Abstract
Astrocytes, which have various important functions, have previously been associated with Parkinsons disease (PD), particularly in 1-methyl-4-phenylpyridinium (MPP+) and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) models of PD. MPP+ is the toxic metabolite of MPTP and is generated by the enzymatic activity of monoamine oxidase B, which is predominantly located in astrocytes. MPP+ acts as a mitochondrial complex I inhibitor. Autophagy is an evolutionarily conserved self-digestion pathway in eukaryotic cells, which occurs in response to various types of stress, including starvation and oxidative stress. Lithium treatment has previously been shown to induce autophagy in astrocytes by inhibiting the enzyme inositol monophosphatase, which may aid in the treatment of neurodegenerative diseases, including Huntington's disease, in which the toxic protein is an autophagy substrate. Therefore, using western blotting and MTT assay, the present study aimed to investigate the protective effects of lithium-induced autophagy against astrocyte injury caused by MPP+ treatment, as well as the potential underlying mechanisms. The results of the present study suggested that lithium was able to induce autophagy in astrocytes treated with MPP+, and this likely occurred via activation of the phosphoinositide 3-kinase/AKT pathway.
Keywords: astrocyte, Parkinson's disease, autophagy, 1-methyl-4-phenylpyridinium, microtubule-associated protein light chain-3, lithium, 3-methyladenine, phosphorylated AKT
Introduction
Parkinsons disease (PD), which is the second most common neurodegenerative disease among the aging human population after Alzheimer's disease (AD), is thought to be caused by genetic factors and environmental agents, including 1-methyl-4-phenyl-1,2,3,6 -tetrahydropyridine (MPTP) (1). MPTP was initially discovered to be a dopaminergic neurotoxin in the early 1980s after it contaminated a source of synthetic heroin (2), although it is predominantly used to produce animal models of PD. MPTP is converted to its metabolite 1-methyl-4-phenylpyridinium ion (MPP+) by monoamine oxidase B (MAO-B), which is predominantly localized within astrocytes (3). Previous studies have reported a role for autophagy in PD, and a defect in autophagy, particularly in mitochondrial autophagy (mitophagy), has emerged as a potential novel pathogenic mechanism underlying the development of PD (4–7). Previous studies have suggested that MPP+ may initiate dopaminergic neuronal cell death, and induce autophagy in SH-SY5Y cells, SK-N-SH cells, neuronal PC12 cells and the rat brain (8–12).
Astrocytes have numerous essential functions in the healthy central nervous system (CNS), including responding to oxidative stress-induced injury (13). Various neurological disease states, including PD, AD and ischemic injury, have previously been associated with varying degrees of astrocyte activation or astrogliosis (14,15). Autophagy has been shown to be activated in rat cortical astrocytes following permanent middle cerebral artery occlusion (pMCAO), and in primary astrocytes following oxygen and glucose deprivation (OGD) injury, in which autophagy markedly decreased cell survival following OGD and focal cerebral ischemia (16). Astrocytes express NF-E2-related factor (Nrf2), which binds to the antioxidant response element (ARE) in order to induce the expression of antioxidant enzymes. Overexpression of Nrf2 in astrocytes has been reported to protect against 6-hydroxydopamine damage in mice (17), thus suggesting a potential therapeutic strategy for the treatment of PD. Chen et al (18) demonstrated that Nrf2 expression in astrocytes was able to protect against MPTP, and suggested that modulation of the Nrf2-ARE pathway may be considered a promising target for therapeutics aimed at reducing or preventing neuronal death in patients with PD. These results supported the hypothesis that astrocytes may have a neuroprotective role in PD.
Autophagy is a cellular homeostatic process that involves the sequestration of cytoplasmic material by lysosomes for bulk degradation. Previous studies have suggested that autophagy may have an important role in the pathogenic process of PD (19–22). However, whether activation of autophagy exerts beneficial or detrimental effects in PD is currently unclear, since both protective and destructive effects have previously been reported (22,23). Lithium has been shown to induce autophagy by inhibiting inositol monophosphatase, which in turn leads to depletion of free inositol and decreased levels of inositol 1,4,5-trisphosphate (24,25). Previous studies have detected therapeutic and protective effects of lithium compounds in various models of neuronal disease, including brain ischemia, AD, affective bipolar disease and kainate-induced neuronal cell death (26–28).
Astrocytes have important functions and may be beneficial to neurons under certain conditions. In addition, MPP+ may trigger oxidative stress, which may subsequently induce autophagy. However, to the best of our knowledge, no previous study has investigated whether MPP+ is able to induce autophagy in astrocytes and the underlying mechanisms. Therefore, the present study aimed to investigate whether MPP+ was able to induce autophagy in astrocytes and its function. Furthermore, the ability of lithium to protect astrocytes treated with MPP+, and its potential underlying mechanisms, were analyzed.
Materials and methods
Ethics statement
The present study was approved by the Institutional Animal Ethical Committee of Sun Yat-sen University (Guangzhou, China), in accordance with recommendations in the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MA, USA).
Primary astrocyte culture
A total of 100 specific-pathogen-free neonatal male C57BL/6 mice (Guangzhou University of Chinese Medicine, Guangzhou, China), aged 1-day-old, were maintained at 25°C. Following sacrifice via an overdose of 10% chloral hydrate (0.03 ml; Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) via intraperitoneal injection and disinfection with 75% alcohol, astrocyte-enriched cultures were prepared from the cerebral cortex. The meninges were removed from dissected cerebral cortexes and tissues were cut into ~1 mm3 sections, which were subsequently digested using 0.25% trypsin (Gibco; Thermo Fisher Scientific, Inc. Waltham, MA, USA) at 37°C for 15 min. Digestion was terminated using Dulbecco's modified Eagle's medium/nutrient F12 (DMEM/F12) supplemented with 10% fetal bovine serum (FBS) and penicillin/streptomycin (50 U/ml; 50 µg/ml) (all Gibco; Thermo Fisher Scientific, Inc.). Following centrifugation at 112 × g for 5 min, the astrocytes were gently forced through a sterile 70 µm Nitex mesh, after which they were resuspended in DMEM/F12 containing 10% heat-inactivated FBS, 2 mM L-glutamine, 50 U/ml penicillin and 50 mg/ml streptomycin (all Gibco; Thermo Fisher Scientific, Inc.). Subsequently, astrocytes (1×106 cells/ml) were seeded into a poly-lysine-coated flask, which was stored in a humidified atmosphere containing 5% CO2 and 95% air at 37°C. The culture medium was replaced after 24 h, and was subsequently replaced every 2–3 days. Upon reaching confluence (typically 12–14 days later), microglia were detached from the astrocytes by agitation at 260 rpm for 16 h. Astrocytes were subsequently detached using trypsin-ethylenediaminetetraacetic acid solution (Gibco; Thermo Fisher Scientific, Inc.), and were seeded in the same culture medium. Following three or more consecutive passages, cells were seeded into 96-well plates (105 cells/well) or dishes for further experimentation. The purity of the astrocytes was determined using glial fibrillary acidic protein (GFAP) immunocytochemistry using rabbit anti-GFAP polyclonal antibody (1:5,000; ab7260; Abcam, Cambridge, UK), which indicated that 98% of the cultured cells were GFAP-positive, using a microscope (Bx51; Olympus Corporation, Tokyo, Japan).
Cell treatment
In order to measure the toxicity of MPP+, the cells were divided into seven groups, including one control group and six groups treated with MPP+, which were treated with 50, 100, 200, 400, 800 or 1,200 µM MPP+ (Sigma-Aldrich, St. Louis, MO, USA), respectively. In order to measure the induction of autophagy in the astrocytes, the cells were divided into several groups, including the control group (treated with FBS), the starvation group (incubated in DMEM/F12 without FBS or MPP+; positive control), MPP+ groups (0.2, 0.4 and 0.8mM MPP+, respectively), and MPP+ and inhibitor groups, which were treated with 10mM Pepstain A lysosomal inhibitor (Sigma-Aldrich). Furthermore, in order to explore the function of the induced autophagy, the cells were pretreated with autophagy inhibitors, 10 mM 3-methyladenine (3-MA), 0.01 mM chloroquine (CQ), 10 mM lysosomal inhibitor Pepstain A and 10 mM lithium (all Sigma-Aldrich) for 1 h, after which MPP+ was added for an additional 24 h.
Cell viability assay
Cell viability was analyzed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich) assay, as outlined in a previous study (29). Briefly, 24 h following treatment with the various concentrations of MPP+, astrocytes in the 96-well plates were washed with phosphate-buffered saline, after which the cells were incubated with MTT (5 mg/ml) at 37°C for 4 h. Subsequently, the supernatants were removed, 100 µl dimethyl sulfoxide was added to each well, and the plates were agitated on a microplate shaker in order to dissolve the blue MTT-formazan. Absorbance was measured at 570 nm using a ELx800 microplate reader (BioTek Instruments, Inc., Winooski, VT, USA). Cell viability was expressed as the ratio of the signal obtained from the treated cultures to the control cultures.
Western blotting
Cells were harvested and lysed using the Mammalian Cell Extraction kit (BioVision, Inc., Milpitas, CA, USA). The resulting lysates were subjected to the Bradford Protein assay (Pierce Biotechnology, Inc., Rockford, IL, USA), in order to determine protein concentrations and to ensure equal protein loading. Protein samples (30 µg) were separated by 8, 10 or 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and were subsequently transferred onto polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA). The membranes were blocked using Tris-buffered saline Tween 20 [50 mM Tris-HCl, 154 mM NaCl, 0.1% Tween 20 (pH 7.5)], containing 5% nonfat dry milk for 1 h, and were subsequently probed with rabbit anti-microtubule-associated protein light chain-3 (LC3; 1:5,000; NB100-2220; Novus Biologicals, LLC, Littleton, CO, USA) polyclonal antibody or anti-phosphorylated (p)AKT (1:5,000; ab81283; Abcam) monoclonal antibody overnight at 4°C, according to the manufacturer's protocol. Following primary antibody incubation, the membranes were washed and incubated with either horseradish peroxidase-conjugated anti-mouse (1:1,000; 7076) or anti-rabbit immunoglobulin G (1:1,000; 7074; both Cell Signaling Technology, Inc., Billerica, MA, USA) for 1 h at room temperature. Chemiluminescence reactions were conducted according to the manufacturer's protocol (EMD Millipore, Bedford, MA, USA). The intensity (INT × area) of each band was measured and analyzed using the ChemiDoc XRS+ Imaging system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Data are presented as the percentage of the vehicle control, and β-actin (1:4,000; ab-32092; Novus Biologicals, LLC) was used as an internal control.
Statistical analysis
Data were analyzed using SPSS 13.0 for Windows (SPSS, Inc., Chicago, IL, USA) and presented as the mean ± standard deviation. All experiments were repeated at least three times. Statistical analysis was conducted using one-way analysis of variance, followed by Student's t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
Toxicity of MPP+
Primary astrocyte cells were treated with increasing concentrations of MPP+, ranging from 0 to 1,200 µM, for 24 h, and the cell viability was determined in order to identify the optimal concentration of MPP+ (Fig. 1). MPP+ decreased cell viability in a concentration-dependent manner. The viability of cells treated with >200 µM MPP+ was significantly decreased, particularly at 800 and 1,200 µM MPP+ (P<0.05; Fig 1). The median lethal dose of MPP+ was ~430 µM. Therefore, 400 and 800 µM MPP+ concentrations were selected for further experimentation.
Figure 1.
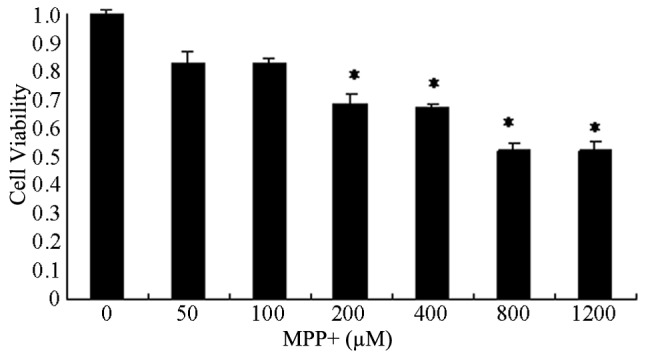
Concentration-dependent effects of MPP+ on the cell viability of primary astrocyte cells. The cells were treated with 0, 50, 100, 200, 400, 800 or 1,200 µM MPP+ for 24 h, and the cell viability was determined using MTT assays. Absorbance was measured using a microplate reader. Data are presented as the mean ± standard error of the mean of five experiments. *P<0.05 vs. the untreated controls. MPP+, 1-methyl-4-phenylpyridinium.
Induction of autophagy in MPP+-treated primary astrocytes
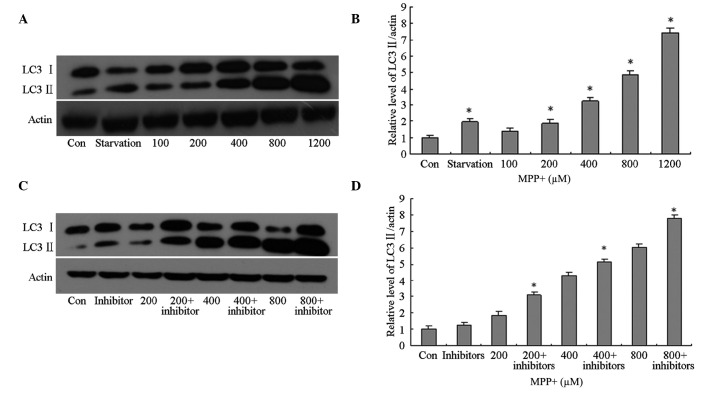
In order to investigate autophagy induction in the control cells, as well as those treated with various concentrations of MPP+, total protein was extracted from the astrocytes and western blotting using anti-LC3 primary antibodies was conducted. In the primary astrocytes treated with >200 µM MPP+, the protein expression levels of LC3 II were significantly increased in a concentration-dependent manner (P<0.05; Fig. 2A and B), as compared with the control cells. However, it was unclear whether the increase in LC3 II protein expression levels was associated with autophagy induction or lysosomal dysfunction; therefore, the cells were pretreated with lysosomal inhibitors. The protein expression levels of LC3 II in the MPP+-treated cells pretreated with lysosomal inhibitors were significantly increased in a concentration-dependent manner (P<0.05; Fig. 2C and D), as compared with the control cells; thus suggesting that LC3 II protein expression levels increased in MPP+-treated astrocytes due to the induction of autophagy and not the impairment of lysosomes.
Figure 2.
Effects of MPP+ on the protein expression levels of LC3 II in primary astrocytes. (A and B) The cells were treated with FBS (Con), without FBS (starvation) or MPP+ (100, 200, 400, 800 or 1,200 µM) for 24 h. Autophagy was determined by detecting the protein expression levels of LC3 II using western blotting. Protein concentrations were quantified by measuring the band density. (C and D) The cells were treated with FBS (Con), without FBS (starvation) or MPP+ (200, 400 or 800 µM), and a lysosomal inhibitor for 24 h. The protein expression levels of LC3 II were detected using western blotting. Protein concentrations were quantified by measuring the band density. Data are presented as the mean ± standard error of the mean of triplicate experiments. *P<0.05 vs. the control group. MPP+, 1-methyl-4-phenylpyridinium; LC3, microtubule-associated protein light chain-3; FBS, fetal bovine serum; Con, control group.
Effects of autophagy inhibitors on the viability of astrocytes treated with MPP+
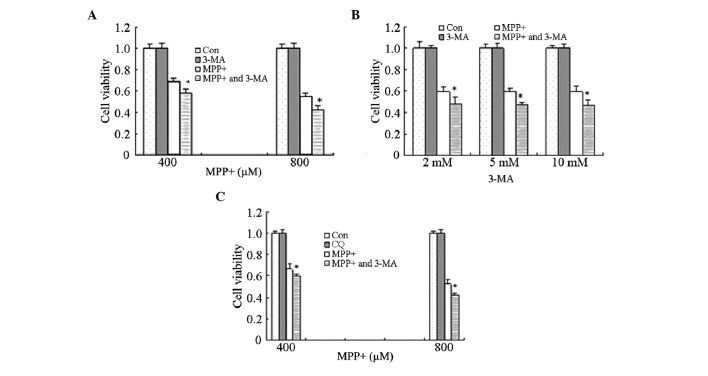
In order to investigate the effects of MPP+-induced autophagy, astrocytes were treated with 10 mM 3-MA for 1 h, prior to treatment with MPP+ for 24 h. Cell viability was significantly decreased in the cells treated with MPP+ and 3-MA, as compared with the cells treated with MPP+ alone (P<0.05; Fig. 3A). These results suggest that MPP+-induced autophagy exerts potential protective effects on astrocytes.
Figure 3.
Effects of autophagy inhibitors on the cell viability of MPP+-treated astrocytes. (A) The effects of 3-MA on the viability of MPP+-treated cells. Astrocytes were pretreated with 3-MA (10 mM) for 1 h, after which they were treated with MPP+ (400 or 800 µM) for 24 h. (B) The effects of various doses of 3-MA on the viability of MPP+-treated cells. Astrocytes were pretreated with 3-MA (2, 5 or 10 mM) for 1 h, after which they were treated with MPP+ (800 µM) for 24 h. (C) The effects of CQ on the viability of MPP+-treated cells. Astrocytes were pretreated with CQ (0.01 mM) for 1 h, after which they were treated with MPP+ (400 or 800 mM) for 24 h. The cell viability was determined using MTT assays, and absorbance was measured using a microplate reader. Data are presented as the mean ± standard error of the mean of triplicate experiments. *P<0.05 vs. the Con and MPP+-treated astrocytes. MPP+, 1-methyl-4-phenylpyridinium; 3-MA, 3-methyladenine; CQ, chloroquine; Con, control group.
However, previous studies reported that high doses of 3-MA were lethal to cells, and therefore the effects of three different doses of 3-MA (2, 5 and 10 mM) were analyzed in the present study. Treatment with 3-MA (2, 5 or 10 mM) alone did not affect cell viability; however, cell viability was significantly decreased following treatment of astrocytes with all three concentrations of 3-MA in combination with MPP+, as compared with MPP+ treatment alone (P<0.05; Fig. 3B). These results suggest that the ability of 3-MA to decrease cell viability is not associated with the dose but with other mechanisms, such as the inhibition of autophagy.
Astrocytes were also treated with CQ in order to corroborate the effects of autophagy inhibitors on the cell viability of MPP+-treated astrocytes. Consistent with the results for 3-MA, astrocytes pretreated with CQ for 1 h exhibited decreased cell viability, as compared with the MPP+ treatment alone (P<0.05; Fig. 3C). These results suggest that MPP+-induced autophagy exerts protective effects.
Potential mechanisms underlying the effects of 3-MA on MPP+-treated cells
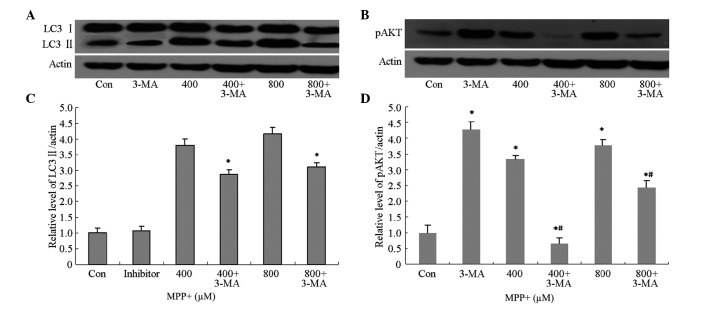
3-MA is a selective inhibitor of class III phosphatidylinositol kinase (30,31), and has been accepted as a specific inhibitor of autophagy. In order to investigate whether 3-MA was able to inhibit MPP+-induced autophagy, the protein expression levels of autophagy-associated proteins, including LC3 II and pAkt, were analyzed by western blotting. The protein expression levels of LC3 II and pAkt in astrocytes pretreated with 3-MA were markedly decreased (Fig. 4A and B), and were shown to be significantly decreased by quantification of optical density (OD) values (P<0.05; Fig. 4C and D). These results suggest that 3-MA is able to inhibit MPP+-induced autophagy via inhibition of the phosphoinositide 3-kinase (PI3K)/AKT pathway.
Figure 4.
Effects of 3-MA pretreatment on LC3 II and pAkt protein expression levels in astrocytes treated with MPP+. Astrocytes were pretreated with 3-MA (10 mM) for 1 h, after which they were treated with MPP+ (400 or 800 µM) for 24 h. Protein expression levels of (A) LC3 II and (B) pAkt were detected using western blotting. The relative protein expression levels of (C) LC3 II and (D) pAkt were quantified by calculating OD values. Data are presented as the mean ± standard error of the mean of triplicate experiments. *P<0.05 vs. the control group; #P<0.05 vs. the MPP+-treated astrocytes. 3-MA, 3-methyladenine; LC3 II, microtubule-associated protein light chain-3; MPP+, 1-methyl-4-phenylpyridinium; OD, optical density; Con, control.
Protective effects of lithium on astrocytes treated with MPP+
Lithium exerts protective effects in numerous diseases, including AD and Huntington's disease, and its underlying mechanism has been shown to involve the inhibition of glycogen synthase kinase-3β, activation of the PI3K/Akt pathway, and the induction of autophagy (24,32). In order to investigate the effects of lithium on astrocytes treated with MPP+, astrocytes were pretreated with lithium for 1 h and cell viability was determined. The viability of cells pretreated with lithium significantly increased, as compared with the cells treated with MPP+ alone (P<0.05; Fig. 5). These results suggest that lithium is able to protect astrocytes against the effects MPP+.
Figure 5.
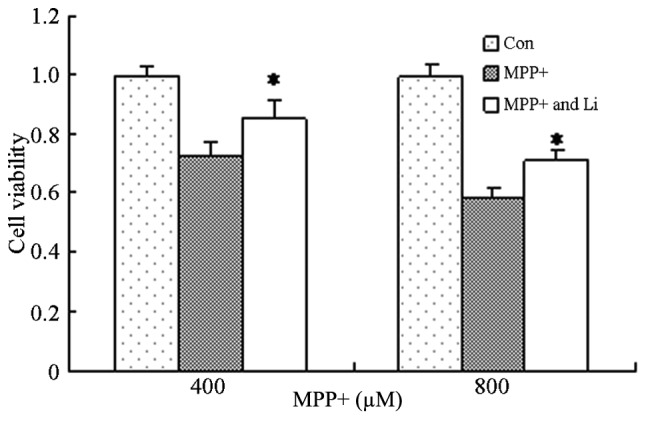
Protective effects of Li on MPP+-treated astrocytes. Astrocytes were pretreated with Li (10 mM) for 1 h, after which they were treated with MPP+ (400 or 800 µM) for 24 h. Cell viability was determined using MTT assays and the absorbance was measured using a microplate reader. Data are presented as the mean ± standard error of the mean of triplicate experiments. *P<0.05 vs. the control group. MPP+, 1-methyl-4-phenylpyridinium; Li, lithium; Con, control.
Potential mechanisms underlying the protective effects of lithium
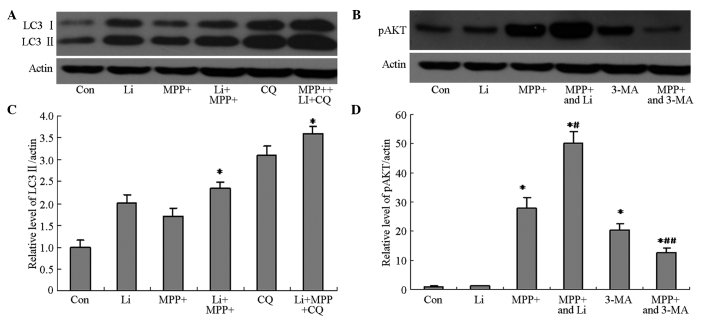
The ability of lithium to induce autophagy in MPP+-treated astrocytes was investigated, in order to elucidate the mechanism underlying the protective effects of lithium. The protein expression levels of LC3 II and pAkt in cells pretreated with lithium were markedly increased, as compared with the cells treated with MPP+ alone (Fig. 6A and B), and were shown to be significantly increased by quantification of OD values (P<0.05; Fig. 6C and D). Furthermore, pretreatment with CQ, as well as lithium, markedly attenuated the lithium-induced upregulation of LC3 II and pAkt protein expression (Fig. 6), and viability (Fig. 7) of cells treated with MPP+. These results suggest that the protective effects of lithium may be associated with its ability to induce autophagy in astrocytes treated with MPP+ via activation of the PI3K/Akt pathway.
Figure 6.
Effects of Li pretreatment on LC3 II and pAkt protein expression levels in astrocytes treated with MPP+. Astrocytes were pretreated with Li (10 mM) for 1 h, after which they were treated with MPP+ (400 µM) and CQ (0.01 mM) for 24 h. (A) The protein expression levels of LC3 II were detected using western blotting. (B) Astrocytes were pretreated with Li (10 mM) for 1 h, after which they were treated with MPP+ (400 µM) for 24 h. The protein expression levels of pAkt were detected using western blotting. The relative protein expression levels of (C) LC3 II and (D) pAkt were determined by quantifying OD values. Data are presented as the mean ± standard error of the mean of triplicate experiments. *P<0.05 vs. the control group; #P<0.05 vs. MMP+ alone; ##P<0.05 vs. 3-MA alone. Li, lithium; LC3 II, microtubule-associated protein light chain-3; MPP+, 1-methyl-4-phenylpyridinium; CQ, chloroquine; OD, optical density; Con, control.
Figure 7.
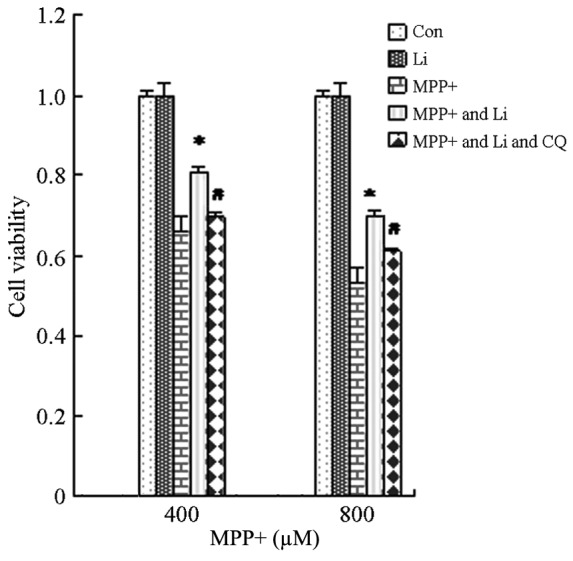
Li-induced autophagy is protective to astrocytes. Astrocytes were pretreated with Li (10 mM) for 1 h, after which they were treated with MPP+ (400 or 800 µM) and CQ (0.01 mM) for 24 h. Cell viability was determined using MTT assays and the absorbance was measured using a microplate reader. Data are presented as the mean ± standard error of the mean of triplicate experiments. *P<0.05 vs. the control group. Li, lithium; MPP+, 1-methyl-4-phenylpyridinium; CQ, chloroquine; Con, control.
Discussion
Glial cells have an active role in normal brain functioning, and their interactions with neuronal cells are important for maintaining homeostasis in the extracellular microenvironment (33–35). The function of glial cells is particularly important following a CNS insult, such as ischemia. Following injury, astrocytes are responsible for the removal of accumulated excitotoxic neurotransmitters and ions, including glutamate, lactate, hydrogen, and potassium ions, which have previously been associated with brain injury (36). High levels of glutamine synthetase have been detected in astrocytes, which, alongside a specific glutamate transporter, acts to remove and detoxify extracellular glutamate and generate the neuronal substrate glutamine, as part of the glutamate/glutamine cycle (37,38). Astrocytes store glycogen and are able to provide lactate as an alternative aerobic substrate for neuronal energy production during recovery, and also maintain the osmotic environment (39–41). Astrocytes produce various cytokines and growth factors, which function in the CNS as mediators of immune and inflammatory responses, and may exert neurotoxic and neuroprotective effects (42). High levels of reduced glutathione, which is an important antioxidant in the CNS, have previously been detected in astrocytes, but not in neurons (43). Astrocytes support and protect themselves, and other cellular elements with which they are in intimate contact, and also actively participate in the destruction of brain tissue following ischemia; thus suggesting that a disturbance of astroglial activities may induce neuronal dysfunction.
The present study hypothesized that the MPP+ neurotoxin may induce autophagy in primary astrocytes, and aimed to investigate the effects of the induced autophagy. In addition, the ability of lithium treatment to enhance autophagy and exert protective effects on astrocytes, as well as the potential underlying mechanisms, was investigated. The results of the present study suggested that MPP+ was able to induce autophagy in astrocytes, likely via the induction of oxidative stress.
In the present study, astrocytes pretreated with autophagy inhibitors, including 3-MA and CQ, exhibited decreased cell viability, as compared with cells treated with MPP+ alone, thus suggesting that autophagy induced by MPP+ exerts protective effects on astrocytes. These results were inconsistent with Zhu et al (9), who demonstrated that neither 3-MA nor wortmannin (WT) were able to inhibit the increase in autophagic vacuoles (AV)/late AVs induced by MPP+. In addition, 3-MA (5 mmol/l) and WT (50–5 mol/l) had no significant effects on basal SH-SY5Y cell viability.
Previous studies have demonstrated that lithium may induce autophagy (24,25). Lithium compounds have been shown to exert therapeutic and protective effects in various models of neuronal disease, including brain ischemia and AD. In addition, LiCl was demonstrated to attenuate a reduction in the cell viability of PC12 cells induced by treatment with MPP+ via the induction of autophagy (44). In the present study, the cell viability of astrocytes pretreated with lithium for 1 h were significantly increased, as compared with the cells treated with MPP+ only. These results were consistent with our hypothesis and the results from Youdim and Arraf (44); however, they were inconsistent with previous studies, which were unable to demonstrate cytoprotective effects for lithium in astroglial cells and cerebellar granule neurons (45,46). However, this inconsistency may be attributed to the analysis of different cell types among the studies. In addition, the results of the present study suggested that the protective effects of lithium on astrocytes treated with MPP+ were associated with the induction of autophagy and activation of the PI3K/AKT pathway, which was consistent with previous studies (24,47).
In the present study, MPP+ was able to induce autophagy in astrocytes, which was shown to exert protective effects on the cells. In addition, lithium was able to protect astrocytes from the toxic effects of MPP+ by enhancing the rate of autophagy; thus supporting the hypothesis that induced autophagy in astrocytes treated with MPP+ may exert protective effects. The results of the present study may help to elucidate the important role of astrocytes in the pathophysiology of PD, and direct the development of a novel strategy to treat patients with PD. Future studies should endeavor to elucidate the effects of induced autophagy in astrocytes on neurons.
Acknowledgements
The present study was supported by grants from the National Basic Research Program of China (grant no. 2011CB504100), the National Natural Science Foundation of China (grant no. 30770766), the Guangdong Natural Science Foundation (grant no. 10151008901000187) and the Guangdong Science and Technology Foundation (grant nos. 2008B080703021, 2006B36004021 and 2006B60501023).
References
- 1.Dauer W, Przedborski S. Parkinson's disease: Mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/S0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 2.Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 3.Vives BC, Zhou C, Huang Y, Cui M, de Vries RL, Kim J, May J, Tocilescu MA, Liu W, Ko HS, et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci USA. 2010;107:378–383. doi: 10.1073/pnas.0911187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, Youle RJ. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8:e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geisler S, Holmström KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, Springer W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol. 2010;12:119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- 6.Kawajiri S, Saiki S, Sato S, Sato F, Hatano T, Eguchi H, Hattori N. PINK1 is recruited to mitochondria with parkin and associates with LC3 in mitophagy. FEBS Lett. 2010;584:1073–1079. doi: 10.1016/j.febslet.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Levitt P, Pintar JE, Breakefield XO. Immunocytochemical demonstration of monoamine oxidase B in brain astrocytes and serotonergic neurons. Proc Natl Acad Sci USA. 1982;79:6385–6389. doi: 10.1073/pnas.79.20.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zigmond MJ, Stricker EM. Animal models of parkinsonism using selective neurotoxins: Clinical and basic implications. Int Rev Neurobiol. 1989;31:1–79. doi: 10.1016/S0074-7742(08)60277-9. [DOI] [PubMed] [Google Scholar]
- 9.Zhu JH, Horbinski C, Guo F, Watkins S, Uchiyama Y, Chu CT. Regulation of autophagy by extracellular signal-regulated protein kinases during 1-methyl-4-phenylpyridinium-induced cell death. Am J Pathol. 2007;170:75–86. doi: 10.2353/ajpath.2007.060524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nopparat C, Porter JE, Ebadi M, Govitrapong P. 1-methyl-4-phenylpyridinium-induced cell death via autophagy through a Bcl-2/Beclin 1 complex-dependent pathway. Neurochem Res. 2014;39:225–232. doi: 10.1007/s11064-013-1208-8. [DOI] [PubMed] [Google Scholar]
- 11.Rodríguez-Blanco J, Martín V, García-Santos G, Herrera F, Casado-Zapico S, Antolín I, Rodriguez C. Cooperative action of JNK and AKT/mTOR in 1-methyl-4-phenylpyridinium-induced autophagy of neuronal PC12 cells. J Neurosci Res. 2012;90:1850–1860. doi: 10.1002/jnr.23066. [DOI] [PubMed] [Google Scholar]
- 12.Hung KC, Huang HJ, Lin MW, Lei YP, Lin AM. Roles of autophagy in MPP+ -induced neurotoxicity in vivo: The involvement of mitochondria and α-synuclein aggregation. PLoS One. 2014;9:e91074. doi: 10.1371/journal.pone.0091074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch EC, Breidert T, Rousselet E, Hunot S, Hartmann A, Michel PP. The role of glial reaction and inflammation in Parkinson's disease. Ann N Y Acad Sci. 2003;991:214–228. doi: 10.1111/j.1749-6632.2003.tb07478.x. [DOI] [PubMed] [Google Scholar]
- 15.Teismann P, Schulz JB. Cellular pathology of Parkinson's disease: Astrocytes, microglia and inflammation. Cell Tissue Res. 2004;318:149–161. doi: 10.1007/s00441-004-0944-0. [DOI] [PubMed] [Google Scholar]
- 16.Qin AP, Liu CF, Qin YY, Hong LZ, Xu M, Yang L, Liu J, Qin ZH, Zhang HL. Autophagy was activated in injured astrocytes and mildly decreased cell survival following glucose and oxygen deprivation and focal cerebral ischemia. Autophagy. 2010;6:738–753. doi: 10.4161/auto.6.6.12573. [DOI] [PubMed] [Google Scholar]
- 17.Jakel RJ, Townsend JA, Kraft AD, Johnson JA. Nrf2-mediated protection against 6-hydroxy-dopamine. Brain Res. 2007;1144:197–201. doi: 10.1016/j.brainres.2007.01.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen PC, Vargas MR, Pani AK, Smeyne RJ, Johnson DA, Kan YW, Johnson JA. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson's disease: Critical role for the astrocyte. Proc Natl Acad Sci USA. 2009;106:2933–2938. doi: 10.1073/pnas.0813361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plowey ED, Cherra SJ, III, Liu YJ, Chu CT. Role of autophagy in G2019S-LRRK2-associated neurite shortening in differentiated SH-SY5Y cells. J Neurochem. 2008;105:1048–1056. doi: 10.1111/j.1471-4159.2008.05217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA, Sou YS, Saiki S, Kawajiri S, Sato F, et al. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol. 2010;189:211–221. doi: 10.1083/jcb.200910140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tong Y, Yamaguchi H, Giaime E, Boyle S, Kopan R, Kelleher RJ, III, Shen J. Loss of leucine-rich repeat kinase 2 causes impairment of protein degradation pathways, accumulation of alpha-synuclein and apoptotic cell death in aged mice. Proc Natl Acad Sci USA. 2010;107:9879–9884. doi: 10.1073/pnas.1004676107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alegre-Abarrategui J, Christian H, Lufino MM, Mutihac R, Venda LL, Ansorge O, Wade-Martins R. LRRK2 regulates autophagic activity and localizes to specific membrane microdomains in a novel human genomic reporter cellular model. Hum Mol Genet. 2009;18:4022–4034. doi: 10.1093/hmg/ddp346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan T, Rawal P, Wu Y, Xie W, Jankovic J, Le W. Rapamycin protects against rotenone-induced apoptosis through autophagy induction. Neuroscience. 2009;164:541–551. doi: 10.1016/j.neuroscience.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 24.Sarkar S, Floto RA, Berger Z, Imarisio S, Cordenier A, Pasco M, Cook LJ, Rubinsztein DC. Lithium induces autophagy by inhibiting inositol monophosphatase. J Cell Biol. 2005;170:1101–1111. doi: 10.1083/jcb.200504035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarkar S, Rubinsztein DC. Inositol and IP3 levels regulate autophagy: Biology and therapeutic speculations. Autophagy. 2006;2:132–134. doi: 10.4161/auto.2387. [DOI] [PubMed] [Google Scholar]
- 26.Chuang DM. The antiapoptotic actions of mood stabilizers: molecular mechanisms and therapeutic potentials. Ann NY Acad Sci. 2005;1053:195–204. doi: 10.1111/j.1749-6632.2005.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 27.Cappuccio I, Calderone A, Busceti CL, Biagioni F, Pontarelli F, Bruno V, Storto M, Terstappen GT, Gaviraghi G, Fornai F, et al. Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is required for the development of ischemic neuronal death. J Neurosci. 2005;25:2647–2657. doi: 10.1523/JNEUROSCI.5230-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Busceti CL, Biagioni F, Aronica E, Riozzi B, Storto M, Battaglia G, Giorgi FS, Gradini R, Fornai F, Caricasole A, et al. Induction of the Wnt inhibitor, Dickkopf-1, is associated with neurodegeneration related to temporal lobe epilepsy. Epilepsia. 2007;48:694–705. doi: 10.1111/j.1528-1167.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- 29.Loo DT, Rilleman JR. Measurement of cell death. Methods Cell Biol. 1998;57:251–264. doi: 10.1016/S0091-679X(08)61583-6. [DOI] [PubMed] [Google Scholar]
- 30.Seglen PO, Gordon PB. 3-Methyladenine: Specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc Natl Acad Sci USA. 1982;79:1889–1892. doi: 10.1073/pnas.79.6.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schu PV, Takegawa K, Fry MJ, Stack JH, Waterfield MD, Emr SD. Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science. 1993;260:88–91. doi: 10.1126/science.8385367. [DOI] [PubMed] [Google Scholar]
- 32.Sarkar S, Krishna G, Imarisio SA, Saiki S, O'Kane CJ, Rubinsztein DC. A rational mechanism for combination treatment of Huntington's disease using lithium and rapamycin. Hum Mol Genet. 2008;17:170–178. doi: 10.1093/hmg/ddm294. [DOI] [PubMed] [Google Scholar]
- 33.Norenberg MD. Astrocyte responses to CNS injury. J Neuropathol Exp Neurol. 1994;53:213–220. doi: 10.1097/00005072-199405000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Norenberg MD. Active and passive roles of astrocytes in neurologic disease: Commentary on forum position paper. Neurotoxicology. 1998;19:23–26. [PubMed] [Google Scholar]
- 35.Ridet JL, Malhotra SK, Privat A, Gage FH. Reactive astrocytes: Cellular and molecular cues to biological function. Trends Neurosci. 1997;20:570–577. doi: 10.1016/S0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- 36.Amédée T, Robert A, Coles JA. Potassium homeostasis and glial energy metabolism. Glia. 1997;21:46–55. doi: 10.1002/(SICI)1098-1136(199709)21:1<46::AID-GLIA5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 37.Sonnewald U, Westergaard N, Schousboe A. Glutamate transport and metabolism in astrocytes. Glia. 1997;21:56–63. doi: 10.1002/(SICI)1098-1136(199709)21:1<56::AID-GLIA6>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 38.Tansey FA, Farooq M, Cammer W. Glutamine synthetase in oligodendrocytes and astrocytes: New biochemical and immunocytochemical evidence. J Neurochem. 1991;56:266–272. doi: 10.1111/j.1471-4159.1991.tb02591.x. [DOI] [PubMed] [Google Scholar]
- 39.Schurr A, Payne RS, Miller JJ, Rigor BM. Brain lactate is an obligatory aerobic energy substrate for functional recovery after hypoxia: Further in vitro validation. J Neurochem. 1997;69:423–426. doi: 10.1046/j.1471-4159.1997.69010423.x. [DOI] [PubMed] [Google Scholar]
- 40.Gerhart DZ, Enerson BE, Zhdankina OY, Leino RL, Drewes LR. Expression of monocarboxylate transporter MCT1 by brain endothelium and glia in adult and suckling rats. 1. Am J Physiol. 1997;273(1):E207–E213. doi: 10.1152/ajpendo.1997.273.1.E207. [DOI] [PubMed] [Google Scholar]
- 41.Koehler-Stec EM, Simpson IA, Vannucci SJ, Landschulz KT, Landschulz WH. Monocarboxylate transporter expression in mouse brain. 1. Am J Physiol. 1998;275(3):E516–E524. doi: 10.1152/ajpendo.1998.275.3.E516. [DOI] [PubMed] [Google Scholar]
- 42.Aschner M. Astrocytic functions and physiological reactions to injury: The potential to induce and/or exacerbate neuronal dysfunction-a forum position paper. Neurotoxicology. 1998;19:7–17. [PubMed] [Google Scholar]
- 43.Raps SP, Lai JC, Hertz L, Cooper AJ. Glutathione is present in high concentrations in cultured astrocytes but not in cultured neurons. Brain Res. 1989;493:398–401. doi: 10.1016/0006-8993(89)91178-5. [DOI] [PubMed] [Google Scholar]
- 44.Youdim MB, Arraf Z. Prevention of MPTP (N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) dopaminergic neurotoxicity in mice by chronic lithium: Involvements of Bcl-2 and Bax. Neuropharmacology. 2004;46:1130–1140. doi: 10.1016/j.neuropharm.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 45.Lai JS, Zhao C, Warsh JJ, Li PP. Cytoprotection by lithium and valproate varies between cell types and cellular stresses. Eur J Pharmacol. 2006;539:18–26. doi: 10.1016/j.ejphar.2006.03.076. [DOI] [PubMed] [Google Scholar]
- 46.Yeste M, Alvira1 D, Verdaguer E, Tajes M, Folch J, Rimbau V, Pallàs M, Camins A. Evaluation of acute antiapoptotic effects of Li+ in neuronal cell cultures. J Neural Transm. 2007;114:405–416. doi: 10.1007/s00702-006-0557-8. [DOI] [PubMed] [Google Scholar]
- 47.King TD, Bijur GN, Jope RS. Caspase-3 activation induced by inhibition of mitochondrial complex I is facilitated by glycogen synthase kinase-3beta and attenuated by lithium. Brain Res. 2001;919:106–114. doi: 10.1016/S0006-8993(01)03005-0. [DOI] [PubMed] [Google Scholar]