Abstract
Insulin autoimmune syndrome (IAS) is a disease characterized by hyperinsulinemic hypoglycemia associated with autoantibodies against endogenous insulin. A 56-year-old man was admitted to Ningbo First Hospital for the treatment of spontaneous hypoglycemia. He was found to have elevated fasting insulin level (>1,000 mIU/l) and presence of insulin autoantibodies, and after appropriate workup, was diagnosed with IAS. After treating with prednisone for 2 months, his insulin level started decreasing. In patients with repeated hypoglycemia, IAS should be considered in the differential diagnosis. Prednisone may be effective for the treatment of hypoglycemia in patients with IAS.
Keywords: insulin autoimmune syndrome, insulin autoantibodies, continuous glucose monitoring system
Introduction
Insulin autoimmune syndrome (IAS) is an uncommon cause of spontaneous hypoglycemia, and it was first reported by Hirata et al in 1970 (1). In patients with IAS, insulin levels were found to be significantly elevated, usually higher than 100 mIU/ml, along with positive test results for insulin autoantibodies. Insulin autoimmune syndrome is usually diagnosed in patients with a negative history of exposure to exogenous insulin. The occurrence of insulin-induced IAS is rare. Herein, we report a case study of IAS induced by exogenous insulin.
Case report
A 56-year-old Han Chinese man presented to Ningbo First Hospital (Ningbo, China) on March 24, 2014 with a complaint of repeated episodes of hypoglycemia. He had been diagnosed with type 2 diabetes mellitus in 2004 and was subsequently treated with oral antidiabetic agents, including metformin 500 mg BID (Bristol-Myers Squibb, New York, NY, USA) and gliclazide 60 mg QD (Servier, Beijing, China). He had been prescribed Novo Mix 30R therapy (insulin aspart 30, 12 U before breakfast and 10 U before dinner) for the past 5 years. In 2012, he was admitted in a local hospital in a comatose state after a short prodrome of syncope, diaphoresis, hunger sensation and palpitation. At that time, his blood glucose level decreased to 2.3 mmol/l (normal range, 3.9–6.1 mmol/l). His hypoglycemic symptoms were relieved quickly with intravenous glucose injection and discontinuation of insulin therapy. Thereafter, he experienced three more episodes of symptomatic hypoglycemia even after the treatment. The patient was admitted to Ningbo First Hospital for further evaluation and treatment on March 24, 2014. On admission, his random blood glucose level was 2.2 mmol/l. He had a history of poliomyelitis and was not aware of any medical history of hypoglycemic episodes in his family members.
His physical vital signs were as follows: Height, 163 cm; weight, 50.5 kg; body mass index, 19 kg/m2; and blood pressure, 122/78 mm Hg. Hyperinsulinemia was initially suspected, and then confirmed by 75 g oral glucose tolerance test (Table I). Laboratory investigations showed positive insulin autoantibodies (Table II). Abdominal contrast-enhanced computed tomography (CT) revealed a normal pancreas and gastrointestinal tract, with no suspicious masses resembling insulinomas. Thus, IAS was considered as the probable cause of repeated hypoglycemia.
Table I.
75 g oral glucose tolerance test.
| Indicators | 0 min | 120 min | Normal range |
|---|---|---|---|
| One day after admission | |||
| Blood glucose (mmol/l) | 3.41 | 16.64 | 3.9–6.1 |
| Insulin (mIU/l) | >1,000 | >1,000 | 1.9–23.0 |
| C-peptide (ng/ml) | 5.69 | 6.39 | 1.1–5.0 |
| One week after admission | |||
| Blood glucose (mmol/l) | 3.3 | 15.3 | 3.9–6.1 |
| Insulin (mIU/l) | >1,000 | >1,000 | 1.9–23.0 |
| C-peptide (ng/ml) | 6.51 | 6.62 | 1.1–5.0 |
| 15 days after discharge | |||
| Blood glucose (mmol/l) | 6.72 | 10.2 | 3.9–6.1 |
| Insulin (mIU/l) | 642.1 | 650.2 | 1.9–23.0 |
| C-peptide (ng/ml) | 4 | 7.26 | 1.1–5.0 |
| Six months after discharge | |||
| Blood glucose (mmol/l) | 6.67 | 11.3 | 3.9–6.1 |
| Insulin (mIU/l) | 16.5 | 53.8 | 1.9–23.0 |
| C-peptide (ng/ml) | 4.3 | 6.89 | 1.1–5.0 |
Table II.
Laboratory investigations of the patient.
| Parameter | Value | Normal range |
|---|---|---|
| Hemoglobin A1c (%) | 7.6 | 4.0–6.0 |
| Total cholesterol (mmol/l) | 4.62 | 2.8–5.67 |
| High density lipoprotein-cholesterol (mmol/l) | 1.33 | 0.8–1.92 |
| Low density lipoprotein-cholesterol (mmol/l) | 2.45 | 2.1–3.3 |
| Triglycerides (mmol/l) | 0.63 | 0.1–1.8 |
| Homocysteine (µmol/l) | 6 | 0–10 |
| Creatinine (µmol/l) | 57.3 | 40–104 |
| Blood urea nitrogen (mmol/l) | 8.96 | 1.79–7.14 |
| Alanine aminotransferase (IU/l) | 45 | 9–50 |
| Aspartate aminotransferase (IU/l) | 35 | 15–40 |
| Total thyroxin (µg/dl) | 7.6 | 6.09–12.23 |
| Free thyroxin (ng/dl) | 1.01 | 0.61–1.12 |
| Total triiodothyronine (ng/ml) | 0.97 | 0.87–1.76 |
| Free triiodothyronine (pg/ml) | 3.09 | 2.5–3.9 |
| Thyroid-stimulating hormone (mIU/l) | 0.85 | 0.34–5.6 |
| Anti-tiroperoxidasa (IU/ml) | 1.1 | 0–9 |
| Follicle-stimulating hormone (IU/l) | 28.74 | 1.27–19.26 |
| Luteinizing hormone (IU/l) | 9.73 | 1.24–8.62 |
| Prolactin (µg/l) | 7.83 | 4–14.4 |
| Testosterone (ng/ml) | 6.06 | 1.75–7.81 |
| Growth hormone (ng/ml) | 0.06 | 0.01–1 |
| Cortisol, 8 AM (µg/dl) | 9.54 | 8.7–22.4 |
| Cortisol, 4 PM (µg/dl) | 4.89 | 0–10 |
| Adrenocorticotropic hormone, 8 AM (pg/ml) | 20.05 | 9.89–79.14 |
| Adrenocorticotropic hormone, 4 PM (pg/ml) | 13.92 | 4.95–39.57 |
| Insulin autoantibodies | Positive | Negative |
| Islet cell antibodies | Negative | Negative |
| Glutamic acid decarboxylase antibodies | Negative | Negative |
| Tumor markers | ||
| Alpha-fetoprotein | 2.98 | 0–9.00 |
| Carcinoembryonic antigen | 0.86 | 0–5.00 |
| Carbohydrate antigen 19–9 | 4.4 | 0–25.0 |
| Cancer antigen 125 | 8.7 | 0–35.0 |
| Cancer antigen 15–3 | 9.9 | 0–14.0 |
| Neuron-specific enolase | 9.04 | 0–15.20 |
| CYF211 | 1.07 | 0–3.30 |
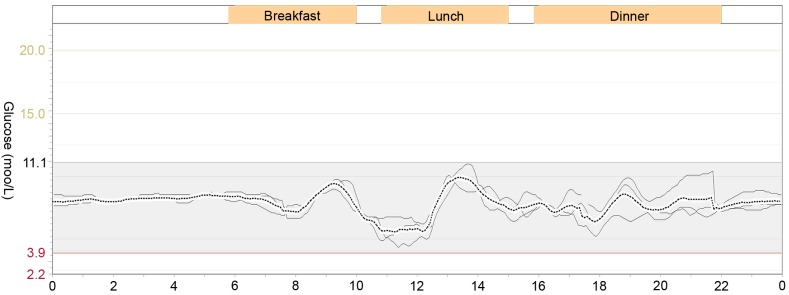
The patient was prescribed oral antidiabetic agents only, including repaglinide (1 mg at 6:00 a.m., 1 mg at 10:00 a.m., and 2 mg at 4:00 a.m.; Novo Nordisk, Bagsværd, Denmark), acarbose (50 mg TID; Huadong Medicine Group Co., Ltd., Hangzhou, China) and pioglitazone (15 mg QD; Huadong Medicine Group Co., Ltd.) for 10 days. However, no signs of remission of hypoglycemia were detected. His fasting blood glucose level was normal, but postprandial blood glucose level was elevated (Fig. 1). One week after admission, prednisone (10 mg PO QD) was added to control his hypoglycemia. His symptoms disappeared and there was no recurrence of hypoglycemia (Fig. 1). Then, his prednisone dose was maintained at 5 mg PO QD after 4 weeks. At day 15 after discharge, he had elevated blood glucose and insulin levels (Table I) and positive insulin autoantibodies, but had no symptomatic hypoglycemia. Six months after discharge, the glucose excursion was evaluated using a continuous glucose monitoring system (MRT; Medtronic, Ltd., Dublin, Ireland) without prednisone on three consecutive days (2). He had a stable blood glucose level without any episode of hypoglycemia or hyperglycemia (Fig. 2). The parameters of blood glucose variability were calculated as follows: Mean absolute glucose excursion was 2.4 mmol/l, mean of daily difference was 0.69 mmol/l. The mean blood glucose on three consecutive days was 7.1±1.5 mmol/l, and his fasting insulin level was 16.5 mIU/l. The initially positive result for insulin autoantibodies turned to negative.
Figure 1.
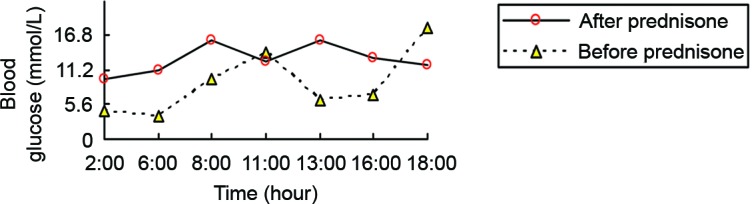
Average blood glucose level of the patient before and after prednisone treatment.
Figure 2.
Data of glucose excursions using a continuous glucose monitoring system on three consecutive days, 24 h per day. The black line represents the average glucose level for 3 days.
Discussion
Insulin autoimmune syndrome, following insulinoma and extrapancreatic neoplasms, is the third leading cause of spontaneous hypoglycemia in Japan (3). The incidence of IAS is similar in men and women, and it is more frequently observed in patients aged >40 years (3). In the present study, we excluded the possibility of insulinoma or extrapancreatic neoplasms by abdominal contrast-enhanced CT. The classical IAS is characterized by postprandial hypoglycemic episodes, elevated insulin levels, and positive insulin autoantibodies. In this case, the patient did not have postprandial hypoglycemic episodes, suggesting an atypical presentation of IAS. Considering that the patient was given insulin therapy, the source of insulin autoantibodies should be distinguished. Usually, autoantibodies against exogenous insulin are relatively weak and disappear spontaneously (4). However, in the present patient insulin autoantibodies tested positive for a relatively long period after discontinuing insulin therapy; it indicates that these insulin autoantibodies may have been endogenous.
Insulin receptor autoimmune diseases may cause insulin resistance and paradoxical hypoglycemia, which are also considered in the differential diagnosis (5). In this condition, hypoglycemia usually occurs when the stomach is empty and disappears after taking food (5). These patients may have other autoimmune diseases, such as acanthosis nigricans and hyperandrogenism. Importantly, insulin receptor antibodies can be positive while insulin autoantibodies are negative in these cases, which are helpful in the differential diagnosis; however, these were unavailable in our hospital. Considering a high level of insulin and positive insulin autoantibodies, in this case, IAS seems to be the more likely diagnosis.
At present, the exact mechanism underlying IAS is unknown. The commonly accepted hypothesis is use of medications containing a sulfhydryl group might induce insulin autoantibodies and influence the binding and release of insulin by autoantibodies (6). Alpha-lipoic acid, a nutritional supplement for treating diabetic neuropathy, has also been described to cause IAS (7). Insulin secretes when glucose concentration rises in the blood. However, autoantibodies can prevent normal action of insulin by binding with it (6). When the glucose concentration decreases, the autoantibodies gradually dissociate from insulin, resulting in a surplus of insulin, which may contribute to hypoglycemia (7). Certain diseases such as Graves' disease, systemic lupus erythematosus and rheumatoid arthritis, may also trigger the production of autoantibodies (8). Insulin aspart, insulin glulisine and endogenous insulin may induce IAS, but the association among them remains unclear (9,10). In the present case, the patient did not have a history of immune diseases or taking any of the oral drugs mentioned above. However, he received long-term insulin treatment, and continued to experience hypoglycemic attacks even after discontinuation of his insulin treatment.
As the majority of patients with IAS can achieve a remission soon after drug withdrawal, surgery is not the first choice (11). Small, frequent meals are recommended to reduce or avoid hypoglycemic episodes (12). In addition, glucocorticoids and immunosuppressants may be useful as adjuvant therapies for IAS control (13). A Japanese study showed that corticosteroid therapy reduced the amount of insulin receptor binding sites and avoided hypoglycemic attacks, suggesting the usefulness of corticosteroid therapy in the treatment of IAS (14). In the present case, a small dose of prednisone was used, with an initial dosage of 10 mg QD and maintaining at 5 mg QD. The patient achieved a remission and did not experience symptomatic hypoglycemia again. His insulin autoantibodies turned negative after prednisone treatment for 12 weeks. Acarbose, diazoxide, octreotide, pancreatectomy and plasmapheresis have also been the treatment of choice for the successful management of IAS (15).
Insulin autoimmune syndrome should be considered in any patient who suffers hyperinsulinemic hypoglycemia, particularly in a patient in which a neoplasm was not detected inside or outside of the pancreas. Insulin-induced IAS rarely occurs. The causal association between insulin and IAS as well as the underlying mechanisms require further investigation.
References
- 1.Hirata Y, Ishizu H, Ouchi N. Insulin autoimmunity in a case with spontaneous hypoglycaemia. J Japan Diab Soc. 1970;13:312–320. [Google Scholar]
- 2.Philippon M, Sejil S, Mugnier M, Rocher L, Guibergia C, Vialettes B, Delenne B. Use of the continuous glucose monitoring system to treat insulin autoimmune syndrome: Quantification of glucose excursions and evaluation of treatment efficacy. Diabet Med. 2014;31:e20–e24. doi: 10.1111/dme.12418. [DOI] [PubMed] [Google Scholar]
- 3.Savas-Erdeve S, Agladioglu S Yilmaz, Onder A, Kendirci HN Peltek, Bas VN, Sagsak E, Cetinkaya S, Aycan Z. An uncommon cause of hypoglycemia: Insulin autoimmune syndrome. Horm Res Paediatr. 2014;82:278–282. doi: 10.1159/000362758. [DOI] [PubMed] [Google Scholar]
- 4.Lamy PJ, Sault C, Renard E. High fasting serum insulin level due to autoantibody interference in insulin immunoassay discloses autoimmune insulin syndrome: A case report. Ann Biol Clin. 2016;74:490–494. doi: 10.1684/abc.2016.1168. [DOI] [PubMed] [Google Scholar]
- 5.Wang YL, Yao PW, Zhang XT, Luo ZZ, Wu PQ, Xiao F. Insulin autoimmune syndrome: 73 Cases of clinical analysis. Chin Med J. 2015;128:2408–2409. doi: 10.4103/0366-6999.163376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ismail AA. The insulin autoimmune syndrome (IAS) as a cause of hypoglycaemia: An update on the pathophysiology, biochemical investigations and diagnosis. Clin Chem Lab Med. 2016 Apr 12; doi: 10.1515/cclm-2015-1255. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 7.Wong SL, Priestman A, Holmes DT. Recurrent hypoglycemia from insulin autoimmune syndrome. J Gen Intern Med. 2014;29:250–254. doi: 10.1007/s11606-013-2588-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eisenbarth GS. Immunoendocrinology: Scientific and Clinical Aspects. Humana Press; New York, NY: 2011. [Google Scholar]
- 9.Kawasaki M, Oikawa Y, Katsuki T, Kabeya Y, Tomita M, Okisugi M, Shimada A. Insulin glulisine may cause a disease resembling insulin autoimmune syndrome: Case report. Diabetes Care. 2013;36:e195–e196. doi: 10.2337/dc13-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki K, Hirayama S, Ito S. A case of a non-insulin dependent diabetic patient with regular spontaneous hypoglycemic attacks, which were due to insulin-binding antibodies induced by human insulin therapy. Tohoku J Exp Med. 1997;182:163–173. doi: 10.1620/tjem.182.163. [DOI] [PubMed] [Google Scholar]
- 11.Kandaswamy L, Raghavan R, Pappachan JM. Spontaneous hypogly hypoglycemia: diagnostic evalution and management. Endocrine. 2016;53:47–57. doi: 10.1007/s12020-016-0902-0. [DOI] [PubMed] [Google Scholar]
- 12.Lanas A, Paredes A, Espinosa C, Caamaño E, Pérez-Bravo F, Pinto R, Iñiguez G, Martínez D, Soto N. Insulin autoimmune syndrome: Report of two cases. Rev Med Chil. 2015;143:938–942. doi: 10.4067/S0034-98872015000700016. (in Spanish) [DOI] [PubMed] [Google Scholar]
- 13.Saxon DR, McDermott MT, Michels AW. Novel management of insulin autoimmune sydrome with rituximab and continuous glucose monitoring. J Clin Endocrinol Metab. 2016;101:1931–1934. doi: 10.1210/jc.2016-1097. [DOI] [PubMed] [Google Scholar]
- 14.Ohtsuka Y, Kondo T, Shimada M, Murakami K, Ide H, Kawakami Y. Erythrocyte insulin receptor in insulin autoimmune syndrome: Effects of corticosteroid therapy. Tohoku J Exp Med. 1987;151:181–190. doi: 10.1620/tjem.151.181. [DOI] [PubMed] [Google Scholar]
- 15.Lupsa BC, Chong AY, Cochran EK, Soos MA, Semple RK, Gorden P. Autoimmune forms of hypoglycemia. Medicine (Baltimore) 2009;88:141–153. doi: 10.1097/MD.0b013e3181a5b42e. [DOI] [PubMed] [Google Scholar]