Abstract
The aim of the current study was to examine the effects of chronic consumption of soft drinks (SDs) on hepatic oxidative stress and cytochrome P450 enzymes (CYPs) expression in the livers of Wistar rats. For 3 consecutive months, the rats had free access to three different soft drinks, Coca-Cola, Pepsi-Cola and 7-UP. The rats were subsequently compared with control group rats that had consumed water. Blood and hepatic tissue samples were assayed for the changes in antioxidants, liver function biomarkers and hepatic gene expression for different isoforms of hepatic CYP. The results indicated that SD consumption (SDC) decreased serum antioxidant levels and increased malondialdehyde secretion, and increased liver biomarkers (glutamate pyruvate transaminase and glutamate oxaloacetate). SD induced alterations in mRNA expression of hepatic antioxidants and cytochrome isoforms. The expression of peroxidase, catalase, CYP1A2, CYP3A2 and CYP2C11 in the liver were upregulated following SDC. By contrast, CYP2B1 was downregulated after 3 months of SDC in liver tissue samples. Thus, the present findings indicate that SDs induced oxidative stress in the liver of Wistar rats and for the first time, to the best of our knowledge, indicate that SDC disrupts hepatic CYP enzymes that may affect drug metabolism. Therefore, drug-dosing programs should be carefully designed to take these novel findings into consideration for the treatment of diseases.
Keywords: soft drinks, oxidative stress, hepatic cytochromes
Introduction
During the last 30 years, consumption of soft drinks (SDs) has increased worldwide (1). SDs predominantly contain water, phosphoric acid, caffeine, sugar as a sweetener and other preservatives, colorings, as well as flavors (2). The increase in consumption of SDs is not good for health and is concerning (2–4), as SDs contain high levels of caffeine, which cause addiction as it is rapidly absorbed from the intestine (5). A correlation between SDs and incidence of certain diseases, such as obesity, diabetes mellitus and cardiovascular disease has been confirmed (6–8). In addition, it has been shown that Coca-Cola consumption delayed elimination of methotrexate and, therefore, is a predisposing factor for acute renal failure (9).
Cytochrome P450 enzymes (CYPs) are capable of catalyzing the oxidative biotransformation of the majority of therapeutic agents and other lipophilic xenobiotics, therefore, they are particularly relevant for clinical pharmacology (10). Drug and metabolite clearance depends on CYP enzyme activities; their inhibition leads to drug-drug interaction and toxicity (11), while their induction increases drug and elimination (12). A large number of factors, such as components of food cause CYP modulation in rats (13). Therefore, SD consumption (SDC) may affect CYP profiles, and induce oxidative and hepatic stress.
A positive association between caffeine and coffee consumption and ovarian cancer was suggested to be modified by the CYP1A2 genotype and associated with consumption of certain foods (14). In Saudi Arabia and the Middle East, consumption of SDC (three times per day) with meals (15) is common. Therefore, the current study was conducted to examine the effect of chronic SDC on serum oxidative stress biomarkers and alteration in hepatic enzyme activity. Furthermore, mRNA expression of hepatic antioxidants [catalase and glutathione peroxidase (GSH-Px)] and hepatic CYP isoforms (CYP1A2, CYP3A2, CYP2B1 and CYP2C11) were examined.
Materials and methods
Chemicals, materials and kits
Ethidium bromide for agarose preparation and Tris-Borate-EDTA buffer were purchased from Sigma-Aldrich (St. Louis, MO, USA) and agarose was purchased from Bio Basic Int. (Markham, ON, Canada). The Wistar rats (n=40) were purchased from King Abdel-Aziz University, King Fahd Center for Scientific Research (Jeddah, Saudi Arabia). The malondialdehyde (MDA), glutathione peroxidase (GSH-Px) and catalase kits were purchased from Bio-Diagnostic Co., (Giza, Egypt). Coca-Cola (The World of Coca-Cola, Atlanta, GA, USA), Pepsi-Cola (Pepsi Co, Purchase, NY, USA) and 7-UP (Dr Pepper Snapple Group, Inc., Plano, TX, USA) were used and were purchased from Ta'if markets in Saudi Arabia. The DNA 100-bp ladder was purchased from MBI Fermentas (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Oligo dT primer and dNTPs were obtained from SibEnzyme Ltd. (Novosibirsk, Russia). Qiazol reagent (for RNA extraction) was bought from Qiagen, Inc. (Valencia, CA, USA).
Experimental animals, design and sampling
The current study was approved by the Ethics Committee of the College of Applied Medical Sciences, Ta'if University (Turabah, Saudi Arabia; project no. 3792/34/1). Forty male Wistar rats (age, 12 weeks), weighing 200–280 g were allocated into four groups. The rats were handled every day at 9:00 a.m., and kept under observation for 1 week for complete acclimatization. Rats were housed at 20±50°C under a 12-h light/dark cycle and received free access to food and water for the first week. Next, the rats were divided into four groups as follows: Control group, no treatment; Coca-Cola group (group 2); Pepsi-Cola (group 3) and 7-UP group (group 4). Rats in groups 2–4 received SD ad libitum for 3 consecutive months. After 3 months of SDC, all rats were anesthetized by inhalation of diethyl ether. Blood (~5 ml) and small samples (50 mg) of liver tissues were collected from the anesthetized rats into sterilized vacutainer tubes. Serum was extracted following centrifugation of the clotted blood for 15 min at 3,000 × g at 40°C, and maintained at −20°C until biochemical measurements were obtained. For mRNA expression of hepatic genes, liver samples were maintained at −80°C in QIAzol reagent for RNA extraction.
Serum biochemical assays
MDA, GSH-Px, and catalase were assayed spectrophotometrically using the available commercial ELISA kits, and glutamate pyruvate transaminase (GPT) and glutamate oxaloacetate (GOT) were assayed spectrophotometrically using commercial kits (all kits were purchased from Bio-Diagnostic, Co.). Methods were performed according to the manufacturer's instructions.
cDNA preparation, synthesis and gene expression analysis
Total RNA was extracted from tissue samples as previously described (16). The integrity of RNA was visualized and confirmed after running in denaturated agarose gel (1.5%), then stained with ethidium bromide. Oligo dT primer (0.5 ng) was added to 2 µg total RNA to induce denaturation and was then used for cDNA synthesis (16). For semi-quantitative gene expression analysis, specific primers were designed for genes (Table I) using the Oligo-4 computer program by (Macrogen Co., Seoul, South Korea). Semi-quantitative polymerase chain reaction (PCR) was conducted in a total volume of 25 µl as previously described (15). Using a Bio-Rad T100™ Thermal Cycle machine, PCR was performed with the following cycling conditions: 95°C for 4 min (1 cycle), followed by 27 cycles (to achieve optimal gene expression), each consisting of denaturation at 95°C for 60 sec, annealing as presented in Table I for 60 sec and extension at 72°C for 60 sec, with an additional final extension at 72°C for 10 min. Glyceraldehyde-3-phosphate dehydrogenase expression served as an internal standard and as a reference. The PCR products were electrophoresed at 100 V for 30 min after running in 1.5% agarose gel and stained with ethidium bromide in Tris-Borate-EDTA buffer. The PCR products were visualized using the InGenius 3.0 gel documentation system (Syngene, Frederick, MD, USA) and under ultraviolet light. The densitometric analysis for PCR bands was performed using ImageJ software version 1.47 (http://imagej.en.softonic.com/).
Table I.
PCR conditions for examined genes in the liver.
| Name | Sense 5′-3′ | Anti-sense 5′-3′ | Annealing temperature | Product size (bp) |
|---|---|---|---|---|
| β-actin | ATGTACGTAGCCATCCAGGC | TCCACACAGAGTACTTGCGC | 56°C | 628 |
| CYP1A2 | GCAGGTCAACCATGATGAGAA | CGGCCGATGTCTCGGCCATCT | 56°C | 334 |
| CYP2C11 | TGCCCCTTTTTACGAGGCT | GGAACAGATGACTCTGAATTCT | 55°C | 368 |
| CYP3A2 | TTGATCCGTTGTTCTTGTCA | GGCCAGGAAATACAAGCAA | 52°C | 342 |
| CYP2B1 | TCTCACTCAACACTACGTTC | CTGGGAAAGGATCCAAGCCTGGG | 58°C | 450 |
| Catalase | ACGAGATGGCACACTTTGACAG | TGGGTTTCTCTTCTGGCTATGG | 55°C | 341 |
| Glutathione peroxidase | AAGGTGCTGCTCATTGAGAATG | CGTCTGGACCTACCAGGAACTT | 57°C | 406 |
CYP, cytochrome P450; PCR, polymerase chain reaction.
Statistical analysis
Data are presented as the mean ± standard error of the mean. One-way analysis of variance (ANOVA) was used to analyze data together with post hoc descriptive tests using SPSS software version 11.5 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Carbonated soft drinks alter serum levels of MDA, GSH-Px, catalase and hepatic biomarkers in Wistar rats
SDC for 3 consecutive months disrupted liver activity, as demonstrated by the significant increase in serum levels of MDA (P<0.05; Table II) when compared with control rats. By contrast, antioxidants activity of GSH-Px and catalase were significantly decreased (P<0.05) in the serum of rats in the Coca-Cola, Pepsi-Cola and 7-UP groups when compared with the control rats (Table II). As shown in Table III, Coca-Cola and Pepsi-Cola consumption significantly increased the serum levels GPT and GOT (P<0.05; Table III).
Table II.
Serum changes in MDA, and tissue levels of GSH-Px and catalase levels in the liver of rats after chronic consumption of soft drinks.
| Group | MDA (nmol/g protein) | GSH-Px (U/g protein) | Catalase (U/g protein) |
|---|---|---|---|
| Control | 32.7±2.7 | 52.1±4.8 | 31.7±1.9 |
| Coca-Cola | 63.7±7.6a | 41.9±4.2a | 18.6±1.1a |
| Pepsi-Cola | 96.7±9.8a | 38.3±4.8a | 22.3±2.1a |
| 7-UP | 59.1±3.7a | 36.3±2.9a | 17.5±1.9a |
Values are presented as means ± standard error of the mean for three independent experiments per treatment.
P<0.05 vs. control. MDA, malondialdehyde; GSH-Px, glutathione peroxidase.
Table III.
Serum changes in GPT and GOT levels following chronic consumption of soft drinks.
| Group | GPT (U/l) | GOT (U/l) |
|---|---|---|
| Control | 55.4±2.8 | 130.6±2.6 |
| Coca-Cola | 68.6±3.0a | 158.1±9.5a |
| Pepsi-Cola | 84.9±3.5b | 212.7±31.4b |
| 7-UP | 60.4±3.7 | 145.9±12.1 |
Values are presented as means ± standard error of the mean for three independent experiments per treatment.
P<0.05 vs. control
P<0.05 vs. Coca-Cola and 7-UP. GPT, glutamate pyruvate transaminase; GOT, glutamate oxaloacetate.
Carbonated SDs downregulate catalase and peroxidase mRNA expression in Wistar rats
SDC for 3 months in Wistar rats caused downregulation in mRNA expression of hepatic catalase. The downregulation was more apparent in the Coca-Cola and 7-UP groups compared with the Pepsi-Cola group (Fig. 1A). In addition, mRNA expression of hepatic peroxidase was downregulated in all SDC treated groups. The magnitude was greater in Coca-Cola, followed by Pepsi-Cola then 7-UP (Fig. 1B).
Figure 1.
Semi-quantitative reverse transcription-polymerase chain reaction analysis of (A) catalase and (B) glutathione peroxidase in liver tissue samples from Wistar rats following chronic administration of three soft drinks for 3 months. Densitometric analysis was performed for five different rats per group. Values are presented as means ± standard error of the mean (n=5 rats per group). *P<0.05 vs. CNT. CNT, control; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Carbonated SDs affect mRNA expression of hepatic CYP1A2 in Wistar rats
Hepatic CYP1A2 mRNA expression in the liver tissue samples was upregulated significantly (P<0.05) following SDC for 3 months. The upregulation of CYP1A2 mRNA expression was 0.5-, 1- and 1.3-fold in the Coca-Cola, Pepsi-Cola and 7-UP groups, respectively (Fig. 2).
Figure 2.
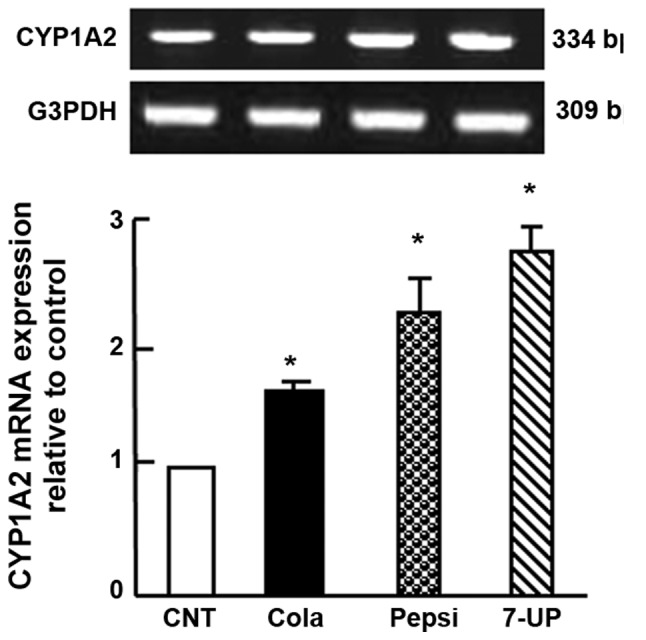
Semi-quantitative reverse transcription-polymerase chain reaction analysis of CYP1A2 in liver tissues after chronic administration of soft drinks for 3 months in Wistar rats. Densitometric analysis was performed for five different rats per group. Values are presented as means ± standard error of the mean (n=5 rats per group). *P<0.05 vs. CNT group. CYP, cytochrome P450; CNT, control; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Carbonated SDs alter mRNA expression of hepatic CYP3A2 and CYP2B1 in Wistar rats
SDC for 3 months in Wistar rats caused upregulation of hepatic CYP3A2 mRNA expression by 1.7-, 3.2- and 2.7-fold in the Coca-Cola, Pepsi-Cola and 7-UP groups, respectively (Fig. 3A). By contrast, SDC for 3 months downregulated hepatic CYP2B1 mRNA expression (P<0.05) when compared with the control. The pattern of downregulation was 0.3-, 0.8- and 0.5-fold when compared with the control group for the Coca-Cola, Pepsi-Cola and 7-UP groups, respectively (Fig. 3).
Figure 3.
Semi-quantitative reverse transcription-polymerase chain reaction analysis of (A) CYP3A2 and (B) CYP2B1 after chronic administration of soft drinks for 3 months in Wistar rats. Densitometric analysis was performed for five different rats per group. Values are presented as means ± standard error of the mean (n=5 rats per group). *P<0.05 vs. CNT group. CYP, cytochrome P450; CNT, control; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Carbonated SDs affected mRNA expression of hepatic CYP2C11 in Wistar rats
SDC for 3 months in Wistar rats caused upregulation in hepatic CYP2C11 mRNA expression (P<0.05) when compared with control rats. The increase in mRNA expression was 1-, 2- and 2.2-fold for the Coca-Cola, Pepsi-Cola and 7-UP groups, respectively (Fig. 4).
Figure 4.
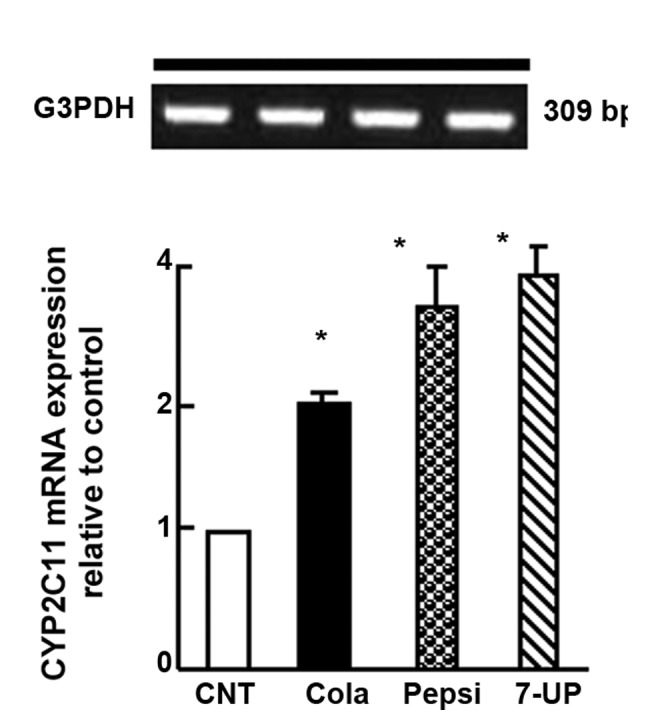
Semi-quantitative reverse transcription-polymerase chain reaction analysis of CYP2C11 in liver tissue samples after chronic administration of soft drinks for 3 months in Wistar rats. Densitometric analysis was performed for five different rats per group. Values are presented as means ± standard error of the mean (n=5 rats per group). *P<0.05 vs. CNT group. CYP, cytochrome P450; CNT, control; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Discussion
In the current study, oxidative stress and alterations in cytochromes expression were demonstrated to be induced in hepatic tissue samples following SDC. The inability of antioxidant defense mechanisms to scavenge excessive levels of reactive oxygen species (ROS) and/or reduction in the basal antioxidant defense mechanisms are the primary causes for oxidative stress in tissues (17). Therefore, degenerative diseases, including hepatopathies are caused by this oxidative stress (17,18). Lipid peroxidation is demonstrated to induce disturbance of membrane function and integrity (19). It is monitored by the increase of serum MDA levels, one of the most commonly used biomarkers for lipid peroxidation (20). Thus, the increased levels of MDA due to consumption of SDs indicate increased lipid peroxidation. Furthermore, it may indicate the potential carcinogenic effect of SDC, as MDA is believed to originate under stress conditions and is highly capable of reacting with multiple biomolecules, such as proteins or DNA that lead to the formation of adducts (21,22). This assumed potential carcinogenic effect of SDC is augmented by the reduction of levels of antioxidant enzymes, GSH-Px and catalase, following SDC. In malignant cells (23) and in primary cancer tissues (24) the level of ROS-scavenging enzymes, such as GSH-Px was significantly decreased.
Furthermore, SDC upregulated CYP1A2 mRNA expression levels and this may indicate the potential carcinogenic effect of SD. The activity of CYP1A2 is a possible risk factor that determines the carcinogenicity of heterocyclic amines in humans (25). In addition, hepatic CYP1A2 is a key enzyme, which exerts an important role in the metabolic clearance of 5% of currently marketed therapeutic agents (26). The induction of CYP1A2 by SDs may also indicate its accelerating effect on the metabolism of certain therapeutic agents that act as substrates for CYP1A2, such as theophylline, caffeine, phenacetin and propranolol (27). As is known, caffeine is a significant component of SDs.
Rat CYP3A2 exhibits a 73% homology to the amino acid sequences of human CYP3A4 (28). The induction of CYP3A2 by carbonated SDs may indicate its potential ability to induce human CYP3A4 due to their close homology (29). A significant correlation was reported between enzyme activity and mRNA expression for CYP3A4 in human liver samples (30). Rat CYP3A2 and human CYP3A4 are involved in the metabolism of erythromycin, nifedipine, lidocaine, testosterone, aflatoxin B1 and benzo[a]pyrene (28,30). Furthermore, CYP3A4 is highly expressed in the adult liver and small intestine (30), and metabolizes xenobiotics and carcinogens (32,33), as well as numerous endogenous compounds, such as bile acids, cholesterol, prostaglandins, fatty acids, retinoids, leukotrienes and biogenic amines (32,34). The induction of CYP3A2 by SDC may be attributed to the presence of taurine, as it was demonstrated that taurine enhanced the induction of CYP3A4 by rifampicin in HepG2 cells (35). SDs contain caffeine as a main ingredient and caffeine was shown to stimulate 5′-AMP-activated protein kinase (AMPK) in the extensor digitorum longus muscle (36). Furthermore, metformin was recently demonstrated to enhance constitutive active/androstane receptor (CAR) phosphorylation in human hepatocytes in part via an AMPK-dependent signaling pathway and suppression of CYP2B6 (37). Thus, the suppressive effect of SDs on CYP2B1 mRNA expression, which was revealed in the current study, may be mediated through caffeine stimulation of AMPK-dependent enhancement of CAR phosphorylation (38).
The upregulation of CYP2C11 by SDC in the current study may be mediated by caffeine. Caffeine is a non-selective adenosine receptor antagonist, which binds with very similar (relatively high) affinity to adenosine A1 and A2A receptors (39). Caffeine was reported to significantly inhibit cyclic adenosine monophosphate (cAMP) in hepatic stellate cells (40). Furthermore, epinephrine, which elevates cAMP via the β2- and the α1-adrenergic receptors in hepatocytes (41) downregulated CYP2C11. In addition, exposure of hepatocytes to increasing concentrations of cAMP for 24 h caused a concentration-dependent suppression of CYP2C11 expression to ~20% of control levels (39). Diclofenac is predominantly metabolized by CYP2C11 in male rats and CYP2C9 in humans (42). CYP2C9 is a major human CYP isoform involved in metabolizing therapeutic agents, such as phenytoin and S-warfarin (42). Therefore the upregulation of CYP2C11 by SDs may increase the elimination of such therapeutic agents that are substrates of CYP2C11 and, thus, decrease the efficacy of those therapeutic agents. Therefore, SDC must be considered during medication prescription and dosing.
In conclusion, the present findings demonstrate the disruption of hepatic CYP enzymes as a result of carbonated SDC, as well as induction of oxidative stress, which may affect drug metabolism. Special care must be taken for patients who consume SDs whilst taking medications for certain diseases.
References
- 1.Nielsen SJ, Popkin BM. Changes in beverage intake between 1977 and 2001. Am J Prev Med. 2004;27:205–210. doi: 10.1016/j.amepre.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Adjene JO, Ezeoke JC, Nwose EU. Histological effects of chronic consumption of soda pop drinks on kidney of adult Wister rats. N Am J Med Sci. 2010;2:215–217. doi: 10.4297/najms.2010.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amato D, Maravilla A, García-Contreras F, Paniagua R. Soft-drinks and health. Rev Invest Clin. 1997;49:387–395. (In Spanish) [PubMed] [Google Scholar]
- 4.Amato D, Maravilla A, Montoya C, Gaja O, Revilla C, Guerra R, Paniagua R. Acute effects of soft drink intake on calcium and phosphate metabolism in immature and adult rats. Rev Invest Clin. 1998;50:185–189. [PubMed] [Google Scholar]
- 5.Rapuri PB, Gallagher JC, Kinyamu HK, Ryschon KL. Caffeine intake increases the rate of bone loss in elderly women and interacts with vitamin D receptor genotypes. Am J Clin Nutr. 2001;74:694–700. doi: 10.1093/ajcn/74.5.694. [DOI] [PubMed] [Google Scholar]
- 6.Swinburn BA, Caterson I, Seidell JC, James WP. Diet, nutrition and the prevention of excess weight gain and obesity. Public Health Nutr 7 (1A) 2004:123–146. doi: 10.1079/phn2003585. [DOI] [PubMed] [Google Scholar]
- 7.Palmer JR, Boggs DA, Krishnan S, Hu FB, Singer M, Rosenberg L. Sugar-sweetened beverages and incidence of type 2 diabetes mellitus in African American women. Arch Intern Med. 2008;168:1487–1492. doi: 10.1001/archinte.168.14.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fung TT, Malik V, Rexrode KM, Manson JE, Willett WC, Hu FB. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr. 2009;89:1037–1042. doi: 10.3945/ajcn.2008.27140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santucci R, Levêque D, Herbrecht R. Cola beverage and delayed elimination of methotrexate. Br J Clin Pharmacol. 2010;70:762–764. doi: 10.1111/j.1365-2125.2010.03744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guengerich FP. Cytochrome p450 and chemical toxicology. Chem Res Toxicol. 2008;21:70–83. doi: 10.1021/tx700079z. [DOI] [PubMed] [Google Scholar]
- 11.Fowler S, Zhang H. In vitro evaluation of reversible and irreversible cytochrome P450 inhibition: Current status on methodologies and their utility for predicting drug-drug interactions. AAPS J. 2008;10:410–424. doi: 10.1208/s12248-008-9042-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studies. JAMA. 1998;279:1200–1205. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 13.Lee JH, Suh OK, Lee MG. Pharmacokinetic changes in drugs during protein-calorie malnutrition: Correlation between drug metabolism and hepatic microsomal cytochrome P450 isozymes. Arch Pharm Res. 2004;27:693–712. doi: 10.1007/BF02980136. [DOI] [PubMed] [Google Scholar]
- 14.Platt DE, Ghassibe-Sabbagh M, Salameh P, Salloum AK, Haber M, Mouzaya F, Gauguier D, Al-Sarraj Y, El-Shanti H, Zalloua PA, et al. Caffeine Impact on Metabolic Syndrome Components Is Modulated by a CYP1A2 Variant. Ann Nutr Metab. 2016;68:1–11. doi: 10.1159/000441481. [DOI] [PubMed] [Google Scholar]
- 15.El-Terras A, Soliman MM, Alkhedaide A, Attia HF, Alharthy A, Banaja AE. Carbonated soft drinks induce oxidative stress and alter the expression of certain genes in the brains of Wistar rats. Mol Med Rep. 2016;13:3147–3154. doi: 10.3892/mmr.2016.4903. [DOI] [PubMed] [Google Scholar]
- 16.Soliman MM, Baiomy AA, Yassin MH. Molecular and histopathological study on the ameliorative effects of curcumin against lead acetate-induced hepatotoxicity and nephrototoxicity in Wistar rats. Biol Trace Elem Res. 2015;167:91–102. doi: 10.1007/s12011-015-0280-0. [DOI] [PubMed] [Google Scholar]
- 17.Hensley K, Robinson KA, Gabbita SP, Salsman S, Floyd RA. Reactive oxygen species, cell signaling, and cell injury. Free Radic Biol Med. 2000;28:1456–1462. doi: 10.1016/S0891-5849(00)00252-5. [DOI] [PubMed] [Google Scholar]
- 18.Alkhedaide A, Soliman MM, Salah-Eldin AE, Ismail TA, Alshehiri ZS, Attia HF. Chronic effects of soft drink consumption on the health state of Wistar rats: A biochemical, genetic and histopathological study. Mol Med Rep. 2016;13:5109–51017. doi: 10.3892/mmr.2016.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niki E. Lipid peroxidation: Physiological levels and dual biological effects. Free Radic Biol Med. 2009;47:469–484. doi: 10.1016/j.freeradbiomed.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 20.Horton AA, Fairhurst S. Lipid peroxidation and mechanisms of toxicity. Crit Rev Toxicol. 1987;18:27–79. doi: 10.3109/10408448709089856. [DOI] [PubMed] [Google Scholar]
- 21.Blair IA. DNA adducts with lipid peroxidation products. J Biol Chem. 2008;283:15545–15549. doi: 10.1074/jbc.R700051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Łuczaj W, Skrzydlewska E. DNA damage caused by lipid peroxidation products. Cell Mol Biol Lett. 2003;8:391–413. [PubMed] [Google Scholar]
- 23.Oberley TD, Oberley LW. Antioxidant enzyme levels in cancer. Histol Histopathol. 1997;12:525–535. [PubMed] [Google Scholar]
- 24.Saydam N, Kirb A, Demir O, Hazan E, Oto O, Saydam O, Güner G. Determination of glutathione, glutathione reductase, glutathione peroxidase and glutathione S-transferase levels in human lung cancer tissues. Cancer Lett. 1997;119:13–19. doi: 10.1016/S0304-3835(97)00245-0. [DOI] [PubMed] [Google Scholar]
- 25.Zaher H, Buters JT, Ward JM, Bruno MK, Lucas AM, Stern ST, Cohen SD, Gonzalez FJ. Protection against acetaminophen toxicity in CYP1A2 and CYP2E1 double-null mice. Toxicol Appl Pharmacol. 1998;152:193–199. doi: 10.1006/taap.1998.8501. [DOI] [PubMed] [Google Scholar]
- 26.Faber MS, Jetter A, Fuhr U. Assessment of CYP1A2 activity in clinical practice: Why, how, and when? Basic Clin Pharmacol Toxicol. 2005;97:125–134. doi: 10.1111/j.1742-7843.2005.pto_973160.x. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez FJ. Molecular genetics of the P-450 superfamily. Pharmacol Ther. 1990;45:1–38. doi: 10.1016/0163-7258(90)90006-N. [DOI] [PubMed] [Google Scholar]
- 28.Gibson GG, Plant NJ, Swales KE, Ayrton A, El-Sankary W. Receptor-dependent transcriptional activation of cytochrome P4503A genes: Induction mechanisms, species differences and interindividual variation in man. Xenobiotica. 2002;32:165–206. doi: 10.1080/00498250110102674. [DOI] [PubMed] [Google Scholar]
- 29.Sumida A, Kinoshita K, Fukuda T, Matsuda H, Yamamoto I, Inaba T, Azuma J. Relationship between mRNA levels quantified by reverse transcription-competitive PCR and metabolic activity of CYP3A4 and CYP2E1 in human liver. Biochem Biophys Res Commun. 1999;262:499–503. doi: 10.1006/bbrc.1999.1233. [DOI] [PubMed] [Google Scholar]
- 30.Nedelcheva V, Gut I. P450 in the rat and man: Methods of investigation, substrate specificities and relevance to cancer. Xenobiotica. 1994;24:1151–1175. doi: 10.3109/00498259409038673. [DOI] [PubMed] [Google Scholar]
- 31.Lamba JK, Lin YS, Thummel K, Daly A, Watkins PB, Strom S, Zhang J, Schuetz EG. Common allelic variants of cytochrome P4503A4 and their prevalence in different populations. Pharmacogenetics. 2002;12:121–132. doi: 10.1097/00008571-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Nelson DR, Koymans L, Kamataki T, Stegeman JJ, Feyereisen R, Waxman DJ, Waterman MR, Gotoh O, Coon MJ, Estabrook RW, et al. P450 superfamily: Update on new sequences, gene mapping, accession numbers and nomenclature. Pharmacogenetics. 1996;6:1–42. doi: 10.1097/00008571-199602000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Shayeganpour A, El-Kadi AO, Brocks DR. Determination of the enzyme(s) involved in the metabolism of amiodarone in liver and intestine of rat: The contribution of cytochrome P450 3A isoforms. Drug Metab Dispos. 2006;34:43–50. doi: 10.1124/dmd.105.006742. [DOI] [PubMed] [Google Scholar]
- 34.Christians U. Transport proteins and intestinal metabolism: P-glycoprotein and cytochrome P4503A. Ther Drug Monit. 2004;26:104–106. doi: 10.1097/00007691-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Matsuda H, Kinoshita K, Sumida A, Takahashi K, Fukuen S, Fukuda T, Takahashi K, Yamamoto I, Azuma J. Taurine modulates induction of cytochrome P450 3A4 mRNA by rifampicin in the HepG2 cell line. Biochim Biophys Acta. 2002;1593:93–98. doi: 10.1016/S0167-4889(02)00345-2. [DOI] [PubMed] [Google Scholar]
- 36.Tsuda S, Egawa T, Kitani K, Oshima R, Ma X, Hayashi T. Caffeine and contraction synergistically stimulate 5′-AMP-activated protein kinase and insulin-independent glucose transport in rat skeletal muscle. Physiol Rep. 2015;3:e12592. doi: 10.14814/phy2.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang H, Garzel B, Heyward S, Moeller T, Shapiro P, Wang H. Metformin represses drug-induced expression of CYP2B6 by modulating the constitutive androstane receptor signaling. Mol Pharmacol. 2014;85:249–260. doi: 10.1124/mol.113.089763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mo SL, Liu YH, Duan W, Wei MQ, Kanwar JR, Zhou SF. Substrate specificity, regulation, and polymorphism of human cytochrome P450 2B6. Curr Drug Metab. 2009;10:730–753. doi: 10.2174/138920009789895534. [DOI] [PubMed] [Google Scholar]
- 39.Iber H, Li-Masters T, Chen Q, Yu S, Morgan ET. Regulation of hepatic cytochrome P450 2C11 via cAMP: Implications for down-regulation in diabetes, fasting, and inflammation. J Pharmacol Exp Ther. 2001;297:174–180. [PubMed] [Google Scholar]
- 40.Wang H, Guan W, Yang W, Wang Q, Zhao H, Yang F, Lv X, Li J. Caffeine inhibits the activation of hepatic stellate cells induced by acetaldehyde via adenosine A2A receptor mediated by the cAMP/PKA/SRC/ERK1/2/P38 MAPK signal pathway. PLoS One. 2014;9:e92482. doi: 10.1371/journal.pone.0092482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morgan NG, Blackmore PF, Exton JH. Age-related changes in the control of hepatic cyclic AMP levels by alpha 1- and beta 2-adrenergic receptors in male rats. J Biol Chem. 1983;258:5103–5109. [PubMed] [Google Scholar]
- 42.Yasuda K, Ueno S, Ueda E, Nishikawa M, Takeda K, Kamakura M, Ikushiro S, Sakaki T. Influence of sesamin on CYP2C-mediated diclofenac metabolism: In vitro and in vivo analysis. Pharmacol Res Perspect. 2015;3:e00174. doi: 10.1002/prp2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]