslo, Norway (Eggesbø, Iszatt); Department of Epidemiology, Lazio Regional Health System, Rome, Italy (Forastiere); Generation R Study Group, Department of Epidemiology, Erasmus University Medical Centre, Rotterdam, Netherlands (Gaillard, Jaddoe); Institute for Risk Assessment Sciences, Utrecht University, Utrecht, Netherlands (Gehring, Porta); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Govarts); Department of Environmental Epidemiology, Nofer Institute of Occupational Medicine, Lodz, Poland (Hanke); School of Public Health, Physiotherapy, and Population Science, University College Dublin, Dublin, Ireland (Kelleher, Viljoen); Department of Epidemiology, CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Mommers, Polańska, Thijs); Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana–Universitat Jaume I, Universitat de València Joint Research Unit of Epidemiology and Environmental Health, Valencia, Spain (Murcia); Cancer Epidemiology Unit, Department of Medical Sciences, University of Turin and Reference Centre for Epidemiology and Cancer Prevention in Piemonte, Turin, Italy (Pizzi, Richiardi); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Schoeters); University of Antwerp, Antwerp, Belgium; University of Southern Denmark, Odense, Denmark (Schoeters); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Sunyer, Vrijheid); Pompeu Fabra University, Barcelona, Spain (Sunyer); Department of Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain (Vrijheid); Department of Public Health, Academic Medical Centre, University of Amsterdam, Amsterdam, Netherlands (Vrijkotte); Centre for Nutrition, Prevention and Health Services, National Institute for Public Health and the Environment, Bilthoven, Netherlands (Wijga); CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Zeegers); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Kogevinas); Institut Hospital del Mar d'Investigacions Mèdiques, Barcelona, Spain (Kogevinas); National School of Public Health, Athens, Greece (Kogevinas)
1,
Alet H Wijga
Alet H Wijga, PhD
1Department of Social Medicine, Faculty of Medicine, University of Crete, Heraklion, Greece (Stratakis, Roumeliotaki, Chatzi); Section of Complex Genetics, Department of Genetics and Cell Biology, NUTRIM School of Nutrition and Translational Research in Metabolism, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Stratakis, Zeegers); Obesity Prevention Program, Harvard Pilgrim Health Care Institute, Department of Population Medicine, Harvard Medical School, Boston, Massachusetts (Oken, Rifas-Shiman); Department of Clinical Epidemiology, Predictive Medicine and Public Health, University of Porto Medical School, Porto, Portugal (Barros); Epidemology Research Unit, Institute of Public Health, University of Porto, Porto, Portugal (Barros); Public Health Division of Gipuzkoa, Basque Government; Health Research Institute, Biodonostia, San Sebastián, Spain (Basterrechea, Oliveira); Centros de Investigación Biomédica en Red Epidemiología y Salud Pública, Spain (Basterrechea, Murcia, Oliveira, Sunyer, Vrijheid, Kogevinas); Centre for Research in Epidemiology and Biostatistics Paris Sorbonne Cité, Institut National de la Santé et de la Recherche Medicale, Early Origin of the Child Development and Health Team, Villejuif, France (Charles, Heude); Université Paris Descartes, Villejuif, France (Charles, Heude); Norwegian Institute of Public Health, Oslo, Norway (Eggesbø, Iszatt); Department of Epidemiology, Lazio Regional Health System, Rome, Italy (Forastiere); Generation R Study Group, Department of Epidemiology, Erasmus University Medical Centre, Rotterdam, Netherlands (Gaillard, Jaddoe); Institute for Risk Assessment Sciences, Utrecht University, Utrecht, Netherlands (Gehring, Porta); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Govarts); Department of Environmental Epidemiology, Nofer Institute of Occupational Medicine, Lodz, Poland (Hanke); School of Public Health, Physiotherapy, and Population Science, University College Dublin, Dublin, Ireland (Kelleher, Viljoen); Department of Epidemiology, CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Mommers, Polańska, Thijs); Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana–Universitat Jaume I, Universitat de València Joint Research Unit of Epidemiology and Environmental Health, Valencia, Spain (Murcia); Cancer Epidemiology Unit, Department of Medical Sciences, University of Turin and Reference Centre for Epidemiology and Cancer Prevention in Piemonte, Turin, Italy (Pizzi, Richiardi); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Schoeters); University of Antwerp, Antwerp, Belgium; University of Southern Denmark, Odense, Denmark (Schoeters); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Sunyer, Vrijheid); Pompeu Fabra University, Barcelona, Spain (Sunyer); Department of Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain (Vrijheid); Department of Public Health, Academic Medical Centre, University of Amsterdam, Amsterdam, Netherlands (Vrijkotte); Centre for Nutrition, Prevention and Health Services, National Institute for Public Health and the Environment, Bilthoven, Netherlands (Wijga); CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Zeegers); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Kogevinas); Institut Hospital del Mar d'Investigacions Mèdiques, Barcelona, Spain (Kogevinas); National School of Public Health, Athens, Greece (Kogevinas)
1,
Maurice P Zeegers
Maurice P Zeegers, PhD
1Department of Social Medicine, Faculty of Medicine, University of Crete, Heraklion, Greece (Stratakis, Roumeliotaki, Chatzi); Section of Complex Genetics, Department of Genetics and Cell Biology, NUTRIM School of Nutrition and Translational Research in Metabolism, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Stratakis, Zeegers); Obesity Prevention Program, Harvard Pilgrim Health Care Institute, Department of Population Medicine, Harvard Medical School, Boston, Massachusetts (Oken, Rifas-Shiman); Department of Clinical Epidemiology, Predictive Medicine and Public Health, University of Porto Medical School, Porto, Portugal (Barros); Epidemology Research Unit, Institute of Public Health, University of Porto, Porto, Portugal (Barros); Public Health Division of Gipuzkoa, Basque Government; Health Research Institute, Biodonostia, San Sebastián, Spain (Basterrechea, Oliveira); Centros de Investigación Biomédica en Red Epidemiología y Salud Pública, Spain (Basterrechea, Murcia, Oliveira, Sunyer, Vrijheid, Kogevinas); Centre for Research in Epidemiology and Biostatistics Paris Sorbonne Cité, Institut National de la Santé et de la Recherche Medicale, Early Origin of the Child Development and Health Team, Villejuif, France (Charles, Heude); Université Paris Descartes, Villejuif, France (Charles, Heude); Norwegian Institute of Public Health, Oslo, Norway (Eggesbø, Iszatt); Department of Epidemiology, Lazio Regional Health System, Rome, Italy (Forastiere); Generation R Study Group, Department of Epidemiology, Erasmus University Medical Centre, Rotterdam, Netherlands (Gaillard, Jaddoe); Institute for Risk Assessment Sciences, Utrecht University, Utrecht, Netherlands (Gehring, Porta); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Govarts); Department of Environmental Epidemiology, Nofer Institute of Occupational Medicine, Lodz, Poland (Hanke); School of Public Health, Physiotherapy, and Population Science, University College Dublin, Dublin, Ireland (Kelleher, Viljoen); Department of Epidemiology, CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Mommers, Polańska, Thijs); Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana–Universitat Jaume I, Universitat de València Joint Research Unit of Epidemiology and Environmental Health, Valencia, Spain (Murcia); Cancer Epidemiology Unit, Department of Medical Sciences, University of Turin and Reference Centre for Epidemiology and Cancer Prevention in Piemonte, Turin, Italy (Pizzi, Richiardi); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Schoeters); University of Antwerp, Antwerp, Belgium; University of Southern Denmark, Odense, Denmark (Schoeters); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Sunyer, Vrijheid); Pompeu Fabra University, Barcelona, Spain (Sunyer); Department of Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain (Vrijheid); Department of Public Health, Academic Medical Centre, University of Amsterdam, Amsterdam, Netherlands (Vrijkotte); Centre for Nutrition, Prevention and Health Services, National Institute for Public Health and the Environment, Bilthoven, Netherlands (Wijga); CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Zeegers); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Kogevinas); Institut Hospital del Mar d'Investigacions Mèdiques, Barcelona, Spain (Kogevinas); National School of Public Health, Athens, Greece (Kogevinas)
1,
Manolis Kogevinas
Manolis Kogevinas, PhD
1Department of Social Medicine, Faculty of Medicine, University of Crete, Heraklion, Greece (Stratakis, Roumeliotaki, Chatzi); Section of Complex Genetics, Department of Genetics and Cell Biology, NUTRIM School of Nutrition and Translational Research in Metabolism, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Stratakis, Zeegers); Obesity Prevention Program, Harvard Pilgrim Health Care Institute, Department of Population Medicine, Harvard Medical School, Boston, Massachusetts (Oken, Rifas-Shiman); Department of Clinical Epidemiology, Predictive Medicine and Public Health, University of Porto Medical School, Porto, Portugal (Barros); Epidemology Research Unit, Institute of Public Health, University of Porto, Porto, Portugal (Barros); Public Health Division of Gipuzkoa, Basque Government; Health Research Institute, Biodonostia, San Sebastián, Spain (Basterrechea, Oliveira); Centros de Investigación Biomédica en Red Epidemiología y Salud Pública, Spain (Basterrechea, Murcia, Oliveira, Sunyer, Vrijheid, Kogevinas); Centre for Research in Epidemiology and Biostatistics Paris Sorbonne Cité, Institut National de la Santé et de la Recherche Medicale, Early Origin of the Child Development and Health Team, Villejuif, France (Charles, Heude); Université Paris Descartes, Villejuif, France (Charles, Heude); Norwegian Institute of Public Health, Oslo, Norway (Eggesbø, Iszatt); Department of Epidemiology, Lazio Regional Health System, Rome, Italy (Forastiere); Generation R Study Group, Department of Epidemiology, Erasmus University Medical Centre, Rotterdam, Netherlands (Gaillard, Jaddoe); Institute for Risk Assessment Sciences, Utrecht University, Utrecht, Netherlands (Gehring, Porta); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Govarts); Department of Environmental Epidemiology, Nofer Institute of Occupational Medicine, Lodz, Poland (Hanke); School of Public Health, Physiotherapy, and Population Science, University College Dublin, Dublin, Ireland (Kelleher, Viljoen); Department of Epidemiology, CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Mommers, Polańska, Thijs); Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana–Universitat Jaume I, Universitat de València Joint Research Unit of Epidemiology and Environmental Health, Valencia, Spain (Murcia); Cancer Epidemiology Unit, Department of Medical Sciences, University of Turin and Reference Centre for Epidemiology and Cancer Prevention in Piemonte, Turin, Italy (Pizzi, Richiardi); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Schoeters); University of Antwerp, Antwerp, Belgium; University of Southern Denmark, Odense, Denmark (Schoeters); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Sunyer, Vrijheid); Pompeu Fabra University, Barcelona, Spain (Sunyer); Department of Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain (Vrijheid); Department of Public Health, Academic Medical Centre, University of Amsterdam, Amsterdam, Netherlands (Vrijkotte); Centre for Nutrition, Prevention and Health Services, National Institute for Public Health and the Environment, Bilthoven, Netherlands (Wijga); CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Zeegers); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Kogevinas); Institut Hospital del Mar d'Investigacions Mèdiques, Barcelona, Spain (Kogevinas); National School of Public Health, Athens, Greece (Kogevinas)
1,
Leda Chatzi
Leda Chatzi, PhD
1Department of Social Medicine, Faculty of Medicine, University of Crete, Heraklion, Greece (Stratakis, Roumeliotaki, Chatzi); Section of Complex Genetics, Department of Genetics and Cell Biology, NUTRIM School of Nutrition and Translational Research in Metabolism, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Stratakis, Zeegers); Obesity Prevention Program, Harvard Pilgrim Health Care Institute, Department of Population Medicine, Harvard Medical School, Boston, Massachusetts (Oken, Rifas-Shiman); Department of Clinical Epidemiology, Predictive Medicine and Public Health, University of Porto Medical School, Porto, Portugal (Barros); Epidemology Research Unit, Institute of Public Health, University of Porto, Porto, Portugal (Barros); Public Health Division of Gipuzkoa, Basque Government; Health Research Institute, Biodonostia, San Sebastián, Spain (Basterrechea, Oliveira); Centros de Investigación Biomédica en Red Epidemiología y Salud Pública, Spain (Basterrechea, Murcia, Oliveira, Sunyer, Vrijheid, Kogevinas); Centre for Research in Epidemiology and Biostatistics Paris Sorbonne Cité, Institut National de la Santé et de la Recherche Medicale, Early Origin of the Child Development and Health Team, Villejuif, France (Charles, Heude); Université Paris Descartes, Villejuif, France (Charles, Heude); Norwegian Institute of Public Health, Oslo, Norway (Eggesbø, Iszatt); Department of Epidemiology, Lazio Regional Health System, Rome, Italy (Forastiere); Generation R Study Group, Department of Epidemiology, Erasmus University Medical Centre, Rotterdam, Netherlands (Gaillard, Jaddoe); Institute for Risk Assessment Sciences, Utrecht University, Utrecht, Netherlands (Gehring, Porta); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Govarts); Department of Environmental Epidemiology, Nofer Institute of Occupational Medicine, Lodz, Poland (Hanke); School of Public Health, Physiotherapy, and Population Science, University College Dublin, Dublin, Ireland (Kelleher, Viljoen); Department of Epidemiology, CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Mommers, Polańska, Thijs); Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana–Universitat Jaume I, Universitat de València Joint Research Unit of Epidemiology and Environmental Health, Valencia, Spain (Murcia); Cancer Epidemiology Unit, Department of Medical Sciences, University of Turin and Reference Centre for Epidemiology and Cancer Prevention in Piemonte, Turin, Italy (Pizzi, Richiardi); Environmental Risk and Health, Flemish Institute for Technological Research, Mol, Belgium (Schoeters); University of Antwerp, Antwerp, Belgium; University of Southern Denmark, Odense, Denmark (Schoeters); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Sunyer, Vrijheid); Pompeu Fabra University, Barcelona, Spain (Sunyer); Department of Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain (Vrijheid); Department of Public Health, Academic Medical Centre, University of Amsterdam, Amsterdam, Netherlands (Vrijkotte); Centre for Nutrition, Prevention and Health Services, National Institute for Public Health and the Environment, Bilthoven, Netherlands (Wijga); CAPHRI School for Public Health and Primary Care, Faculty of Health, Medicine and Life Sciences, Maastricht University Medical Centre+, Maastricht, Netherlands (Zeegers); Centre for Research in Environmental Epidemiology, Barcelona, Spain (Kogevinas); Institut Hospital del Mar d'Investigacions Mèdiques, Barcelona, Spain (Kogevinas); National School of Public Health, Athens, Greece (Kogevinas)
1