Abstract
Due to the safety, convenience and efficacy of subcutaneous administration of bortezomib (scBor), it is becoming increasingly common to treat multiple myeloma (MM) using this treatment method. The current retrospective study suggested a lower incidence of peripheral neuropathy (PN) outcomes and superior efficacy following treatment with scBor combined with thalidomide and dexamethasone (VTD) in MM when compared with intravenous Bor (ivBor) treatment. The data of 81 patients from the Affiliated Hospital of Nantong University between September 2011 and February 2014 were analyzed, including 37 scBor and 44 ivBor patients administered a median (range) of 5.5 (3–8) and 6 (3–10) chemotherapy cycles, respectively. Adverse events (AEs) were assessed according to the National Cancer Institute (NCI) Common Terminology Criteria for AEs, and response and progression were assessed by the International Myeloma Working Group criteria. Evidence of histopathology using transmission electron microscopy (TEM) was obtained from an in vivo model of adult Sprague Dawley (SD) rats. Following bortezomib-based VTD chemotherapy, patients had achieved very good partial remission or demonstrated no significant difference between the scBor and ivBor treatment groups (75.6 vs. 84.1%, respectively; P=0.350). The 1-year progression-free survival (83.8 vs. 84.1%, scBor vs. ivBor; P=0.921) and 1-year overall survival (OS) (91.9 vs. 90.9%, respectively; P=0.926) were also similar. PN rates of all the NCI grades were 51.3 and 61.3% (P=0.371); grade ≥2, 35.1 and 56.8% (P=0.052); and grade ≥3, 32.7 and 20.5% (P=0.015) in the subcutaneous and intravenous treatment groups, respectively, which suggests that severe PN may be less common following scBor treatment. There were no severe injection site reactions in the scBor-treated group. The incidence of adverse events were comparable between the two groups, including thrombocytopenia, anemia, fatigue and gastrointestinal symptoms such as nausea and vomiting. Furthermore, TEM images of the SD rat sciatic nerves revealed that all rats suffered PN to varying degrees, except the control group, and that the PN of ivBor-treated rats (in the presence and the absence of thalidomide) was more severe than that of scBor-treated rats. It was concluded that a subcutaneous dose of bortezomib of 1.3 mg/m2 may result in a lower incidence and severity of PN, with equivalent efficacy, as a component of combination VTD chemotherapy.
Keywords: peripheral neuropathy, bortezomib, subcutaneous injection, thalidomide, multiple myeloma, VTD
Introduction
Multiple myeloma is the second most common hematological malignancy, with an incidence of 3.29–4.82 cases per 100,000 individuals a year globally, an incidence that is increasing due to population ageing (1). The proteasome inhibitor bortezomib was, at first use, applied to a relapsed or refractory multiple myeloma (MM) at a 1.3 mg/m2 dose, twice per week (2). It then demonstrated its efficacy via >10 years of clinical use (2,3). Bortezomib acts upon multiple myeloma by affecting signaling cascades, leading to a toxic buildup of misfolded proteins (4,5). However, the precise mechanism underlying the action of this agent has yet to be fully elucidated. Intravenous injection was previously the standard way of administering bortezomib (2), but subcutaneous administration is increasingly common in the treatment of MM due to the comparative efficacy and improved safety profile of this method of administration, particularly with regard to the lower incidence and severity of peripheral neuropathy (PN). PN primarily affects the sensory nerves, causing symptoms such as a burning sensation, loss of response to external stimuli, paresthesia, discomfort or neuralgia, interfering with patient quality-of-life (6). Use of a single agent, bortezomib, prolonged the median time of progression of MM from 3 to 9 months (3,7). Furthermore, it has been revealed that bortezomib-based combination chemotherapy has an improved efficacy from that of bortezomib alone (2). However, the resultant PN following combination therapy, which includes agents such as thalidomide, is of key importance to address (8). Thalidomide is popular in MM treatment due to its low cost and efficacy profile; this drug suppresses vascular regeneration, improves immune regulation and interferes with the protective bone marrow microenvironment surrounding myeloma cells, promoting apoptosis of tumor cells. PN resulting from thalidomide treatment is common (8,9). Bortezomib-thalidomide-dexamethasone (VTD) is one of the most common chemotherapy regimens; however, the systemic safety profile, particularly regarding the inevitable PN, requires additional attention. In the present study, the incidence of PN following treatment with subcutaneous bortezomib and its efficacy when combined with thalidomide was retrospectively analyzed.
Patients and methods
Patients and methods
The present study was approved by the Ethics Committee of the Affiliated Hospital of Nantong University, Nantong, China, and written informed consent was obtained from the patient or patients' families. The clinical data of 81 patients (37 subcutaneously administered and 44 intravenously administered treatment) of newly diagnosed (n=77) or refractory (n=4) MM following 1–3 prior therapies were comprehensively analyzed. These patients were all admitted to the Affiliated Hospital of Nantong University, Nantong, China between September 2011 and February 2014. All patients received a 21-day cycle of 1.3 mg/m2 bortezomib (Takeda Oncology, Cambridge, MA, USA) on days 1, 4, 8 and 11 by subcutaneous injection (scBor) or intravenous infusion (ivBor), 75–125 mg thalidomide (Changzhou Pharmaceutical Factory, Jiangsu, China) daily and 20 mg dexamethasone (Wuhan Yuancheng Pharmaceutical Co., Ltd., Hubei, China) on days 1, 2, 4, 5, 8, 9, 11 and 12. Neuroprotective therapy, including fursultimine or mecobalamine was administered upon the development of PN. The patients were administered the recommended scBor concentration (3) of 2.5 mg/ml (1.75 mg bortezomib reconstituted with 0.7 ml normal 0.9% saline) or 1.0 mg/ml ivBor. The subcutaneous injection sites were the thighs and the abdomen, regularly switching side of injection to prevent injection site reaction (ISR). All patients provided written informed consent. Baseline demographics, disease characteristics and chemotherapy regimens were comparable between the two treatment groups (Table I; P>0.05 for all baseline demographics).
Table I.
Patient demographics and number of cases demonstrating specific characteristics.
| Characteristic | Subcutaneous bortezomib (n=37 patients) | Intravenous bortezomib (n=44 patients) |
|---|---|---|
| Age, years (range) | 63 (43–85) | 64 (36–83) |
| Age ≥65 years | 15 (40.5%) | 18 (40.9%) |
| Male patients | 28 (75.7%) | 31 (70.5%) |
| Newly diagnosed patients | 35 (94.6%) | 38 (86.4%) |
| Myeloma type | ||
| IgG | 15 (40.5%) | 17 (38.6%) |
| IgA | 11 (29.7%) | 13 (29.5%) |
| IgD | 0 (0.0%) | 2 (4.5%) |
| IgM | 2 (5.4%) | 2 (4.5%) |
| Light chain | Lam5 (13.5%), kap4 (10.8%) | Lam6 (13.6%), kap4 (9.1%) |
| ISS stage | ||
| I–II | 15 (40.5%) | 17 (38.6%) |
| III | 22 (59.5%) | 27 (61.4%) |
| No. patients with lytic bone lesions | 24 (64.9%) | 29 (65.9%) |
| Serum albumin, g/l (range) | 34.3 (20–40) | 32.5 (21–38) |
| Plasma β2 microglobulin, mg/l (range) | 4.58 (2.9–23) | 5.77 (1.8–26.5) |
| No. patients using cyclophosphamide | 13 (35.1%) | 15 (34.1%) |
| Renal insufficiency | 6 (16.2%) | 9 (20.5%) |
| Diabetes | 3 (8.1%) | 5 (11.4%) |
| Diabetic neuropathy, grade 1 | 1 (2.7%) | 2 (4.5%) |
Data are presented as number of cases (percentage of total cases), or mean value (range), where indicated. Ig, immunoglobulin; ISS, International Staging System.
The response and progression were assessed by the International Myeloma Working Group criteria (10). Adverse events (AEs) were assessed according to the National Cancer Institute Common Terminology Criteria for AEs version 3.0 (11). The primary aim of this was to investigate the comparative incidence of PN, and the comparative efficacy, following treatment with scBor and ivBor. Grading of neuropathic pain, a symptom of PN, was as follows: Grade 1, pain not interfering with functioning; grade 2, moderate pain, in which pain or analgesics are interfering with functioning, but not interfering with activities of daily living (ADL); grade 3, severe pain, in which pain or analgesics are severely interfering with ADL; grade 4, disabling pain (11). Efficacy was predominantly determined by the complete remission rate or very good partial remission (CR/VGPR) (10), progression-free survival and overall survival rate (OS) after a median follow-up time of 13 (6–16) and 16 months (3–19) in the scBor and ivBor groups, respectively.
Sprague Dawley (SD) rat model
Due to an absence of evidence of PN histopathology in patients, an in vivo model of PN was established in adult SD rats. A total of 18 SD female rats (weight, 200±5 g; age, 2 months; sourced from the Animal Laboratory, Nantong University, Nantong, China) were randomly assigned to 6 groups of 3 rats as follows: i) scBor; ii) ivBor; iii) scBor combined with thalidomide; iv) ivBor combined with thalidomide; v) thalidomide only; and vi) untreated control group. These rats were maintained at 24°C with a 12/12 h light:dark cycle, and were administered food and water at regular intervals. A dose of 1.3 mg/m2 (0.2 mg/kg) bortezomib (Takeda Oncology) was administered to the relevant groups on days 1, 4, 8 and 11, and 100 mg (10.5 mg/kg) thalidomide (Changzhou Pharmaceutical Factory) was administered daily to the relevant groups. Bortezomib was administered via subcutaneous abdominal or tail vein injection, and thalidomide was administered orally. On the 14th day of treatment, rats were sacrificed by cervical dislocation and sciatic nerve samples were extracted by the Animal Laboratory, Nantong University,. These were examined to determine changes with transmission electron microscopy (TEM).
Statistical methods
Data analysis was performed with the Statistical Package for Social Science software (SPSS version 17.0; SPSS, Inc., Chicago, IL, USA). Continuous variables are expressed as median and range and were compared using a t-test. Categorical variables are reported as percentages and compared using a χ2 test. Overall survival rate, determined from diagnosis to mortality, was estimated by the Kaplan-Meier method and curve comparison was conducted using log-rank analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
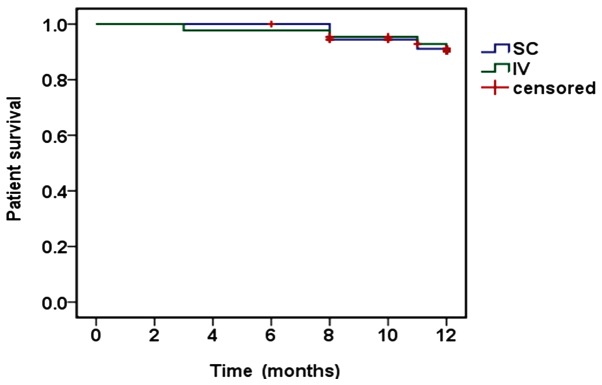
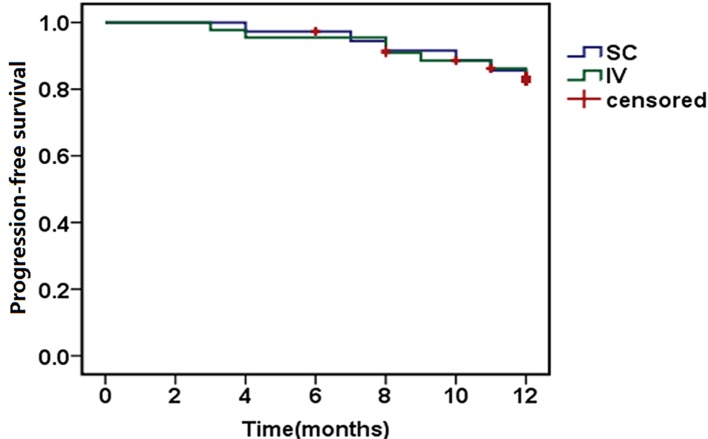
The median number of cycles of bortezomib administered to patients were 5.5 (3–8) and 6 (3–10) in the scBor and ivBor groups, respectively. This included 33 of 37 (89.2%) scBor-treated patients that had completed ≥4 cycles of treatment, with comparable numbers of 40 of 44 (90.9%) patients administered ivBor. Patients who had PN or neuropathic pain of grade ≥2 (10) at baseline were excluded from the study. Among the 37 and 44 participating patients, 28 (75.6%) and 37 (84.1%) achieved VGPR or better (P=0.350), including 13 (35.1%) and 17 (38.6%) with CR, from the scBor and ivBor groups, respectively (P=0.749). The median time to reach VGPR was 2 months in both groups. At the final time point, 3 years after the study commencement, 34 (91.9%) and 40 (90.9%) patients survived for ≥1 year (P=0.926; Fig. 1) and 31 (83.8%) and 37 (84.1%) reached 1-year progression-free survival (P=0.921; Fig. 2), in the scBor and ivBor groups, respectively. It is of note that 6 patients (2 scBor and 4 ivBor) succumbed to MM by the end of the study. These data collectively suggest an equivalent efficacy of bortezomib administered by either route.
Figure 1.
Kaplan-Meier estimates of overall survival following SC and IV bortezomib treatment. SC, subcutaneous; IV, intravenous.
Figure 2.
Kaplan-Meier estimates of progression-free survival following SC and IV bortezomib treatment. SC, subcutaneous; IV, intravenous.
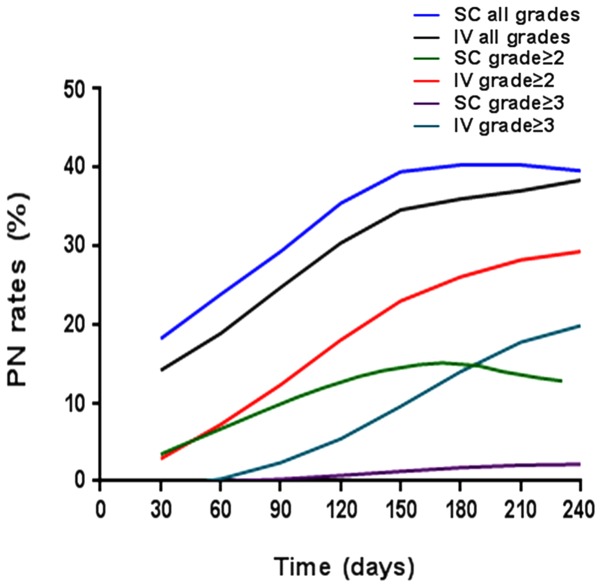
Following supplementation with thalidomide and dexamethasone, the incidence of PN, the most detrimental adverse event, arising due to the treatment remained lower in the scBor-treated group than the ivBor-treated group (all cases: 51.3 vs. 61.3%, P=0.371; grade ≥2: 35.1 vs. 56.8%, P=0.052; grade ≥3: 2.7 vs. 20.5%, P=0.015, respectively; Fig. 3). Following treatment with thalidomide, the median time until onset of any grade of PN was 3.0 (1–7) and 4.0 (1–10) months, median time until onset of grade ≥2 was 3.5 (2–11) and 4.0 (2–12) months in the scBor and ivBor groups, respectively, and time until the onset of grade ≥3 PN was 5.5 (4–10) months in the ivBor group (as no patients demonstrated grade ≥3 PN in the scBor group). In the scBor and ivBor groups, 28 (75.7%) and 29 (65.9%) patients, respectively, were additionally administered fursultimine or mecobalamine upon the development of PN. A total of 0 and 3 (6.8%) patients in the scBor and ivBor groups, respectively, discontinued thalidomide use or were administered a reduced dose of 50 mg thalidomide for ~1 month to prevent or attenuate severe PN. Other common side effects were thrombocytopenia, anemia and feeble and gastrointestinal symptoms, including nausea and vomiting (Table II).
Figure 3.
Increasing PN rates following SC and IV bortezomib treatment. PN, peripheral neuropathy; SC, subcutaneous; IV, intravenous.
Table II.
Hematology parameters and rate of other adverse events, expressed as no. cases (% of total cases).
| Subcutaneous bortezomib (n=37 patients) | Intravenous bortezomib (n=44 patients) | |||
|---|---|---|---|---|
| Side effects | All grades | Grade ≥3 | All grades | Grade ≥3 |
| Hematology laboratory data | ||||
| White blood cell count | 19 (51.4%) | 2 (5.4%) | 20 (45.5%) | 9 (20.5%) |
| Hemoglobin level decrease | 28 (75.7%) | 17 (45.9%) | 35 (79.5%) | 22 (50.0%) |
| Platelet count decrease | 17 (45.9%) | 9 (24.3%) | 26 (59.1%) | 17 (38.6%) |
| Diarrhea | 4 (10.8%) | 0 (0.0%) | 5 (11.4%) | 0 (0.0%) |
| Abdominal bloating | 4 (10.8%) | 0 (0.0%) | 7 (15.9%) | 0 (0.0%) |
| Nausea/vomiting | 7 (18.9%) | 0 (0.0%) | 9 (20.5%) | 0 (0.0%) |
| Constipation | 13 (35.1%) | 0 (0.0%) | 15 (34.1%) | 2 (4.5%) |
| Fatigue | 17 (45.9%) | 2 (5.4%) | 29 (65.9%) | 3 (6.8%) |
| Fever | 7 (18.9%) | 0 (0.0%) | 10 (22.7%) | 2 (4.5%) |
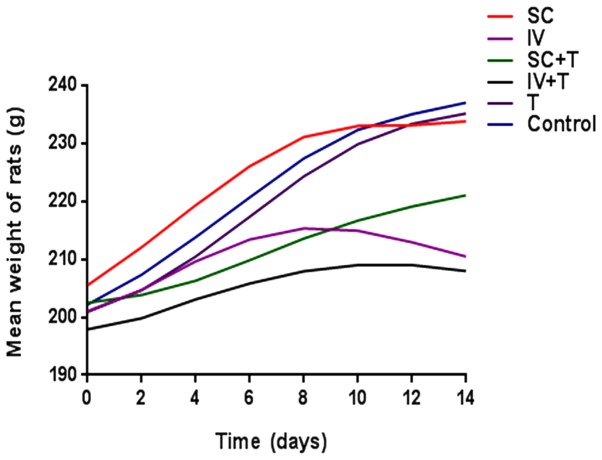
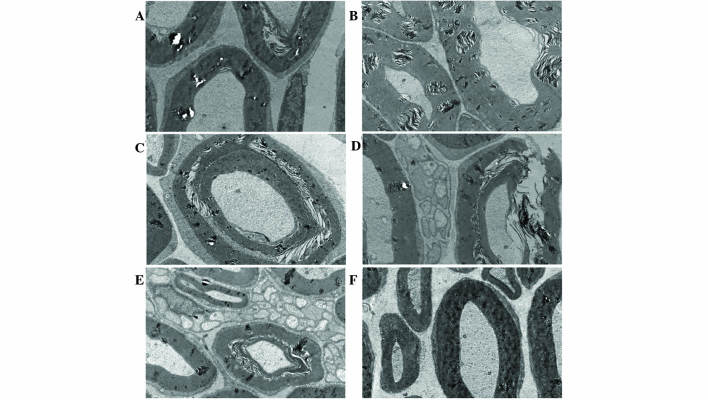
Adult SD rats subjected to ivBor treatment gained weight more slowly (Fig. 4) and were less active compared with the untreated control group, in both the presence and absence of thalidomide treatment, indicating systemic poisoning. There were no evident symptoms of systemic poisoning in the scBor-treated and control groups of rats. TEM images indicated PN due to the layered structures (Fig. 5A and B), discontinuous (Fig. 5C) or ruptured tissue (Fig. 5D) of the myelin sheath and vacuolation (Fig. 5E). Furthermore, these observations were more common in the ivBor-treatment group than the scBor group, in both the presence and absence of thalidomide. In addition, only minor changes to the dorsal root nerve of scBor-treated rats were observed.
Figure 4.
Mean weights of Sprague-Dawley rats in the six groups. SC, subcutaneous; IV, intravenous.
Figure 5.
Transmission electron microscope images (magnification, ×10,000) of left sciatic nerves in rats from six groups, as follows: (A) scBor; (B) scBor combination treatment with thalidomide; (C) ivBor; (D) ivBor combination treatment with thalidomide; (E) thalidomide only and (F) control. sc, subcutaneous; Bor, bortezomib; iv, intravenous.
Discussion
The present study revealed the superior efficacy, and lower incidence and severity of PN following treatment with scBor compared with ivBor when combined with thalidomide and dexamethasone. Despite several minor differences between this and previous therapies, the similarity between the scBor and ivBor groups across several efficacy indicators (VGPR, CR and 1-year overall survival/progression-free survival) was apparent, and revealed a significant improvement compared with a single-agent therapy (3,7). This efficacy may be associated with a higher percentage of patients receiving additional cyclophosphamide, a lower percentage of patients with an ISS stage III (12) or an age ≥65 years old and a lower level of β2 microglobulin in the scBor-treated group.
The intravenous method of administration is the most common method of bortezomib treatment. However, subcutaneous injection of bortezomib is becoming increasingly used, and the side effects of scBor single treatment are well understood (3,7,13,14). A previous study by Arnulf et al (7) of treatment with bortezomib alone demonstrated that the incidence of PN at all grades, grade ≥2 and grade ≥3 were 38 and 53%, 24 and 41% and 6 and 16% in the scBor and ivBor groups, respectively. This was in agreement with the 51.3 and 61.3%, 35.1 and 56.8% and 2.7 and 20.5% observed in the scBor and ivBor groups, respectively, in the present study. Garderet et al (15) demonstrated a cumulative, dose-associated incidence of grade ≥3, exacerbated PN following treatment with bortezomib and thalidomide combinatorial treatment; this presented at an incidence of 29% for VTD treatment and 12% for thalidomide and dexamethasone treatment. The median time of onset of all grades of PN of the patients in the present study was similar, and there were no cases of grade ≥3 PN associated with scBor treatment. These data suggested a decrease in severity of PN following scBor treatment, which may be associated with a lower maximum plasma concentration of bortezomib, neuroprotective effects and thalidomide withdrawal or with dose reduction when severe PN appeared in 3 cases (3). PN occurred more frequently following subcutaneous delivery of bortezomib, but was less severe following this method of bortezomib treatment. In addition, bortezomib-associated PN typically became apparent by the end of therapy cycles 4 or 5, was dose-associated and was reversible in the majority of patients. The baseline characteristics of the age or number or type of prior therapies were not risk factors associated with PN, and the efficacy of bortezomib was not adversely affected by the grade ≥2 PN (16) or the dose regimen (17). Furthermore, there were no obvious ISRs in the current patients, and the low severity of ISRs was similar to that of a previous report (18). Subcutaneous administration of bortezomib therefore provides an important alternative method of treatment, particularly for the elderly or patients with poor vascular conditions, due to its improved safety profile.
Detailed information with regard to other side effects of bortezomib have been sufficiently documented whether this is administered independently or as part of a combination therapy (18–23). Upon treatment with the VTD combination therapy, a decreased white blood cell count, hemoglobin level and platelet count, and gastrointestinal symptoms were the most common side effects in both groups, but these were not severe in the present study. These data were consistent with previous studies (22,23) and presented additional information about the scBor severity as part of a VTD combination therapy.
From the results of previous in vivo studies, it has been hypothesized that bortezomib predominantly causes direct sciatic nerve and dorsal root ganglia toxicity due to ubiquitin aggregates accumulating in the cytoplasm (24,25). In the current study, TEM images of the sciatic nerve of the SD rats indicated that changes to the myelin sheath were less common following scBor compared with ivBor treatment. Furthermore, PN is more severe in combination treatments containing thalidomide; however, this effect was lessened with scBor combination compared with the ivBor combination treatment. Symptoms of systemic poisoning, such as a slower weight increase and lower activity, were more evident following ivBor treatment, in both the presence and absence of thalidomide.
In conclusion, treatment with scBor demonstrated a lower incidence and severity of PN compared with ivBor administration, but did not adversely affect the efficacy of treatment when combined with thalidomide and dexamethasone. Previous studies have indicated that a weekly dose of bortezomib should be used within combinatorial treatments in order to achieve the lowest PN rates (26–28). However, the most efficient dose schedule of bortezomib under varying ISS stages and patient demographics requires additional research.
References
- 1.Phekoo KJ, Schey SA, Richards MA, Bevan DH, Bell S, Gillett D, Møller H. Consultant Haematologists, South Thames Haematology Specialist Committee: A population study to define the incidence and survival of multiple myeloma in a National Health Service Region in UK. Br J Haematol. 2004;127:299–304. doi: 10.1111/j.1365-2141.2004.05207.x. [DOI] [PubMed] [Google Scholar]
- 2.Pantani L, Zamagni E, Zannetti BA, Pezzi A, Tacchetti P, Brioli A, Mancuso K, Perrone G, Rocchi S, Tosi P, Cavo M. Bortezomib and dexamethasone as salvage therapy in patients with relapsed/refractory multiple myeloma: Analysis of long-term clinical outcomes. Ann Hematol. 2014;93:123–128. doi: 10.1007/s00277-013-1828-8. [DOI] [PubMed] [Google Scholar]
- 3.Moreau P, Pylypenko H, Grosicki S, Karamanesht L, Leleu X, Grishunina M, Rekhtman G, Masliak Z, Robak T, Shubina A, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: A randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12:431–440. doi: 10.1016/S1470-2045(11)70081-X. [DOI] [PubMed] [Google Scholar]
- 4.Soriano GP, Besse L, Li N, Kraus M, Besse A, Meeuwenoord N, Bader J, Everts B, den Dulk H, Overkleeft HS, Florea BI, Driessen C. Proteasome inhibitor-adapted myeloma cells are largely independent from proteasome activity and show complex proteomic changes, in particular in redox and energy metabolism. Leukemia. 2016 doi: 10.1038/leu.2016.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ri M. Mechanism of action and determinants of sensitivity to the proteasome inhibitor bortezomib in multiple myeloma therapy. Rinsho Ketsueki. 2016;57:537–45. doi: 10.11406/rinketsu.57.537. [DOI] [PubMed] [Google Scholar]
- 6.Dimopoulos MA, Beksac M, Benboubker L, Roddie H, Allietta N, Broer E, Couturier C, Mazier MA, Angermund R, Facon T. Phase II study of bortezomib-dexamethasone alone or with added cyclophosphamide or lenalidomide for sub-optimal response as second-line treatment for patients with multiple myeloma. Haematologica. 2013;98:1264–72. doi: 10.3324/haematol.2013.084376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnulf B, Pylypenko H, Grosicki S, Karamanesht L, Leleu X, van de Velde H, Feng H, Cakana A, Deraedt W, Moreau P. Updated survival analysis of a randomized phase III study of subcutaneous versus intravenous bortezomib in patients with relapsed multiple myeloma. Haematologica. 2012;97:1925–1928. doi: 10.3324/haematol.2012.067793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glasmacher A, Hahn C, Hoffmann F, Naumann R, Goldschmidt H, von Lilienfeld-Toal M, Orlopp K, Schmidt-Wolf I, Gorschlüter M. A systematic review of phase-II trials of thalidomide monotherapy in patients with relapsed or refractory multiple myeloma. Br J Haematol. 2006;132:584–593. doi: 10.1111/j.1365-2141.2005.05914.x. [DOI] [PubMed] [Google Scholar]
- 9.Palumbo A, Davies F, Kropff M, Bladé J, Delforge M, da Costa F Leal, Sanz R Garcia, Schey S, Facon T, Morgan G, Moreau P. Consensus guidelines for the optimal management of adverse events in newly diagnosed, transplant-ineligible patients receiving melphalan and prednisone in combination with thalidomide (MPT) for the treatment of multiple myeloma. Ann Hematol. 2010;89:803–811. doi: 10.1007/s00277-010-0925-1. [DOI] [PubMed] [Google Scholar]
- 10.Durie BG, Harousseau JL, Miguel JS, Bladé J, Barlogie B, Anderson K, Gertz M, Dimopoulos M, Westin J, Sonneveld P, Ludwig H, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 11.National Cancer Institute, corp. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. Common terminology criteria for adverse events, version 3.0. 2011 Apr 11; Accessed.
- 12.Greipp PR, San MJ, Durie BG, Crowley JJ, Barlogie B, Bladé J, Boccadoro M, Child JA, Avet-Loiseau H, Kyle RA, Lahuerta JJ, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412–20. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 13.Hoy SM. Subcutaneous bortezomib: In multiple myeloma. Drugs. 2013;73:45–54. doi: 10.1007/s40265-013-0006-6. [DOI] [PubMed] [Google Scholar]
- 14.Grosicki S. Subcutaneous bortezomib as a new promising way to successful maintenance therapy in multiple myeloma. Wiad Lek. 2012;65:167–173. (In Polish) [PubMed] [Google Scholar]
- 15.Garderet L, Iacobelli S, Moreau P, Dib M, Niederwieser D, Masszi T, Fontan T, Michallet M, Gratwohl A, Lafon I, et al. Superiority of the triple combination of bortezomib-thalidomide-dexamethasone over the dual combination of thalidomide-dexamethasone in patients with multiple myeloma progressing or relapsing after autologous transplantation: The MMVAR/IFM 2005-04 Randomized phase III trial from the chronic leukemia working party of the European group for blood and marrow transplantation. J Clin Oncol. 2012;30:2475–2482. doi: 10.1200/JCO.2011.37.4918. [DOI] [PubMed] [Google Scholar]
- 16.Tacchetti P, Terragnac C, Galli M, Zamagni E, Petrucci MT, Pezzi A, Montefusco V, Martello M, Tosi P, Baldini L, et al. Bortezomib- and thalidomide-induced peripheral neuropathy in multiple myeloma: Clinical and molecular analyses of a phase 3 study. Am J Hematol. 2014;89:1085–1091. doi: 10.1002/ajh.23835. [DOI] [PubMed] [Google Scholar]
- 17.Richardson PG, Sonneveld P, Schuster MW, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, Goldschmidt H, Reece D, et al. Reversibility of symptomatic peripheral neuropathy with bortezomib in the phase III APEX trial in relapsed multiple myeloma: Impact of a dose-modification guideline. Br J Haematol. 2009;144:895–903. doi: 10.1111/j.1365-2141.2008.07573.x. [DOI] [PubMed] [Google Scholar]
- 18.Lamm W, Drach-Schauer B, Eder S, Drach J. Bortezomib administered subcutaneously is well tolerated in bortezomib-based combination regimens used in patients with multiple myeloma. Oncology. 2013;85:223–227. doi: 10.1159/000355197. [DOI] [PubMed] [Google Scholar]
- 19.San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kroptt M, Spicka I, Petrucci MT, Palumbo A, Samoilova OS, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 20.Wright JJ. Combination therapy of bortezomib with novel targeted agents: An emerging treatment strategy. Clinical Cancer Research. 2010;16:4094–4104. doi: 10.1158/1078-0432.CCR-09-2882. [DOI] [PubMed] [Google Scholar]
- 21.San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, Spicka I, Petrucci MT, et al. Continued overall survival benefit after 5 years' follow-up with bortezomib-melphalan-prednisone (VMP) versus melphalan-prednisone (MP) in patients with previously untreated multiple myeloma and no increased risk of second primary malignancies: Final results of the phase 3 VISTA trial. Blood. 2011;118:476. [Google Scholar]
- 22.Hrusovsky I, Emmerich B, von Rohr A, Voegeli J, Taverna C, Olie RA, Pliskat H, Frohn C, Hess G. Bortezomib retreatment in relapsed multiple myeloma - results from a retrospective multicentre survey in Germany and Switzerland. Oncology. 2010;79:247–54. doi: 10.1159/000322866. [DOI] [PubMed] [Google Scholar]
- 23.Sher T, Ailawadhi S, Miller KC, Manfredi D, Wood M, Tan W, Wilding G, Czuczman MS, Hernandez-llizaliturri FJ, Hong F, et al. A steroid-independent regimen of bortezomib, liposomal doxorubicin and thalidomide demonstrate high response rates in newly diagnosed multiple myeloma patients. Br J Haematol. 2011;154:104–110. doi: 10.1111/j.1365-2141.2011.08703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cavaletti G, Gilardini A, Canta A, Rigamonti L, Rodriguez-Menendez V, Ceresa C, Marmirili P, Bossi M, Oggioni N, D'Incalci M, De Coster R. Bortezomib-induced peripheral neurotoxicity: A neurophysiological and pathological study in the rat. Exp Neurol. 2007;204:317–325. doi: 10.1016/j.expneurol.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Csizmadia V, Raczynski A, Csizmadia E, Fedyk ER, Rottman J, Alden CL. Effect of an experimental proteasome inhibitor on the cytoskeleton, cytosolic protein turnover and induction in the neuronal cells in vitro. Neurotoxicology. 2008;29:232–243. doi: 10.1016/j.neuro.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Mangiacavalli S, Pochintesta L, Pascutto C, Cocito F, Cazzola M, Corso A, Pompa A. Good clinical activity and favorable toxicity profile of once weekly bortezomib, fotemustine and dexamethasone (B-MuD) for the treatment of relapsed multiple myeloma. Am J Hematol. 2013;88:102–106. doi: 10.1002/ajh.23358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bringhen S, Larocca A, Rossi D, Cavalli M, Genuardi M, Ria R, Gentili S, Patriarca F, Nozzoli C, Levi A, et al. Efficacy and safety of once-weekly bortezomib in multiple myeloma patients. Blood. 2010;116:4745–4753. doi: 10.1182/blood-2010-07-294983. [DOI] [PubMed] [Google Scholar]
- 28.Moore S, Atwal S, Sachchithanantham S, Streetly M, Khan I, Percy L, Narat S, Rabin N, Johnston R, D'Sa S, et al. Weekly intravenous bortezomib is effective and well tolerated in relapsed/refractory myeloma. Eur J Haematol. 2013;90:420–425. doi: 10.1111/ejh.12070. [DOI] [PubMed] [Google Scholar]