Abstract
OBJECTIVES: To explore the pharmacokinetic (PK) profile and safety of ezogabine (EZG)/retigabine (RTG) as adjunctive therapy for uncontrolled partial-onset seizures (POS) in adolescents.
METHODS: In this multiple-dose study (NCT01494584), adolescents with POS received EZG/RTG immediate-release tablets three times daily (TID) as adjunctive therapy to 1 to 3 concurrent antiepileptic drugs. The study comprised a screening phase, and a 5- to 8-week treatment phase starting with 100 mg TID up-titrated once weekly by ≤50 mg TID to a maximum dosage of 300 mg TID. There were 8 venous blood samples and 2 finger-prick blood samples collected for PK analysis during 8-hour time periods at the target dosages of 100, 200, and 300 mg TID.
RESULTS: This study was terminated prematurely on US Food and Drug Administration advice due to pigmentation/discoloration findings in long-term, open-label extension studies in adults. Five participants (ages 13–16 years) had enrolled in the study. For the EZG/RTG 100-, 200-, and 300-mg doses, the area under the concentration-time curve during the dosage intervals was 1680, 2559, and 3784 ng/hr/mL; maximum plasma concentrations were 370, 536, and 751 ng/mL, and minimum plasma concentrations were 105, 200, and 287 ng/mL, respectively. Venous and finger-prick concentrations of EZG/RTG were similar. No significant adverse events were observed during treatment (133–213 days).
CONCLUSIONS: EZG/RTG PK appeared linear across the dosage range of 100 to 300 mg TID in adolescents with POS, and were consistent with adult observations. The small sample size and short study duration preclude conclusions regarding the safety and efficacy of EZG/RTG.
INDEX TERMS: adolescent, anticonvulsant, antiepileptic drug, epilepsy, ezogabine, pharmacokinetics, retigabine, seizures
INTRODUCTION
More than 10 million children worldwide are estimated to have active epilepsy.1 Childhood epilepsy has a broad clinical spectrum and a wide range of causes. Children and adolescents can present with seizures of a similar type to those seen in adults, but epilepsy in childhood differs from that in adults by the occurrence of seizures in a structurally and functionally maturing brain. In childhood epilepsy, generalized seizures tend to predominate in the first few years of life, with partial seizures becoming more common after the age of 5 years. Seizure types not observed in adults may also be seen, and seizures may occur as part of a specific unique epilepsy syndrome, such as Lennox-Gastaut Syndrome (LGS), a severe form of intractable generalized epilepsy that accounts for 3% to 5% of childhood epilepsies.1 New antiepileptic drugs (AEDs) could improve the management of epilepsy in children and young people.
Ezogabine (EZG; US adopted name)/retigabine (RTG; international non-proprietary name; GlaxoSmithKline, Research Triangle Park, NC) is an AED that facilitates the opening of neuronal potassium channels. EZG/RTG is approved in the United States, the European Union, and other countries for the adjunctive treatment of partial-onset seizures (POS) in adults 18 years and older who have responded inadequately to other AEDs and for whom the benefits outweigh the risks of retinal abnormalities and potential decline in visual acuity.2–4 The safety and efficacy of EZG/RTG (600–1200 mg/day) as adjunctive therapy in adults with POS were demonstrated in 3 pivotal studies.5–7
The PK profile of EZG/RTG in individuals younger than 18 years has not previously been determined and, as part of the pediatric/adolescent development program for EZG/RTG, 2 studies of EZG/RTG in adolescent individuals with POS or LGS were planned. The PK study (NCT01494584) was intended to characterize the steady-state PK profile of EZG/RTG and to assess the safety, tolerability, and efficacy of repeat doses of adjunctive treatment with EZG/RTG; systemic exposure of the N-acetyl metabolite of EZG/RTG (NAMR) after dosage with EZG/RTG to steady state was also assessed. A long-term, open-label extension (OLE) study (NCT01668654) was planned to evaluate the long-term safety, tolerability, and efficacy of EZG/RTG as adjunctive treatment in individuals completing the PK study.
Both studies were terminated prematurely in April 2013 when the US Food and Drug Administration (FDA) placed a clinical hold on the pediatric/adolescent program because of reports of retinal pigmentation and discoloration of nails, lips, skin, and/or mucosa in adults taking EZG/RTG following long-term dosage in OLE studies.8,9 These adverse events (AEs) are thought to be related to long-term use of EZG/RTG10,11; however, because their exact cause and specific risk factors (including patient age) remain unclear, the pediatric/adolescent program was put on hold while GlaxoSmithKline continues to review the safety of EZG/RTG. All adolescents receiving EZG/RTG in the PK and OLE studies discontinued treatment, and enrollment was stopped. All participants received comprehensive eye examinations, and examination of nails, lips, skin, and oral mucosa for pigmentation or discoloration. At the time of termination of the studies, no individuals with LGS had been recruited, and insufficient data were obtained for efficacy and health outcome end points to be evaluated. The results of the PK study, and the safety data that were obtained before study termination, are reported here.
MATERIALS AND METHODS
Study Design
This study was an open-label, multiple-dose, phase 2 study of EZG/RTG to evaluate the PK, safety, and tolerability in adolescents ages 12 to <18 years, with POS or LGS, conducted at 4 sites in the United States. EZG/RTG tablets were administered orally three times daily (TID) as adjunctive therapy to at least 1, but no more than 3, concurrent AEDs. Every effort was made while designing the study to accommodate the needs of the adolescent study population and parents or caregivers (such as flexibility of PK sampling day), to minimize the impact on schooling and family life while maintaining adherence to study procedures and appropriate safety monitoring.
After a screening period of up to a maximum of 14 days, EZG/RTG was up-titrated to the target maximum dosage (TMD) of 300 mg TID during a 5- to 8-week treatment period using the titration regimen indicated in Table 1. The initial dosage of EZG/RTG was the same as the adult starting dosage of 100 mg TID,3 and was up-titrated by a maximum of 50 mg TID no more than once weekly. The TMD was the highest tolerated dosage or the dosage that achieved systemic exposure similar to that observed at the dosage of 300 mg TID in adults, whichever was reached first (the maximum recommended dosage of EZG/RTG in adults is 400 mg TID).
Table 1.
Ezogabine/Retigabine (EZG/RTG) Dosage Regimen
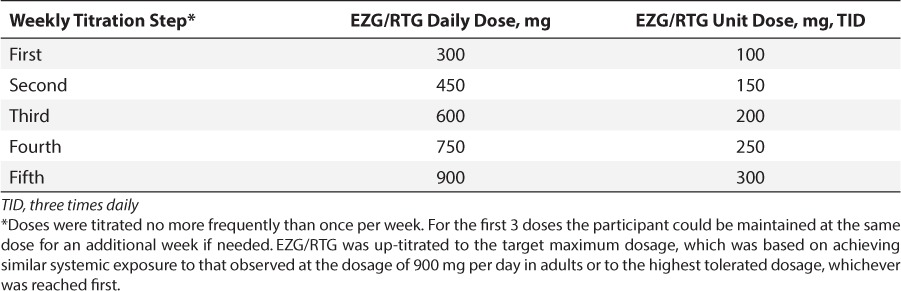
For the first 3 titration steps, the participant could be maintained at the same dosage for an additional week if needed (e.g., because of inability to attend the clinic, waiting for PK results before up-titrating the dosage, safety concerns, or repetition of safety laboratory results). For the first 4 participants, the PK data collected at the end of the first week were evaluated on a fast-turnaround basis. If the EZG/RTG dosage during the first week was well tolerated, participants continued to the second dosage level. Pharmacokinetics and tolerability data were reviewed by the clinical study team and investigator prior to subsequent titration steps, and dosages could be adjusted on an individual basis. Participants/caregivers completed a diary to capture seizures, compliance with the EZG/RTG regimen, and concurrent AEDs. Site staff contacted the participant and/or parent by telephone at least every other day to verify treatment compliance and to inquire about tolerability.
On day 7 of each week of treatment, participants returned to the site where medical examinations were conducted. These included assessments of any AEs related to bladder function or central nervous system symptoms, electrocardiogram (ECG), vital signs, Pediatric Lower Urinary Tract Symptoms scale, Columbia Suicide Severity Rating Scale, and postvoid residual bladder ultrasound (titration step 3 only). It was preferable that these examinations were conducted on day 7 of each week, although a visit window of −3 days or +1 day was allowed. On withdrawal from the study, or at the end of the study, all participants received comprehensive eye examinations, and examination of nails, lips, skin, and oral mucosa for pigmentation or discoloration.
Participants who, in the opinion of the clinical investigator, might benefit from the continuation of EZG/RTG treatment were offered the option to continue treatment beyond 12 months in an OLE study. Otherwise, participants were tapered off EZG/RTG by reduction of their daily dosage by approximately one third each week. On PK study completion or withdrawal, all participants who did not enter the OLE study attended a follow-up visit 7 to 11 days after the last dose of EZG/RTG.
Ethics
The study protocols, amendments, consent forms, and other relevant information were reviewed and approved by the relevant Institutional Review Board/institutional ethics committee. The PK and OLE studies were conducted in accordance with the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Good Clinical Practice, and all applicable local regulatory requirements, and the guiding principles of the Declaration of Helsinki. Written informed consent was obtained from each participant's parent or guardian prior to participation in both studies, and accompanying assent was obtained from the participant.
Participants
The target sample size was 8 participants in order to obtain evaluable PK data from approximately 6 participants.
PK Study
Participants (male or female) were eligible to be included in the study if they were ages 12 to <18 years at the time informed consent was obtained and had received a diagnosis of uncontrolled POS (with or without secondarily generalized seizures) or LGS. Participants were required to be taking from 1 to 3 AED(s) at a stable dosage for at least 1 month before enrollment. Participants with seizures for at least 6 months that had been incompletely controlled by adequate doses of at least 2 approved AEDs were also included in the study. Female participants were eligible to participate if they were of non-childbearing potential or, if of child-bearing potential, they were abstinent or using an accepted method of contraception. Male participants were eligible to participate if they were abstinent from intercourse, or if they used an accepted method of contraception.
Key exclusion criteria were: diagnosis of epilepsy secondary to progressive cerebral disease, tumor, or any progressive neurodegenerative disease; status epilepticus in the 6 months before or during screening; treatment with felbamate or treatment with vigabatrin within 6 months before screening; and evidence of visual field constriction following discontinuation of treatment with vigabatrin. Other exclusion criteria included: following a ketogenic diet; any clinically significant medical condition that could potentially affect participant safety or the study outcome; clinically significant disorders of the urinary tract or urinalysis results; active suicidal ideation in the past 6 months; a history of suicide attempt in the past 2 years, or >1 lifetime suicide attempt; clinically significant arrhythmias, or an average of 3 QT intervals corrected with Fridericia's formula (QTcF) ≥450 milliseconds; a history of alcohol consumption within 6 months of the study; and use of prescription or non-prescription drugs (excluding the prescribed concurrent AEDs, acute use of benzodiazepines per protocol, and daily multivitamins).
Study End Points
The primary end point was the steady-state EZG/RTG PK, including area under the concentration-time curve over the dosage interval (AUC0-τ), maximum (Cmax) and minimum (Cmin) observed plasma concentration, apparent clearance following oral dose (CL/F), and apparent volume of distribution. Secondary end points were NAMR (AUC0-τ) and Cmin, and EZG/RTG time to Cmax (tmax) and half-life at steady state (if data allowed).
Study Assessments
PK Assessments
Whole-blood samples for PK analysis of EZG/RTG and NAMR were collected on 3 occasions during the up-titration phase at target dosages of 100, 200, and 300 mg TID (i.e., at weeks 1, 3, and 5 of the titration period). Blood samples were collected via an indwelling cannula or by direct venipuncture during an 8-hour dosage interval at predose and approximately 0.5, 1.0, 1.5, 2, 4, 6, and 8 hours postdose. Two microsamples (finger prick) were also obtained at ≤30 minutes predose (trough) and at 2 hours postdose (approximate tmax) at each target dosage level (100, 200, and 300 mg TID) to assess the validity of an assay for EZG/RTG and NAMR using a blood-spot technique. Samples were intended to be collected on day 7 of dosage at each target PK dosage level. However, the study included flexibility to allow collection of PK samples from day 4 to day 8 after commencement of each target PK dose level.
Participants were asked, wherever possible, to follow an 8-hourly (TID) EZG/RTG dosage regimen on the day before the PK assessments and to have an interval of approximately 8 to 10 hours between the previous dose and the in-clinic PK sampling-day dose. Participants were advised that this could be achieved by attending the clinic using either of the two following scenarios: 1) PK collection following first daily dose. On the day preceding the PK sampling day, participants would take their third daily dose of EZG/RTG as close to midnight as possible (no earlier than 10 pm and no later than 2 am). The predose blood sample would be taken at the clinic, and participants would take their first daily dose of EZG/RTG within approximately 30 minutes of the predose blood draw and as close to 8 to 10 hours after the previous dose as possible. Or 2) PK collection following second daily dose. Participants would take their first dose of EZG/RTG as usual in the morning of the PK sampling day. A predose blood sample would be taken at the clinic approximately 30 minutes before the second dose of the day. The second dose was taken as close as possible to 8 to 10 hours after the morning dose.
Analytic Methods for Assay of PK Samples
A series of plasma samples (10-μL aliquots) were analyzed for EZG/RTG and NAMR by use of a validated bioanalytic method based on liquid-liquid extraction, followed by ultra-high performance liquid chromatography–tandem mass spectrometry analysis. The lower limit of quantification for both analytes was 5 ng/mL when a 10-μL aliquot of plasma was used with a higher limit of quantification of 2500 ng/mL.
Quality control samples, prepared at 3 different analyte concentrations and stored with study samples, were analyzed with each batch of samples against separately prepared calibration standards. For the analysis to be acceptable, no more than one third of the total quality control results and no more than one half of the results from each concentration level were to deviate from the nominal concentration by more than 15%. The applicable analytic runs met all pre-defined run acceptance criteria.
PK and Statistical Analyses
The EZG/RTG and NAMR concentration–time data were analyzed by non-compartmental methods with WinNonlin version 6.3 (Pharsight, St. Louis, MO). Calculations were based on the actual sampling times recorded in the study. The following EZG/RTG and NAMR PK parameter calculations were planned: Cmax, tmax, AUC(0-τ), and trough concentration (Cτ). In addition, EZG/RTG CL/F was determined as Dose/AUC(0-τ), and apparent volume of distribution was to be determined as MRT*CL/F, where MRT is the mean residence time, which was calculated as AUMC(0-τ)/AUC(0-τ), where AUMC(0-τ) is the area under the first moment curve determined as the area under the concentration * time versus time curve. The apparent terminal elimination half-life (t1/2) for EZG/RTG was planned to be obtained as the ratio of ln2/λz, where λz is the terminal phase rate constant estimated by linear regression analysis of the log-transformed concentration–time data.
Safety and Tolerability Assessments
Safety and tolerability were assessed from the start of study treatment until the follow-up visit (7–11 days after the last dose of EZG/RTG). Adverse events were recorded, and parameters including vital signs, ECG, postvoid residual bladder ultrasound and urine volume, sexual maturation, and clinical laboratory variables, were monitored. Additional assessments of the eyes, nails, lips, skin, and oral mucosa were performed following the protocol amendment at study termination.
RESULTS
Study Population and Participant Withdrawal
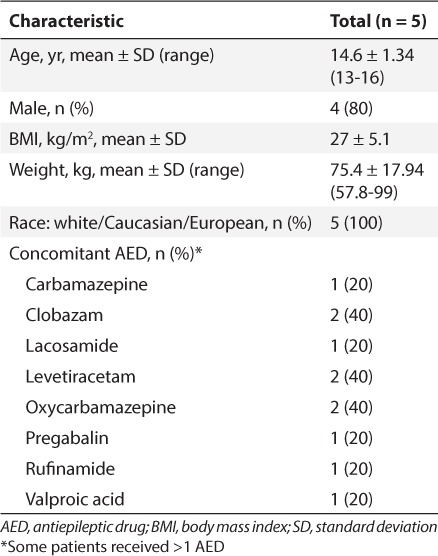
A total of 5 participants (mean age range, 13–16 years) were enrolled in the study. Mean weight of participants was 75.38 kg (range, 57.8–99.0 kg). Baseline demographics and AEDs at study entry are described in Table 2. No enrolled participants had a diagnosis of LGS. Four participants completed the study (35–37 days' treatment with EZG/RTG); the fifth participant was withdrawn from treatment (after 22 days' treatment with EZG/RTG) because of the clinical hold.
Table 2.
Baseline Participant Demographics and Characteristics
Pharmacokinetics
At the time of study termination, PK data were available from the 5 participants enrolled. For 3 participants, PK data were available at all 3 target EZG/RTG dosages (100, 200, and 300 mg TID). However, for one of these participants at the first week's visit, the dosage was up-titrated in error to 150 mg before the PK assessment. The PK data for this participant, therefore, represent the first dosage of 150 mg instead of the intended 100 mg TID at steady state. For the remaining 2 participants, 1 participant had PK data at 100 mg TID, and 1 participant had PK data at 100 mg and 200 mg TID (Table 3). The PK profile of EZG/RTG was approximately linear across the target dosage range of 100 to 300 mg TID (Table 3 and Figure 1). Based on geometric mean values, the ratio of AUC(0-τ) for NAMR to EZG/RTG was approximately 1.33, indicating that systemic exposure to NAMR was approximately 33% higher than exposure to EZG/RTG (Table 3). The t1/2 of EZG/RTG could not be accurately determined for most of the PK profiles; therefore, summary statistics of t1/2 and apparent volume of distribution are not presented.
Table 3.
Pharmacokinetic Parameters (Geometric Mean [CV%]) of Ezogabine/Retigabine (EZG/RTG) and N-Acetyl Metabolite of EZG/RTG (NAMR) Following Repeat Dose Administration of EZG/RTG (100, 200, and 300 mg Three Times Daily [TID]) to Adolescent Participants
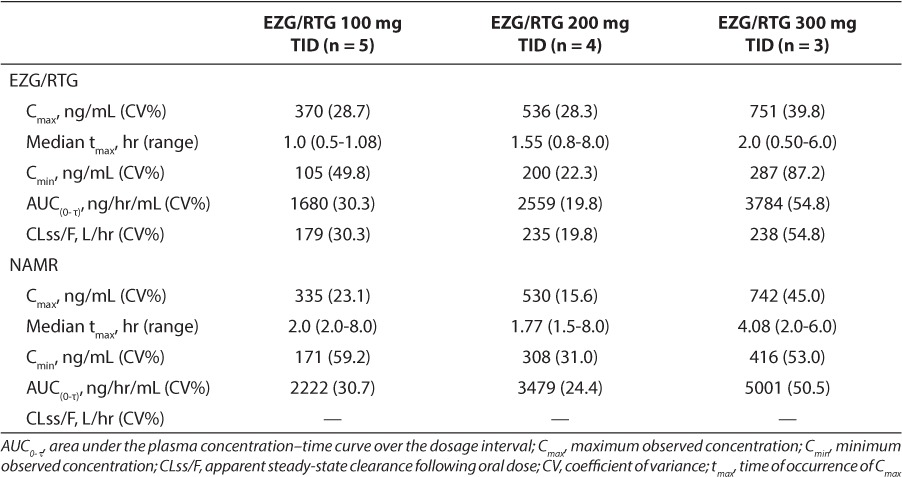
Figure 1.
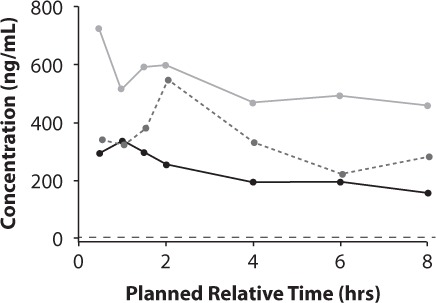
Mean plasma ezogabine/retigabine (EZG/RTG) concentration–time plots by EZG/RTG daily dosage.
 300 mg (100 mg TID);
300 mg (100 mg TID);
 600 mg (200 mg TID);
600 mg (200 mg TID);
 900 mg (300 mg TID)
900 mg (300 mg TID)
The EZG/RTG concentrations obtained from venous blood and finger prick were similar for both sampling methods (Figure 2).
Figure 2.
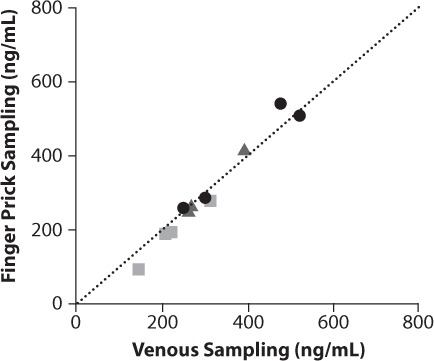
Comparison of ezogabine/retigabine (EZG/RTG) concentrations in blood samples collected by standard EZG/RTG venous sampling and finger-prick sampling.
TID, three times daily.
 EZG/RTG 300 mg (100 mg TID) (n=4);
EZG/RTG 300 mg (100 mg TID) (n=4);
 EZG/RTG 600 mg (200 mg TID) (n=3); • EZG/RTG 900 mg (300 mg TID) (n=4);
EZG/RTG 600 mg (200 mg TID) (n=3); • EZG/RTG 900 mg (300 mg TID) (n=4);
 Line of unity representing no difference between the values for EZG/RTG concentration obtained by venous and finger-prick sampling.
Line of unity representing no difference between the values for EZG/RTG concentration obtained by venous and finger-prick sampling.
Safety and Tolerability
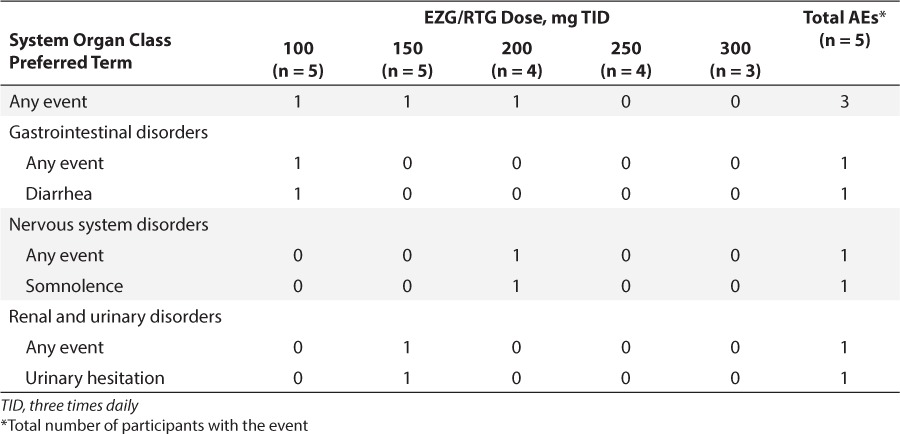
Of the 5 participants, 3 experienced AEs (Table 4). One participant had diarrhea, insomnia, and skin irritation pretrial, and diarrhea during the first treatment period (300 mg daily dose [100 mg TID]). The same participant also had diarrhea, insomnia, and skin irritation during the follow-up period, when they resolved on the same day as onset. During the second treatment period (450 mg daily dose), 1 participant had mild urinary hesitation, which occurred intermittently during a 6-day period and resolved spontaneously. Another participant had a single episode of somnolence during the third treatment period (600 mg daily dose). No abnormalities in vital signs or ECG findings were reported as AEs. Two participants had ECGs that were abnormal but were not considered to be clinically significant by the investigator. No participants showed any signs or symptoms of suicidality.
Table 4.
Number of Participants with Adverse Events (AEs) Following Administration of Ezogabine/Retigabine (EZG/RTG): Safety Population
One AE (urinary hesitation) occurring in 1 participant was considered by the investigator to be related to EZG/RTG. At screening, 1 participant had a residual urine volume of 38 mL and noted that the bladder “always” felt empty after voiding. Residual urine volume increased to 58 mL on day 7 of the third titration step (EZG/RTG 200 mg TID) in this participant, who noted on day 7 of the fourth titration step (EZG/RTG 250 mg TID) that the bladder “never” felt empty. A second participant had residual urine volumes of 1.3 mL and 43 mL at screening and on day 7 of the third titration step (EZG/RTG 200 mg TID), respectively, and noted the bladder “always” felt empty after voiding throughout the study. One participant had measurable residual urine in the bladder at screening (4 mL) but no residual urine on day 7 of the third titration step (EZG/RTG 200 mg TID). No participant had pigmentation of the retina or other ocular tissues or discoloration of nails, lips, skin, or mucosa.
DISCUSSION
This study of EZG/RTG in an adolescent population was the first study planned to evaluate the PK, safety, and efficacy of EZG/RTG in pediatric patients. The program was intended to study adolescents ages 12 to <18 years, followed by children ages 2 to <12 years, and then infants and toddlers ages 28 days to <24 months. In this PK study, the PK profile of EZG/RTG at therapeutic exposures in adolescent participants appeared linear across the daily dosage range of 100 to 300 mg TID.
In adults, the absolute bioavailability of EZG/RTG following oral administration is ~60%.4 EZG/RTG is rapidly absorbed, reaching maximum concentrations 1 to 2 hours post dosage. It is eliminated with a median half-life of 6 to 8 hours.4 The PK of EZG/RTG is essentially linear across the adult therapeutic dosage range of 200 to 400 mg TID.12
Metabolism of EZG/RTG is via N-acetylation and N-glucuronidation, with the formation of NAMR, and N-glucuronidated metabolites of EZG/RTG and NAMR.4 The systemic exposure and elimination half-life of NAMR are similar to EZG/RTG. NAMR is not considered to contribute to the efficacy of EZG/RTG because it has one third to one fifth of the activity of EZG/RTG in rodent models of electroshock protection, and even lower activity than EZG/RTG (1/30th) in rodent kindling models of partial seizures (data on file; Valeant Pharmaceuticals, Bridgewater, NJ). The N-glucuronide metabolites of EZG/RTG and NAMR are the predominant circulating metabolites, accounting for ~90% of circulating drug-related material.4 EZG/RTG and its metabolites are eliminated mainly renally, with ~36% of the administered dose excreted as unchanged drug and ~18% as NAMR.4
It was expected that the PK profile of EZG/RTG in adolescents would be similar to that observed in adults because the clearance mechanisms of EZG/RTG are fully matured in the adolescent population. Therefore, the starting dosage of 100 mg TID was based on the same starting dosage used in the adult population. However, because EZG/RTG was the first potassium channel opener to be studied in an adolescent population, the TMD was limited to 300 mg TID as a precautionary measure (rather than the recommended maximum adult dosage of 400 mg TID). None of the concomitant AEDs are known to affect the PK of EZG/RTG.13
The PK profile of EZG/RTG in the adolescent participants in this study was consistent with that observed previously in healthy adult participants receiving EZG/RTG at dosage levels of 200 mg TID and 300 mg TID following the same up-titration schedule (50 mg TID every week; Figure 3).9 The age (range, 13–16 years), weight, and body mass index of the participant appeared to have no impact on AUC(0-τ) of EZG/RTG.
Figure 3.
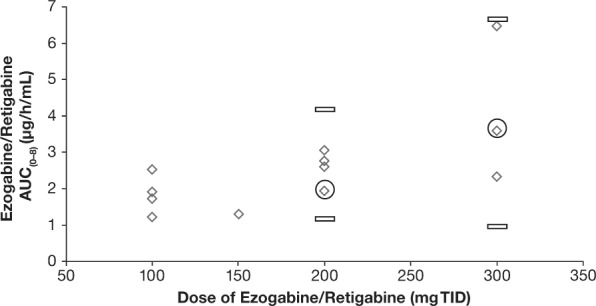
Ezogabine/retigabine (EZG/RTG) area under the concentration-time curve from time zero (predose) to 8 hours postdose (AUC(0-8)) values in adolescent participants compared with adult participants9 by EZG/RTG daily dosage 100 mg, 200 mg, and 300 mg three times daily (TID).
⋄ Adolescent AUC(0–8); ○ Median Adult; ▭ Minimum/Maximum
In these adolescent participants, the ratio of systemic exposure to NAMR relative to EZG/RTG based on AUC(0–τ) was higher in adolescent participants than that observed in the adult population (1.33 versus 0.8, respectively).10 However, given the small number of adolescent participants in this study (n = 5), no firm conclusions about the magnitude of the NAMR to EZG/RTG ratio can be drawn. Nevertheless, because NAMR has substantially less activity than EZG/RTG in in vitro and in vivo pharmacology models, the apparently slightly higher exposure to NAMR in adolescent participants compared with adult participants is unlikely to be of clinical relevance.
The use of EZG/RTG as an adjunctive therapy to other AEDs in adolescent participants was not associated with any safety and tolerability findings of significant clinical concern across the dosage range studied. The early termination of this study following the placement of a clinical hold by the FDA resulted in insufficient data and too few participants for an accurate assessment of safety; however, safety findings in the adolescent participants in this short study were similar to those in adults.10
The PK and safety findings of this study suggest that at adult dosage recommendations, EZG/RTG could be clinically effective for the management of drug-resistant POS in adolescents aged 12 to <18 years.
ACKNOWLEDGMENTS
These studies were supported by GlaxoSmithKline (GlaxoSmithKline study numbers RTG113284 and RTG113388). Editorial support in the form of writing, drafting tables, and collating author comments was provided by Linda Brown, BSc (Hons) (Caudex, Oxford, United Kingdom), and funded by GlaxoSmithKline. These data have not been previously presented or published publicly except as required by regulation (i.e., summary data have been posted per the FDA Amendments Act).
Glossary
Abbreviations
- AE
adverse event
- AED
antiepileptic drug
- AUC0-τ
area under the plasma concentration-time curve over the dosage interval
- AUMC(0-τ)
area under the first moment curve determined as the area under the concentration * time versus time curve
- CL/F
clearance following oral dose
- Cmax
maximum observed concentration
- Cmin
minimum observed concentration
- CLss/F
apparent steady-state clearance following oral dose
- CV
coefficient of variance
- ECG
electrocardiogram
- EZG
ezogabine
- FDA
Food and Drug Administration
- LGS
Lennox-Gastaut Syndrome
- MRT
mean residence time
- NAMR
N-acetyl metabolite of EZG/RTG
- OLE
open-label extension
- PK
pharmacokinetic
- POS
partial-onset seizure
- RTG
retigabine
- tmax
time of occurrence of Cmax
- t1/2
half-life
- TID
three times daily
- TMD
target maximum dose
Footnotes
Disclosure All authors have had full access to the study data and take responsibility for the integrity of the data and the accuracy of the data analysis. D.J.T. is an employee and shareholder of GlaxoSmithKline. M.B. is an employee and shareholder of GlaxoSmithKline. S.M.A. is an employee and shareholder of GlaxoSmithKline. J.W.W. receives research funding from GlaxoSmithKline.
REFERENCES
- 1. Guerrini R. Epilepsy in children. Lancet. 2006; 367( 9509): 499– 524. [DOI] [PubMed] [Google Scholar]
- 2. Blackburn-Munro G, Dalby-Brown W, Mirza NR. et al. Retigabine: chemical synthesis to clinical application. CNS Drug Rev. 2005; 11( 1): 1– 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. GlaxoSmithKline. . POTIGA (ezogabine) prescribing information. Research Triangle Park, NC: GlaxoSmithKline; 2015. Available from: https://www.gsksource.com/pharma/content/gsk/source/us/en/brands/potiga.html. Accessed January 5, 2016. [Google Scholar]
- 4. GlaxoSmithKline. . Trobalt (retigabine) summary of product characteristics. Uxbridge, United Kingdom: GlaxoSmithKline; 2015. Available from: http://www.medicines.org.uk/EMC/medicine/24527/SPC/Trobalt/. Accessed January 5, 2016. [Google Scholar]
- 5. Brodie MJ, Lerche H, Gil-Nagel A. et al. Efficacy and safety of adjunctive ezogabine (retigabine) in refractory partial epilepsy. Neurology. 2010; 75( 20): 1817– 1824. [DOI] [PubMed] [Google Scholar]
- 6. French JA, Abou-Khalil BW, Leroy RF. et al. Randomized, double-blind, placebo-controlled trial of ezogabine (retigabine) in partial epilepsy. Neurology. 2011; 76( 18): 1555– 1563. [DOI] [PubMed] [Google Scholar]
- 7. Porter RJ, Partiot A, Sachdeo R, Nohria V, Alves WM. Randomized, multicenter, dose-ranging trial of retigabine for partial-onset seizures. Neurology. 2007; 68: 1197– 1204. [DOI] [PubMed] [Google Scholar]
- 8. US Food and Drug Administration. . FDA Drug Safety Communication: anti-seizure drug Potiga (ezogabine) linked to retinal abnormalities and blue skin discoloration. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm349538.htm. Accessed January 5, 2016.
- 9. Prescott JS, Evans CA. Pigmentary abnormalities (discoloration) associated with ezogabine/retigabine treatment: nonclinical aspects. Poster 2.324. 68th Annual Meeting of the American Epilepsy Society (AES), Seattle, WA, December 5–9, 2014. [Google Scholar]
- 10. Garin Shkolnik T, Feuerman H, Didkovsky E. et al. Blue-gray mucocutaneous discoloration: a new adverse effect of ezogabine. JAMA Dermatol. 2014; 150( 9): 984– 989. [DOI] [PubMed] [Google Scholar]
- 11. Beacher NG, Brodie MJ, Goodall C. A case report: retigabine induced oral mucosal dyspigmentation of the hard palate. BMC Oral Health. 2015; 15( 1): 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tompson DJ, Crean CS. Clinical pharmacokinetics of retigabine/ezogabine. Curr Clin Pharmacol. 2013; 8( 4): 319– 331. [DOI] [PubMed] [Google Scholar]
- 13. Tompson DJ, Crean CS. The interaction potential of retigabine (ezogabine) with other antiepileptic drugs. Curr Clin Pharmacol. 2014; 9( 2): 148– 156. [DOI] [PubMed] [Google Scholar]


