Abstract
Purpose
We explored the diagnostic use of circulating tumor cells in patients with neoadjuvant bladder cancer using enumeration and next generation sequencing.
Materials and Methods
A total of 20 patients with bladder cancer who were eligible for cisplatin based neoadjuvant chemotherapy were enrolled in an institutional review board approved study. Subjects underwent blood draws at baseline and after 1 cycle of chemotherapy. A total of 11 patients with metastatic bladder cancer and 13 healthy donors were analyzed for comparison. Samples were enriched for circulating tumor cells using the novel IsoFlux™ System microfluidic collection device. Circulating tumor cell counts were analyzed for repeatability and compared with Food and Drug Administration cleared circulating tumor cells. Circulating tumor cells were also analyzed for mutational status using next generation sequencing.
Results
Median circulating tumor cell counts were 13 at baseline and 5 at followup in the neoadjuvant group, 29 in the metastatic group and 2 in the healthy group. The concordance of circulating tumor cell levels, defined as low—fewer than 10, medium—11 to 30 and high—greater than 30, across replicate tubes was 100% in 15 preparations. In matched samples the IsoFlux test showed 10 or more circulating tumor cells in 4 of 9 samples (44%) while CellSearch® showed 0 of 9 (0%). At cystectomy 4 months after baseline all 3 patients (100%) with medium/high circulating tumor cell levels at baseline and followup had unfavorable pathological stage disease (T1-T4 or N+). Next generation sequencing analysis showed somatic variant detection in 4 of 8 patients using a targeted cancer panel. All 8 cases (100%) had a medium/high circulating tumor cell level with a circulating tumor cell fraction of greater than 5% purity.
Conclusions
This study demonstrates a potential role for circulating tumor cell assays in the management of bladder cancer. The IsoFlux method of circulating tumor cell detection shows increased sensitivity compared with CellSearch. A next generation sequencing assay is presented with sufficient sensitivity to detect genomic alterations in circulating tumor cells.
Keywords: urinary bladder neoplasms; neoplastic cells, circulating; neoadjuvant therapy; pathology, molecular; genomics
Bladder cancer accounts for 15,500 deaths annually.1 In the absence of metastases radical cystectomy offers the possibility of cure but with limited success.2 Pathological stage, lymphovascular invasion and histological grade are prognostic factors for survival. pStage is associated with excellent 5-year disease-free survival (85% to 90%).2–4
Perioperative chemotherapy has been investigated to improve survival in bladder cancer. Adjuvant cisplatin based chemotherapy is only applicable to 20% of patients due to the high incidence of renal insufficiency and perioperative complications. Patients generally have better performance status and organ function before cystectomy, providing the rationale for neoadjuvant therapy. Neoadjuvant chemotherapy prior to cystectomy results in pathological down-staging at surgery with a 38% frequency of pT0 compared to 15% in control patients who did not receive neoadjuvant chemotherapy in a randomized phase III trial.3 It also provides an overall survival benefit.2,5–7 Unfortunately cisplatin based chemotherapy is only feasible in 50% of patients with bladder cancer due to inadequate renal, cardiac and neurological function.8 Cisplatin also carries a risk of kidney damage and neuropathy. Overall survival with cisplatin based neoadjuvant chemotherapy is only 5% at 5 years. The critical unmet need in the management of muscle invasive bladder cancer is the identification of minimally invasive biomarkers to stratify patients who would benefit from neoadjuvant chemotherapy while sparing others needless toxicity.9
CTCs are detectable in most epithelial cancers and may enable early assessment for neoadjuvant chemotherapy in bladder cancer.10–13 CTC counts predict progression-free and overall survival in metastatic breast cancer,14 and overall survival in colon cancer and castration resistant prostate cancer.15,16 CTCs were shown to be predictive biomarkers in patients with castration resistant prostate cancer treated with enzalutamide.17,18
The CellSearch CTC Test™ detects CTCs in nonmetastatic and metastatic bladder cancer.19–22 CellSearch has low sensitivity for localized bladder cancer, demonstrating CTCs in only 17% to 23% of patients before cystectomy.19,22 Nevertheless, the presence of CTCs preoperatively was an independent adverse prognostic factor for cancer-free survival in patients undergoing cystectomy. One recent study showed that CTCs remained an independent predictor of cancer specific mortality in patients treated with cystectomy without chemotherapy.23
The low sensitivity of CellSearch limits its usefulness for bladder cancer. The IsoFlux System has previously been shown to increase sensitivity for CTC detection and molecular profiling in prostate and colorectal cancer.24 This platform uses immunomagnetic isolation of CTCs in a microfluidic environment to enhance CTC capture and minimize white blood cell carryover. The magnetic beads can be functionalized with multiple antibodies, although EpCAM alone was used in this study for a direct comparison with CellSearch. The resulting CTC samples are suitable for enumeration and NGS.
This study explored the potential role of CTCs in identifying patients who might be better served by moving directly to cystectomy to avoid additional chemotherapy.
Materials and Methods
Study Aims
1) We explored the use of CTCs as a bladder cancer biomarker. 2) We compared assay performance of the IsoFlux System to that of CellSearch. 3) We tested the repeatability of the IsoFlux assay. 4) We established the feasibility of using CTCs for NGS (fig. 1).
Figure 1.
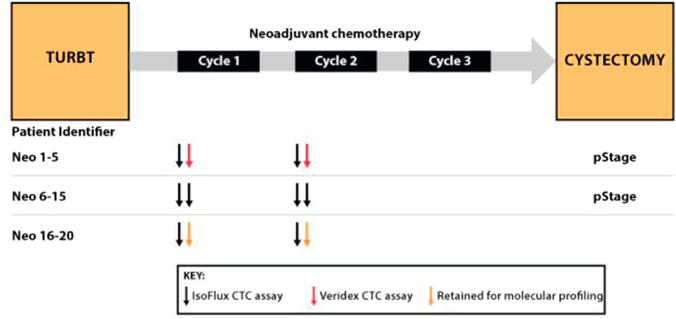
Outline of neoadjuvant study endpoints. At time of transurethral resection of bladder tumor (TURBT) 20 patients with neoadjuvant bladder cancer were enrolled. In all patients 2 tubes of blood were drawn before first neoadjuvant chemotherapy cycle and 2 were drawn after first cycle approximately 1 month later. First 5 patients had 1 blood tube drawn and tested on IsoFlux System and second blood tube tested on CellSearch assay. Next 10 patients had 2 tubes analyzed on IsoFlux System to assess repeatability. Remaining 6 patients had 2 tubes processed on IsoFlux System but 1 was retained for molecular profiling. Pathological stage was available in first 17 patients.
Patient Selection
Two prospective pilot studies in a total of 30 patients with bladder cancer received institutional review board approval at University of Michigan and University of California-San Francisco. One cohort of 20 patients had locally advanced, resectable bladder cancer. These patients had blood collections before and during neoadjuvant chemotherapy. Eligible patients met certain criteria, including 1) histological evidence of urothelial carcinoma invading the muscularis propria, 2) no radiological evidence of metastases, 3) candidacy for cisplatin based chemotherapy and 4) candidacy for radical cystectomy with curative intent. Two patients were lost to followup. Of the patients 16 underwent pathological staging at cystectomy. A second cohort of 11 patients with metastasis was used for comparison and 13 healthy donor samples (AllCells®) were collected to serve as controls.
Sample Collection
Neoadjuvant patient blood samples were collected at baseline and after the first cycle of chemotherapy. The second blood draw was taken 3 or 4 weeks after baseline in those on the dose dense MVAC, MVAC or classic cisplatin-gemcitabine regimen. In the metastatic and healthy groups only 1 blood draw was taken. Samples were collected in 10 ml BD Vacutainer® ethyl-enediaminetetraacetic acid tubes and shipped overnight to Fluxion Biosciences for processing within 36 hours. Actual blood volume was 5 to 10 ml for IsoFlux enumeration tests. CellSearch CTC Test samples were collected in CellSearch CellSave tubes and processed at University of Michigan within 36 hours. All results were normalized to 7.5 ml.
CTC Enrichment and Enumeration
IsoFlux System samples were enriched using the IsoFlux CTC Enrichment Kit. CTCs were defined as morphologically intact, cytokeratin positive, CD45 negative and nucleated according to the IsoFlux CTC Enumeration Kit. In a subset of 15 samples 2 tubes were collected at the same time point for repeatability testing. Samples sent for CellSearch enumeration were prepared according to manufacturer instructions. This test relies on a similar definition of CTCs (CK+, CD45− or nucleated) but differs in exact reagents and equipment.
NGS Analysis
A subset of 8 samples had a second blood tube drawn for NGS analysis using the IsoFlux NGS DNA Kit. This kit uses a column to further deplete leukocytes from the enriched CTCs, followed by whole genome amplification based on REPLI-g DNA polymerase (Qiagen®). The resulting gDNA went through target enrichment, library preparation and sequencing using a targeted cancer panel with AmpliSeq™ CHPv2 and the Ion PGM™ System. Final variants were filtered using IonReporter™ software and a specialized set of filters according to manufacturer instructions using the IsoFlux NGS Kit, including percent mutant reads, mutant read counts, strand bias, homo-polymer length and exclusion of common germline mutations. Analytical validation of this filter set was performed using the MDA-MB-231 model tumor cell line (ATCC®) with known mutations spiked into healthy whole blood. This cell line was chosen for its low EpCAM expression and mesenchymal-like properties to more closely approximate clinical samples.
Statistical Analysis
CTC values and pathological T/N stage after cystectomy were analyzed in a 2 × 2 table. Sensitivity, specificity, and positive and negative predictive values are reported. Concordance is reported with the corresponding κ statistic and the 95% CI.
Results
Patient Demographics
Clinicopathological variables are presented for the 3 cohorts, including 21 patients in the neoadjuvant group, 11 in the metastatic group and 13 healthy controls (tables 1 and 2). Clinical staging in the neoadjuvant group was T2N0M0 in 71% of cases, T3N0M0 in 14%, T4N0M0 in 10% and T4N1M0 in 5%. In the metastatic group 10% of patients were chemonaïve while the remaining 90% had received 1 or more lines of chemotherapy and 36% (4 of 11) had undergone prior cystectomy.
Table 1. Demographics and clinicopathological variables of neoadjuvant bladder cancer cohort.
| Pt Identifier—Age—Sex | c Stage | pStage | Bladder Tumor Transurethral Resection Lymphovascular Invasion | Neoadjuvant Chemotherapy Regimen | Cockroft-Gault eGFR (ml/min/1.73 m2) |
|---|---|---|---|---|---|
| Neo1–82–M | T2N0M0 | pT3aN1Mx | Present | Cisplatin + gemcitabine | 72 |
| Neo2–58–M | T2N0M0 | pT2N0Mx | Absent | MVAC | 99 |
| Neo3–79–M | T4aN1M0 | pT4aN2Mx | Absent | Cisplatin + gemcitabine | 81 |
| Neo4–59–M | T2N0M0 | pTON0Mx | Absent | MVAC | 86 |
| Neo5–50–M | T2N0M0 | pT1N0Mx | Present | MVAC | 71 |
| Neo6–49–M | T2N0N0 | pT2bN0Mx | Absent | MVAC | 103 |
| Neo7–62–M | T4aN0M0 | pT4N1Mx | Absent | MVAC | 62 |
| Neo8–62–M | T3N0M0 | pT3aN0(0/17)Mx | Absent | Cisplatin + gemcitabine | 61 |
| Neo9–65–M | T2N0M0 | pT1N0(0/11)Mx | Absent | MVAC | 97 |
| Neo10–73–M | T2N0M0 | pTisN0(0/11)Mx | Absent | Cisplatin + gemcitabine | 73 |
| Neoll–71–F | T2N0M0 | pT3aN0(0/9)Mx | Not determined | Cisplatin + gemcitabine | Greater than 60 |
| Neo12–55–F | T2N0M0 | pT0N0(0/22)Mx | Absent | Dose dense MVAC | 52 |
| Neo13–76–M | T2N0M0 | Not applicable | Present | Cisplatin + gemcitabine | Greater than 60 |
| Neo14–76–F | T2N0M0 | pT0N0(0/10)M0 | Absent | MVAC | Greater than 60 |
| Neo15–52–F | T2N0M0 | pT0N0(0/22)Mx | Absent | MVAC | Greater than 60 |
| Neo16–69–M | T3N0M0 | pT3aN0(0/9)Mx | Present | Split dose cisplatin + gemcitabine | 58 |
| Neo17–62–M | T4N0M0 | pT4aN0Mx | Present | MVAC | Greater than 60 |
| Neo18–82–M | T2N0M0 | Not applicable | Absent | Cisplatin + gemcitabine | Greater than 60 |
| Neo19–65–F | T2N0M0 | Not applicable | Absent | MVAC | 59 |
| Neo20–57–M | T2N0M0 | Not applicable | Absent | Cisplatin + gemcitabine | 59 |
Table 2. Demographics and clinicopathological variables of metastatic bladder cancer cohort and healthy donor controls.
| Pt Identifier—Age—Sex | Chemotherapy History | Metastatic Sites | Prior Cystectomy |
|---|---|---|---|
| Metastatic: | |||
| Met1–61–M | 1st Line | Lymph nodes, bone, viscera | No |
| Met2–68–M | More than 2 lines | Lymph nodes, bone. viscera | Yes |
| Met3-–6–F | 1st Line | Lymph nodes, viscera | No |
| Met4–70–M | 2nd Line | Lymph nodes | No |
| Met5–66–M | 1st Line | Lymph nodes, bone, viscera | Yes |
| Met6–71–M | Not applicable | No | |
| Met7–73–F | 2nd Line | Lymph nodes, bone | Yes |
| Met8–61–M | Naïve | Viscera | No |
| Met9–81–M | Naïve | Lymph nodes, bone | No |
| Met10–43–M | 2nd Line | Lymph nodes, viscera | Yes |
| Met11–75–M | 1st Line | Viscera | No |
| Control: | – | – | – |
| HD1–22–M | |||
| HD2–22–M | |||
| HD3–44–M | |||
| HD4–44–M | |||
| HD5–26–M | |||
| HD6–30–M | |||
| HD7–30–M | |||
| HD8–54–M | |||
| HD9–27–M | |||
| HD10–26–M | |||
| HD11–24–M | |||
| HD12–36–M | |||
| HD13–36–M | |||
CTC Enumeration
CTC counts are reported at baseline for all cohorts and following 1 cycle of chemotherapy, the latter in the neoadjuvant group only (table 3). For patients with multiple tubes tested at the same time point CTC counts are shown as the mean of the 2 values. The median CTC value was 13 in the neoadjuvant group at baseline, 5 in the neoadjuvant group at followup, 29 in the metastatic group and 2 in the healthy group (fig. 2). There was a low number of CTC positive events in the healthy group (median 2, range 0 to 8, 99% CI of the mean 0.14–4.02). To simplify the overall analysis CTC counts were grouped into bins, including low—CTC less than 10, medium—10 to 30 and high—greater than 30. In the neoadjuvant group 60% of baseline samples were CTC medium or high, which decreased to 33% after 1 cycle of chemotherapy. CTC counts were grouped by pStage, showing that adverse pStage was associated with increased CTCs (greater than 10) (fig. 3). In the metastatic cohort 73% of samples were medium or high and in the healthy group the rate was 0%.
Table 3. CTC count normalized to 7.5 ml blood in patients in all 3 groups.
| Pt Identifier | CTC Count |
|---|---|
| Neoadjuvant (baseline/followup):* | |
| Neo1 | 50/60 |
| Neo2 | 10/Not applicable |
| Neo3 | 60/7 |
| Neo4 | 8/2 |
| Neo5 | 0/4 |
| Neo6 | 0/2 |
| Neo7 | 11/750 |
| Neo8 | 2/0 |
| Neo9 | 19/6 |
| Neo10 | 1/3 |
| Neo11 | 179/0 |
| Neo12 | 128/0 |
| Neo13 | 88/3 |
| Neo14 | 1/0 |
| Neo15 | 3/34 |
| Neo16 | 15/9 |
| Neo17 | 358/33 |
| Neo18 | 183/100 |
| Neo19 | 233/Not applicable |
| Neo20 | 8/14 |
| Metastatic: | |
| Met1 | 32 |
| Met2 | 26 |
| Met3 | 146 |
| Met4 | 88 |
| Met5 | 26 |
| Met6 | 1 |
| Met7 | 33 |
| Met8 | 0 |
| Met9 | 154 |
| Met10 | 34 |
| Met11 | 6 |
| Control: | |
| HD1 | 1 |
| HD2 | 0 |
| HD3 | 2 |
| HD4 | 0 |
| HD5 | 3 |
| HD6 | 4 |
| HD7 | 4 |
| HD8 | 8 |
| HD9 | 2 |
| HD10 | 1 |
| HD11 | 2 |
| HD12 | 0 |
| HD13 | 0 |
CTC averaged in subset with multiple tubes tested at same time point.
Figure 2.
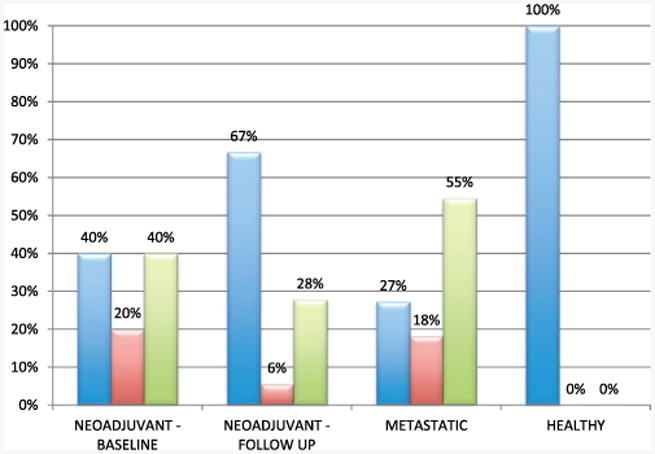
Enumeration results. CTC count in each cohort was grouped into bins of CTC low—less than 10 (blue bars), medium—10 to 30 (red bars) and high—greater than 30 (green bars), and reported as percent of each cohort.
Figure 3.
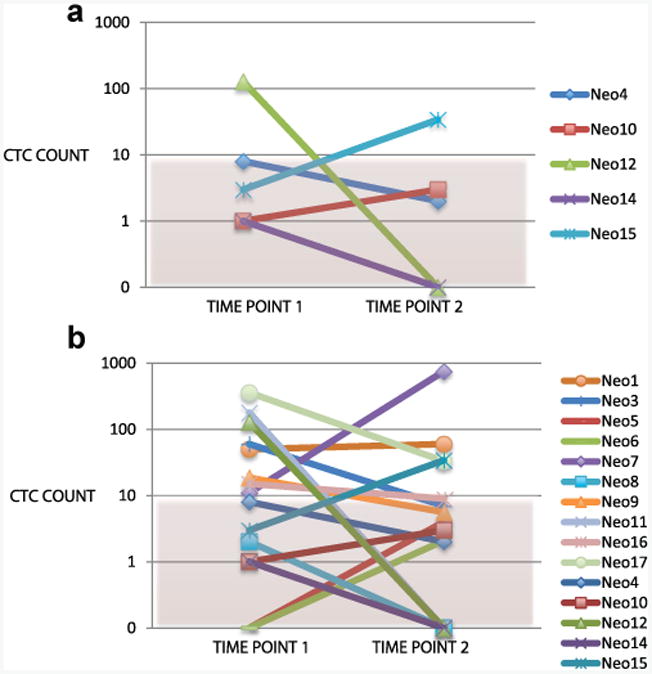
Relative change in CTC count between baseline and 1-month followup in patients in neoadjuvant group by pathological stage. a, subset with pStage T0 and N0 at cystectomy. b, subset with pStage T1 or N1 or greater at cystectomy.
Assay Reproducibility
A subset of 15 neoadjuvant samples had 2 blood tubes submitted at the same time point to assess the reproducibility of the enumeration assay (fig. 4, a). When using the low, medium and high scheme, the concordance between tube 1 and tube 2 samples was 15 of 15 (100%).
Figure 4.
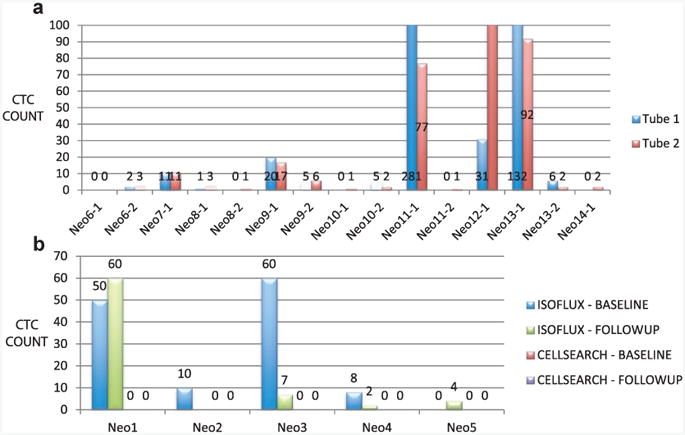
a, assay repeatability. In patient subset duplicate blood tubes were collected at same time point for repeatability analysis. Normalized CTC counts are shown on log scale with reference to low/medium/high classification. Concordance among these 15 matched samples using this scheme was 100%. b, comparison to CellSearch. First 5 patients had matched samples sent for analysis on IsoFlux System and CellSearch CTC Test at baseline and after 1 chemotherapy cycle. In 5 patients tested no cells were detected using CellSearch.
Comparison to CellSearch
In 5 patients in the neoadjuvant group blood tubes were collected for parallel analysis between IsoFlux and CellSearch at baseline and followup (fig. 4, b). This resulted in 9 comparisons since 1 patient was lost to followup. The IsoFlux test showed that 4 of 9 samples (44%) had 10 or more CTCs. None of the 10 samples tested using the CellSearch system had a CTC count above 0.
CTC Correlation to Pathological Staging
In a subset of 16 patients in the neoadjuvant group pathological stage was determined at cystectomy 4 months after baseline. Table 4 shows CTC low, medium or high levels at baseline and after 1 cycle of chemotherapy. Analysis was performed to see how CTC counts before and after chemotherapy were associated with pStage. Nodal involvement (N1-N2) and tumor size (T1-4) strongly correlated with poor prognosis. These parameters were used to assess whether the tumor had responded well to the chemotherapy regimen. These data are presented to determine whether there was initial evidence that CTCs could predict which patients might benefit from proceeding straight to cystectomy rather than receiving further chemotherapy.
Table 4. CTC count by pStage.
| No. T0/Tis (%)* | No. T1-4 or N1/2 (%)* | No. Concordance/Total No. (%) | κ (95% CI) | |
|---|---|---|---|---|
| Baseline: | 5 | 11 | 12/16 (75) | 0.48 (0.05—0.9) |
| Low | 4 (57) | 3 (43) | ||
| Medium/high | 1 (11) | 8 (89) | ||
| 1-Mo followup:† | 5 | 10 | 7/15 (46.7) | 0.08 (−0.3–0.4) |
| Low | 4 (36) | 7 (64) | ||
| Medium/high | 1 (25) | 3 (75) | ||
| Baseline vs followup paired samples: | 5 | 10 | – | – |
| Low vs low | 3 (50) | 3 (50) | ||
| Low vs medium/high | 1 (100) | 0 | ||
| Medium/high vs low | 1 (20) | 4 (80) | ||
| Medium/high vs medium/high | 0 | 3 (100) |
Subset of neoadjuvant group underwent cystectomy and had p stage available (T0/Tis—CTC low and T1-4 or N1/2—CTC medium/high).
After 1 chemotherapy cycle.
Baseline CTC with a cutoff of less than 10 had 73% sensitivity and 80% specificity. The positive predictive value of baseline CTCs (medium/high baseline CTCs and unfavorable pStage) was 89% (8 of 9 patients). Negative predictive value (low baseline CTCs and favorable pStage) was 57% (4 of 7 patients). Concordance, defined as 1) low CTC, pT0/Tis and N0 or 2) medium/high CTC and T1-T4 or N1/2, was 75% (12 of 16 patients) using baseline CTCs with a κ statistic of 0.48 (95% CI 0.05–0.9, table 4). Followup CTCs using the same cut point had 30% sensitivity, 80% specificity, 75% positive predictive value and 36.4% negative predictive value. Concordance was 46.7% with a κ statistic of 0.08 (95% CI −0.3–0.4).
Next Generation Sequencing
Analytical
A total of 12 analytical samples were prepared using 4 spike-in concentrations, including 0, 3, 6 or 12 cells per ml blood in 3 samples each. One sample per concentration was enumerated to assess final CTC purity in NGS samples. CTC recovery was 0, 2, 4 and 8 cells per ml, respectively, representing 70% average recovery for MDA-MB-231. This represented a tumor cell purity of 0%, 5%, 8% and 15%, respectively. Three single nucleotide variants in the BRAF, KRAS and TP53 genes were detected down to the lowest CTC purity (5%) (supplementary table, http://jurology.com/). These variants were detected in pure cell line samples with BRAF and KRAS showing a heterozygous frequency of almost 50% and TP53 showing a homozygous frequency of almost 100%.
At the primary test site 96% of true positive calls (23 of 24) were made across these 8 samples. One false-positive variant was called in 1 of the 8 samples. Six DNA samples were run at a second sequencing site to assess reproducibility. The same true positive calls were made (94%, 17 of 18) with closely correlated allelic frequency and 2 false-positive results detected (fig. 5).
Figure 5.
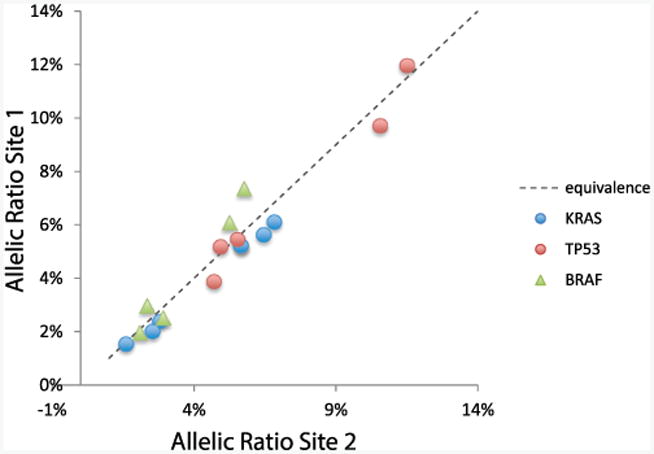
Analytical validation of NGS assay showing correlation between allelic frequencies from runs at 2 sites. MDA-MB-231 model tumor cell line was spiked into healthy donor blood, resulting in final CTC concentrations of 0 to 8 CTCs/ml blood. Cell line has 3 known somatic variants in BRAF, KRAS and TP53 genes. NGS workflow detected each variant for 17 of 18 positive calls with only 1 false-positive call in test group.
Clinical
Eight clinical bladder cancer samples and positive/negative controls were analyzed with the same validated NGS workflow. All 6 positive control calls were correct with no false-positive calls in any positive controls or healthy samples. A matched blood tube was used for CTC enumeration and determination of CTC purity, defined as the ratio of tumor cells to the total number of cells (table 5). The same variant filtering criteria applied to analytical samples was used for clinical sample analysis to produce high confidence variant calls. Clinical samples showed a median count of 27 CTCs recovered at a median purity of 12%. Four of the 8 patient samples (50%) tested had at least 1 variant detected using the CHPv2 cancer hotspot panel. All 3 expected variants in the 2 positive controls were detected and no false-positive findings were detected in negative controls. Of the 8 patient samples 4 (50%) had at least 1 somatic variant detected.
Table 5. NGS analysis of clinical samples in 8 patients in neoadjuvant and metastatic settings, and in positive (spiked) and negative (healthy) controls.
| Pt Identifier | Ca Stage | CTC Count (% purity) | Gene Sequence Variants | Location + Defects |
|---|---|---|---|---|
| Healthy | – | 0 | None | Not applicable |
| Healthy | – | 0 | None | Not applicable |
| Healthy | – | 0 | None* | Not applicable |
| Healthy | – | 0 | None | Not applicable |
| N17 (1-mo followup) | Neoadjuvant | 40 (10) | FGFR2 | Chr10:123279681 G>A p.Arg251Ter |
| N18 (1-mo followup) | Neoadjuvant | 100 (19) | PDGFRA* | Chr4:55144562 A>G p.Tyr679Cys |
| N22* | Neoadjuvant | 20 (12) | None | – |
| N22 (1-mo followup)* | Neoadjuvant | 21 (8) | EGFR | Chr7:55221802 G>A p.(=) |
| M9 | Metastatic | 154 (25) | JAK2* | Chr9:5073770, T>G, p.Val617Phe |
| M10 | Metastatic | 34 (4) | None* | – |
| M12* | Metastatic | 11 (15) | None | – |
| M13 | Metastatic | 8 (not determined) | None | – |
| MDA-MB-231 cell spike | Pos control | Not determined | KRAS, BRAF, TP53* | Chr7:140481417 C>A, p.Gly464Val, chr12:25398281 C>T p.Gly13Asp, chr17:7577099 G>T p.Gly207Trp |
| MDA-MB-231 cell spike | Pos control | 60 (8) | KRAS, BRAF, TP53 | Chr7:140481417 C>A p.Gly464Val, chr12:25398281 C>T p.Gly13Asp, chr17:7577099 G>T p.Gly207Trp |
Parallel NGS analysis was performed at 2 independent laboratories for sample subset that showed concordant calls.
Discussion
This pilot study explored the usefulness of CTC analysis in the diagnostic assessment of bladder cancer. CellSearch, which has FDA (Food and Drug Administration) clearance for counting CTCs in breast, prostate and colorectal cancers, has low sensitivity in bladder cancer with detectable CTCs in only 17% to 23% of patients. A more sensitive methodology is needed to further pursue CTC biomarker applications in the clinic. The IsoFlux System was used in this study and compared to CellSearch in a subset of patients. The IsoFlux System recovered greater than 10 CTCs in 44% of the matched clinical samples (4 of 9) tested compared to 0% for CellSearch, which identified no CTCs in the same 9 samples. This suggests a gain in sensitivity to detect patients diagnosed with bladder cancer compared to healthy controls. This improvement is due to more uniform flow and magnetic forces applied in the microfluidic IsoFlux cartridge.
The IsoFlux test was reproducible across the low/medium/high classification with 100% concordance (15 of 15 matched time point samples). It demonstrated the ability to recover medium/high levels of CTCs in 60% of neoadjuvant cases and 73% of metastatic cases (fig. 2). The latter was consistent with a likely higher tumor burden. The decrease from 60% to 33% in CTC high/medium cases after the first chemotherapy cycle was consistent with the presumed cytolytic effect of chemotherapy.
A matrix of associations was examined to determine whether baseline or followup CTCs could predict pathological stage at cystectomy. The data suggested that baseline CTC levels were a better predictor of pStage with 73% sensitivity while followup CTC levels after 1 chemotherapy cycle were not as indicative with 30% sensitivity. While the small study size limits the statistical analysis of the association, this has helped refine the test plan for the planned expansion of this study.
This study also explored the use of NGS to profile somatic variants in CTCs. NGS has already shown benefit in tumor tissue samples by identifying patients likely to respond to the emerging class of molecular therapies. NGS samples must be of sufficient purity to detect somatic variants. This is readily achievable for tumor biopsies, which routinely exceed 50% purity, but it poses a challenge for CTCs, which are often in the less than 1% purity range. Methods used in this study demonstrated the ability to produce samples with greater than 5% purity in 85% of the patients tested and greater than 10% purity in 71% of patients. For NGS analysis 4 neoadjuvant and 4 metastatic cases were selected. At purity levels near 5% the known mutations in the analytical samples were detectable using a commercially available sequencer and a targeted gene panel. For the clinical sample set 7 of 8 samples had CTC purity above 5% and variants were detected in 4 of the 8 samples. The same variants were detected at different NGS service laboratories, thereby increasing confidence in the NGS workflow.
However, an absence of detected mutations cannot be equated to the lack of mutations in the patient tumor. Mutation detection depends on the presence of CTCs of sufficient purity and the concordance between mutations harbored by CTCs and different parts of the tumor tissue. Although it was not performed in this study, comparison to the primary tumor tissue would add confidence that the mutations detected in CTCs are represented in the primary tumor. A recent study comparing single cell CTC exome sequencing to data on multiple biopsy sites demonstrated high concordance between CTC mutations and metastatic trunk mutations.25 This feasibility study establishes a workflow to detect and analyze CTCs without the need for single cell picking, a process that can be challenging at most clinical laboratories.
Conclusions
This study demonstrates a potential role for CTCs in the management of neoadjuvant bladder cancer. The IsoFlux method increases the sensitivity of CTC detection and enables molecular profiling of recovered cells via NGS. A larger prospective trial is planned to explore these aims further.
Acknowledgments
Kirsten Copren, Kathryn Thompson and Jennifer Dang, University of California-San Francisco, assisted with sequencing. NGS was done at the University of California-San Francisco Genome Core and Thermo Fisher, South San Francisco, California.
Supported by Michigan Institute for Clinical and Health Research, and Clinical and Translational Science Award Grant UL1TR000433.
Abbreviations and Acronyms
- CTC
circulating tumor cell
- MVAC
methotrexate, vinblastine, doxorubicin and cisplatin
- NGS
next generation sequencing
- pStage
pathological stage at cystectomy
Footnotes
Study received University of Michigan and University of California-San Francisco institutional review board approval.
References
- 1.Surveillance, Epidemiology, and End Results. [Accessed July 23, 2014];SEER Stat Fact Sheets: Bladder Cancer. Available at http://seer.cancer.gov/statfacts/html/urinb.html.
- 2.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 3.Sonpavde G, Goldman BH, Speights VO, et al. Quality of pathologic response and surgery correlate with survival for patients with completely resected bladder cancer after neoadjuvant chemotherapy. Cancer. 2009;115:4104. doi: 10.1002/cncr.24466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 5.Advanced Bladder Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis. Lancet. 2003;361:1927. doi: 10.1016/s0140-6736(03)13580-5. [DOI] [PubMed] [Google Scholar]
- 6.Neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: a randomised controlled trial. International Collaboration of Trialists. Lancet. 1999;354:533. [PubMed] [Google Scholar]
- 7.Splinter TA, Scher HI, Denis L, et al. The prognostic value of the pathological response to combination chemotherapy before cystectomy in patients with invasive bladder cancer. European Organization for Research on Treatment of Cancer-Genitourinary Group. J Urol. 1992;147:606. doi: 10.1016/s0022-5347(17)37318-4. [DOI] [PubMed] [Google Scholar]
- 8.Galsky MD, Hahn NM, Rosenberg J, et al. A consensus definition of patients with metastatic urothelial carcinoma who are unfit for cisplatin-based chemotherapy. Lancet Oncol. 2011;12:211. doi: 10.1016/S1470-2045(10)70275-8. [DOI] [PubMed] [Google Scholar]
- 9.Kruk S, Gakis G, Stenzl A. Disseminated and circulating tumor cells for monitoring chemotherapy in urological tumors. Anticancer Res. 2011;31:2053. [PubMed] [Google Scholar]
- 10.Gallagher DJ, Milowsky MI. Circulating tumor cells: a potential bladder cancer biomarker? Eur J Clin Med Oncol. 2010;2:105. [Google Scholar]
- 11.Friedlander TW, Premasekharan G, Paris PL. Looking back to the future of CTCs. Pharmacol Ther. 2014;142:271. doi: 10.1016/j.pharmthera.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Ashworth T. A case of cancer in which cells similar to those in the tumors were seen in the blood after death. Aust Med J. 1869;14:146. [Google Scholar]
- 13.Poste G, Fidler IJ. The pathogenesis of cancer metastasis. Nature. 1980;283:139. doi: 10.1038/283139a0. [DOI] [PubMed] [Google Scholar]
- 14.Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 15.Cohen SJ, Punt CJ, Iannotti N, et al. Prognostic significance of circulating tumor cells in patients with metastatic colorectal cancer. Ann Oncol. 2009;20:1223. doi: 10.1093/annonc/mdn786. [DOI] [PubMed] [Google Scholar]
- 16.de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 17.Scher HI, Beer TM, Higano CS, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1-2 study. Lancet. 2010;375:1437. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flaig TW, Wilson S, van Bokhoven A, et al. Detection of circulating tumor cells in metastatic and clinically localized urothelial carcinoma. Urology. 2011;78:863. doi: 10.1016/j.urology.2011.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallagher DJ, Milowsky MI, Ishill N, et al. Detection of circulating tumor cells in patients with urothelial cancer. Ann Oncol. 2009;20:305. doi: 10.1093/annonc/mdn627. [DOI] [PubMed] [Google Scholar]
- 21.Naoe M, Ogawa Y, Morita J, et al. Detection of circulating urothelial cancer cells in the blood using the CellSearch System. Cancer. 2007;109:1439. doi: 10.1002/cncr.22543. [DOI] [PubMed] [Google Scholar]
- 22.Rink M, Chun FK, Dahlem R, et al. Prognostic role and HER2 expression of circulating tumor cells in peripheral blood of patients prior to radical cystectomy: a prospective study. Eur Urol. 2012;61:810. doi: 10.1016/j.eururo.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 23.Soave A, Minner S, Engel O, et al. Implications for circulating tumor cells on adjuvant chemotherapy decision-making in patients with urothelial carcinoma of the bladder treated with radical cystectomy. Presented at annual congress of European Association of Urology; Amsterdam, The Netherlands. April 11-15, 2014; abstract 47554. [Google Scholar]
- 24.Harb W, Fan A, Tran T, et al. mutational analysis of circulating tumor cells using a novel micro-fluidic collection device and qPCR assay. Transl Oncol. 2013;6:528. doi: 10.1593/tlo.13367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lohr JG, Adalsteinsson VA, Cibulskis K, et al. Whole-exome sequencing of circulating tumor cells provides a window into metastatic prostate cancer. Nat Biotechnol. 2014;32:479. doi: 10.1038/nbt.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]


