Abstract
IMPORTANCE
This is the first study to date to examine volumetric alterations in the anterior insula (AI) as a potential biomarker for the course of childhood major depressive disorder (MDD).
OBJECTIVES
To examine whether children with a history of preschool-onset (PO) MDD show reduced AI volume, whether a specific symptom of PO MDD (pathological guilt) is related to AI volume reduction (given the known relationship between AI and guilt processing), and whether AI volumes predict subsequent likelihood of having an episode of MDD.
DESIGN, SETTING, AND PARTICIPANTS
In a prospective longitudinal study, 306 children (age range, 3.00–5.11 years) and caregivers completed DSM diagnostic assessments at 6 annual time points during 10 years as part of the Preschool Depression Study. Magnetic resonance imaging was completed on a subset of 145 school-age children (age range, 6.11–12.11 years).
MAIN OUTCOMES AND MEASURES
Whole-brain–adjusted AI volume measured using magnetic resonance imaging at school age and children’s diagnosis of MDD any time after their imaging.
RESULTS
Compared with children without a history of PO MDD, school-age children previously diagnosed as having PO MDD had smaller left and right AI volumes (Wilks Λ = 0.94, F2,124 = 3.37, P = .04, Cohen d = 0.23). However, the effect of PO MDD on reduced AI volumes was better explained by children’s experience of pathological guilt during preschool (Λ = 0.91, F2,120 = 6.17, P = .003, d = .30). When covarying for children’s lifetime history of MDD episodes, their experience of pathological guilt during preschool, as well as their sex and age at the time of imaging, schoolchildren’s right-side AI volume was a significant predictor of being diagnosed as having an MDD episode after imaging (odds ratio, 0.96; 95% CI, 0.01–0.75; P = .03).
CONCLUSIONS AND RELEVANCE
These results provide evidence that structural abnormalities in AI volume are related to the neurobiology of depressive disorders starting in early childhood. The present findings are consistent with mounting research in adult MDD suggesting that insula function and structure may be a target biomarker for major depression.
The search for early neurobehavioral markers for depression has been the focus of intense investigation for several decades.1,2 While important advances have been made in understanding atypical structural and functional brain correlates of emotion processing and regulation in depressed individuals, the identification of specific regions or networks associated with symptom manifestations and illness onset and course remains an important and somewhat elusive goal. The identification of early symptom–specific neurobehavioral markers of a chronic and recurrent course of depression could inform which symptom domains and therefore which individuals to target for early interventions. Furthermore, understanding brain-behavior relationships in this risk trajectory could be critical to illuminating the mechanisms of risk, information that is essential for the design of targeted early interventions.3
Investigation of brain-behavior relationships in depressed preschool-age children is a new direction that has the potential to elucidate trajectories of risk and the development of preventive interventions.4 A growing body of literature has established construct and discriminant validity for a form of depression in preschool children that shows continuity with DSM-5 major depressive disorder (MDD) at school age and early adolescence.5 More specifically, findings indicated that approximately 50% of children diagnosed as having a preschool-onset (PO) form of MDD (ie, developmentally modified duration and symptom manifestations) went on to develop full DSM-5 criteria for a major depressive episode (with no modifications). Preschool-onset MDD is a specific and stable syndrome that has been identified in several independent study samples and is characterized by age-adjusted core DSM symptoms of depression (but excludes 2-week duration).6,7 Preschool depression has been detected in several epidemiological samples, and a 1% to 2% prevalence rate has been estimated.8–10 Furthermore, PO MDD has been associated with alterations in stress cortisol reactivity, altered neural functioning, atypical neural system connectivity, and volumetric brain alterations consistent with established findings in adult depression.11–16
To date, one of the most consistent and robust correlates of PO depression has been the tendency for pathological guilt.17–19 This includes both the experience of excessive guilt and infrequent or chronic maladaptive attempts to repair, amend, or correct wrongdoings (real or imagined) from which a sense of guilt emerged. For example, toddlers (between 12 and 35 months old) who manifest pathological forms of guilt before age 3 years were on average 10 times as likely as same-age peers without pathological guilt to be diagnosed as having MDD at age 5 years.19 Notably, high levels of guilt in conjunction with the chronic use of maladaptive reparation strategies (eg, rumination) to reduce excessive feelings of guilt have been shown to be a highly specific marker of preschool depression, differentiating it from other disorders, including anxiety disorders.17,20 While the etiology of the early development of guilt remains understudied, empirical data have established the important influence of caregiving behaviors, genetic factors, and experiences of adversity, stress, and trauma.21–33
Given the central and specific role of guilt in preschool depression, it is critical to understand the neurobiological correlates of guilt in this group. Therefore, the aim of the present study was to examine pathological vs nonpathological guilt within the context of early childhood depression. There is mounting evidence from social neuroscience research indicating that structural and functional features of the anterior insula (AI) serve as a neural substrate for experiences and regulation of self-conscious emotions in general and guilt in particular.34,35 For example, researchers have used a guilt-focused autobiographical narrative task using neuroimaging methods to demonstrate the role of the AI in the experience of guilt.36,37 Investigations using functional magnetic resonance imaging have also implicated the insula in other complex social emotions such as empathy.38 Relevant to these basic brain-emotion relationships, atypical structural and activation properties of the AI have been identified in adults with past, current, and future episodes of MDD.39,40 Decreased volume of left and right AI has been detected in acutely depressed and remitted depressed adults.40–48 More specifically, variation in AI volume has been associated with MDD episode number and duration, symptom severity, and prognosis in older samples.3,43,44,49 Therefore, findings from disparate but highly related areas of social, affective, and clinical neuroscience provide empirical support for our hypothesis that preschool depression would predict AI volume reduction when measured at school age and that the early experience of pathological guilt may be an important symptom in the expected relationship between PO MDD and reduced AI volumes.
Although numerous other cortical and subcortical regions have been implicated in the processing and regulation of emotion in depressive and healthy samples, the AI is consistently implicated in the learning, processing, and regulation of social emotions such as guilt, a highly specific symptom of PO MDD. Furthermore, our focus on the AI as opposed to the posterior insula is based on extant findings that the anterior and not posterior portion of the insula has a prominent role in emotion processing. Therefore, the anterior portion of the insula was the focus of the present study based on mounting evidence for its role in depressotypic cognitions and emotion processing and its involvement in the complex social emotion of guilt.
In the present prospective longitudinal study, we investigated volume differences of the AI in a population of children who experienced depression during the preschool period compared with children who were without this history. Based on findings in older children and adults, we hypothesized that children with a history of PO MDD would have significantly smaller AI volumes than same-age peers without PO MDD, even after controlling for comorbid anxiety disorders. If AI volumes differed in relation to PO MDD, we aimed to test whether specific symptoms of MDD, particularly guilt, could be identified as a link between PO MDD and decreased AI volume. Preschool-onset pathological guilt (PO guilt) was tested as a moderator of the expected relationship between PO MDD and AI volume. That is, schoolchildren with a history of PO MDD and PO guilt were expected to have smaller insula volume than children with only one of these characteristics. The temporal nature of the data collection did not allow us to test guilt as a mediator of the hypothesized relationship between PO MDD and AI volume. However, we tested whether the expected relationship between PO MDD or preschool pathological guilt and AI volume remained significant when covarying for children’s experience of stressful or traumatic life events, which are known to effect both guilt development and brain function and structure.50–52 The second major aim of the present study was to test AI volume as a candidate structural neuro-marker of childhood MDD course. We hypothesized that reduced left and right AI volume would predict the likelihood of a recurrent course of MDD in later childhood.
Methods
Participants
Parental written consent and child assent were obtained before study participation. The institutional review board at Washington University in St Louis approved all procedures in accord with institutional ethical guidelines. Data were analyzed from 145 participants in the Preschool Depression Study, a prospective longitudinal study of 306 preschool-age children conducted at the Washington University School of Medicine in St Louis Early Emotional Development Program. For the original study, children 3.00 to 5.11 years old and their primary caregivers were recruited from day cares, preschools, and primary care sites in the St Louis, Missouri, area using the Preschool Feelings Checklist53 to oversample children with depression or at risk for depression. Children underwent 6 annual clinical assessments during 10 years (ie, approximately every 12 months), and a subset will have completed 3 neuroimaging sessions (ie, approximately every 18 months) between the ages of 6.11 and 12.11 years (eFigure 1 in the Supplement).
Original Preschool Depression Study participants who met all inclusion criteria based on data quality and availability were included in the present analyses. Of 306 children in the Preschool Depression Study, 145 completed the neuroimaging session and had complete data on all variables in the present analyses. Nine participants were excluded based on being born at less than 34 weeks’ gestation, the mother reporting drinking during all 3 trimesters, and the child having an IQ of less than 80. Of the 136, an additional 7 children did not have diagnostic data available after imaging at the time of analyses, resulting in a final sample size of 129 for all proceeding analyses.
Measures
DSM Psychiatric Diagnoses
Trained staff conducted up to 6 in-person assessments with children and their primary caregivers from study enrollment through the time of imaging. For assessments before age 8 years, a reliable and age-appropriate semistructured parent-reported diagnostic interview (the Preschool Age Psychiatric Assessment [PAPA]54) was used to assess psychiatric symptoms. After age 8 years, the Child and Adolescent Psychiatric Assessment (CAPA)55,56 was used, which includes child-reported and caregiver-reported psychiatric symptoms to inform diagnostic classification. Interviews were audiotaped, reviewed for reliability, and calibrated for accuracy.57 Four dichotomous diagnostic variables (absent or present) were created based on the caregivers’ completed PAPA and the parents’ and children’s completed CAPA. First was PO MDD (yes or no) and MDD before age 6 years (PO MDD variable [n = 47]). This is the independent variable of primary interest. Second was ever diagnosed as having an anxiety disorder (yes or no) (ie, general anxiety disorder, posttraumatic stress disorder, separation anxiety disorder, obsessive-compulsive disorder, panic attack, panic disorder with agoraphobia, panic disorder without agoraphobia, agoraphobia, agoraphobia without a history of panic disorder, and social phobia) from baseline through imaging (anxiety diagnosis up to the time of imaging variable [n = 62]). The variable is used only as a covariate, Third was ever diagnosed as having MDD (yes or no) from baseline up to and including the day of imaging (MDD diagnosis up to the time of imaging variables [n = 65]). This variable is used as a covariate, Fourth was MDD diagnosed after the time of imaging (yes or no) (MDD after imaging variable [n = 24]). This variable is used as the dependent variable in the final analysis.
Key PO MDD Symptoms
Preschool-onset pathological guilt was based on the caregiver endorsing this item of the PAPA MDD module before the child turned 6 years. Pathological guilt in the present study is operationalized as a child perseverating on feeling guilt for minor misbehaviors or feeling guilt about behaviors that happened long ago. Pathological guilt could also include a child’s statements to her parents about feeling as though she is a bad kid, as well as blaming herself for things that were not her fault. To be coded as clinically significant, the parent must have reported that the child’s feelings of guilt are typically not modifiable and involve excessive self-blame. Multiple questions were asked as probes to determine children’s experience of pathological guilt (yes or no). Therefore, guilt and the additional preschool symptoms examined are coded as dichotomous and do not have a dimensional equivalent obtained by the PAPA interview. Preschool-onset vegetative symptoms included caregivers’ endorsement of 1 or more of the following: child displaying significant reduction in appetite, weight loss, increased need for sleep, and excessive fatigability. Preschool-onset somatic symptoms included children’s frequent complaints of headaches or stomach pains not associated with any medical or nutritional basis. Each PO symptom was coded as absent or present.
Stressful or Traumatic Life Events
It has been suggested that children who experience more traumatic life events are at greater risk for becoming guilt prone.21 Children’s experience of stressful or traumatic life events from baseline up until the day of their imaging were assessed using the PAPA and CAPA stressful or traumatic life events modules. There are 18 stressful life events (eg, change in day care or school) and 21 traumatic life events (eg, death of a loved one) assessed in the PAPA and CAPA. The frequencies of occurrences of all types of stressful or traumatic life events were summed to create an overall stressful life event frequency and an overall traumatic life event frequency. These modules of the PAPA and CAPA have established reliability and acceptable psychometric properties.54,58
Magnetic Resonance Imaging Acquisition and AI Volume Analysis
Structural images were collected as part of a longer imaging session that also included acquisition of task-based and functional connectivity data. Imaging data were collected using a 3-T imaging system (TIM TRIO; Siemens). The T1-weighted structural images were acquired in the sagittal plane using an MPRAGE 3-dimensional sequence (repetition time, 2400 milliseconds; echo time, 3.16 milliseconds; flip angle, 8°; slab, 176 mm; 176 sections; 256 × 256–pixel matrix; field of view, 256 mm; and voxel size, 1 × 1 × 1 mm).
A software program (FreeSurfer version 5.1.0; http://surfer.nmr.mgh.harvard.edu/) was used to segment each participant’s anatomical image using the atlas by Destrieux et al,59 allowing estimation of left and right anterior gray matter volume (excluding the posterior portion of the insula). The white and pial FreeSurfer surfaces were visually inspected and were regenerated with manual intervention to correct errors when necessary. The AI volume was taken from the “S_circular_insula_ant + G_insular_short” parcellation of the Destrieux cortical atlas. Whole-brain volume (total gray plus cortical white matter volume) was also obtained from FreeSurfer. Consistent with existing published literature,60 AI volumes were adjusted by the total segmented whole-brain volume (structure divided by whole-brain volume, times 1000) before all analyses. A Shapiro-Wilk test61,62 (P > .05) and visual inspections of their histograms, normal Q-Q plots, and box plots showed that left and right hemisphere AI volumes were approximately normally distributed for children in the PO MDD and non–PO MDD groups and that neither group differed significantly from normal.
Statistical Analysis
Clinical and Demographic Differences Between Groups
The primary analyses focused on comparisons of children with PO MDD vs those without (ie, non–PO MDD). Similarity of these 2 groups on demographic and clinical variables was evaluated using t tests and χ2 analyses (Table). The non–PO MDD group included children with other psychiatric diagnoses, children without PO MDD but who had school-age–onset MDD, and healthy children. Given our present objectives, we did not formally test a model using a 4-level diagnostic group variable. However, further descriptive details of the subgroups are provided in eTable 1 in the Supplement and in the other supplementary material.
Table.
Demographic, Clinical, and Imaging Characteristics of Diagnostic Groups
| Variable | Preschool Depression (n = 47) |
No Preschool Depression (n = 82) |
Group Comparison |
|---|---|---|---|
| Child Demographic Factors | |||
| Female sex, No. (%) | 20 (42.6) | 42 (51.2) | OR, −0.71; 95% CI, −1.10 to 0.37; P = .34 |
| Age at baseline, mean (SD), y | 4.6 (0.8) | 4.4 (0.7) | F1,127 = 3.16; P = .08 |
| Age at the time of imaging, mean (SD), y | 9.9 (1.2) | 9.8 (1.3) | F1,127 = 0.30; P = .59 |
| IQ, mean (SD) | 103 (14) | 109 (14) | F1,119 = 5.47; P = .02 |
| Prepubertal status at the time of imaging, No. (%) | 23 (48.9) | 43 (52.4) | OR, 1.08; 95% CI, −0.65 to 0.81; P = .67 |
| White race/ethnicity, No. (%) | 20 (42.6) | 55 (67.1) | OR, 2.75; 95% CI, 1.30 to 5.76; P = .007 |
| Right-handedness, No. (%) | 44 (93.6) | 76 (92.7) | OR, 0.86; 95% CI, −1.58 to 1.28; P = .84 |
| Psychotropic medication ever use, No. (%) | 15 (31.9) | 11 (13.4) | OR, 3.03; 95% CI, 1.25 to 7.32; P = .01 |
| Psychotropic medication use within 48 h of imaging, No. (%) | 4 (8.5) | 4 (4.9) | OR, 1.81; 95% CI, −0.84 to 2.03; P = .42 |
| Family Demographic Factors, No. (%) | |||
| Gross annual income at the time of imaging <$60 000 | 29 (61.7) | 39 (47.6) | OR, 0.56; 95% CI, −1.31 to 0.16; P = .12 |
| Education ≥4-y degree | 20 (42.6) | 47 (57.3) | OR, 0.11; 95% CI, −1.32 to 0.13; P = .11 |
| Clinical Variables, No. (%) | |||
| Endorsed pathological guilt | 26 (55.3) | 16 (19.5) | OR, 5.11; 95% CI, 2.31 to 11.29; P < .001 |
| Endorsed somatic symptoms | 31 (66.0) | 64 (78.0) | OR, 0.55; 95% CI, −1.41 to 0.19; P = .14 |
| Endorsed vegetative symptoms | 19 (40.4) | 7 (8.5) | OR, 7.30; 95% CI, 2.76 to 19.16; P < .001 |
| Anxiety ever from baseline to the time of imaging | 30 (63.8) | 32 (39.0) | OR, 2.76; 95% CI, 1.30 to 5.80; P = .007 |
| Depressed at the time of imaging | 12 (25.5) | 11 (13.4) | OR, 2.21; 95% CI, −0.12 to 1.71; P = .09 |
| Depression diagnosed after imaging | 15 (31.9) | 9 (11.0) | OR, 4.00; 95% CI, 1.56 to 10.10; P = .004 |
| Imaging and Diagnostic Timing, Mean (SD) | |||
| Years from baseline to imaging | 5.73 (0.92) | 5.88 (1.04) | F1,127 = 0.65; P = .42 |
| Days from imaging until MDD diagnosis after imaging | 367 (171) | 527 (351) | F1,127 = 2.27; P = .15 |
| Years from PO MDD diagnosis to the time of imaging | 5.45 (1.01) | NA | NA |
Abbreviations: MDD, major depressive disorder; NA, not applicable; OR, odds ratio; PO, preschool-onset.
Potential Covariates
Children’s age at imaging and sex were included as covariates in all analyses. The following variables were also tested as possible covariates using separate multivariate analyses of variance with left and right AI volumes as the dependent variables: children’s handedness, pubertal status (prepubertal vs pubertal), children’s history of psychotropic medication use up until the time of imaging (yes or no), gross family income at the time of imaging, and caregivers’ highest level of education completed (eTable 2 in the Supplement). If AI volume differed significantly in relation to the covariates, then the significant variable was included as a covariate in the multivariate analyses of covariance (MANCOVAs) described below.
AI Volume Differences in Relation to PO MDD
A 2 × 2 MANCOVA was conducted to test for a main effect of PO MDD on left or right AI volume, while controlling for children’s age and sex. The same MANCOVA was repeated using anxiety diagnosis up to the time of imaging (described above) as an additional covariate. The aim was to determine whether the expected effect of PO MDD on AI volume was persistent when accounting for past or current anxiety disorders.
AI Volume Differences in Relation to Specific Symptoms of PO MDD
Three separate 2 × 2 × 2 MANCOVAs were conducted to test for main effects of PO MDD and PO guilt, as well as the interaction effect of PO MDD × PO guilt on left or right AI volume using age and sex as covariates. This same MANCOVA design was repeated using PO vegetative and PO somatic symptoms. Symptoms identified as having a significant effect on AI volume were further tested after covarying for children’s experience of stressful or traumatic events from baseline up until the time of imaging.
AI Volume as a Predictor of Full MDD After Imaging
Binary logistic regression analysis was used to test whether schoolchildren’s right or left AI volume predicted the likelihood of being diagnosed as having MDD any time after their imaging. This analysis included children’s age, sex, MDD up to the time of imaging (yes or no), and any PO symptom (yes or no) that predicted AI volume differences.
All analyses were conducted using statistical software. We used IBM SPSS 21.0 for Macintosh (SPSS Inc).
Results
Demographic and Clinical Characteristics
The Table summarizes demographic and clinical information for the PO MDD group vs the non–PO MDD group. The PO MDD status did not differ significantly in relation to children’s sex, age, handedness, pubertal status, family income, or caregivers’ education.
Covariates
None of the covariates tested had a significant effect on AI volume and thus were excluded from all remaining analyses (eTable 2 in the Supplement). In addition to the covariate analyses, we also tested whether whole-brain volume at school age differed in relation to children’s prior PO MDD diagnosis, and no differences were found (F1,127 = 0.51, P = .48). This analysis was conducted to investigate whether the results could be explained by diagnostic group differences at the whole-brain volume level.
Does PO MDD Predict Left or Right AI Volume?
There was a significant multivariable main effect of PO MDD status on AI volume (Wilks Λ = 0.94, F2,124 = 3.37, P = .04, Cohen d = 0.23). As summarized in Figure 1 and in eFigure 2 in the Supplement, Bonferroni-adjusted pairwise comparisons demonstrated that school-age children with a history of PO MDD (left: mean [SD], 2.83 [0.22]; right: 2.86 [0.31]) compared with same-age peers without a history of PO MDD (left: mean [SD], 2.95 [0.26]; right: 2.96 [0.28]) had significantly smaller left AI volume (F1,125 = 6.29, P = .01, d = .22) but not right AI volume (F1,125 = 2.83, P = .10, d = .15), although the trend was clearly in the same direction for the right side.
Figure 1. Main Effect of Preschool Depression on Whole-Brain Adjusted Anterior Insula Volumes.
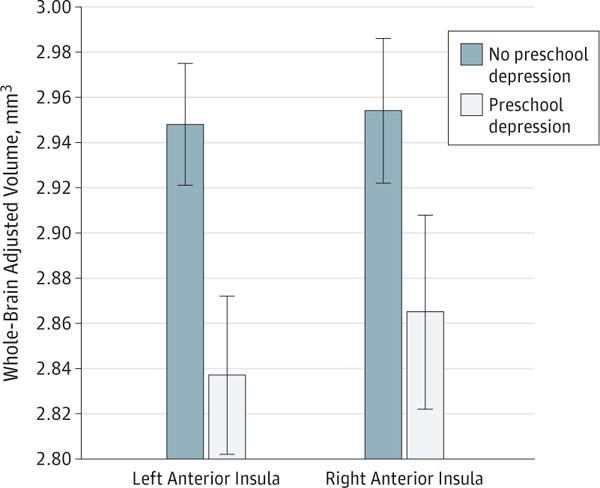
The vertical lines show the SD.
Does PO MDD Predict AI Volume When Covarying for Other Internalizing Disorders?
Prior diagnosis of an anxiety disorder did not have a significant multivariable effect on AI volume (Λ = 0.97, F2,123 = 2.25, P = .11, d = .18). The multivariable effect of PO MDD on AI volume was significant at a trend level even when including children’s diagnosis of anxiety up to the time of imaging as a covariate (Λ = 0.96, F2,123 = 2.81, P = .06, d = .21). Most important, the effect size of PO MDD remained consistent whether anxiety was or was not included as a covariate. Follow-up comparisons indicated that PO MDD was associated with significantly smaller left AI volume (F1,124 = 5.61, P = .02, d = .21) but not right AI volume (F1,124 = 1.39, P = .24, d = .10).
Are There Specific Symptoms of PO MDD That Account for Its Effect on Insula?
When the main effects of PO MDD and PO guilt, as well as their interaction effect, were tested using MANCOVA, PO guilt had the only significant multivariable effect (Λ = 0.88, F2,122 = 7.69, P = .001, d = .33). Independent of POMDD status, school-age children who exhibited pathological guilt during preschool age (left: mean [SD], 2.79 [0.25]; right: 2.79[0.31])vs those without PO guilt (left: mean [SD], 2.97 [0.23]; right: 2.98 [0.27]) had significantly smaller left AI volumes (F1,123 = 10.71, P = .001, d = .28) and right AI volumes (F1,123 = 10.34, P = .002, d = .28) (Figure 2). With PO guilt included in the model, PO MDD no longer had a significant effect on insula volume (Λ = 0.99, F2,122 = 0.47, P = .63), and the effect size was substantially smaller (d = .09) than it was before PO guilt was included in the model (d = 0.23). The PO MDD × PO guilt interaction effect was not significant (Λ = 0.99, F2,122 = 0.41, P = .66, d = .08).
Figure 2. Main Effect of Preschool-Onset Pathological Guilt on Anterior Insula Volumes.
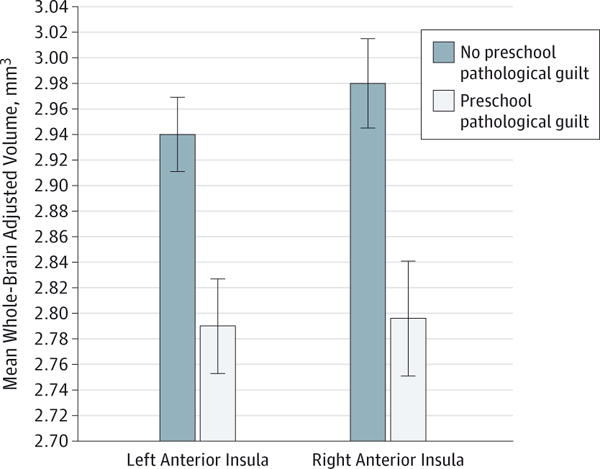
The vertical lines show the SD.
These identical analyses were repeated using PO vegetative and PO somatic symptoms instead of guilt. The significant main effect of PO MDD on insula remained when the PO vegetative symptom was included in the model (Λ = 0.94, F2,122 = 3.71, P = .03, d = .24). There was no significant main effect of vegetative symptom (Λ = 0.96, F2,122 = 2.57, P = .08, d = .20) on AI volume, and the interaction effect of PO vegetative symptom × PO MDD on AI volume was nonsignificant (Λ = 0.99, F2,122 = 0.05, P = .95, d = .03). Similarly, there was no significant main effect (Λ = 0.98, F2,122 = 0.97, P = .38, d = .13) or interaction effect of PO somatization × PO MDD on AI volume (Λ = 0.99, F2,122 = 0.004, P > .99, d = .003). The main effect of PO MDD on insula was at a trend level when PO somatization was included in the model (Λ = 0.96, F2,122 = 2.46, P = .09, d = .20). Again, the effect size of PO MDD on left and right AI volumes remained comparable to the results without somatization in the model.
Do AI Volumes Differ in Relation to MDD Symptoms When Covarying for Stressful or Traumatic Events?
We conducted a follow-up MANCOVA to test whether AI volumes differed significantly in relation to PO MDD or PO guilt when children’s experiences of stressful or traumatic life events from the time of study enrollment up until the time of imaging were included as covariates. When the main effects of PO MDD and PO guilt, as well as their interaction effect, were tested using children’s sex, age, and experiences of stressful or traumatic events as covariates using MANCOVA, PO guilt had the only significant multivariable effect (Λ = 0.91, F2,120 = 6.17, P = .003, d = .30). Independent of PO MDD status and after covarying for age, sex, and stressful or traumatic experiences, school-age children who exhibited pathological guilt during preschool age had significantly smaller left AI volumes (F1,121 = 7.85, P = .006, d = .25) and right AI volumes (F1,121 = 9.00, P = .003, d = .26).
Do AI Volumes Predict a Subsequent Depression Diagnosis?
To test whether AI volume predicted MDD diagnosis after imaging, it was necessary to include children’s prior diagnosis of MDD up to the time of imaging as a covariate. Preschool-onset guilt was also included as a covariate to ensure that MDD after imaging was specific to AI volume and not to a history of MDD or PO guilt up to the time of imaging. Children with an MDD diagnosis up to the time of imaging were approximately 11 times as likely as same-age peers without a prior diagnosis of MDD to have an MDD diagnosis after their imaging (odds ratio [OR], 11.38; 95% CI, 2.88–44.94; P < .01). However, even when including the robust effect of MDD up to the time of imaging, as well as children’s age, sex, and PO guilt symptoms (OR, 0.86; 95% CI, 0.28–2.65; P = .79), children with larger right-side AI volumes were significantly less likely to be diagnosed as having full MDD after their imaging (diagnosed on average 14 months after the imaging date [OR, 0.96; 95% CI, 0.01–0.75; P = .03]) (Figure 3). Left-side AI volume was not significantly associated with children’s MDD diagnosis after the time of imaging (OR, 2.70; 95% CI, 0.24–29.88; P = .42). Given the known association between stressful or traumatic events and greater risk for recurrence of MDD, a follow-up analysis was conducted to examine whether AI volumes predicted MDD recurrence after imaging when children’s stressful or traumatic life events were included in the model as covariates. Results indicated that smaller right AI volume remained a significant predictor (OR, 0.12; 95% CI, 0.01–0.99; P = .04) of DSM-5 MDD diagnosis after imaging when covarying for MDD from baseline up until the time of imaging, age, sex, PO guilt, and children’s stressful or traumatic life events.
Figure 3. Whole-Brain Adjusted Right Anterior Insula Volume Predicting Depression Diagnosis a Mean of 1.5 Years After Imaging.
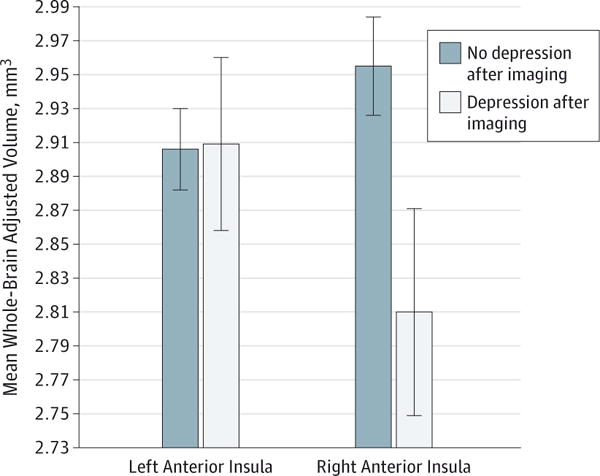
The vertical lines show the SD.
Discussion
Our findings demonstrated that PO MDD was associated with decreased AI volume at school age even when covarying for the effects of sex, age at imaging, stressful or traumatic life events, and co-occurring anxiety disorders. Although PO MDD was also associated with decreased volume in the right AI at a trend level, the association was not statistically significant. When preschool guilt (an emotion consistently linked to the AI) was included in the model, it significantly predicted smaller left-side and right-side AI volume at school age. Furthermore, when guilt was included in the model, PO MDD was no longer a significant predictor of AI volume, reducing the effect of PO MDD on AI volume by almost half. Contrary to expectations, preschool guilt did not significantly moderate the effect of PO MDD on AI volume. The unique role of preschool guilt on AI volume was further supported by the finding that other symptoms of preschool depression (ie, somatic or vegetative) were not significantly related to AI volume, nor did they serve to reduce the effect size of PO MDD on AI volume when included in the model. This suggests that the association between PO MDD and smaller AI volume may be partially explained by the experience of pathological guilt before age 6 years. However, future studies using continuous measures of guilt that can better assess variation in guilt severity are needed to further test this association.
Study findings also demonstrated that reduced right-side AI volume at school age was a significant predictor of risk for future occurrences of depression in later childhood and early adolescence. This finding is consistent with data in adults using neuroimaging and lesion investigations that report aberrations in AI volume in samples with MDD, obsessive-compulsive disorder, and eating disorders.63 Most important, right-side AI volume was a significant and robust predictor of DSM-5 MDD diagnosis after imaging even when MDD diagnosis up to the time of imaging, preschool guilt, age, and sex were included as covariates. This is consistent with adult literature suggesting that AI volume reduction may represent a biological marker of depression (as well as other disorders).3,64 Extending the adult literature, these study findings provide evidence for the first structural brain biomarker to date of risk for recurrent depression in childhood. Future studies that follow up children into later adolescence and early adulthood are needed to more fully inform this risk trajectory.
Preschool guilt emerged as a unique symptom in the prediction of AI volume even over and above the diagnosis of PO MDD itself. This finding supports a risk model in which high levels of guilt experienced early in life might have an effect on the development of the AI, and reduced AI volumes might then serve as a risk biomarker for a later recurrent course of depression. Alternatively, smaller insula volumes may have pre dated the experience of high levels of guilt in the risk trajectory, an issue our study cannot inform because the children were not imaged during the preschool period. Regardless of whether reduced AI volume preceded or followed preschool guilt, it served as a statistically significant biomarker of later depression recurrence. Given the specific relationships found among guilt, AI volume, and the course of MDD, future prospective longitudinal studies with larger sample sizes and longer follow-up periods should be designed to further test the direction and magnitude of these effects.
As noted above, the present findings are limited by the absence of magnetic resonance imaging data obtained during the preschool period to determine whether reduced AI volume was present before the experiences of elevated guilt. Furthermore, children’s manifestation of pathological guilt was assessed using a single item from the caregiver, a potentially important limitation given developmental findings of poor convergence between behavioral and maternal report of children’s guilt expressions.65 Future studies that carefully track guilt experiences using multiple methods and reporters (eg, teachers and day care providers) and later course of depression into late adolescence and early adulthood are needed. While guilt has shown specificity to depression in early childhood, guilt in older children, adolescents, and adults has also been significantly associated with other disorders such as obsessive-compulsive, eating, and anxiety disorders; therefore, investigations of other psychopathological outcomes in preschool samples would be of interest.66,67 Furthermore, it would be important in future research to distinguish between different forms and functions of guilt68 because at least 2 forms of guilt have been identified in older populations. Deontological guilt is the intrapsychic sense of guilt,51 arising out of the assumption of having wronged a moral authority, broken one’s moral code, or deviated from social norms.28,67 Altruistic guilt is the interpersonal sense of guilt, associated with the tendency to feel empathy, often arising from the distress of others.69,70 In adults, deontological guilt and altruistic guilt activate different neural systems.42,71 Altruistic guilt is often related to depression in adolescents and adults,31,72 whereas deontological guilt is thought to have a stronger role in other disorders such as obsessive-compulsive, eating, and anxiety disorders.66
Conclusions
The effects of early interventions that target reductions in pathological guilt and enhancement of adaptive guilt on brain development and later depression risk represent a promising future research direction. More specifically, examining structural and functional neurodevelopment of the insula of young people at high risk for depression could inform neurobiological models of the developmental psychopathology of MDD. Such developmental models are necessary to inform the earliest possible detection, targeted preventive intervention strategies, and perhaps estimates of therapeutic prognosis. Understanding the earliest antecedents in this risk trajectory will inform how to target interventions during this early developmental period of relatively high neuroplasticity.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported by grants 2R01 MH064769-06A1 (Dr Luby) and PA-07-070 NIMH R01 (Drs Barch, Botteron, and Luby) from the National Institutes of Health. Dr Belden’s work on this article was supported by grant 5K01MH090515-04 from the National Institutes of Health.
Role of the Funder/Sponsor: The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Drs Belden and Luby had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Belden, Barch, Botteron, Luby.
Acquisition, analysis, or interpretation data: Belden, Barch, Harms, Botteron, Luby.
Drafting of the manuscript: Belden, Barch, Oakberg, April, Luby.
Critical revision of the manuscript for important intellectual content: Belden, Barch, Harms, Luby.
Statistical analysis: Belden, Barch.
Obtained funding: Belden, Barch, Botteron, Luby.
Administrative, technical, or material support: Oakberg, April, Harms.
Study supervision: Belden, Barch, Botteron, Luby.
Conflict of Interest Disclosures: None reported.
Additional Contributions: We acknowledge our child participants and their parents, whose participation and cooperation made this research possible.
References
- 1.Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends Cogn Sci. 1999;3(1):11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- 2.Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11(2):240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- 3.McGrath CL, Kelley ME, Holtzheimer PE, et al. Toward a neuroimaging treatment selection biomarker for major depressive disorder. JAMA Psychiatry. 2013;70(8):821–829. doi: 10.1001/jamapsychiatry.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaffrey MS, Luby JL, Barch DM. Towards the study of functional brain development in depression: an interactive specialization approach. Neurobiol Dis. 2013;52:38–48. doi: 10.1016/j.nbd.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luby JL, Gaffrey MS, Tillman R, April LM, Belden AC. Trajectories of preschool disorders to full DSM depression at school age and early adolescence: continuity of preschool depression. Am J Psychiatry. 2014;171(7):768–776. doi: 10.1176/appi.ajp.2014.13091198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luby JL, Mrakotsky C, Heffelfinger A, Brown K, Spitznagel E. Characteristics of depressed preschoolers with and without anhedonia: evidence for a melancholic depressive subtype in young children. Am J Psychiatry. 2004;161(11):1998–2004. doi: 10.1176/appi.ajp.161.11.1998. [DOI] [PubMed] [Google Scholar]
- 7.Bufferd SJ, Dougherty LR, Carlson GA, Rose S, Klein DN. Psychiatric disorders in preschoolers: continuity from ages 3 to 6. Am J Psychiatry. 2012;169(11):1157–1164. doi: 10.1176/appi.ajp.2012.12020268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wichstrøm L, Berg-Nielsen TS, Angold A, Egger HL, Solheim E, Sveen TH. Prevalence of psychiatric disorders in preschoolers. J Child Psychol Psychiatry. 2012;53(6):695–705. doi: 10.1111/j.1469-7610.2011.02514.x. [DOI] [PubMed] [Google Scholar]
- 9.Lavigne JV, Lebailly SA, Hopkins J, Gouze KR, Binns HJ. The prevalence of ADHD, ODD, depression, and anxiety in a community sample of 4-year-olds. J Clin Child Adolesc Psychol. 2009;38(3):315–328. doi: 10.1080/15374410902851382. [DOI] [PubMed] [Google Scholar]
- 10.Egger HL, Angold A. Common emotional and behavioral disorders in preschool children: presentation, nosology, and epidemiology. J Child Psychol Psychiatry. 2006;47(3–4):313–337. doi: 10.1111/j.1469-7610.2006.01618.x. [DOI] [PubMed] [Google Scholar]
- 11.Luby JL, Heffelfinger A, Mrakotsky C, Brown K, Hessler M, Spitznagel E. Alterations in stress cortisol reactivity in depressed preschoolers relative to psychiatric and no-disorder comparison groups. Arch Gen Psychiatry. 2003;60(12):1248–1255. doi: 10.1001/archpsyc.60.12.1248. [DOI] [PubMed] [Google Scholar]
- 12.Barch DM, Gaffrey MS, Botteron KN, Belden AC, Luby JL. Functional brain activation to emotionally valenced faces in school-aged children with a history of preschool-onset major depression. Biol Psychiatry. 2012;72(12):1035–1042. doi: 10.1016/j.biopsych.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaffrey MS, Luby JL, Botteron K, Repovš G, Barch DM. Default mode network connectivity in children with a history of preschool onset depression. J Child Psychol Psychiatry. 2012;53(9):964–972. doi: 10.1111/j.1469-7610.2012.02552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luking KR, Repovs G, Belden AC, et al. Functional connectivity of the amygdala in early-childhood–onset depression. J Am Acad Child Adolesc Psychiatry. 2011;50(10):1027–1041.e3. doi: 10.1016/j.jaac.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pagliaccio D, Luby J, Gaffrey M, et al. Anomalous functional brain activation following negative mood induction in children with pre-school onset major depression. Dev Cogn Neurosci. 2012;2(2):256–267. doi: 10.1016/j.dcn.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki H, Botteron KN, Luby JL, et al. Structural-functional correlations between hippocampal volume and cortico-limbic emotional responses in depressed children. Cogn Affect Behav Neurosci. 2013;13(1):135–151. doi: 10.3758/s13415-012-0121-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luby J, Belden A, Sullivan J, Hayen R, McCadney A, Spitznagel E. Shame and guilt in preschool depression: evidence for elevations in self-conscious emotions in depression as early as age 3. J Child Psychol Psychiatry. 2009;50(9):1156–1166. doi: 10.1111/j.1469-7610.2009.02077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belden A, Sullivan J, Pautsch J, Williams M, Luby J. Preschool mood disorders and unique development of self-conscious emotion. Poster presented at: American Academy of Child and Adolescent Psychiatry 53rd Annual Meeting; October 24–29, 2006; San Diego, California. [Google Scholar]
- 19.Luby J, Belden A. Depressive-symptom onset during toddlerhood in a sample of depressed preschoolers: implications for future investigations of major depressive disorder in toddlers. Infant Ment Health J. 2012;33(2):139–147. doi: 10.1002/imhj.21314. [DOI] [PubMed] [Google Scholar]
- 20.Luby JL, Belden AC, Pautsch J, Si X, Spitznagel E. The clinical significance of preschool depression: impairment in functioning and clinical markers of the disorder. J Affect Disord. 2009;112(1–3):111–119. doi: 10.1016/j.jad.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiss J, Sampson H, Mount Zion Psychotherapy Research Group . The Psychoanalytic Process: Theory, Clinical Observation, and Empirical Research. New York, NY: Guilford Press; 1986. [Google Scholar]
- 22.Tangney JP, Wagner P, Gramzow R. Proneness to shame, proneness to guilt, and psychopathology. J Abnorm Psychol. 1992;101(3):469–478. doi: 10.1037//0021-843x.101.3.469. [DOI] [PubMed] [Google Scholar]
- 23.Tilghman-Osborne C, Cole D, Felton J, Ciesla J. Relation of guilt, shame, behavioral and characterological self-blame to depressive symptoms in adolescents over time. J Soc Clin Psychol. 2008;27(8):809–842. doi: 10.1521/jscp.2008.27.8.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zahn-Waxler C, Robinson J. Empathy and guilt: early origins of feelings of responsibility. In: Tangney JP, Fischer KW, editors. Self-conscious Emotions: The Psychology of Shame, Guilt, Embarrassment, and Pride. New York, NY: Guilford Press; 1995. pp. 143–173. [Google Scholar]
- 25.Baker E, Baibazarova E, Ktistaki G, Shelton KH, van Goozen SH. Development of fear and guilt in young children: stability over time and relations with psychopathology. Dev Psychopathol. 2012;24(3):833–845. doi: 10.1017/S0954579412000399. [DOI] [PubMed] [Google Scholar]
- 26.Carnì S, Petrocchi N, Del Miglio C, Mancini F, Couyoumdjian A. Intrapsychic and interpersonal guilt: a critical review of the recent literature. Cogn Process. 2013;14(4):333–346. doi: 10.1007/s10339-013-0570-4. [DOI] [PubMed] [Google Scholar]
- 27.Eisenberg N. Emotion, regulation, and moral development. Annu Rev Psychol. 2000;51(1):665–697. doi: 10.1146/annurev.psych.51.1.665. [DOI] [PubMed] [Google Scholar]
- 28.Gangemi A, Mancini F. Guilt and Guilts: Re-constructing Emotional Spaces: From Experience to Regulation. Prague, Czechoslovakia: Prague College of Psychosocial Studies Press; 2011. pp. 169–188. [Google Scholar]
- 29.Kim S, Thibodeau R, Jorgensen RS. Shame, guilt, and depressive symptoms: a meta-analytic review. Psychol Bull. 2011;137(1):68–96. doi: 10.1037/a0021466. [DOI] [PubMed] [Google Scholar]
- 30.Masi G, Favilla L, Mucci M, Poli P, Romano R. Depressive symptoms in children and adolescents with dysthymic disorder. Psychopathology. 2001;34(1):29–35. doi: 10.1159/000049277. [DOI] [PubMed] [Google Scholar]
- 31.O’Connor L, Berry J, Weiss J. Interpersonal guilt, shame, and psychological problems. J Soc Clin Psychol. 1999;18(2):181–203. [Google Scholar]
- 32.Viding E, Blair RJR, Moffitt TE, Plomin R. Evidence for substantial genetic risk for psychopathy in 7-year-olds. J Child Psychol Psychiatry. 2005;46(6):592–597. doi: 10.1111/j.1469-7610.2004.00393.x. [DOI] [PubMed] [Google Scholar]
- 33.Soria V, Cardoner N, Gratacos M, et al. Mapping structural impact of Val66Met BDNF genotype in melancholic depression: influence on time to clinical remission. Eur Neuropsychopharmacol. 2011;21:S444–S445. [Google Scholar]
- 34.Gu X, Gao Z, Wang X, et al. Anterior insular cortex is necessary for empathetic pain perception. Brain. 2012;135(pt 9):2726–2735. doi: 10.1093/brain/aws199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamm C, Singer T. The role of anterior insular cortex in social emotions. Brain Struct Funct. 2010;214(5–6):579–591. doi: 10.1007/s00429-010-0251-3. [DOI] [PubMed] [Google Scholar]
- 36.Shin LM, Dougherty DD, Orr SP, et al. Activation of anterior paralimbic structures during guilt-related script-driven imagery. Biol Psychiatry. 2000;48(1):43–50. doi: 10.1016/s0006-3223(00)00251-1. [DOI] [PubMed] [Google Scholar]
- 37.Wagner U, N’Diaye K, Ethofer T, Vuilleumier P. Guilt-specific processing in the prefrontal cortex. Cereb Cortex. 2011;21(11):2461–2470. doi: 10.1093/cercor/bhr016. [DOI] [PubMed] [Google Scholar]
- 38.Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci. 2009;13(8):334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Manoliu A, Meng C, Brandl F, et al. Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Front Hum Neurosci. 2013;7:930. doi: 10.3389/fnhum.2013.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sliz D, Hayley S. Major depressive disorder and alterations in insular cortical activity: a review of current functional magnetic imaging research. Front Hum Neurosci. 2012;6:323. doi: 10.3389/fnhum.2012.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Craig AD. How do you feel—now? the anterior insula and human awareness. Nat Rev Neurosci. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 42.Basile B, Mancini F, Macaluso E, Caltagirone C, Frackowiak RS, Bozzali M. Deontological and altruistic guilt: evidence for distinct neurobiological substrates. Hum Brain Mapp. 2011;32(2):229–239. doi: 10.1002/hbm.21009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hatton SN, Lagopoulos J, Hermens DF, Naismith SL, Bennett MR, Hickie IB. Correlating anterior insula gray matter volume changes in young people with clinical and neurocognitive outcomes: an MRI study. BMC Psychiatry. 2012;12:45. doi: 10.1186/1471-244X-12-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mayberg HS, Brannan SK, Tekell JL, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000;48(8):830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- 45.Soriano-Mas C, Hernández-Ribas R, Pujol J, et al. Cross-sectional and longitudinal assessment of structural brain alterations in melancholic depression. Biol Psychiatry. 2011;69(4):318–325. doi: 10.1016/j.biopsych.2010.07.029. [DOI] [PubMed] [Google Scholar]
- 46.Sprengelmeyer R, Steele JD, Mwangi B, et al. The insular cortex and the neuroanatomy of major depression. J Affect Disord. 2011;133(1–2):120–127. doi: 10.1016/j.jad.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi T, Malhi GS, Wood SJ, et al. Insular cortex volume in established bipolar affective disorder: a preliminary MRI study. Psychiatry Res. 2010;182(2):187–190. doi: 10.1016/j.pscychresns.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 48.Wiebking C, Bauer A, de Greck M, Duncan NW, Tempelmann C, Northoff G. Abnormal body perception and neural activity in the insula in depression: an fMRI study of the depressed “material me”. World J Biol Psychiatry. 2010;11(3):538–549. doi: 10.3109/15622970903563794. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi T, Yücel M, Lorenzetti V, et al. Volumetric MRI study of the insular cortex in individuals with current and past major depression. J Affect Disord. 2010;121(3):231–238. doi: 10.1016/j.jad.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 50.O’Connor LE, Berry JW, Weiss J, Schweitzer D, Sevier M. Survivor guilt, submissive behaviour and evolutionary theory: the down-side of winning in social comparison. Br J Med Psychol. 2000;73(pt 4):519–530. doi: 10.1348/000711200160705. [DOI] [PubMed] [Google Scholar]
- 51.Stuewig J, McCloskey LA. The relation of child maltreatment to shame and guilt among adolescents: psychological routes to depression and delinquency. Child Maltreat. 2005;10(4):324–336. doi: 10.1177/1077559505279308. [DOI] [PubMed] [Google Scholar]
- 52.Tilghman-Osborne C, Cole DA, Felton JW. Inappropriate and excessive guilt: instrument validation and developmental differences in relation to depression. J Abnorm Child Psychol. 2012;40(4):607–620. doi: 10.1007/s10802-011-9591-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luby JL, Heffelfinger A, Koenig-McNaught AL, Brown K, Spitznagel E. The Preschool Feelings Checklist: a brief and sensitive screening measure for depression in young children. J Am Acad Child Adolesc Psychiatry. 2004;43(6):708–717. doi: 10.1097/01.chi.0000121066.29744.08. [DOI] [PubMed] [Google Scholar]
- 54.Egger HL, Erkanli A, Keeler G, Potts E, Walter BK, Angold A. Test-retest reliability of the Preschool Age Psychiatric Assessment (PAPA) J Am Acad Child Adolesc Psychiatry. 2006;45(5):538–549. doi: 10.1097/01.chi.0000205705.71194.b8. [DOI] [PubMed] [Google Scholar]
- 55.Angold A, Costello EJ. A test-retest reliability study of child-reported psychiatric symptoms and diagnoses using the Child and Adolescent Psychiatric Assessment (CAPA-C) Psychol Med. 1995;25(4):755–762. doi: 10.1017/s0033291700034991. [DOI] [PubMed] [Google Scholar]
- 56.Angold A, Prendergast M, Cox A, Harrington R, Simonoff E, Rutter M. The Child and Adolescent Psychiatric Assessment (CAPA) Psychol Med. 1995;25(4):739–753. doi: 10.1017/s003329170003498x. [DOI] [PubMed] [Google Scholar]
- 57.Luby JL, Si X, Belden AC, Tandon M, Spitznagel E. Preschool depression: homotypic continuity and course over 24 months. Arch Gen Psychiatry. 2009;66(8):897–905. doi: 10.1001/archgenpsychiatry.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Costello EJ, Angold A, March J, Fairbank J. Life events and post-traumatic stress: the development of a new measure for children and adolescents. Psychol Med. 1998;28(6):1275–1288. doi: 10.1017/s0033291798007569. [DOI] [PubMed] [Google Scholar]
- 59.Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 2010;53(1):1–15. doi: 10.1016/j.neuroimage.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lopez-Larson MP, King JB, Terry J, McGlade EC, Yurgelun-Todd D. Reduced insular volume in attention deficit hyperactivity disorder. Psychiatry Res. 2012;204(1):32–39. doi: 10.1016/j.pscychresns.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shapiro SS, Wilk MB. An Analysis of Variance Test for Normality (Complete Samples) New York, NY: JSTOR; 1964. [Google Scholar]
- 62.Razali NM, Wah YB. Power comparisons of Shapiro-Wilk, Kolmogorov-Smirnov, Lilliefors and Anderson-Darling tests. J Stat Modeling Analytics. 2011;2(1):21–33. [Google Scholar]
- 63.Nagai M, Kishi K, Kato S. Insular cortex and neuropsychiatric disorders: a review of recent literature. Eur Psychiatry. 2007;22(6):387–394. doi: 10.1016/j.eurpsy.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 64.Liu CH, Jing B, Ma X, et al. Voxel-based morphometry study of the insular cortex in female patients with current and remitted depression. Neuroscience. 2014;262:190–199. doi: 10.1016/j.neuroscience.2013.12.058. [DOI] [PubMed] [Google Scholar]
- 65.Kochanska G, Gross JN, Lin MH, Nichols KE. Guilt in young children: development, determinants, and relations with a broader system of standards. Child Dev. 2002;73(2):461–482. doi: 10.1111/1467-8624.00418. [DOI] [PubMed] [Google Scholar]
- 66.Tilghman-Osborne C, Cole DA, Felton JW. Definition and measurement of guilt: implications for clinical research and practice. Clin Psychol Rev. 2010;30(5):536–546. doi: 10.1016/j.cpr.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Basile B, Mancini F, Macaluso E, Caltagirone C, Bozzali M. Abnormal processing of deontological guilt in obsessive-compulsive disorder. Brain Struct Funct. 2014;219(4):1321–1331. doi: 10.1007/s00429-013-0570-2. [DOI] [PubMed] [Google Scholar]
- 68.Orth U, Berking M, Burkhardt S. Self-conscious emotions and depression: rumination explains why shame but not guilt is maladaptive. Pers Soc Psychol Bull. 2006;32(12):1608–1619. doi: 10.1177/0146167206292958. [DOI] [PubMed] [Google Scholar]
- 69.Hoffman ML. Affect and moral development. New Dir Child Adolesc Dev. 1982;1982(16):83–103. [Google Scholar]
- 70.Thompson RA, Hoffman ML. Empathy and the development of guilt in children. Dev Psychol. 1980;16(2):155. [Google Scholar]
- 71.Morey RA, McCarthy G, Selgrade ES, Seth S, Nasser JD, LaBar KS. Neural systems for guilt from actions affecting self versus others. Neuroimage. 2012;60(1):683–692. doi: 10.1016/j.neuroimage.2011.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O’Connor LE, Berry JW, Weiss J, Gilbert P. Guilt, fear, submission, and empathy in depression. J Affect Disord. 2002;71(1–3):19–27. doi: 10.1016/s0165-0327(01)00408-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


