Abstract
Background
Aggressive periodontitis is associated with the presence of Aggregatibacter actinomycetemcomitans, a leukotoxin (Ltx)-producing periodontal pathogen. Ltx has the ability to lyse white blood cells including neutrophils.
Objectives
This study was aimed at investigating the interactions between neutrophils and Ltx with regard to the chemotactic properties of Ltx and the release of neutrophil extracellular traps (NETs).
Methods
Neutrophils from healthy blood donors were isolated and incubated for 30 min and 3 h with increasing concentrations of Ltx (1, 10, and 100 ng/mL) as well as with A. actinomycetemcomitans strains (NCTC 9710 and HK 1651) producing different levels of Ltx. Formation of NETs and cell lysis were assessed by microscopy, fluorescence-based assays, and measurement of released lactate dehydrogenase. Neutrophil migration in response to different Ltx gradients was monitored by real-time video microscopy, and image analysis was performed using ImageJ software.
Results
Although Ltx (10 and 100 ng/mL) and the leukotoxic A. actinomycetemcomitans strain HK 1651 lysed some neutrophils, other cells were still capable of performing NETosis in a concentration-dependent manner. Low doses of Ltx and the weakly leukotoxic strain NCTC 9710 did not lead to neutrophil lysis, but did induce some NETosis. Furthermore, all three concentrations of Ltx enhanced random neutrophil movement; however, low directional accuracy was observed compared with the positive control (fMLP).
Conclusions
The results indicate that Ltx acts both as a neutrophil activator and also causes cell death. In addition, Ltx directly induces NETosis in neutrophils prior to cell lysis. In future studies, the underlying pathways involved in Ltx-meditated neutrophil activation and NETosis need to be investigated further.
Keywords: leukotoxin, Aggregatibacter actinomycetemcomitans, neutrophils, NETs, migration, chemotaxis, aggressive periodontitis
Aggressive periodontitis (AgP) is a severe, rapidly progressing form of periodontitis, which frequently causes alveolar bone and tooth loss in early adulthood (1). Aggregatibacter actinomycetemcomitans, a Gram-negative facultative anaerobic rod, is known to play a pivotal role in the development of this disease (2). Deep periodontal pockets provide a suitable environment for strictly anaerobic periodontal bacteria; however, A. actinomycetemcomitans is a facultative anaerobic organism which produces a white blood cell lysing agent called ‘leukotoxin’ (Ltx), which in addition to forming pores in leukocytes also activates neutrophil degranulation and a pro-inflammatory response in macrophages (3, 4). A. actinomycetemcomitans exhibits two leukotoxic phenotypes: a minimally leukotoxic (non-JP2 genotype) and a highly leukotoxic (JP2 genotype) (5, 6). However, it has been shown that a subgroup of serotype b of the non-JP2 genotype also is highly leukotoxic (7). DNA sequence analysis of the Ltx promoter regions from these two clones revealed that the minimally leukotoxic genotype harbours a full-length promoter region, while the highly leukotoxic JP2 genotype is characterised by missing a 530 bp sequence of the Ltx promoter region (8). A higher prevalence of JP2 carriers with clinically evident AgP has been found in some countries, particularly in North- and West-Africa (9).
A. actinomycetemcomitans actively transports Ltx from the cell, and Ltx has been detected in outer membrane-like vesicles (10) as well as attached to their cell surfaces (11). The toxin also features amphipathic helices at the N-terminus which interact with host cell membranes (12). In 1997, Lally et al. (13) identified leukocyte function antigen-1 (LFA-1) as the cellular receptor for Ltx, which is expressed on neutrophilic polymorphonuclear leukocytes (neutrophils). Neutrophils, the most abundant white blood cells, present in the oral cavity are first-line defenders and their number in oral tissues increases during inflammation (14). They reach the periodontal tissues by migrating towards the highest concentration of a chemical compound, for example, bacteria-derived N-formyl-met-leu-phe (fMLP), a process known as chemotaxis. Neutrophil dysfunction has been associated with chronic as well as aggressive forms of periodontitis (15, 16); however, the underlying mechanisms remain elusive. We hypothesise that local neutrophil-mediated periodontal tissue damage may be induced by A. actinomycetemcomitans Ltx.
A recently discovered innate defence strategy of neutrophils is the release of DNA to the extracellular environment, where the web-like DNA threads trap and kill microorganisms by means of DNA-bound antimicrobial proteins and peptides (17). These neutrophil extracellular traps (NETs) are also known to arise in periodontal tissues and purulent pockets, which are often found in AgP (18–20). NETs represent a host defence mechanism, but may also cause host tissue injury, as NET-bound proteins and enzymes have the ability to damage tissues and to further enhance inflammation (21). This study was aimed at investigating the potential of Ltx to activate neutrophils, induce migration, and elicit NET formation in addition to triggering neutrophil lysis.
Methods
Recruitment of volunteers and experimental setup
Healthy blood donors were recruited from the Blood Donation Center of the University Hospital Bonn as well as from staff of the Birmingham Dental School and Hospital. All study participants provided written informed consent as approved by the ethics committee of the University Hospital of Bonn and Birmingham (approval numbers 336/13 and 14/SW/1148). Bacterial cultures, cell isolation, and NET microscopy as well as lactate dehydrogenase (LDH) assays were performed in Bonn. Ltx extraction was conducted in the Department of Odontology at the Umeå University, and the chemotaxis assays and fluorescence-based NET quantification were performed at the Birmingham Dental School and Hospital.
Bacteria
The strains of A. actinomycetemcomitans employed in this study were JP2 genotype serotype b strain HK 1651 and low-leukotoxicity serotype c strain NCTC 9710 (non-JP2 genotype). The bacteria were maintained on tryptic soy agar and grown in tryptic soy broth (Becton Dickinson, Heidelberg, Germany) at 37°C in a 5% CO2 atmosphere. The leukotoxicity of the highly and minimally leukotoxic strains HK 1651 and NCTC 9710 was determined as described elsewhere (22). LDH release into the cell culture supernatants from phorbol-12-myristate-13-acetate (PMA)-differentiated THP-1 cells was measured in response to bacteria, with moieties of infection (MOIs) ranging from 0 to 300.
Ltx extraction
Purified Ltx was obtained as described elsewhere (23). In brief, HK 1651 was grown in sterile-filtered peptone yeast extract glucose broth (3) at 37°C in a 5% CO2 atmosphere. The cells were harvested in the early stationary phase of growth by centrifugation (10,000× g for 10 min). The cell pellet was resuspended in 20 mM phosphate buffer and incubated at 4°C for 1 h under rocking to extract outer membrane proteins other than Ltx. The cells were pelleted by centrifugation (10,000× g for 20 min), resuspended in phosphate buffer with 0.3 M NaCl, and incubated for 1 h under rocking at 4°C to extract the Ltx. The suspension was ultra-centrifuged (100,000× g for 60 min) and the Ltx-containing supernatant separated by gel filtration. The Ltx-fraction (300 µg/mL) was aliquoted and stored at −80°C.
Visualisation and quantification of NETs
NET microscopy and fluorescence-based NETosis assays were conducted as described elsewhere (24, 25). In brief, neutrophils were isolated by Ficoll/Histopaque gradient centrifugation. Neutrophils were of high purity (90–95%) and viability (>95%) as confirmed by H&E and trypan blue staining. The cells were seeded onto 12-well adhesive slides (5×103 per spot) for microscopy (Marienfeld, Lauda-Königshofen, Germany) or into 96-well plates for NET quantification assays (105 per well) and allowed to attach for 30 min in serum-free RPMI. Next, bacteria (MOI 1, 10, and 100) or Ltx (1, 10, and 100 ng/mL) as well as a negative (RPMI) and a positive (100 ng/mL PMA) controls were added to the neutrophils, and the samples were incubated for 30 min or 3 h for microscopy and for 3 h for NET quantification assays, respectively. After the incubation period, the microscopy samples were fixed and stained with propidium iodide and assessed under a fluorescence microscope (Olympus IX 81). For NET quantification, micrococcal nuclease (MNase, Thermo Fisher, Rugby, UK) was pipetted into each well and, after an incubation time of 10 min, Sytox Green nucleic acid fluorescent stain (Thermo Fisher, Rugby, UK) was added. Measurements of fluorescence intensity (arbitrary fluorescent units, AFU) were carried out in triplicate using a fluorescence plate reader (Berthold Twinkle LB 970, Harpenden, UK). All experiments were carried out in serum-free RPMI at 37°C in a 5% CO2 atmosphere, as serum nucleases are known to degrade NETs (26).
Measurement of LDH release
The assays were performed using 96-well plates, into which 107 neutrophils were seeded per well. Cells were allowed to settle for 30 min, and the bacterial strains were added in MOIs 1, 10, and 100. After an incubation time of 30 min, the plates were centrifuged at 2,000× g for 5 min. Supernatants were collected and directly assessed by measurement of LDH release into the supernatants using a commercially available kit (LDH-Cytotoxicity Assay Kit II, Abcam, Cambridge, UK). The spectrophotometric reading measurements (OD450) were calculated in percent change compared with the negative controls (unstimulated neutrophils). Samples containing bacteria only were employed as additional negative controls.
Chemotaxis assay
In order to assess the ability of Ltx to recruit neutrophils, a chemotaxis assay was performed under aerobic conditions using the Insall chamber as described elsewhere (27, 28). Briefly, isolated neutrophils were added to BSA-blocked coverslips, which were then incubated at RT for 20 min to allow the cells to adhere. The coverslip was inverted and placed onto the chemotaxis chamber. Ltx (1, 10, and 100 ng/mL), fMLP (10 nM, Sigma Aldrich, Dorset, UK) as a positive control, or RPMI medium as a negative control was injected into the chemoattractant channels. The chemoattractant fMLP was shown to strongly induce directed neutrophil migration in our previous studies and is therefore a suitable positive control. Cell movement was analysed over a period of 20 min after a preincubation phase with the chemotactic agent for 5 min, using a Zeiss Primovert microscope (Carl Zeiss Imaging, Thornwood, NY), and images were captured every 30 s for 40 frames per condition using a Q Imaging Retiga 2000R camera (Qimaging, Surrey, Canada). The images generated by video microscopy were processed as described elsewhere (27). Briefly, Q pro-imaging software (Surrey, Canada) and ImageJ 1.45SR software (National Institutes of Health, Bethesda, MD) and the manual tracking plug-in (MtrackJ) were used for analysis of cell speed, cell velocity, and chemotactic index (CI) per experiment with 15 cells per each frame. Velocity is defined as a vector quantity that refers to the rate at which a cell changes its position in a particular direction of movement, whereas the CI is the total dislocation in the direction of the gradient, divided by the total path length, that is, the directional angle or accuracy of movement.
Statistical analysis
Two-sample two-tailed t-testing for unpaired data was performed to calculate significant differences of the means of two groups, assuming unequal variances (Welch's t-test). One-way ANOVA and Tukey's multiple comparisons testing were conducted for chemotactic assay data using GraphPad PRISM software (La Jolla, CA). Statistical significance was defined as p≤0.05.
Results
Ltx induces NET release
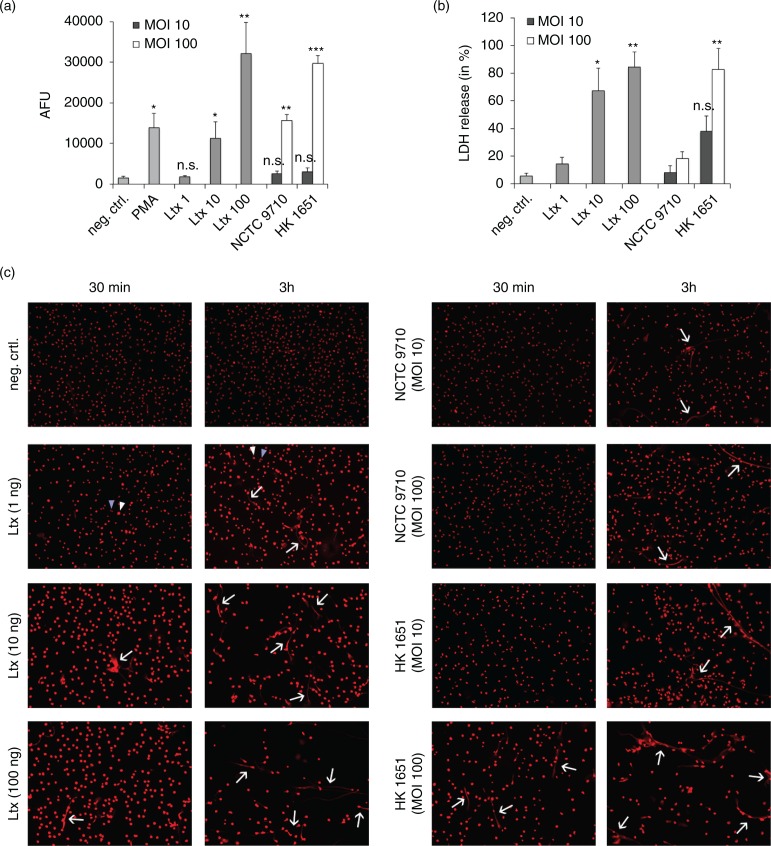
Fluorescence-based quantification showed that low concentrations of Ltx (1 ng/mL) did not cause significant NETosis after 3 h, whereas a concentration of 10 ng/mL Ltx led to NET formation comparable with the positive control (PMA). High Ltx concentrations (100 ng/mL) strongly induced NETosis, and the highly leukotoxic strain HK1651 led to similar results as high concentrations of Ltx when applied at an MOI of 100. The low leukotoxic strain NCTC 9710 at an MOI of 100 caused moderate NETosis with AFU measurements comparable with the samples incubated with 10 ng/mL of Ltx. Both strains applied at an MOI of 10 caused significantly less NET formation compared with MOI 100 (Fig. 1a).
Fig. 1.
Ltx effects on NET formation and neutrophil lysis. (a) NET quantification after 3 h of incubation as assessed by fluorescent 96-well plate-based assays. AFU=arbitrary fluorescent units. ***p=0.001, **p≤0.01, *p=0.02, asterisks indicate significant differences to the negative control. (b) Release of lactate dehydrogenase (LDH) from neutrophils as an indicator of cell lysis after 30 min of incubation. **p≤0.01, *p=0.05, asterisks indicate significant differences in comparison to the negative control. All experiments were conducted in triplicate and using three different donors, and Welch's t-test was applied to calculate significant differences. (c) Fluorescence microscope images of neutrophils after exposure to either controls [RPMI, PMA (100 ng/mL)], Ltx (1, 10, and 100 ng/mL), or A. actinomycetemcomitans strains (MOI 10 and 100) for 30 min and 3 h (magnification 10×). Arrows indicate NETs, and white arrowheads indicate swollen cells versus normal cells (blue arrowheads). Swollen cells suggest Ltx-mediated cell injury with osmotic influx of water. An increased rate of cell detachment can be seen in samples with higher Ltx concentrations and with HK 1651 in an MOI of 100 after 3 h, indicating cell death.
Ltx-mediated neutrophil lysis
Neutrophil lysis, as assessed by the release of LDH, was visible after 30 min of incubation with Ltx or bacteria (Fig. 1b). While 1 ng/mL of Ltx caused only minimal, non-significant neutrophil lysis, higher Ltx concentrations (10 and 100 ng/mL) as well as the highly leukotoxic strain HK1651 at an MOI of 100 led to significant releases of LDH. Strain NCTC 9710 did not cause any notable lysis after 30 min.
Ltx-dependent neutrophil swelling
The findings regarding NETosis and lysis could be visualised and confirmed by means of microscopy (Fig. 1c): an increase in cell size of nearly all neutrophils could already be observed after 30 min in the samples containing higher concentrations (10 and 100 ng/mL) of Ltx and in the HK 1651 (MOI 100) sample. This cell enlargement indicates osmotic swelling subsequently to pore formation evoked by Ltx. The fact that NETs were seen after 3 h of incubation further suggests that cytoplasmic swelling is a slow process that allows neutrophils to perform NETosis and leads to cell death later on, causing a detachment of neutrophils from the surface, which was seen after 3 h in samples with higher Ltx concentrations. Ltx at 1 ng/mL and strain HK 1651 at an MOI of 10 caused visible cell enlargement of some, but not all cells after 30 min, whereas an increased amount of swollen cells could be seen after 3 h in these samples. The low leukotoxic strain NCTC 9710 only caused a slight increase in cell size at an MOI of 100 and after 3 h.
Ltx enhances neutrophil movement
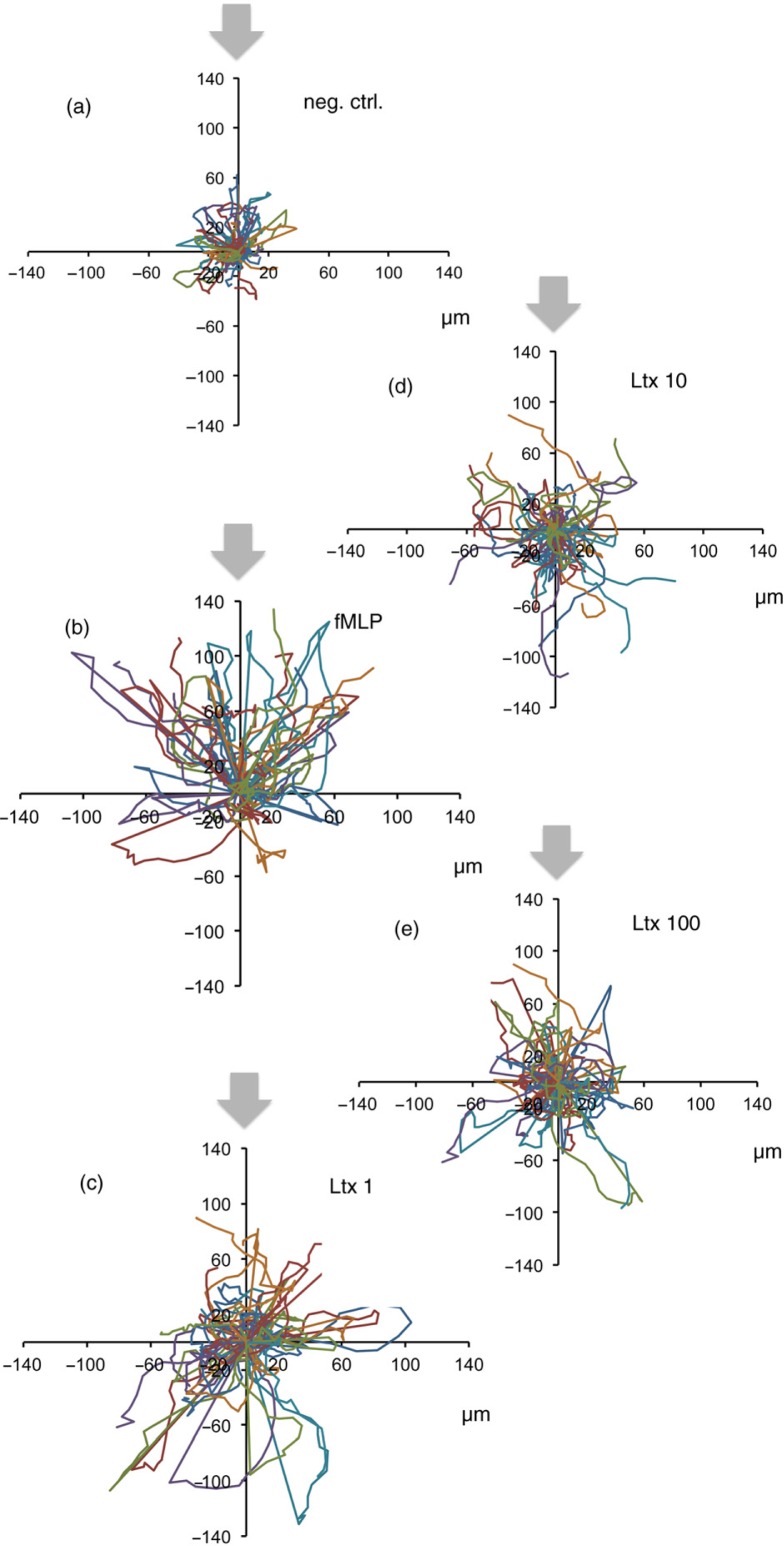
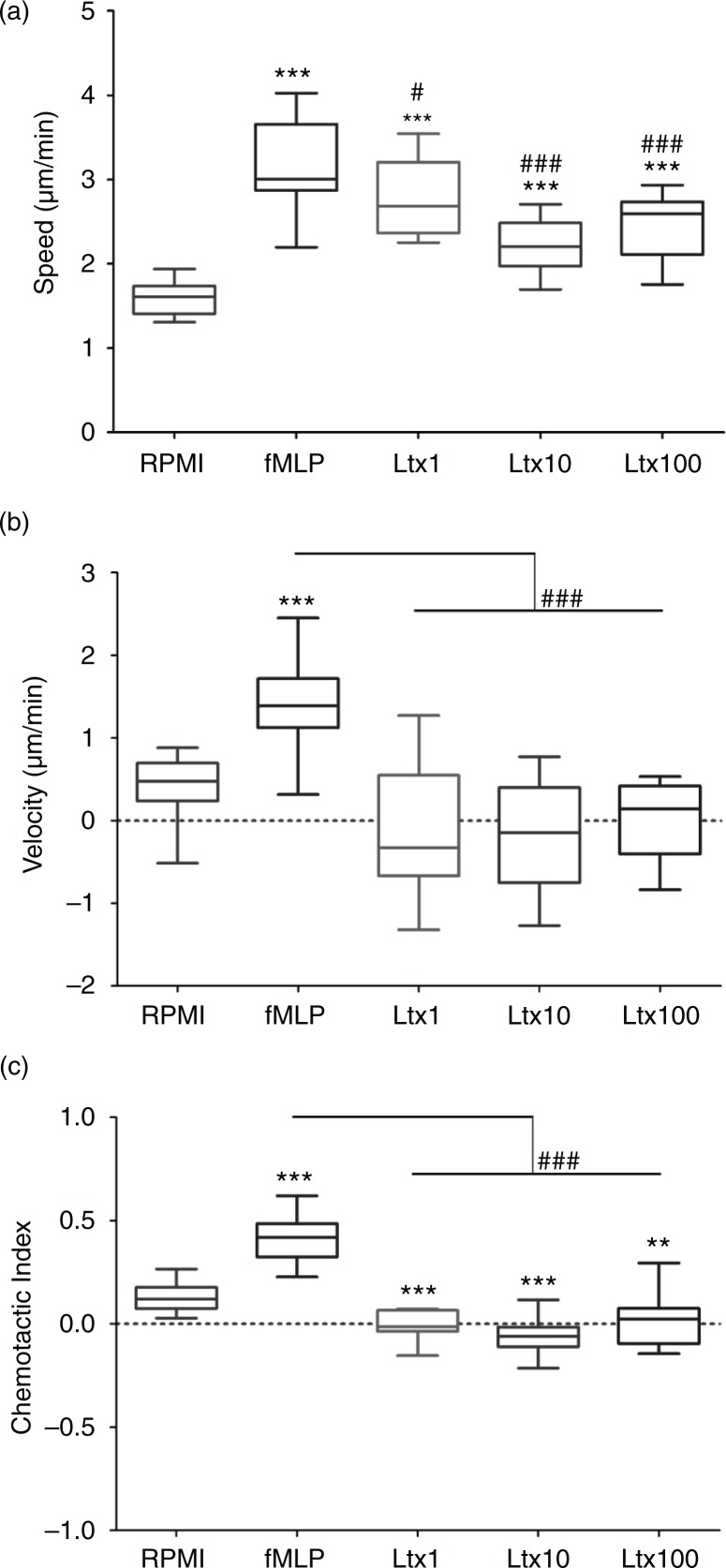
The chemotaxis assay revealed an enhanced random speed of movement of neutrophils induced by Ltx at 1, 10, and 100 ng/mL (Fig. 2a–e). After 5 min of preincubation, neutrophils started to crawl, however, with very low directional accuracy and velocity compared with the fMLP positive control, indicating that while Ltx activated neutrophil movement, the lack of directionality of movement indicated that it does not act as a chemoattractant. Even at very low concentrations of Ltx (1 ng/mL), the cells migrated with a significantly high speed compared with the negative controls, however, with a significantly lower velocity compared with positive controls, and therefore exhibited a low CI (Fig. 3a–c). Notably, neutrophil speed was decreased at higher Ltx concentrations (10 and 100 ng/mL), and cell swelling was observed during the imaging procedure of 20 min (data not shown), indicating pore formation and initiation of cell death by Ltx.
Fig. 2.
Neutrophil migration induced by Ltx (1, 10, and 100 ng/mL). (a–e) Neutrophil motion is depicted in spider diagrams, where each line represents a cell. Grey arrows denote the origin of the putative chemotactic agent, and cells should be migrating towards this arrow (migration rate in µm/min). All diagrams are represented at the same scale to aid comparison. Chemotactic assays were conducted in three independent experiments using three different blood donors, and 15 cells per donor were tracked.
Fig. 3.
Measures of neutrophil motion. (a) Speed and (b) velocity, respectively (in µm/min), of neutrophils in response to each agent. Asterisks indicate significant differences in comparison to the negative control and hashes represent significant differences in comparison to the positive control; **p=0.01, ***p≤0.001, # p≤0.05, ### p≤0.001. (c) The chemotactic index, which is the total shift in the direction of the gradient, divided by the total path length. Asterisks indicate significant differences in comparison to the negative control, and hashes represent significant differences in comparison to the positive control; ***p≤0.001, **p=0.006, ### p≤0.001.
Discussion
A. actinomycetemcomitans is a pathogenic facultative anaerobic bacterium frequently found in patients with AgP, a destructive inflammation that is thought to be associated with impaired neutrophil function. In this study, we demonstrate that Ltx acts as a neutrophil activator and NET inducer. Although Ltx is strongly leukotoxic, some neutrophils were still capable of eliciting anti-bacterial immune responses in the form of NETosis against A. actinomycetemcomitans producing high amounts of Ltx. Our results confirm those by Aulik et al. (29) and support the generally accepted finding that only approximately 30% of neutrophils will enter NETosis when stimulated (20). Moreover, we show for the first time that Ltx induces a non-directional neutrophil migration, which may be associated with increased tissue transit times of neutrophils in vivo (27).
NET-associated DNA has a distinct appearance as strands or web-like morphologies, whereas DNA secreted during apoptosis as a consequence of lysis (30) is fragmented and does not present as a visible structure. Therefore, our microscopy images provide evidence that NETs form, alongside with lysis. Moreover, NETosis is an active process that takes several hours to develop (31), whereas cell lysis occurs within the first 30 min (29). The fact that most NET structures were seen after 3 h of incubation and that fluorescence-based measurements of extracellular DNA only reached distinct differences after 3 h indicates that NETosis took place and that lysis-associated extracellular DNA was not detectable. This may be due to early DNA fragmentation and degradation, which apoptosis is characterised by (32). Moreover, these findings suggest that cytoplasmic swelling and lysis are slow processes that allow neutrophils to perform NETosis and do not lead to cell death immediately. This supports previous findings by Johansson et al. (33), who demonstrated that neutrophil degranulation is an active process taking place independently of and before Ltx-mediated lysis.
The fact that Ltx caused remarkable NET release suggests that CD11/CD18 (LFA-1), a binding site for Ltx on neutrophil surfaces, is involved in a downstream signalling cascade that may also involve the generation of reactive oxygen species (ROS), since ROS formation is necessary for NET release (34). LFA-1-dependent NET release and LFA-1-associated ROS release were demonstrated in previous studies (35, 36). Importantly, we have formerly demonstrated that LFA-1 is responsible for Ltx-mediated neutrophil lysis (33). Moreover, LFA-1 is strongly involved in neutrophil migration and chemotaxis (37). Whether the LFA-1 pathway is responsible for Ltx-triggered NETosis requires to be further elucidated in future studies employing LFA-1 antagonists. As expected, the release of LDH occurred in an Ltx-concentration-dependent manner, corroborating previous findings from our group and providing evidence for Ltx-induced neutrophil lysis (33).
Enhanced inflammation, as found in untreated AgP, could be the result of increased neutrophil recruitment to sites of bacterial overgrowth. It has been reported that A. actinomycetemcomitans induces gingival epithelial cells to rapidly release the neutrophil chemoattractant interleukin 8 (38). The chemotactic properties of Ltx reported in a study by Totani et al. (39) could be only partially confirmed by our results, where Ltx induced strong neutrophil movement, indicating neutrophil activation, but without detectable directionality. However, the Ltx used in their experiments had not been isolated from A. actinomycetemcomitans. We here observed that even at very low concentrations (1 ng/mL), the cells migrated significantly. At higher Ltx concentrations (10 and 100 ng/mL), the cells increased in size and moved at a reduced speed compared with 1 ng/mL, indicating that Ltx-mediated osmotic swelling and therefore reduced functionality may have occurred within the incubation time employed. Further studies are needed to extend our understanding of the exact mechanism, by which Ltx induces this non-directional movement in neutrophils. In vivo, it seems plausible that many neutrophils become lysed even before reaching the site of A. actinomycetemcomitans colonisation within periodontal pockets, as the Ltx molecule was found to penetrate periodontal tissues (40).
This study was aimed at investigating bacterial factors influencing neutrophil behaviour under exclusion of host factors; therefore, neutrophils from healthy donors were used. Van Dyke et al. (41) showed that neutrophils of patients with AgP featured a different chemotactic behaviour in comparison with healthy subjects, strongly suggesting the neutrophil phenotype as a host-derived influence factor for the disease. It is not known, however, what leads to these differential phenotypes. It is possible that neutrophil dysfunction such as impaired chemotaxis may be due to very low levels of systemically circulating Ltx, priming and disturbing these cells. This is also supported by the findings of Kumar and Prakash (42), who demonstrated a beneficial effect of periodontal treatment, which lowers the bacterial burden, on neutrophil chemotaxis. Previous studies have confirmed a systemic Ltx burden in A. actinomycetemcomitans-infected individuals, accompanied by anti-Ltx serum antibodies (43, 44). The differences in the neutrophil function between individuals with and without AgP could also be due to a variability in anti-Ltx antibody production and clonality. These antibodies can inactivate Ltx (45), but interindividual differences have not been investigated yet. Moreover, it is likely that other host factors, such as serum components, play a role in enhancing Ltx systemically and, thus, modify the virulence of this toxin (46, 47). Evidence from the literature exists that carriers of the highly leukotoxic JP2 clone of A. actinomycetemcomitans have a significantly increased risk of attachment loss in the context of AgP, suggesting that Ltx may play a key role in this inflammatory tissue destruction (48).
Elevated levels of Ltx, as found in the periodontium of affected patients, may finally activate and lyse neutrophils present in the periodontal tissues. Unfavourably, neutrophils release cytotoxic agents into their environment upon membrane damage as well as upon NETosis. Moreover, remnants of dead cells attract further neutrophils and macrophages in order to effect neutrophil efferocytosis. As these newly recruited leukocytes become subject to lysis (3), this may lead to a vicious pro-inflammatory cycle within the periodontal tissues. Further studies are warranted to investigate the ability of Ltx to prime and activate neutrophils at different concentrations locally and systemically as well as to assess the types and concentrations of systemically circulating anti-Ltx antibodies in patients carrying highly leukotoxic A. actinomycetemcomitans strains.
Our observations suggest an altered functional neutrophil behaviour in patients challenged with highly leukotoxic A. actinomycetemcomitans, in which neutrophils exert a timely reduced arsenal of defence modalities but show pronounced NET formation alongside cell death. Our finding that some neutrophils were capable of performing NETosis although exhibiting osmotic swelling requires further attention in future research, but has in part been investigated by Ting-Beall et al. (49), who reported that neutrophils can tolerate a certain range of osmolalities and regulate their volume in response to osmotic stress. Importantly, it is known that NETosis occurs only under conditions where oxygen is present and that the enzyme NADPH-oxidase, which requires oxygen to form ROS, is required for NET formation (34, 50). Therefore, it can be concluded that in an oxygenated setting, some neutrophils mount ROS-mediated defences to A. actinomycetemcomitans, while others are lysed by Ltx.
The findings presented here further indicate that highly leukotoxic strains of A. actinomycetemcomitans are likely to cause an imbalance of neutrophil-mediated defences, whereas strains with a lower leukotoxic profile may lead to an enhanced inflammatory response without causing excessive neutrophil lysis. The susceptibility of neutrophils to highly leukotoxic A. actinomycetemcomitans, becoming lysed within minutes before reaching the site of bacterial accumulation, may well be a contributing factor in the progression of AgP. However, a comprehensive study comparing the reactivity of neutrophils from healthy and AgP-affected individuals towards the Ltx stimulus is needed to further understand the importance of Ltx in dysbalancing neutrophil responses.
Acknowledgements
We would like to thank Prof. John Matthews for his help with improving the manuscript and Prof. Robert H. Insall for kindly providing the Insall chamber for chemotactic assays. We further thank Prof. Dr. Johannes Oldenburg for supplying buffy coats at the Blood Donation Center of the University Hospital Bonn, Prof. Dr. Achim Hoerauf for providing research facilities, and Prof. Dr. Isabelle Bekeredjian-Ding from the Paul-Ehrlich Institute in Langen as well as Prof. Dr. James Deschner from the Department of Experimental Dento-Maxillo-Facial Medicine in Bonn for their valuable input and support. We are also grateful to Dr. Naomi Hubber for helping with collecting blood at the Birmingham Dental School and Hospital.
Conflict of interest and funding
This study was financially supported by the German Society of Dental and Oral Medicine (DGZMK), the Västerbotten County of Sweden (TUA), and the University of Birmingham.
References
- 1.Stabholz A, Soskolne WA, Shapira L. Genetic and environmental risk factors for chronic periodontitis and aggressive periodontitis. Periodontol 2000. 2000;53:138–53. doi: 10.1111/j.1600-0757.2010.00340.x. [DOI] [PubMed] [Google Scholar]
- 2.Fine DH, Markowitz K, Furgang D, Fairlie K, Ferrandiz J, Nasri C, et al. Aggregatibacter actinomycetemcomitans and its relationship to initiation of localized aggressive periodontitis: longitudinal cohort study of initially healthy adolescents. J Clin Microbiol. 2007;45:3859–69. doi: 10.1128/JCM.00653-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johansson A. Aggregatibacter actinomycetemcomitans leukotoxin: a powerful tool with capacity to cause imbalance in the host inflammatory response. Toxins (Basel) 2011;3:242–59. doi: 10.3390/toxins3030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelk P, Abd H, Claesson R, Sandstrom G, Sjostedt A, Johansson A. Cellular and molecular response of human macrophages exposed to Aggregatibacter actinomycetemcomitans leukotoxin. Cell Death Dis. 2011;2:e126. doi: 10.1038/cddis.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kachlany SC. Aggregatibacter actinomycetemcomitans leukotoxin: from threat to therapy. J Dent Res. 2010;89:561–70. doi: 10.1177/0022034510363682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spitznagel J, Jr, Kraig E, Kolodrubetz D. Regulation of leukotoxin in leukotoxic and nonleukotoxic strains of Actinobacillus actinomycetemcomitans . Infect Immun. 1991;59:1394–401. doi: 10.1128/iai.59.4.1394-1401.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Höglund Aberg C, Haubek D, Kwamin F, Johansson A, Claesson R. Leukotoxic activity of Aggregatibacter actinomycetemcomitans and periodontal attachment loss. PLoS One. 2014;9:e104095. doi: 10.1371/journal.pone.0104095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brogan JM, Lally ET, Poulsen K, Kilian M, Demuth DR. Regulation of Actinobacillus actinomycetemcomitans leukotoxin expression: analysis of the promoter regions of leukotoxic and minimally leukotoxic strains. Infect Immun. 1994;62:501–8. doi: 10.1128/iai.62.2.501-508.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haubek D, Johansson A. Pathogenicity of the highly leukotoxic JP2 clone of Aggregatibacter actinomycetemcomitans and its geographic dissemination and role in aggressive periodontitis. J Oral Microbiol. 2014;6 doi: 10.3402/jom.v6.23980. 23980, doi: http://dx.doi.org/10.3402/jom.v6.23980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kato S, Kowashi Y, Demuth DR. Outer membrane-like vesicles secreted by Actinobacillus actinomycetemcomitans are enriched in leukotoxin. Microb Pathog. 2002;32:1–13. doi: 10.1006/mpat.2001.0474. [DOI] [PubMed] [Google Scholar]
- 11.Berthold P, Forti D, Kieba IR, Rosenbloom J, Taichman NS, Lally ET. Electron immunocytochemical localization of Actinobacillus actinomycetemcomitans leukotoxin. Oral Microbiol Immunol. 1992;7:24–7. doi: 10.1111/j.1399-302x.1992.tb00015.x. [DOI] [PubMed] [Google Scholar]
- 12.Lally ET, Hill RB, Kieba IR, Korostoff J. The interaction between RTX toxins and target cells. Trends Microbiol. 1999;7:356–61. doi: 10.1016/s0966-842x(99)01530-9. [DOI] [PubMed] [Google Scholar]
- 13.Lally ET, Kieba IR, Sato A, Green CL, Rosenbloom J, Korostoff J, et al. RTX toxins recognize a beta2 integrin on the surface of human target cells. J Biol Chem. 1997;272:30463–9. doi: 10.1074/jbc.272.48.30463. [DOI] [PubMed] [Google Scholar]
- 14.Hirschfeld J. Dynamic interactions of neutrophils and biofilms. J Oral Microbiol. 2014;6:26102. doi: 10.3402/jom.v6.26102. doi: http://dx.doi.org/10.3402/jom.v6.26102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kantarci A, Oyaizu K, Van Dyke TE. Neutrophil-mediated tissue injury in periodontal disease pathogenesis: findings from localized aggressive periodontitis. J Periodontol. 2003;74:66–75. doi: 10.1902/jop.2003.74.1.66. [DOI] [PubMed] [Google Scholar]
- 16.Bhansali RS, Yeltiwar RK, Bhat KG. Assessment of peripheral neutrophil functions in patients with localized aggressive periodontitis in the Indian population. J Indian Soc Periodontol. 2013;17:731–6. doi: 10.4103/0972-124X.124485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–5. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 18.Vitkov L, Klappacher M, Hannig M, Krautgartner WD. Extracellular neutrophil traps in periodontitis. J Periodontal Res. 2009;44:664–72. doi: 10.1111/j.1600-0765.2008.01175.x. [DOI] [PubMed] [Google Scholar]
- 19. Kamma JJ, Nakou M, Gmur R, Baehni PC. Microbiological profile of early onset/aggressive periodontitis patients. Oral Microbiol Immunol. 2004;19:314–21. doi: 10.1111/j.1399-302x.2004.00161.x. [DOI] [PubMed] [Google Scholar]
- 20.Cooper PR, Palmer LJ, Chapple IL. Neutrophil extracellular traps as a new paradigm in innate immunity: friend or foe? Periodontol 2000. 2013;63:165–97. doi: 10.1111/prd.12025. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan MJ, Radic M. Neutrophil extracellular traps: double-edged swords of innate immunity. J Immunol. 2012;189:2689–95. doi: 10.4049/jimmunol.1201719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Claesson R, Gudmundson J, Aberg CH, Haubek D, Johansson A. Detection of a 640-bp deletion in the Aggregatibacter actinomycetemcomitans leukotoxin promoter region in isolates from an adolescent of Ethiopian origin. J Oral Microbiol. 2015;7:26974. doi: 10.3402/jom.v7.26974. doi: http://dx.doi.org/10.3402/jom.v7.26974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johansson A, Hänström L, Kalfas S. Inhibition of Actinobacillus actinomycetemcomitans leukotoxicity by bacteria from the subgingival flora. Oral Microbiol Immunol. 2000;15:218–25. doi: 10.1034/j.1399-302x.2000.150402.x. [DOI] [PubMed] [Google Scholar]
- 24.Hirschfeld J, Dommisch H, Skora P, Horvath G, Latz E, Hoerauf A, et al. Neutrophil extracellular trap formation in supragingival biofilms. Int J Med Microbiol. 2015;305:453–63. doi: 10.1016/j.ijmm.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Roberts H, White P, Dias I, McKaig S, Veeramachaneni R, Thakker N, et al. Characterization of neutrophil function in Papillon-Lefevre syndrome. J Leukoc Biol. 2016;100:433–44. doi: 10.1189/jlb.5A1015-489R. [DOI] [PubMed] [Google Scholar]
- 26.von Köckritz-Blickwede M, Chow OA, Nizet V. Fetal calf serum contains heat-stable nucleases that degrade neutrophil extracellular traps. Blood. 2009;114:5245–6. doi: 10.1182/blood-2009-08-240713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts HM, Ling MR, Insall R, Kalna G, Spengler J, Grant MM, et al. Impaired neutrophil directional chemotactic accuracy in chronic periodontitis patients. J Clin Periodontol. 2015;42:1–11. doi: 10.1111/jcpe.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muinonen-Martin AJ, Veltman DM, Kalna G, Insall RH. An improved chamber for direct visualisation of chemotaxis. PLoS One. 2010;5:e15309. doi: 10.1371/journal.pone.0015309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aulik NA, Hellenbrand KM, Klos H, Czuprynski CJ. Mannheimia haemolytica and its leukotoxin cause neutrophil extracellular trap formation by bovine neutrophils. Infect Immun. 2010;78:4454–66. doi: 10.1128/IAI.00840-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelk P, Johansson A, Claesson R, Hanstrom L, Kalfas S. Caspase 1 involvement in human monocyte lysis induced by Actinobacillus actinomycetemcomitans leukotoxin. Infect Immun. 2003;71:4448–55. doi: 10.1128/IAI.71.8.4448-4455.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yipp BG, Kubes P. NETosis: how vital is it? Blood. 2013;122:2784–94. doi: 10.1182/blood-2013-04-457671. [DOI] [PubMed] [Google Scholar]
- 32.Zhang JH, Xu M. DNA fragmentation in apoptosis. Cell Res. 2000;10:205–11. doi: 10.1038/sj.cr.7290049. [DOI] [PubMed] [Google Scholar]
- 33.Johansson A, Claesson R, Hänström L, Sandström G, Kalfas S. Polymorphonuclear leukocyte degranulation induced by leukotoxin from Actinobacillus actinomycetemcomitans . J Periodontal Res. 2000;35:85–92. doi: 10.1034/j.1600-0765.2000.035002085.x. [DOI] [PubMed] [Google Scholar]
- 34.Palmer LJ, Cooper PR, Ling MR, Wright HJ, Huissoon A, Chapple IL. Hypochlorous acid regulates neutrophil extracellular trap release in humans. Clin Exp Immunol. 2012;167:261–8. doi: 10.1111/j.1365-2249.2011.04518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDonald B, Urrutia R, Yipp BG, Jenne CN, Kubes P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe. 2012;12:324–33. doi: 10.1016/j.chom.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 36.Decleva E, Dri P, Menegazzi R, Busetto S, Cramer R. Evidence that TNF-induced respiratory burst of adherent PMN is mediated by integrin alpha(L)beta(2) J Leukoc Biol. 2002;72:718–26. [PubMed] [Google Scholar]
- 37.Heit B, Colarusso P, Kubes P. Fundamentally different roles for LFA-1, Mac-1 and alpha4-integrin in neutrophil chemotaxis. J Cell Sci. 2005;118:5205–20. doi: 10.1242/jcs.02632. [DOI] [PubMed] [Google Scholar]
- 38.Huang GT, Haake SK, Park NH. Gingival epithelial cells increase interleukin-8 secretion in response to Actinobacillus actinomycetemcomitans challenge. J Periodontol. 1998;69:1105–10. doi: 10.1902/jop.1998.69.10.1105. [DOI] [PubMed] [Google Scholar]
- 39.Totani Y, Saito Y, Ishizaki T, Sasaki F, Ameshima S, Miyamori I. Leukotoxin and its diol induce neutrophil chemotaxis through signal transduction different from that of fMLP. Eur Respir J. 2000;15:75–9. doi: 10.1183/09031936.00.15107500. [DOI] [PubMed] [Google Scholar]
- 40.Johansson A, Claesson R, Hänström L, Kalfas S. Serum-mediated release of leukotoxin from the cell surface of the periodontal pathogen Actinobacillus actinomycetemcomitans . Eur J Oral Sci. 2003;111:209–15. doi: 10.1034/j.1600-0722.2003.00030.x. [DOI] [PubMed] [Google Scholar]
- 41.Van Dyke TE, Horoszewicz HU, Genco RJ. The polymorphonuclear leukocyte (PMNL) locomotor defect in juvenile periodontitis. Study of random migration, chemokinesis and chemotaxis. J Periodontol. 1982;53:682–7. doi: 10.1902/jop.1982.53.11.682. [DOI] [PubMed] [Google Scholar]
- 42.Kumar RS, Prakash S. Impaired neutrophil and monocyte chemotaxis in chronic and aggressive periodontitis and effects of periodontal therapy. Indian J Dent Res. 2012;23:69–74. doi: 10.4103/0970-9290.99042. [DOI] [PubMed] [Google Scholar]
- 43.Brage M, Holmlund A, Johansson A. Humoral immune response to Aggregatibacter actinomycetemcomitans leukotoxin. J Periodontal Res. 2011;46:170–5. doi: 10.1111/j.1600-0765.2010.01325.x. [DOI] [PubMed] [Google Scholar]
- 44.Engström PE, George M, Larsson P, Lally ET, Taichman NS, Norhagen G. Oral and systemic immunoglobulin G-subclass antibodies to Actinobacillus actinomycetemcomitans leukotoxin. Oral Microbiol Immunol. 1999;14:104–8. doi: 10.1034/j.1399-302x.1999.140205.x. [DOI] [PubMed] [Google Scholar]
- 45.Johansson A, Buhlin K, Sorsa T, Pussinen PJ. Systemic Aggregatibacter actinomycetemccomitans leukotoxin-neutralizing antibodies in periodontitis. J Periodontol. 2016:1–15. doi: 10.1902/jop.2016.160193. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 46.Johansson A, Claesson R, Belibasakis G, Makoveichuk E, Hanstrom L, Olivecrona G, et al. Lack of lipoprotein-dependent effects on the cytotoxic interactions of Actinobacillus actinomycetemcomitans leukotoxin with human neutrophils. Apmis. 2002;110:857–62. doi: 10.1034/j.1600-0463.2002.1101203.x. [DOI] [PubMed] [Google Scholar]
- 47.Johansson A, Claesson R, Belibasakis G, Makoveichuk E, Hanstrom L, Olivecrona G, et al. Protease inhibitors, the responsible components for the serum-dependent enhancement of Actinobacillus actinomycetemcomitans leukotoxicity. Eur J Oral Sci. 2001;109:335–41. doi: 10.1034/j.1600-0722.2001.00055.x. [DOI] [PubMed] [Google Scholar]
- 48.Haubek D, Ennibi OK, Poulsen K, Vaeth M, Poulsen S, Kilian M. Risk of aggressive periodontitis in adolescent carriers of the JP2 clone of Aggregatibacter (Actinobacillus) actinomycetemcomitans in Morocco: a prospective longitudinal cohort study. Lancet. 2008;371:237–42. doi: 10.1016/S0140-6736(08)60135-X. [DOI] [PubMed] [Google Scholar]
- 49.Ting-Beall HP, Needham D, Hochmuth RM. Volume and osmotic properties of human neutrophils. Blood. 1993;81:2774–80. [PubMed] [Google Scholar]
- 50.Rohm M, Grimm MJ, D'Auria AC, Almyroudis NG, Segal BH, Urban CF. NADPH oxidase promotes neutrophil extracellular trap formation in pulmonary aspergillosis. Infect Immun. 2014;82:1766–77. doi: 10.1128/IAI.00096-14. [DOI] [PMC free article] [PubMed] [Google Scholar]