Abstract
To investigate the anticancer activities of alkaloids from the Ba lotus seed (BLSA) in human nasopharyngeal carcinoma (NPC) CNE-1 cells, an MTT assay, flow cytometry, reverse transcription-polymerase chain reaction and western blotting were performed. BLSA was found to significantly reduce CNE-1 cell proliferation in a dose-dependent manner at all concentrations compared with the control (P<0.05). In addition, flow cytometry analysis identified that BLSA treatment significantly increased the sub-G1 content in CNE-1 cells (P<0.05). Following BLSA treatment, the mRNA and protein levels of a number of apoptosis-related factors, such as caspase family members (caspase-3, −8 and −9), B-cell lymphoma (Bcl)-2-associated X protein, Fas and Fas ligand were significantly increased compared with the control (P<0.05). This was accompanied by a significant decrease in anti-apoptotic Bcl-2 and Bcl-extra large protein expression compared with the control (P<0.05). Furthermore, BLSA treatment was determined to modulate CNE-1 cell expression of nuclear factor (NF)-κB and NF-κB inhibitor α. The results of the present study indicate that BLSA has anticancer activity through inducing cellular apoptosis. In addition, these results suggest that BLSA can be used as a therapeutic agent in NPC.
Keywords: alkaloids, Ba lotus seed, human nasopharyngeal carcinoma, CNE-1, apoptosis, caspase family
Introduction
Nasopharyngeal carcinoma (NPC) is a type of cancer with a high prevalence rate (2.8/100,000 and 1.9/100,000 people/year in men and women, respectively, in 2008) in Southeast China, particularly in the Guangxi, Guangdong, Hainan and the Hong Kong Special Administrative Region (1). NPC is characterized by a high metastatic potential, frequent initial dissemination to regional lymph nodes and distant metastases, causing patients to succumb to NPC (2). Early diagnosis of NPC and chemoradiotherapy treatment enables the best outcome. The overall five-year survival rate is associated with the NPC stage at diagnosis, ranging from between 58% at stage IV and 90% in stage I. However, in the advanced stages of NPC chemoradiotherapy is impractical (3,4). Therefore, the induction of NPC cell apoptosis is a strategy to control NPC and other malignancies in clinical therapy (5).
B-cell lymphoma 2 (Bcl-2) is part of the Bcl-2 protein family, which regulates cell death by inducing or inhibiting apoptosis. The Bcl-2 family is divided into anti-apoptotic factors, including Bcl-2, Bcl-extra large (Bcl-xL) and Bcl-2-like protein 2, and pro-apoptotic factors, such as Bcl2-associated X protein (Bax), Bcl-2-associated death promoter, Bcl-2-interacting mediator of cell death (Bim), Bcl-2 homologous antagonist/killer and p53 upregulated modulator of apoptosis (6). The extrinsic apoptosis signaling pathway is mediated by receptor-ligand binding. In this signaling pathway, the Fas receptor, Fas ligand (FasL), Fas-associated death domain (FADD) and caspase-8 mediate apoptosis. Alternatively, apoptotic stimuli can cause the depolarization of the inner mitochondrial membrane, leading to the release of cytochrome c (Cyt c) into the cytosol (7). Cyt c molecules induce the activation of apoptotic protease activation factor-1 and procaspase-9. Activated caspase-8 and −9 cleave and activate the final executioner of apoptosis, caspase-3, resulting in chromatin condensation and DNA fragmentation (8–10).
The seed of Nelumbo nucifera (Gaertn), also known as the lotus, is traditionally used in Chinese folk medicine. A number of previous studies have reported that the lotus seed exhibits numerous health benefits and pharmacological effects, such as anti-ischemic (11), antioxidant (12–14), hepatoprotective (12), antiproliferative (15–19), anti-inflammatory (20), anti-infertility (21), anti-arrhythmic (22–26), antifibrotic (27) and antiviral (28) activities. In the present study, the anticancer activity of alkaloids extracted from the Ba lotus seed (BLSA), a new variety of Nelumbo nucifera, which only grows in Chongqing, a city located in the southwest of China, was investigated in human NPC CNE-1 cells. In addition, the mechanism underlying this activity was examined.
Materials and methods
Chemical reagents
TRIzol, OligodT18 primer, murine Maloney leukemia virus (MMLV) reverse transcriptase, RNase inhibitor, ethidium bromide (EtBr) and agarose were purchased from Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA). All other chemical reagents were of analytical grade and purchased from Sigma-Aldrich (Merck Millipore, Darmstadt, Germany).
Preparation of alkaloids from BLSA
Fresh BLSA was purchased from Chongqing Enterprise Engineering Research Center of Ba-lotus Breeding and Deep Processing (Chongqing, China), freeze-dried and ground into a fine powder. Alkaloids were extracted from powdered Ba louts seed (100 g) twice with 1,000 ml of ethanol (80% vol/vol) at 50°C for 1 h. Following filtering, the extraction solution was loaded into a 80 cm cation exchange resin 732 column at 50°C and the filtrate collected 3 h later. Distilled water was used to wash away water-soluble impurities and then an ethanol solution of BLSA extract (80%, v/v) at 3 ml/min was used to elute the alkaloids. The collected ethanol elucent was eluted by 80% ethanol solution (containing 2% of ammonia water) and finely condensed using a vacuum rotary evaporator at 37°C, then freeze-dried and stored at −80°C until required.
Cell culture
Human NPC CNE-1 cells were obtained from the Shanghai Institutes for Biological Sciences (Chinese Academy of Sciences, Shanghai, China). The cells were routinely maintained in Roswell Park Memorial Institute-1640 medium, supplemented with 10% (v/v) fetal bovine serum and 1% penicillin-streptomycin, at 37°C in a humidified 5% CO2 incubator at 95% relative humidity (model 3154; Forma Scientific, Inc., Marietta, OH, USA).
Cell viability assay
Cell viability was measured using the MTT assay. CNE-1 cells were seeded in 96-well plates (Nunc, Rochester, NY, USA) at a density of 1×104 cells/well. Following incubation for 24 h, cells were treated with a number of concentrations (50, 100 and 200 µg/ml) of BLSA for a further 24 h. Then, 0.5 mg/ml of MTT reagent (100 µl; Ekear, Shanghai, China; cat. no. M0105) was added to each well and the cells incubated for 4 h at 37°C. The formazan crystals formed was dissolved in dimethyl sulfoxide (100 µl/well). Then, the absorbance of the wells at 540 nm was measured using a micro plate reader (model 680; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Flow cytometry analysis
BLSA-treated CNE-1 cells were collected following digestion with trypsin, washed twice with cold phosphate buffered saline (PBS) and re-suspended in 2 ml PBS. Then, the DNA of BLSA-treated cells was stained with propidium iodide using a Cycletest Plus DNA Reagent Kit (BD Biosciences; Franklin Lakes, NJ, USA; cat. no. 340242), according to the manufacturer's protocol. Fluorescence intensity was determined using a FACSCalibur flow cytometer and the data analyzed using Cell Quest Pro software (version 5.2.1) (both BD Biosciences).
Reverse transcription polymerase chain reaction (RT-PCR)
RT-PCR was performed for the following genes: Caspase-3, −8 and −9, Bax, Bcl-2, Bcl-xL, Fas, FasL, NF-κB, IkB-α and GADPH. Total RNA was isolated from BLSA-treated CNE-1 cells using TRIzol reagent, according to the manufacturer's recommendations, and centrifuged at 12,000 × g for 15 min at 25°C following the addition of chloroform. Isopropanol was added to the supernatant in a 1:1 ratio and the RNA pelleted by centrifugation (12,000 × g for 15 min at 4°C). The RNA was washed with ethanol, solubilized in diethyl pyrocarbonate-treated RNase-free water and quantified by measuring the absorbance at 260 nm using a UV-1750 spectrophotometer (Shimadzu Corporation, Kyoto, Japan). RNA (1 µg) was reverse transcribed using a PCR master mix [1X reverse transcriptase buffer, dNTPs (1 mM), oligo(dT)18 primers (500 ng), MMLV reverse transcriptase (140 units) and RNase inhibitor (40 units)] for 45 min at 42°C. Then, cDNA (2 µl) was mixed with 1 µl of each primer (10 µM) and 16 µl of DNase-free water in a PCR premix tube (AccuPower PCR PreMix; Bioneer Corporation, Daejeon, Korea) and PCR was performed in an automatic thermocycler (Bioneer Corporation, Daejeon, South Korea) for 40 cycles of 94°C for 5 min, 58°C for 30 sec, and 72°C for 90 sec, followed by a 10 min cycle at 95°C. Sequences of the primers used in PCR are presented at Table I. The PCR products were separated on 2% agarose gels and visualized by EtBr staining. GAPDH was used for normalization of the results. Gene expression was quantified using ImageJ software (version 1.44; National Institutes of Health, Bethesda, MD, USA) and results presented as fold change compared to the control group.
Table I.
RT-PCR primer sequences.
| Gene name | Primer sequences |
|---|---|
| Caspase-3 | Forward: 5′-CAAACTTTTTCAGAGGGGATCG-3′ |
| Reverse: 5′-GCATACTGTTTCAGCATGGCA-3′ | |
| Caspase-8 | Forward: 5′-CCCCACCCTCACTTTGCT-3′ |
| Reverse: 5′-GGAGGACCAGGCTCACTTA-3′ | |
| Caspase-9 | Forward: 5′-GGCCCTTCCTCGCTTCATCTC-3′ |
| Reverse: 5′-GGTCCTTGGGCCTTCCTGGTAT-3′ | |
| Bax | Forward: 5′-AAGCTGAGCGAGTGTCTCCGGCG-3′ |
| Reverse: 5′-CAGATGCCGGTTCAGGTACTCAGTC-3′ | |
| Bcl-2 | Forward: 5′-CTCGTCGCTACCGTCGTGACTTGG-3′ |
| Reverse: 5′-CAGATGCCGGTTCAGGTACTCAGTC-3′ | |
| Bcl-xL | Forward: 5′-CCCAGAAAGGATACAGCTGG-3′ |
| Reverse: 5′-GCGATCCGACTCACCAATAC-3′ | |
| Fas | Forward: 5′-GAAATGAAATCCAAAGCT-3′ |
| Reverse: 5′-TAATTTAGAGGCAAAGTGGC-3′ | |
| FasL | Forward: 5′-GGATTGGGCCTGGGGATGTTTCA-3′ |
| Reverse: 5′-TTGTGGCTCAGGGGCAGGTTGTTG-3′ | |
| NF-κB | Forward: 5′-CACTTATGGACAACTATGAGGTCTCTGG-3′ |
| Reverse: 5′-CTGTCTTGTGGACAACGCAGTGGAATTTTAGG-3′ | |
| IκB-α | Forward: 5′-GCTGAAGAAGGAGCGGCTACT-3′ |
| Reverse: 5′-TCGTACTCCTCGTCTTTCATGGA-3′ | |
| GAPDH | Forward: 5′-CGGAGTCAACGGATTTGGTC-3′ |
| Reverse: 5′-AGCCTTCTCCATGGTCGTGA-3′ |
RT-PCR, reverse transcription polymerase chain reaction; Bax, Bcl2-associated X protein; Bcl-2, B-cell lymphoma 2; Bcl-xL, Bcl-extra large; FasL, Fas ligand; NF-κB, nuclear factor-κB; IκB-α, nuclear factor-κB inhibitor α.
Protein extraction and western blot analysis
For protein extraction, BLSA-treated CNE-1 cells were washed with ice-cold PBS, homogenized with ice-cold radioimmunoprecipitation assay (RIPA) buffer and centrifuged at 13,000 × g for 30 min at 4°C. Protein concentrations were determined using the Bradford Protein Assay kit (Bio-Rad Laboratories, Inc.; cat. no. 5000001). For Western blot analysis, 30 µg of protein extract was separated by SDS-PAGE (10% gel) and then electrotransferred onto a nitrocellulose membrane (Schleicher & Schuell Bioscience, Inc., Keene, NH, USA). Blocking and antibody treatment were conducted in 10% skimmed milk for 2 h at 4°C. The blots were incubated for 4 h at 4°C with primary antibodies against caspase-3 (rabbit monoclonal; 1:1,000; cat. no. 14220S), caspase-8 (rabbit monoclonal; 1:1,000; cat. no. 9478S) and caspase-9 (rabbit monoclonal; 1:1,000; cat. no. 9508S), and Bax (rabbit monoclonal; 1:1,000; cat. no. 14796S), Bcl-2 (rabbit monoclonal; 1:1,000; cat. no. 4223S), Bcl-xL (rabbit monoclonal; 1:1,000; cat. no. 2764S), Fas (mouse monoclonal; 1:1,000; cat. no. 8023S), FasL (rabbit polyclonal; 1:1,000; cat. no. 4273S), NF-κB (mouse monoclonal; 1:1,000; cat. no. 13681S), IκB-α (rabbit monoclonal; 1:1,000; cat. no. 4812S) and β-actin (mouse monoclonal; 1:1,000; cat. no. 12262S) (all Cell Signaling Technology, Inc., Danvers, MA, USA). Following washing with PBS containing 0.05% Tween 20 (PBS-T), the blots were incubated with horseradish peroxidase-conjugated goat anti-rabbit (cat. no. 7074S) or horse anti-mouse antibodies (cat. no. 7076S) at a dilution of 1:5,000 (both Cell Signaling Technology, Inc.) for 1 h at room temperature. Then, blots were washed three times with PBS-T and antibody binding visualized by enhanced chemiluminescence (ECL Western Blotting Detection kit; GE Healthcare Life Sciences, Little Chalfont, UK; cat. no. RPN2108). Protein expression was quantified using ImageJ software (version 1.44; National Institutes of Health).
Statistical analysis
Results are presented as the mean ± standard deviation. Differences between the mean values of groups were assessed by one-way analysis of the variance, followed by a post-hoc Duncan's new multiple range test. P<0.05 was considered to indicate a statistically significant difference. SAS software (version 9.1; SAS Institute, Inc., Cary, NC, USA) was used for statistical analysis.
Results
BLSA decreases CNE-1 cell proliferation
BLSA was found to significantly reduce CNE-1 cell proliferation in vitro, in a dose-dependent manner, at all concentrations tested compared with the control group (P<0.05; Table II). The highest dose of BLSA (200 µg/ml) showed the greatest inhibitory activity (81.3±0.2%; Table II).
Table II.
Growth inhibition of human NPC CNE-1 cells by alkaloids of BLSA evaluated by the MTT assay.
| Treatment (µg/ml) | OD540 | Inhibitory rate (%) |
|---|---|---|
| 0 | 0.471±0.005a | – |
| 50 | 0.376±0.010a | 20.2±0.2a |
| 100 | 0.249±0.014b | 47.1±0.3b |
| 200 | 0.088±0.012b | 81.3±0.2b |
Results are presented as the mean ± the standard deviation of triplicate experiments. Differences between groups were statically analyzed using Duncan's new multiple-range test
P<0.05
P<0.01 vs. the control group. NPC, nasopharyngeal carcinoma; BLSA, Ba lotus seeds; OD540, optical density at 540 nm.
BLSA induces apoptosis in CNE-1 cells
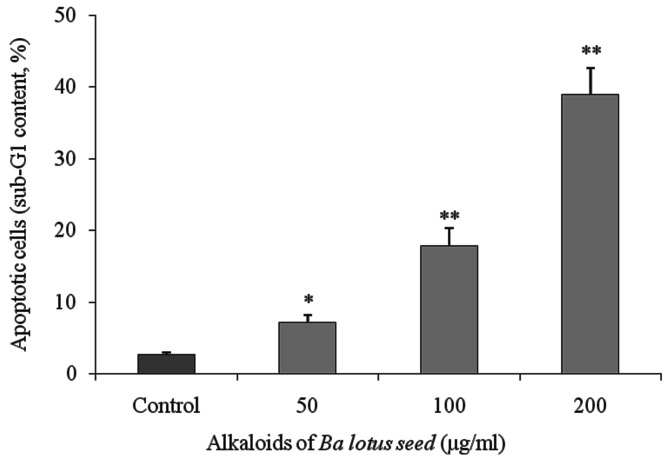
Flow cytometry analysis identified that BLSA increased apoptosis in CNE-1 cells in a dose-dependent manner. BLSA treatment (50, 100 and 200 µg/ml) significantly increased the sub-G1 DNA content of CNE-1 cells to 7.1 (P<0.05), 17.8 (P<0.01) and 38.9% (P<0.01), respectively, compared with 2.6% in the untreated control group (Fig. 1).
Figure 1.
Level of apoptosis (sub-G1 content) induced by alkaloids of BLSA in human NPC CNE-1 cells, evaluated using flow cytometry. Results are presented as the mean ± the standard deviation of triplicate experiments. Differences between groups were statically analyzed using Duncan's new multiple-range test. *P<0.05, **P<0.01 vs. the control group. BLSA, Ba lotus seeds; NPC, nasopharyngeal carcinoma.
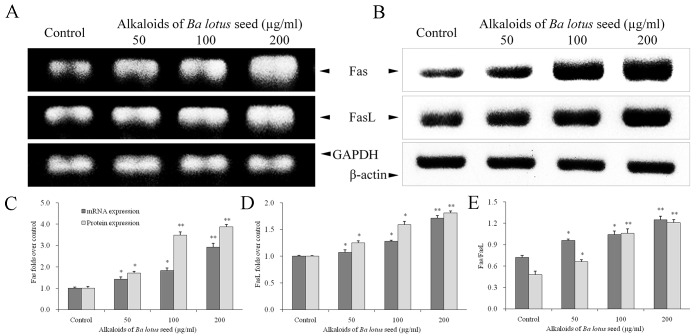
BLSA increases Fas and FasL protein expression in CNE-1 cells
The effect of BLSA on mRNA and protein levels of specific genes was determined by RT-PCR and western blot analysis, respectively. BLSA treatment was identified to significantly increase mRNA and protein levels of Fas and FasL in CNE-1 cells, in a dose-dependent manner, at all concentrations tested compared with the control (P<0.05; Fig. 2). The highest dose of BLSA (200 µg/ml) significantly up-regulated Fas (2.9 fold) and FasL (1.7 fold) mRNA levels, and Fas (1.5 fold) and FasL (3.3 fold) protein levels compared with the control group (all P<0.01; Fig. 2C and D).
Figure 2.
Effect of alkaloids of BLSA on the (A) mRNA and (B) protein expression levels of Fas and FasL in human NPC CNE-1 cells, measured by RT-PCR and western blotting, respectively. Fold change of (C) Fas, (D) FasL and (E) Fas/FasL ratio. Fold change over the control for mRNA was calculated as mRNA expression/GAPDH expression. Fold change over the control for protein was calculated as protein expression/β-actin expression. Results are presented as the mean ± the standard deviation of triplicate experiments. Differences between groups were statically analyzed using Duncan's new multiple-range test. *P<0.05, **P<0.01 vs. the control group. BLSA, Ba lotus seeds; NPC, nasopharyngeal carcinoma, FasL, Fas ligand.
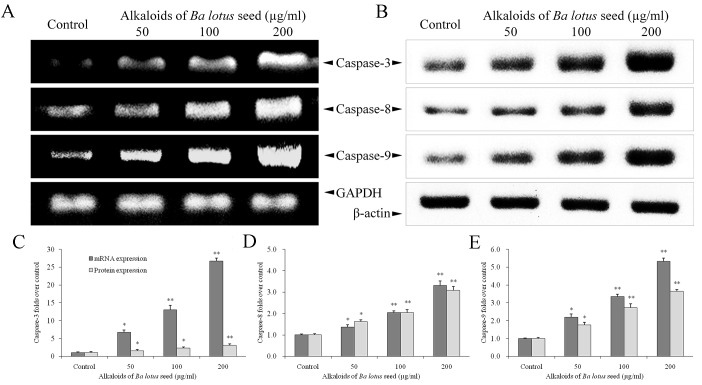
BLSA increases caspase-3, −8 and −9 expression in CNE-1 cells
BLSA treatment significantly increased mRNA and protein expression levels of caspase-3, −8 and −9 in CNE-1 cells, at all concentrations tested compared with the control (P<0.05; Fig. 3). The highest dose of BLSA (200 µg/ml) significantly increased mRNA and protein levels of caspase-3 (26.7 and 1.4 fold, respectively; Fig. 3C), −8 (3.3 and 1.5 fold, respectively; Fig. 3D) and −9 (5.3 and 1.6 fold, respectively; Fig. 3E) compared with the untreated control group (all P<0.01).
Figure 3.
Effect of alkaloids of BLSA on the (A) mRNA and (B) protein expression levels of caspase-3, −8 and −9 in human NPC CNE-1 cells. Fold change of (C) caspase-3, (D) caspase-8 and (E) caspase-9 mRNA and protein expression over the control. Fold change over the control for mRNA was calculated as mRNA expression/GAPDH expression. Fold change over the control for protein was calculated as protein expression/β-actin expression. Results are presented as the mean ± the standard deviation of triplicate experiments. Differences between groups were statically analyzed using Duncan's new multiple-range test. *P<0.05, **P<0.01 vs. the control group. BLSA, Ba lotus seeds; NPC, nasopharyngeal carcinoma.
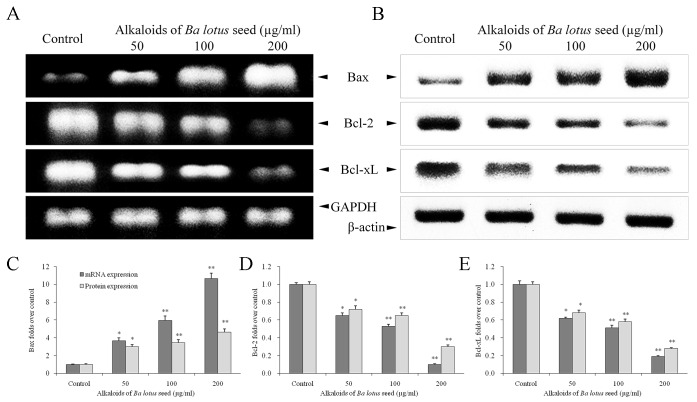
BLSA modulates Bcl-2, Bcl-xL and Bax expression in CNE-1 cells
Compared with the control group, BLSA treatment significantly decreased expression of Bcl-2 and Bcl-xL mRNA and protein, and increased expression of Bax mRNA and protein, in a dose dependent manner, in CNE-1 cells, at all concentrations tested (P<0.05; Fig. 4). At the highest dose (200 µg/ml), BLSA significantly reduced mRNA and protein levels of Bcl-2 (90 and 94%, respectively) and Bcl-xL (81 and 75%, respectively) compared with the control group (P<0.01; Fig. 4D and E). In contrast, 200 µg/ml BLSA enhanced mRNA (10.6 fold; P<0.01) and protein (1.6 fold; P<0.05) levels of Bax in CNE-1 cells (Fig. 4C).
Figure 4.
Effect of alkaloids of BLSA on the (A) mRNA and (B) protein expression levels of Bax, Bcl-2 and Bcl-xL in human NPC CNE-1 cells. Fold change of (C) Bax, (D) Bcl-2 and (E) Bcl-xL mRNA and protein expression over the control. Fold change over the control for mRNA was calculated as mRNA expression/GAPDH expression. Fold change over the control for protein was calculated as protein expression/β-actin expression. Results are presented as the mean ± the standard deviation of triplicate experiments. Differences between groups were statically analyzed using Duncan's new multiple-range test. *P<0.05, **P<0.01 vs. the control group. BLSA, Ba lotus seeds; NPC, nasopharyngeal carcinoma; Bax, Bcl2-associated X protein; Bcl-2, B-cell lymphoma 2; Bcl-xL, B-cell lymphoma-extra large.
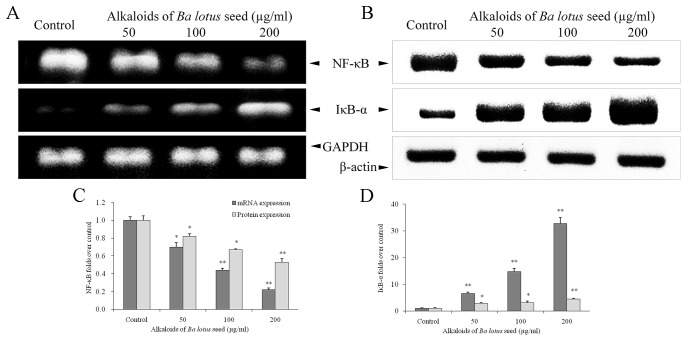
BLSA modulates NF-κB and IκB-α expression in CNE-1 cells
BLSA treatment significantly decreased NF-κB mRNA and protein expression, and increased IκB-α mRNA and protein expression, in a dose dependent manner in CNE-1 cells, at all concentrations tested (P<0.05 vs. the control group; Fig. 5). Following treatment with 200 µg/ml BLSA, NF-κB mRNA and protein levels were significantly decreased by 78% and 35%, respectively, compared with the control group (P<0.01; Fig. 5C). In addition, BLSA increased mRNA and protein levels of IκB-α by 32.7 and 2.3-fold, respectively, compared with the control group (P<0.01; Fig. 5D).
Figure 5.
Effect of alkaloids of BLSA on the (A) mRNA and (B) protein expression levels of NF-κB and IκB-α in human NPC CNE-1 cells. Fold change of (C) NF-κB and (D) IκBα mRNA and protein expression over the control. Fold change over the control for mRNA was calculated as mRNA expression/GAPDH expression. Fold change over the control for protein was calculated as protein expression/β-actin expression. Results are presented as the mean ± the standard deviation of triplicate experiments. Differences between groups were statically analyzed using Duncan's new multiple-range test. *P<0.05, **P<0.01 vs. the control group. BLSA, Ba lotus seeds; NPC, nasopharyngeal carcinoma; NF-κB, nuclear factor-κB; IκB-α, nuclear factor-κB inhibitor α.
Discussion
Alkaloids, isolated from herbs, may possess anti-cancer activities, including induction of cell cycle arrest, apoptosis, autophagy, and inhibition of angiogenesis and metastasis (29). A recent study reported that a number of alkaloids isolated from N. nucifera Gaertn. cv. Rosa-plena exhibited antioxidant and anticancer activity in vitro (30). In the present study, BLSA exhibited anti-cancer effects, associated with the induction of apoptosis, in CNE-1 cells. BLSA significantly reduced CNE-1 cell proliferation and promoted transition into the sub-G1 phase. These results indicate that the anti-CNE-1 effects of BLSA are associated with apoptosis.
In the current study, mRNA and proteins expression levels of a number of apoptosis-associated genes in BLSA-treated CNE-1 cells were investigated using RT-PCR and western blotting, respectively. Following treatment for 24 h with BLSA, mRNA and protein levels of Fas and FasL were significantly increased compared with untreated cells. Fas and FasL are inducers of apoptosis that serve a primary role in death receptor-mediated apoptosis (31). Activation of Fas/FasL recruits FADD and the death domain, which subsequently induce the activation of caspase-8, −9 and −10, key regulators that promote cellar apoptosis (32).
The results of the present study determined that mRNA and protein levels of caspase-3, −8 and −9 were significantly increased in BLSA-treated CNE-1 cells compared with the control group. The caspase signaling cascade is a key event in extrinsic and intrinsic apoptosis, which is characterized by the activation of caspase-8 and −9, respectively (9). Caspase-8, the initiator caspase in Fas signaling, is recruited to the activated Fas receptor and facilitates death receptor-mediated apoptosis (33). Caspase-9, an apoptotic effector molecule in intrinsic apoptosis, initiates programmed cell death following activation (10). Activated caspase-8 and −9 activate caspase-3, an executioner caspase that subsequently induces apoptosis (8). These results indicate that BLSA induces CNE-1 cell apoptosis through activating extrinsic (Fas/FasL) and intrinsic apoptotic signaling pathways.
The Bcl-2 family, a well-known family of apoptosis regulators, serves a primary role in intrinsic apoptosis (34). Bcl-2 and Bcl-xL are typically anti-apoptotic factors that block the release of Cyt c from mitochondria and thus promote cell survival. Bcl-2 can reduce the release of Cyt c from the mitochondria, thus inhibiting apoptosis (6). In contrast, Bax is a pro-apoptotic factor that promotes apoptosis (6,7). The balance between anti- and pro-apoptotic factors influences the occurrence of apoptosis, and is associated with the success rate of chemotherapy in cancer patients (35). In the present study, BLSA treatment significantly increased mRNA and protein levels of pro-apoptotic Bax, and reduced mRNA and protein levels of anti-apoptotic Bcl-2 and Bcl-xL in CNE-1 cells. Activated Bax is directly engaged by Bim to promote apoptosis (36). In addition, caspases-8 may activate Bax and induce the release of Cyt c from the mitochondria, causing the cleavage of caspase-9 and contributing to the activation of caspase-3 (6,37). The results of the current study suggest that BLSA modulates the ratio of anti-apoptotic to pro-apoptotic factors, in particular by enhancing the expression Bax to promote the apoptosis of CNE-1 cells.
NF-κB reduces tumor necrosis factor (TNF)-α-induced cell apoptosis (38) and is an important negative regulator of apoptosis in cancer cells (39). Deregulation of NF-κB expression has been found in a number of human cancers (40,41). Overexpression of NF-κB promotes cell proliferation and reduces cell death (42). In addition, NF-κB can directly activate Bcl-xL (43) and suppress a number of anti-apoptotic factors, such as inhibitor of apoptosis, caspase-8-like FADD-like interleukin-1β-converting enzyme inhibitory protein, TNF receptor associated factor 1 (TRAF1) and TRAF2, to regulate apoptosis (44). Following treatment with BLSA, mRNA and protein levels of NF-κB were significantly reduced in CNE-1 cells. In addition, BLSA treatment significantly increased mRNA and protein levels of IκB-α. Increasing IκB-α levels is a therapeutic strategy to reduce cancer cell growth in clinical chemotherapy (45–47).
In conclusion, the results of the present study indicate that BLSA suppresses the proliferation of human CNE-1 NPC cells in vitro. In addition, the results indicate that BLSA induces apoptosis, through reducing the ratio of anti-apoptotic (Bcl-2 and Bcl-xL) to pro-apoptotic (Bax) factors, increasing mRNA and protein expression levels of Fas/FasL and promoting cleavage of caspase-3, −8 and −9 in CNE-1 cells. BLSA, as an inducer of apoptosis, may have future applications as an adjuvant in clinical therapy for NPC patients.
Acknowledegments
The present study was supported by Chongqing Engineering Research Center for Functional Food (grant no. cstc2015yfpt_gcjsyjzx0027) and the Program for Innovation and Team Building at the Chongqing Institute of Higher Education (grant no. CXTDX201601040).
References
- 1.Cao SM, Simons MJ, Qian CN. The prevalence and prevention of nasopharyngeal carcinoma in China. Chin J Cancer. 2011;30:114–119. doi: 10.5732/cjc.010.10377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lo KW, To KF, Huang DP. Focus on nasopharyngeal carcinoma. Cancer Cell. 2004;5:423–428. doi: 10.1016/S1535-6108(04)00119-9. [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Guo LP, Chen LZ, Zeng YX, Lu SH. Identification of cancer stem cell-like side population cells in human nasopharyngeal carcinoma cell line. Cancer Res. 2007;67:3716–3724. doi: 10.1158/0008-5472.CAN-06-4343. [DOI] [PubMed] [Google Scholar]
- 4.Su J, Xu XH, Huang Q, Lu MQ, Li DJ, Xue F, Yi F, Ren JH, Wu YP. Identification of cancer stem-like CD44+ cells in human nasopharyngeal carcinoma cell line. Arch Med Res. 2011;42:15–21. doi: 10.1016/j.arcmed.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Wong RS. Apoptosis in cancer: From pathogenesis to treatment. J Exp Clin Cancer Res. 2011;30:87. doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ola MS, Nawaz M, Ahsan H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol Cell Biochem. 2011;351:41–58. doi: 10.1007/s11010-010-0709-x. [DOI] [PubMed] [Google Scholar]
- 7.Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011;21:92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peter ME, Krammer PH. The CD95 (APO-1/Fas) DISC and beyond. Cell Death Differ. 2003;10:26–35. doi: 10.1038/sj.cdd.4401186. [DOI] [PubMed] [Google Scholar]
- 9.Cullen SP, Martin SJ. Caspase activation pathways: Some recent progress. Cell Death Differ. 2009;16:935–938. doi: 10.1038/cdd.2009.59. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Y, Lei M, Wang Z, Qiao G, Yang T, Zhang J. TCR-induced, PKC-θ-mediated NF-κB activation is regulated by a caspase-8-caspase-9-caspase-3 cascade. Biochem Biophys Res Commun. 2014;450:526–531. doi: 10.1016/j.bbrc.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JH, Kang M, Cho C, Chung HS, Kang CW, Parvez S, Bae H. Effects of Nelumbinis semen on contractile dysfunction in ischemic and reperfused rat heart. Arch Pharm Res. 2006;29:777–785. doi: 10.1007/BF02974079. [DOI] [PubMed] [Google Scholar]
- 12.Sohn DH, Kim YC, Oh SH, Park EJ, Li X, Lee BH. Hepato-protective and free radical scavenging effects of Nelumbo nucifera. Phytomedicine. 2003;10:165–169. doi: 10.1078/094471103321659889. [DOI] [PubMed] [Google Scholar]
- 13.Rai S, Wahile A, Mukherjee K, Saha BP, Mukherjee PK. Antioxidant activity of Nelumbo nucifera (sacred lotus) seeds. J Ethnopharmacol. 2006;104:322–327. doi: 10.1016/j.jep.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 14.Ling ZQ, Xie BJ, Yang EL. Isolation, characterization, and determination of anti-oxidative activity of oligomericprocyanidins from the seedpod of Nelumbo nucifera Gaertn. J Agric Food Chem. 2005;53:2441–2445. doi: 10.1021/jf040325p. [DOI] [PubMed] [Google Scholar]
- 15.Liu CP, Tsai WJ, Lin YL, Liao JF, Chen CF, Kuo YC. The extracts from Nelumbo nucifera suppress cell cycle progression, cytokine genes expression, and cell proliferation in human peripheral blood mononuclear cells. Life Sci. 2004;75:699–716. doi: 10.1016/j.lfs.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 16.Liu CP, Tsai WJ, Shen CC, Lin YL, Liao JF, Chen CF, Kuo YC. Inhibition of (S)-armepavine from Nelumbo nucifera on autoimmune disease of MRL/MpJ-lpr/lpr mice. Eur J Pharmacol. 2006;531:270–279. doi: 10.1016/j.ejphar.2005.11.062. [DOI] [PubMed] [Google Scholar]
- 17.Liu CP, Kuo YC, Shen CC, Wu MH, Liao JF, Lin YL, Chen CF, Tsai WJ. (S)-armepavine inhibits human peripheral blood mononuclear cell activation by regulating Itk and PLCgamma activation in a PI-3K-dependent manner. J Leukoc Biol. 2007;81:1276–1286. doi: 10.1189/jlb.0106056. [DOI] [PubMed] [Google Scholar]
- 18.Xiao JH, Zhang YL, Feng XL, Wang JL, Qian JQ. Effects of isoliensinine on angiotensin II-induced proliferation of porcine coronary arterial smooth muscle cells. J Asian Nat Pro Res. 2006;8:209–216. doi: 10.1080/1028602042000325609. [DOI] [PubMed] [Google Scholar]
- 19.Yu J, Hu WS. Effects of neferine on platelet aggregation in rabbits. Acta Pharm Sin. 1997;32:1–4. (In Chinese) [PubMed] [Google Scholar]
- 20.Lin JY, Wu AR, Liu CJ, Ying S. Suppressive effects of lotus plumule (Nelumbo nucifera Geartn.) supplementation on LPS-induced systemic inflammation in a BALB/c mouse model. J Food Drug Anal. 2006;14:273–278. [Google Scholar]
- 21.Mazumder UK, Gupta M, Pramanik G, Mukhopadhyay RK, Sarkar S. Antifertility activity of seed of Nelumbo nucifera in mice. Ind J Exp Biol. 1992;30:533–534. [PubMed] [Google Scholar]
- 22.Li G, Li X, Lü F. Effects of neferine on transmembrane potentials of guinea pig myocardium. Zhongguo Yao Li Xue Bao. 1989;10:406–410. (In Chinese) [PubMed] [Google Scholar]
- 23.Li GR, Li XG, Lu FH. Effects of neferine on transmembrane potential in rabbit sinoatrial nodes and clusters of cultured myocardial cells from neonatal rats. Zhongguo Yao Li Xue Bao. 1989;10:328–331. (In Chinese) [PubMed] [Google Scholar]
- 24.Li GR, Qian JQ, Lü FH. Effects of neferine on heart electromechanical activity in anaesthetized cats. Zhongguo Yao Li Xue Bao. 1990;11:158–161. (In Chinese) [PubMed] [Google Scholar]
- 25.Wang JL, Nong Y, Jiang MX. Effects of liensinine on haemodynamics in rats and the physiologic properties of isolated rabbit atria. Yao Xue Xue Bao. 1992;27:881–885. (In Chinese) [PubMed] [Google Scholar]
- 26.Wang JL, Nong Y, Xia GJ, Yao WX, Jiang MX. Effects of liensinine on slow action potentials in myocardium and slow inward current in canine cardiac Purkinje fibers. Yao Xue Xue Bao. 1993;28:812–816. (In Chinese) [PubMed] [Google Scholar]
- 27.Xiao JH, Zhang JH, Chen HL, Feng XL, Wang JL. Inhibitory effect of isoliensinine on bleomycin-induced pulmonary fibrosis in mice. Planta Med. 2005;71:225–230. doi: 10.1055/s-2005-837821. [DOI] [PubMed] [Google Scholar]
- 28.Kuo YC, Lin YL, Liu CP, Tsai WJ. Herpes simplex virus type 1 propagation in HeLa cells interrupted by Nelumbo nucifera. J Biomed Sci. 2005;12:1021–1034. doi: 10.1007/s11373-005-9001-6. [DOI] [PubMed] [Google Scholar]
- 29.Lu JJ, Bao JL, Chen XP, Huang M, Wang YT. Alkaloids isolated from natural herbs as the anticancer agents. Evid Based Complement Alternat Med. 2012;2012:485042. doi: 10.1155/2012/485042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu CM, Kao CL, Wu HM, Li WJ, Huang CT, Li HT, Chen CY. Antioxidant and anticancer aporphine alkaloids from the leaves of Nelumbo nucifera Gaertn. cv. Rosa-plena. Molecules. 2014;19:17829–17838. doi: 10.3390/molecules191117829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O' Reilly LA, Tai L, Lee L, Kruse EA, Grabow S, Fairlie WD, Haynes NM, Tarlinton DM, Zhang JG, Belz GT, et al. Membrane-bound Fas ligand is essential for Fas-induced apoptosis. Nature. 2009;461:659–663. doi: 10.1038/nature08402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waring P, Müllbacher A. Cell death induced by the Fas/Fas ligand pathway and its role in pathology. Immunol Cell Biol. 1999;77:312–317. doi: 10.1046/j.1440-1711.1999.00837.x. [DOI] [PubMed] [Google Scholar]
- 33.Scaffidi C, Medema JP, Krammer PH, Peter ME. FLICE is predominantly expressed as two functionally active isoforms, caspase-8/a and caspase-8/b. J Biol Chem. 1997;272:26953–26958. doi: 10.1074/jbc.272.43.26953. [DOI] [PubMed] [Google Scholar]
- 34.Volkmann N, Marassi FM, Newmeyer DD, Hanein D. The rheostat in the membrane: BCL-2 family proteins and apoptosis. Cell Death Differ. 2014;21:206–215. doi: 10.1038/cdd.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 36.Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/S1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 37.Finucane DM, Bossy-Wetzel E, Waterhouse NJ, Cotter TG, Green DR. Bax-induced caspase activation and apoptosis via cytochrome c released from mitochondria is inhibited by Bcl-xL. J Biol Chem. 1999;274:2225–2233. doi: 10.1074/jbc.274.4.2225. [DOI] [PubMed] [Google Scholar]
- 38.Van Antwerp DJ, Martin SJ, Verma IM, Green DR. Inhibition of TNF-induced apoptosis by NF-κB. Trends Cell Biol. 1998;8:107–111. doi: 10.1016/S0962-8924(97)01215-4. [DOI] [PubMed] [Google Scholar]
- 39.Ohshima K, Sugihara M, Haraoka S, Suzumiya J, Kanda M, Kawasaki C, Shimazaki K, Kikuchi M. Possible immortalization of Hodgkin and Reed-Sternberg cells: Telomerase expression, lengthening of telomere and inhibition of apoptosis by NF-kappaB expression. Leuk Lymphoma. 2001;41:367–376. doi: 10.3109/10428190109057992. [DOI] [PubMed] [Google Scholar]
- 40.Rayet B, Gélinas C. Aberrant rel/nfkb genes and activity in human cancer. Oncogene. 1999;18:6938–6947. doi: 10.1038/sj.onc.1203221. [DOI] [PubMed] [Google Scholar]
- 41.Dolcet X, Llobet D, Pallares J, Matias-Guiu X. NF-kB in development and progression of human cancer. Virchows Arch. 2005;446:475–482. doi: 10.1007/s00428-005-1264-9. [DOI] [PubMed] [Google Scholar]
- 42.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109:S81–S96. doi: 10.1016/S0092-8674(02)00703-1. (Suppl) [DOI] [PubMed] [Google Scholar]
- 43.Chen C, Edelstein LC, Gélinas C. The Rel/NF-kappaB family directly activates expression of the apoptosis inhibitor Bcl-x(L) Mol Cell Biol. 2000;20:2687–2695. doi: 10.1128/MCB.20.8.2687-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin A, Karin M. NF-κB in cancer: A marked target. Semin Cancer Biol. 2003;13:107–114. doi: 10.1016/S1044-579X(02)00128-1. [DOI] [PubMed] [Google Scholar]
- 45.Tergaonkar V, Bottero V, Ikawa M, Li Q, Verma IM. IkappaB kinase-independent IkappaBalpha degradation pathway: Functional NF-kappaB activity and implications for cancer therapy. Mol Cell Biol. 2003;23:8070–8083. doi: 10.1128/MCB.23.22.8070-8083.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee CH, Jeon YT, Kim SH, Song YS. NF-kappaB as a potential molecular target for cancer therapy. Biofactors. 2007;29:19–35. doi: 10.1002/biof.5520290103. [DOI] [PubMed] [Google Scholar]
- 47.Gilmore TD, Garbati MR. Inhibition of NF-κB signaling as a strategy in disease therapy. Curr Top Microbiol Immunol. 2011;349:245–263. doi: 10.1007/82_2010_105. [DOI] [PubMed] [Google Scholar]