EXECUTIVE SUMMARY
A wealth of scientific knowledge is being generated in sleep and circadian science. In order for us to realize the return on investment for such scientific knowledge and to improve the health of the nation, we need to disseminate and implement research findings into practice. An implementation gap—termed a “quality chasm” by the Institutes of Medicine—separates the scientific knowledge we possess and the implementation of such knowledge into preventative interventions or health-care treatments. It is frequently reported that a time lag of 17 years transpires before medical research reaches clinical practice. The rapid development of new therapies and devices for sleep and circadian disorders, the emergence of wearable devices and mobile health, combined with the mounting interest in sleep from the public and technology industries, present a transformative opportunity for sleep and circadian science researchers. In order to capitalize on this opportunity, the Sleep Research Society and the National Institutes of Health partnered to organize a workshop focused on the translation of evidence-based interventions for sleep and circadian disorders into practice strategies that benefit population health and patient outcomes. The workshop drew on the collective expertise of implementation scientists and sleep scientists in the areas of insomnia, sleep-disordered breathing, and adolescent sleep health. Together, they identified implementation gaps, effective interventions, implementation strategies and relevant outcomes and created a set of recommendations that could accelerate late-stage translation of sleep and circadian rhythms research findings to benefit public health. This white paper represents the proceedings and consensus developed at the workshop. The recommendations for high-priority implementation research are targeted at sleep and implementation researchers, educators, patients, professional societies, industry partners, funding-decision and policy makers. The major recommendations for implementation science in sleep and circadian sciences were to address the following high priority future research needs: (1) Costs and economic benefits associated with screening, diagnosing, treating insomnia across different systems (health care system, employers, etc.). (2) Promoting health literacy and education of patients, providers and community stakeholders regarding obstructive sleep apnea. (3) Increase the proportion of students in grades 9 through 12 who get sufficient sleep and (4) Perform trials aimed at improving adherence to treatments for sleep-disordered breathing (particularly evaluating cognitive therapy approaches). The fourth priority area was identified as an important barrier to implementation science efforts in sleep.
INTRODUCTION AND OVERVIEW
“Knowing is not enough; we must apply. Willing is not enough; we must do.”—Goethe
Sleep and circadian disturbances and disorders afflict millions of individuals in the US.1 Nearly 25% of adults in the US report insufficient sleep which, in turn, is associated with increased risk for heart disease, hypertension, obesity, diabetes mellitus, accidents and all-cause mortality.2 Adequate sleep is necessary for healthy infant, child, and adolescent development and both physical and mental health in both children and adults.3 Moreover, sleep and circadian disorders are common with insomnia affecting up to 10% to 15% of adults and sleep-disordered breathing that is conservatively estimated to afflict 7% of adults in the US.4,5 Both insomnia and sleep-disordered breathing are associated with cardiovascular morbidity and mortality.6–13 Furthermore, the public health burden of insomnia and sleep-disordered breathing is increasing due to the aging population and the obesity epidemic, respectively.14 In older adults, the prevalence of sleep-disordered breathing is estimated to be as high as 30% to 40%.5,6,15 Sleep also has important effects on health and function in younger individuals as well. For instance, cross-sectional data in adolescents consistently link reduced sleep duration and delayed sleep timing to risk behaviors such as substance use, violence-related behaviors, risky sexual practices, suicidality, and driving while impaired from alcohol.16–22
In addition to the improved understanding of the public health importance of sleep, circadian rhythms and their disorders on health and safety, there have been major fundamental advances in our understanding of the mechanistic basis for how sleep and circadian rhythms can affect health, disease, and safety.23–28 The wealth of scientific knowledge that is being generated in the fields of sleep and circadian sciences needs to translated to the bedside and communities to benefit the patients and the public.29 An implementation gap—termed a “quality chasm” by the Institutes of Medicine—separates the scientific knowledge we possess and the implementation of such knowledge into preventative interventions or health-care treatments.30 In all of medicine, not just sleep medicine, it is frequently reported that a time lag of 17 years transpires before research evidence reaches clinical practice.31,32 Moreover, at present, Americans receive only half of recommended preventative, acute and long term healthcare.33 Such sobering statistics have led some critics to assert that the translation of basic discoveries and evidence-based clinical interventions, to advance the health and well-being of patients and communities is agonizingly slow.34 The problem is multifaceted and due to lack of access to affordable healthcare resources; patient, provider and healthcare knowledge regarding guidelines; patient-level care seeking behaviors and other factors. However, part of the failure is due to incomplete understanding of how to effectively deliver healthcare guidelines in real-world settings.
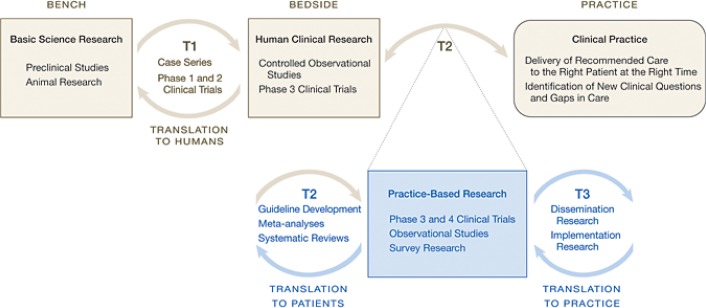
Even if such translation of knowledge to the bedside were to occur, patients and clinicians often face complex and confusing choices when it comes to addressing healthcare concerns. Traditional medical research has not always addressed the questions that patients, families, and their clinicians face daily. Such barriers to dissemination and implementation (D&I) may be overcome only by the full participation of patients in the research process in ways that can make them understand their health and use the available health information more effectively.35 The creation of the Center for Translation Research and Implementation Science (CTRIS) within the National Institutes of Health, the Patient-Centered Outcomes Research Institute (PCORI), and their research networks are meant to tackle these issues head-on to improve the health of the nation.35–37 The ultimate goal of D&I research is to identify ways to extend and adapt generally accepted and effective interventions that have previously been carried out in well-controlled settings to broader populations or settings (i.e., real-world settings like the workplace, schools, community centers and clinics, neighborhoods). D&I research bridges research and practice in real-world settings and strike a balance between rigor and relevance in study designs, methods and outcomes (Figure 1).
Figure 1.
The rapid development of new therapies and devices for sleep and circadian disorders, the emergence of wearable devices and mobile health, and the mounting interest in sleep from the public and technology industries present a transformative opportunity for sleep and circadian science researchers. Recognizing this opportunity, the Sleep Research Society in conjunction with the National Heart Lung Blood Institute organized a workshop in Seattle, Washington, in June 2015 focused on the translation of evidence-based interventions for sleep and circadian disorders to benefit population health. Specifically, the objectives were to set a research agenda for sleep and circadian sciences in the area of implementation science and to enable collaborations between sleep, circadian, and implementation scientists. The workshop drew on the collective expertise of both sleep and implementation scientists who identified implementation gaps, effective interventions, implementation strategies, and relevant outcomes and created a set of recommendations that could accelerate translation of sleep and circadian rhythms research into benefitting the American people. In addition to harnessing the interest of implementation scientists from outside of the sleep field, over 20 young investigators with aspirations in sleep and implementation science were selected through a competitive application process to participate in the workshop. The young investigators culled and weighed the scientific evidence prior to the workshop with the guidance from leaders in the sleep field; presented their synthesized reports at the workshop; and contributed substantially to writing this white paper. This white paper represents the proceedings and consensus development from the workshop. The recommendations for high-priority research were derived from a ranking of future research needs that emerged from the workshop. Such high priority future research needs in this implementation arena will be of substantial interest to sleep and implementation researchers, educators, patients, professional societies, industry partners, funding-decision and policy makers.
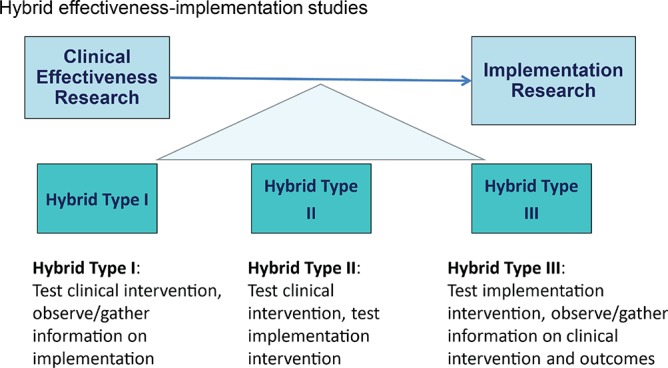
Prior to the workshop, the organizers established major questions that should be addressed by each group domain (Table 1). Each group was tasked with (a) reviewing the state of the science in the specific group domain; (b) identifying interventions that are ready for implementation; (c) clarifying the most critical implementation barriers, questions, gaps; and (d) developing a set of concrete recommendations. When evidence gaps were identified, groups were asked to identify research needed at an earlier phase of translation, such as efficacy utilizing a hybrid design (Figure 2 and Figure 3). For evidence- or guideline-based care delivery, the implementation agenda could include advancing active community-engagement, research networks, and team science; development and promotion of research training in implementation science as it pertains to sleep and circadian sciences; and investigator-initiated implementation research.
Table 1.
Objectives of the sleep and circadian science implementation workshop.

Figure 2.
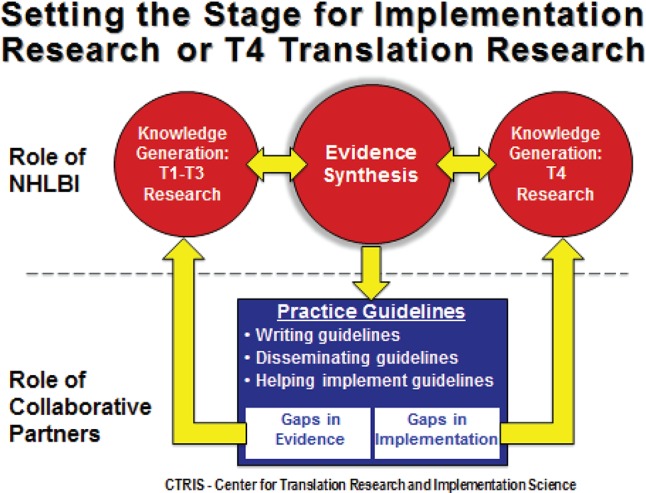
Figure 3.
The following sections review the state of science, deliberations, and recommendations of the three pre-identified tracks on insomnia, sleep-disordered breathing, and adolescent sleep health that were chosen. The workshop focused on three targeted aspects of sleep and circadian science to begin the process towards implementation science considering the large public health burden that they impose. This document can serve as an exemplar for future sleep implementation efforts for other sleep and circadian disorders and sleep duration.
INSOMNIA
Overview
Insomnia disorder is characterized by disturbed sleep and associated impairments during wakefulness. Sleep symptoms may include sleep-onset difficulties, sleep-maintenance problems, or waking up earlier than desired, and must occur at least three nights a week for three months or longer to warrant a diagnosis.38,39 These sleep symptoms must be accompanied by a daytime complaint, such as fatigue, cognitive impairment, or mood disturbance. In the US, prevalence estimates of insomnia range from 10% to 15% in the general population, with significantly higher rates in women, patients with comorbid medical and psychiatric disorders, and first-degree family members of individuals with insomnia.40 Other proposed risk factors, such as old age, low socioeconomic status, and African American race, have less consistent empirical support.41 Insomnia is associated with significant health care costs, as well as occupational impairment in the form of work-place absenteeism, reduced productivity, and health-related quality of life.42,43 It is also a reliable risk factor for various psychiatric and medical conditions, especially depression44 and hypertension.45 A growing body of evidence demonstrates that insomnia is associated with cardiovascular and all-cause mortality.7,9,13,46–55
Promising Interventions: Cognitive Behavioral Therapy for Insomnia (CBT-I)
CBT-I, the most widely studied intervention for insomnia, combines treatment components including stimulus control, sleep restriction, cognitive therapy, relaxation, and sleep hygiene education. Meta-analyses support the efficacy of CBT-I for primary insomnia,56–58 insomnia among older adults,59,60 and insomnia comorbid with medical and/or psychiatric conditions.61–63 CBT-I's positive treatment effects are durable over 12-month follow-up.57 Comparative meta-analyses also suggest CBT-I is superior to benzodiazepine receptor agonist medications for reducing sleep onset latency64,65 and that the effects of CBT-I are more durable over the long term.65 Adverse effects of CBT-I appear to be mild, but have not been investigated as systematically as adverse effects of medications. However, access to CBT-I remains limited due to availability of qualified therapists. Recent research efforts have focused on alternative delivery methods that may increase access to care.66 Compared to control conditions, meta-analyses support the efficacy of group CBT-I,67 self-help modalities (e.g., booklets, videotapes, audiotapes, internet),68 and interactive web programs.69 While each mode of delivery has its limitations, these alternatives are most likely to be viable options for widespread dissemination of CBT-I and increased access to care especially considering recent recommendations that CBT-I is the preferred initial treatment for insomnia.70
Promising Interventions: Pharmacologic Treatments
Benzodiazepine receptor agonists (BzRA), which include benzodiazepine and non-benzodiazepine compounds, are the most widely-used medications for insomnia treatment. Meta-analyses demonstrate their efficacy compared to placebo for subjective (sleep diary) and objective (polysomnography) sleep onset- and sleep maintenance insomnia symptoms.71 BzRA were formerly recommended only for the short-term management of insomnia (≤ 4 weeks), but several double-blind RCTs support their efficacy for up to six months.72,73 Adverse effects include somnolence, headache, cognitive and psycho-motor impairment, and abnormal sleep-related behaviors.71,74 In adults > 60 years of age, the small therapeutic effects may not outweigh the relatively high risk of adverse effects.74 Furthermore, BzRA carry risks of tolerance, dependence, rebound insomnia, and abuse.75 Some evidence suggests increased mortality associated with BzRA use.76 Low-dose sedating antidepressants, which are increasingly prescribed in clinical practice,77 appear to be relatively safe and efficacious in a relatively small number of clinical trials.71,78 However, the risk-benefit ratio of these medications requires further evaluation. The paucity of evidence supporting the efficacy, effectiveness, and safety of the many other medications used to treat insomnia renders them inappropriate for D&I research at this time.
Outcome Measures and Implementation Methods
Numerous psychometrically sound measures are available to assess insomnia treatment outcomes. Objective measures include polysomnography, although not typically recommended outside of clinical research, and actigraphy.79 More common in clinical settings are self-report measures of sleep behaviors,80 insomnia symptoms,81,82 sleep quality,83–85 and sleep-related cognitions.86 An American Academy of Sleep Medicine (AASM) commissioned workgroup recently developed consensus insomnia quality metrics considered vital to a desirable treatment outcome: improved sleep satisfaction/quality (SSQ) and improved daytime functioning.87 Assessment of SSQ is accomplished using self-report measures81–87 and prospective sleep diaries.80 Assessment of daytime functioning can be measured across multiple domains: sleepiness; fatigue, energy, and motivation; family, social, educational, and occupational function; mood; and cognitive function.88 Insomnia treatment outcomes should also be measured at the systemic/organization and the provider level in addition to patient-level outcomes. Measures of successful insomnia treatment implementation include the rate of adoption, penetration of treatment services, patient and provider adherence, sustainability, and cost effectiveness.58 To implement best treatment practices for insomnia all levels of care must be measured with continuous assessment and quality improvement efforts taking place.
Recommendations for Implementation Studies in Insomnia
A large body of evidence describes the diagnosis, health correlates, and treatment of insomnia, and important strides have been made toward implementation of these strategies in specific health care settings. However, a number of barriers and unanswered questions also remain in these areas, creating opportunities for implementation science to bridge existing gaps in insomnia care. Promising interventions ready for implementation are listed in Table 2 and the recommendations for implementation in insomnia are provided in Table 3.
Table 2.
Evidence-based interventions ready for implementation.
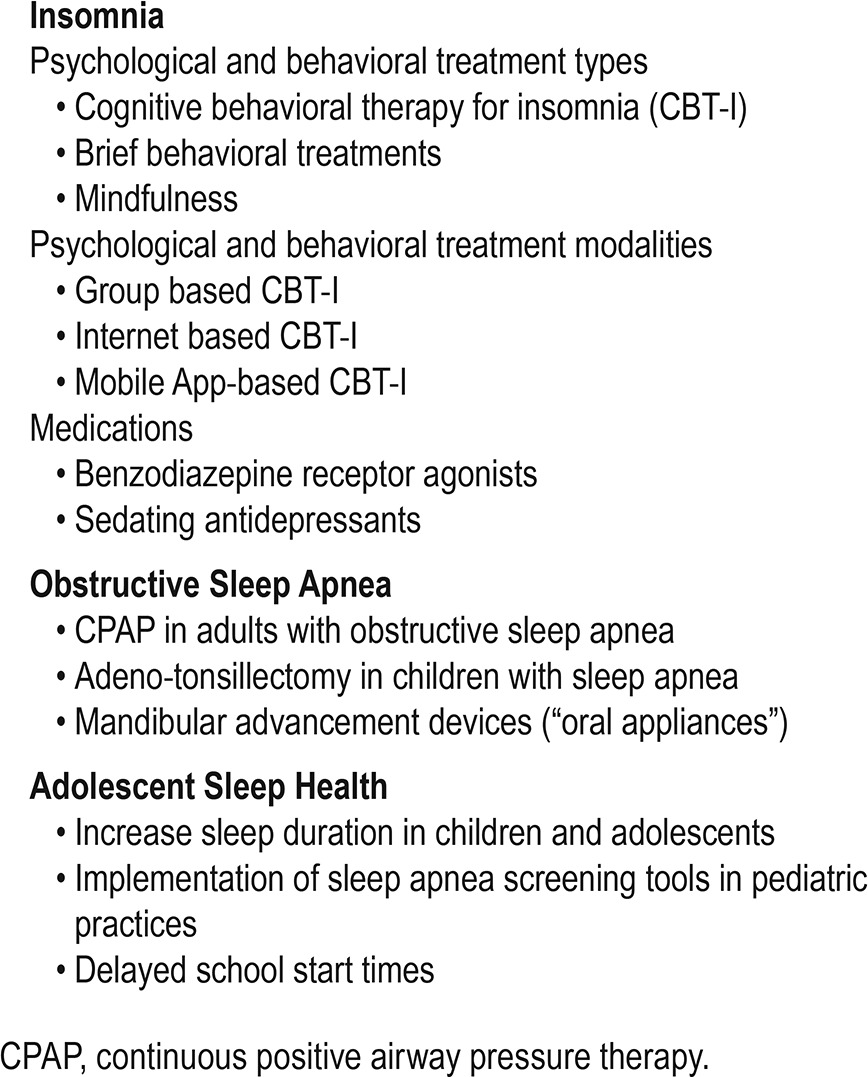
Table 3.
Results of workshop: opportunities for sleep and circadian sciences implementation research for insomnia.

OBSTRUCTIVE SLEEP APNEA
Obstructive sleep apnea (OSA) is a serious condition which involves the cardiovascular, pulmonary and metabolic systems and is associated with increased risk of ischemic stroke and myocardial infarction, particularly in younger individuals.89 Research on OSA has progressed over the last two decades, yet, there are major inadequacies. Overall, positive airway pressure (PAP) therapy appears to be more effective than oral devices such as mandibular advancement devices or oral surgery. However, the benefits with PAP therapy are only realized when such therapy is worn for at least 4–5 hours every night.11,90 Continuous positive airway pressure (CPAP) therapy is generally poorly tolerated,91 although strategies to improve patient adherence such as mask optimization, heated humidification, topical nasal therapy, sleep apnea education, and automatic PAP as compared to continuous PAP therapy may be helpful.92–94 PAP therapy is effective in reducing subjective symptoms of sleepiness, apnea-hypopnea index, and cardiovascular and metabolic health.95,96 Generally, PAP therapy is effective in reducing arterial blood pressure and the benefits are dependent on the population group; and results are better with increasing time of use per night.97,98 In some instances, alternative oral devices also show improved quality of life equivalent to PAP therapy.99 Oral devices including mandibular advancement devices are recommended to be considered as alternatives for PAP treatment only in patients with mild OSA100 and for those who do not tolerate PAP therapy.101 Short-term PAP therapy withdrawal accentuates subjective sleepiness and cardiovascular symptoms thus confirming its use in treating OSA.102 However, over the long term, data on the efficacy of PAP therapy are still forthcoming.103–106 Newer treatment modalities such as hypoglossal nerve stimulation appear to have an advantage over PAP therapy, but the invasive nature, high costs, and the need for more long-term data need to be considered.107 Moreover, such therapies may not work in certain subset of patients with OSA and appropriate selection of treatment responsive individuals remains a challenge.
Health Disparities in OSA
Although men and women are affected by OSA in a ratio of about 2:1,108 very few studies include 33% of women in their sample. Similarly, most studies are performed on obese middle-aged individuals and there is poor representation of races other than Whites, when disease prevalence and severity is greater in minorities.108,109 Therefore, more long-term real-world clinical trials need to be conducted in different populations with adequate representation of women and minorities using different treatment devices. Finally, implementing successful strategies in minority communities in order to increase awareness about OSA and improve adherence to treatment needs action. There are a few ongoing trials which focus on such implementation research in patients with OSA, but more work needs to be done in this area. Moreover, there is a paucity of data on racial or socioeconomic differences or rural versus urban differences in diagnostic and therapy services for OSA.110
Adherence to PAP therapy
While PAP therapy has been shown to be a highly efficacious treatment of OSA in clinical trials, adherence to PAP therapy has been identified as a significant factor limiting real-world effectiveness.111 There are racial differences in PAP adherence.112,113 One study found that PAP users who are black have an average of one hour less use per night than PAP users who are white, with no difference between PAP users who are white and Hispanic.114 Lower socioeconomic status also negatively affects adherence to PAP therapy.115 Additionally, psychological characteristics such as posttraumatic stress disorder, insomnia, and depression reduce PAP adherence.116,117
Given that adherence is a major hurdle to effectiveness of PAP therapy, there have been multiple investigations of interventions to improve patient adherence. Interventions have primarily focused on device or interface factors or educational, supportive, and behavioral interventions. A recent Cochrane review93 showed that automatic PAP therapy appeared to increase PAP use compared to CPAP therapy, but only by 12 minutes per night. In another review which analyzed the impact of educational, behavioral, and supportive interventions on CPAP adherence,94 supportive and educational therapies modestly improved CPAP adherence,while behavioral therapies—such as motivational interviewing and cognitive behavioral therapy— resulted in large improvements in CPAP adherence. There is a clear need for high-quality adequately powered behavioral, educational and supportive interventions, or combinations thereof, on adherence to PAP therapy. Further research in this field is needed to better clarify the efficacy and real-world effectiveness of different interventions, as well as determine the cost-effectiveness and effective implementation strategies for such interventions. The rapid development of mobile health applications, secure messaging, and telemedicine platforms along with patient-centered and patient-navigator strategies are attractive and efficient options for delivering behavioral, educational, and supportive interventions to improve PAP adherence.118–120
Home-Based versus Laboratory-Based Testing
In two large randomized controlled trials, home-based diagnostic testing and PAP titration were comparable to laboratory-based diagnosis and manual PAP titrations in the management of OSA121–123 and may be cost effective alternatives to full night polysomnography.124 However, real-world Markov modelling suggest that laboratory polysomnography may be more cost-effective.125,126 Furthermore, in randomized controlled studies, the effects of treatment on variables such as sleep quality, quality of life, blood pressure, and sleepiness did not differ based on whether the diagnosis for OSA was made in the lab or at-home.127 In terms of acceptance, adherence, time to treatment, and functional improvements, home sleep testing was equivalent to in-laboratory testing.123 Further, ambulatory testing improved test accessibility and reduced waiting times and the total direct cost of OSA management was also reduced.128 However, there is a need for real-world studies without the strict selection criteria that assesses service-related outcomes such as cost-effectiveness, equity, and timeliness when comparing conventional laboratory versus home-based management of OSA.129,130
Impact of Managed Care on OSA Treatment
There are very little data examining the impact of managed care on OSA treatment. In a American Academy of Sleep Medicine (AASM) survey of 245 respondents sleep laboratory staff spent 41–185 minutes per sleep study in order to obtain prior authorization.131 Denial of a laboratory sleep study occurred 21% to 44% of the time, and denial of both in-lab and out of sleep center testing occurred 6% to 15% of the time. These data suggest that there are significant inefficiencies related to management of patients with OSA that would negatively impact the generalizability of cost-effectiveness analysis performed in controlled trials. There is scant literature related to the impact of managed care on patients with OSA. Possible negative impacts of managed care on patients may include the high co-pays associated with sleep diagnostic testing, as well as high payments related to PAP therapy. Additionally, specific hurdles related to Medicare may include the strictly enforced loss of PAP coverage benefits when an arbitrary PAP adherence threshold is not accomplished in a narrow time window. Future research is needed to better identify and overcome barriers to treatment for OSA patients.
Health Services Research, Access to Care, and Health Literacy
Currently there are a paucity of data on issues related to access to care for individuals with OSA. Additionally, little is known about utilization of health services including primary care vs. Sleep specialist for management of OSA, certified sleep clinics vs. non-certified sleep clinics, interventions to promote screening in primary care clinics, and interventions to promote sleep testing.132 There are barriers to care at an individual level, provider level, and at a system level.133,134 Individuals at risk for sleep and circadian disorders including OSA may not be knowledgeable about the symptoms, risk factors as well as diagnosis and treatment regimens. Focus groups have revealed that Blacks often described OSA as being similar to acid reflux or other conditions such as primary cardiac or pulmonary disease.135 It is plausible that such false beliefs and limited knowledge may exist with regards to other sleep and circadian disorders and general sleep hygiene practices.
Health literacy defined as “the degree to which individuals have the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions”136 impacts health status and disease morbidity. There are very few studies that have examined the association between health literacy and sleep and circadian disorders. Nevertheless, the existing studies provide important information, which suggests that patients with sleep and circadian disorders have limited health literacy and that available patient education materials are not easy to understand for most patients.137,138 Moreover, providers may not communicate with their patients about sleep because they may not fully appreciate sleep in the context of the overall health of their patients or be knowledgeable about sleep and circadian disorders such as OSA.139,140 Such lack of knowledge may translate to reduced adherence to treatment such as PAP therapy for OSA, greater discontinuation rates, and consequently greater patient dissatisfaction.132,141 Kramer et al. demonstrated that only 0.13% of primary care physicians referred their patients for OSA screening.142 In cases where a referral was made, patients were more likely to be obese and reported severe obesity.143 Lack of physician referrals is not entirely understood, but may be related to the patient requesting a referral rather than the physician generating the referral based upon a sleep assessment.144 In the US, more health services research aimed at promoting case identification, disease management, and health promotion as it pertains to sleep and circadian disorders needs to be performed.
Data from the National Ambulatory Care Medical Survey (1999–2010) showed that the number of physician office visits for a diagnosis of sleep apnea rose from 1.1 million in 1999 to 8.4 million visits in 2009.145 During the same study period the number of polysomnography and multiple sleep latency testing increased from 500,000 to over 2 million.145 There were significant findings by race for some years and significant differences by gender and age for all years. The sample was primarily white. It is plausible that African Americans and other racial and ethnic groups were not receiving such care in these settings. Lack of geographic distribution of available accredited screening centers poses a barrier and may contribute to health disparities.146
Multi-Level Interventions
Utilizing Brofenbrenner's Ecological Model, multi-level interventions at the individual, provider, community, and health system or national level are needed to improve healthcare delivery to patients with OSA. Interventions targeting individual behavior should include educational interventions that impart knowledge about sleep and circadian disorders. For individuals diagnosed with a disorder such as OSA, interventions should focus on treatment adherence, and education, behavior, motivation and social support.147 There are no known published reports of community level interventions to encourage screening of OSA or other circadian rhythm disorders. Community-level interventions have been effective in addressing hypertension. For example, Victor et al. conducted a study in barbershops to increase hypertension screening among African American men.148 Barbershop owners were trained as lay health educators and conducted blood pressure screening. This approach improved hypertension control and marginally decreased systolic blood pressure.148 Currently there are two ongoing studies to address barriers to OSA screening and adherence to treatment for OSA among African Americans.149
Several interventions are currently underway to raise awareness around the importance of sleep and circadian disorders. For example, the Center for Disease Control and Prevention, the American Academy of Medicine and the Sleep Research Society have developed a partnership to increase knowledge about the importance of adequate sleep.150
Gaps and Future Directions
The review of the literature above highlights the fact there is an abundance of early stage efficacy research, but less later-stage implementation research. Some of the barriers and facilitators to disease detection and treatment have been identified for OSA. However, much less work has been done to translate and implement these findings into real-life settings and systems of care. Most of the translational and implementation work done serve as repositories of information, either in the form of a website with OSA information, support networks for individuals with OSA, patient advocacy, or research databases that scientists tap for more exploratory studies. However, hard to reach groups and underserved communities, who are most at risk for OSA and its medical comorbidities, seldom benefit from the abovementioned translation and implementation efforts because they do not fully engage the population. In conclusion, the areas identified as important for implementation science in the area of sleep-disordered breathing including important barriers that need to be addressed are rank-ordered and listed in Table 4.
Table 4.
Results of workshop: opportunities for sleep and circadian sciences implementation research for sleep-disordered breathing.

ADOLESCENT SLEEP HEALTH
Risk Behavior, Obesity Risk, E-Media Use, and the Role of Primary Care
The recommendations summarized below follow the reviews and workshop discussions of four important components of sleep and health in adolescents: risk behavior, obesity risk, e-media use, and the role of primary care. One promising area for eventual implementation science efforts is the reduction of adolescent risk behaviors through improvements in adolescent sleep health. Adolescence is a uniquely vulnerable period for the development of risk-taking behaviors due to the concurrent structural and functional maturation of relevant brain centers.151,153 Risk behaviors include, but are not limited to, substance use, violence-related behaviors, risky sexual practices, suicidality, and driving while impaired (e.g., by sleepiness or alcohol).154,155 The latter two are perennial top causes of teen death.156 Abundant cross-sectional data consistently link sleep duration and timing to risk behaviors.16,22,157 More limited longitudinal data suggest that sleep loss and sleep and circadian disorders predict later risk behavior, particularly substance involvement, and vice versa.17,158–160 However, with the possible exception of one RCT in progress,161 there are essentially no published experimental or treatment studies that have targeted the sleep-risk behavior pathways in this age group. Such a paucity of scientific data precludes any statements of causality and places constraints on implementation.
Despite this lack of intervention studies, one adolescent risk behavior area identified as promising stems from a small literature suggesting that delaying school start times reduces teen vehicular crashes. Limited experimental evidence has demonstrated a relationship between sleep manipulation and simulated driving in adolescents.162 Moreover, communities with later school start times show fewer teen auto crashes than similar districts with earlier school start times,163,164 and school districts that have enforced delayed school start times have observed concurrent reductions in automobile crashes by as much as 65% to 70%.165,166
While later school start times for middle and high school students that are commensurate with adolescent sleep and circadian biology represent a promising policy-based strategy,167 the need for additional effective interventions that can reduce chronic sleep loss and circadian misalignment in adolescents was identified as an important next step for early stage implementation research.
Obesity Risk
A major clinical concern in adolescence is obesity and downstream medical and psychosocial consequences such as increased cardiovascular morbidity, type 2 diabetes, and decreased quality of life.168 Both cross-sectional and prospective observational studies have linked shorter sleep duration with increased obesity risk in children and adolescents in a dose-dependent fashion.169,170 A smaller body of evidence suggests that sleep timing (e.g., evening chronotype) may also contribute to obesity risk.171–173 Recent experimental studies examining potential mechanisms has pointed to alterations in metabolic regulation (e.g., insulin resistance), and eating behaviors (e.g., calorie consumption and food preference) in sleep restricted teens.174–177 Overall, this research provides a strong theoretical foundation for the development of interventions that target sleep to reduce obesity risk in teens178; however, currently there is no evidence that experimental sleep manipulation results in changes in obesity-related behaviors, metabolic regulation or reduced BMI in normal weight or overweight/obese adolescents.175 Moreover, while there are a handful of RCTs in progress targeting sleep health interventions in younger (i.e., school-aged) obese/overweight children, there are currently no published RCTs in adolescents, nor are there published prospective studies examining the impact of sleep health education in early adolescence on later development of obesity. Therefore, the improvement of sleep health aimed at reducing obesity risk in adolescents is not ready for implementation efforts.
E-Media Use
Electronic media (e-media) may account for a significant amount of adolescents' daily activities, with youth spending > 7 hours/day in front of a screen.179,180 Two recent reviews of this topic have shown a significant adverse association between e-media use and various sleep parameters such as delayed bedtime, longer sleep onset latency, and reduced total sleep time.181,182 Additionally, experimental work has shown the deleterious impact of e-media such as extended video game use on adolescent sleep.183,184 However, the relationship between e-media use, especially in adolescents, is complex and likely bi-directional; for example, studies found that sleep problems in adolescents/young adults is predictive of greater e-media use (not vice versa),185 and screen time is often used as a “sleep-aid.”186
While a number of studies have assessed the impact of interventions to reduce screen time in children,187 and several studies have included limiting screen time as part of recommendations to improve sleep hygiene in general,188 there have been virtually no sleep interventions specifically targeting e-media use in adolescents. Thus, additional studies are needed to further support the causal effect of e-media use on sleep, and to develop and test e-media interventions to improve sleep and associated health outcomes.
The Role of Primary Care in Adolescent Sleep Health
The primary care office represents an important setting for implementation efforts around the identification and management of sleep and circadian disorders and the provision of sleep health education.189,190 While the need to identify and treat sleep and circadian disorders in both children and adolescents is clear,191,192 studies have found low rates of screening, diagnosis, and management of sleep and circadian disorders in youth of all ages in primary care settings.193,194 Moreover, a review of the literature did not identify any implementation studies examining strategies to improve evidence-based identification and management of adolescent sleep and circadian disorders in primary care, highlighting an important gap in the field. OSA identification and treatment in adolescents may be an area that is particularly ripe for implementation, given the body of evidence supporting negative outcomes associated with untreated OSA.192 There are few published guidelines on diagnosis and management of adolescent sleep and circadian disorders for primary care providers and in issued recommendations for OSA diagnosis and management in the primary care setting. Guidelines for evaluation and management of sleep and circadian disorders in adolescents such as those published by the American Academy of Pediatrics (AAP) on OSA,195 should be developed for other disorders (e.g., insomnia, circadian-based disorders, and narcolepsy).196,197
Besides early identification of sleep and circadian disorders, it is critically important for PCPs to provide sleep health education167 and anticipatory guidance to adolescents and their families.197,198 Sleep education programs targeting adolescents in other settings (e.g., schools) have shown promise in improving sleep knowledge, but have not consistently yielded improvements in sleep patterns and behaviors.199 Thus, more work is needed to determine ideal settings and formats to provide sleep guidance resulting in meaningful sleep behavior change. Moreover, recent studies have suggested that there are important racial, ethnic, and socioeconomic factors which increase the risk of unhealthy sleep practices and contribute to sleep health disparities.200–203 Potential topics for sleep health educational interventions in adolescents include drowsy driving, caffeine and stimulant use, irregular sleep-wake patterns, strategic napping, and sleep needs.
In addition, sleep health education interventions would ideally address two critical gaps, improving not only healthy sleep behaviors in families but also sleep health knowledge among pediatric medical care providers. Primary care setting-based interventions must take into consideration barriers identified by primary care providers regarding implementation of sleep health education interventions, including limited time, lack of training in sleep medicine, knowledge gaps and the dearth of published guidelines and practice parameters.198,204–206
While more studies are needed to demonstrate a clear relationship of school start time change (SSTC) to a reduction in health risk behaviors mediated by increased sleep duration and/or reduction in circadian misalignment, healthy school start times (e.g., 08:30 or later167) for middle and high school students as a strategy to improve sleep and health and safety outcomes was identified as a systemic intervention for which a significant amount of empirical evidence is currently available. In addition to improvements in sleep and alertness parameters, a number of positive student outcomes have been identified, including increased academic achievement and engagement, lower depression scores, and decreased rates of health facilities usage.
Research Priorities, Gaps and Future Directions
With these points in mind, a number of priority areas in adolescent sleep health that represent key research gaps and thus provide opportunities for sleep and circadian sciences implementation research were discussed and ranked (Table 5). These research areas were considered to have promise for eventual implementation and dissemination once key gaps are addressed.
Table 5.
Results of workshop: opportunities for sleep and circadian sciences implementation research for adolescent sleep health.

In addition, the group discussed adolescent sleep health research areas that were deemed to have enough empirical evidence to support implementation and dissemination in the near future. Despite the relative paucity of studies documenting the effectiveness of interventions, including RCTs, targeting the key adolescent sleep health issues discussed above, the group was able to identify two major areas. The first was healthy school start times for middle and high school students as a potential means of improving sleep, mood, academic achievement and engagement, and safety, and reducing risk behaviors and cardiovascular and metabolic morbidity in adolescents. The second was application of screening tools and existing guidelines for evidence-based assessment and management of obstructive sleep apnea in primary care settings. Finally, in regards to both of these topics, a number of key remaining research questions critical to address in future research were identified and are summarized below.
For healthy school start times for middle and high school students aimed at reducing proportion of students with insufficient sleep more needs to be done with regards to effective engagement of schools and community stakeholders; identifying tools and strategies (such as effective sleep health education) that could be best adapted to meet the needs of individual communities; derive consensus on the outcome measures that all communities should consider in assessing the impact of SSTC (driving accidents, health and other safety measures such as lower rates of pedestrian accidents and sports and work-related injuries). Moreover, the sustainability of such interventions over time needs to be assessed. Additionally, insight into specific sub-populations of students who may benefit preferentially from SSTC (e.g., high-achieving students, academically and socioeconomically disadvantaged students, racial and ethnic minority students); assessing the impact of SSTC on faculty and other school personnel (e.g., custodial and transportation workers) and other groups of students in the district (e.g., preschool/kindergarten, elementary school, students in special education and vocational programs) needs to be better understood.
Implementation of screening tools and guidelines that would enable identification and treatment of sleep apnea in children and adolescents in primary care settings requires better understanding of the perceived barriers to diagnosing and treating adolescent patients with sleep apnea (e.g., insurance considerations, community availability of and access to appropriate diagnostic and treatment facilities, lack of caregiver knowledge and patient engagement). A critical assessment of current guidelines such as those published by the AAP and whether they are being adequately utilized in primary care as well as an assessment of the optimal methods for assessing the outcomes of enhanced screening, diagnosis and treatment of adolescents with sleep apnea need to be performed.
CONCLUSIONS
Many critical issues confront the future of sleep medicine. Simultaneously, there are many strengths and opportunities that have become available to us. As a community of scientists, clinicians, industry, and policy makers we need to strive to advance the recommendations stemming from this workshop. A complete list of the recommendations are rank-ordered by priority in Tables 3–5. We recognize that there are many topical areas in sleep and circadian sciences that were not comprehensively addressed by this initial endeavor. We need to advance the implementation efforts in these and other areas of sleep and circadian sciences to translate our investments in sleep and circadian research to directly benefit the health of the nation.
CITATION
Parthasarathy S, Carskadon MA, Jean-Louis G, Owens J, Bramoweth A, Combs D, Hale L, Harrison E, Hart CN, Hasler BP, Honaker SM, Hertenstein E, Kuna S, Kushida C, Levenson JC, Murray C, Pack AI, Pillai V, Pruiksma K, Seixas A, Strollo P, Thosar SS, Williams N, Buysse D. Implementation of sleep and circadian science: recommendations from the Sleep Research Society and National Institutes of Health workshop. SLEEP 2016;39(12):2061–2075.
DISCLOSURE STATEMENT
This was not an industry supported study. Funding was provided by the Sleep Research Society, Darien, IL. Dr. Parthasarathy reports grants from NIH/NHLBI (HL095799), grants from Patient Centered Outcomes Research Institute (IHS-1306-02505 and EAIN 3394-UoA), grants from US Department of Defense, grants from NIH (National Cancer Institute; R21CA184920), grants from Johrei Institute, personal fees from American Academy of Sleep Medicine, personal fees from American College of Chest Physicians, non-financial support from National Center for Sleep Disorders Research of the NIH (NHLBI), personal fees from UpToDate Inc., Philips-Respironics, Inc., and Vapotherm, Inc.; grants from Younes Sleep Technologies, Ltd., Niveus Medical Inc., and Philips-Respironics, Inc. outside the submitted work. In addition, Dr. Parthasarathy has a patent UA 14-018 U.S.S.N. 61/884,654; PTAS 502570970 (Home breathing device). The above-mentioned conflicts including the patent are unrelated to the topic of this paper. Dr. Buysse has served as a paid consultant to the following companies, over the past 5 years: Merck, Purdue Pharma, Cerêve, Emmi solutions, Philips Respironics, WebMD, CME Outfitters. He has served as a paid speaker at educational conferences for Astellas and Servier. Dr. Buysse receives research grant support from the National Insitutes of Health. Dr. Thosar was supported by National Space Biomedical Research Institute through NCC 9-58, NIH/NHLBI R01 HL125893, NIH/NHLBI 1F32HL131308-01 during the writing of this manuscript. Dr. Hale reports current grant support from NIH/NICHD (HD073352), NIH/NIA (AG036838) and NIH/NHLBI (HL122460) and has served as a consultant to the BoomShop during the writing of this manuscript. Dr. Hale also sits on the Board of the Directors for the National Sleep Foundation and receives an honorarium for her role as Editor of Sleep Health. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
David Hickam, MD, MPH contributed greatly during the implementation science workshop. Input and editorial assistance from members of the sleep and circadian research community are gratefully acknowledged. The Boards of Directors of the Sleep Research Society are acknowledged for supporting this workshop and for reviewing, providing valuable feedback and approving publication of this white paper. In particular, the logistic support of SRS Executive Director, John Noel, is deeply appreciated.
REFERENCES
- 1.Centers for Disease Control and Prevention, Epidemiology Program Office. Perceived insufficient rest or sleep among adults: United States. MMWR. 2008;58:1175–9. [Google Scholar]
- 2.National Institutes of Health Sleep Disorders Research Plan. 2011. NIH Publication No. 11-7820. http://www.nhlbi.nih.gov/health/prof/sleep/201101011NationalSleepDisordersResearchPlanDHHSPublication11-7820.pdf.
- 3.U.S. Department of Health and Human Services. Office of Disease Prevention and Health Promotion. Healthy People 2020. http://www.healthypeople.gov/2020/topics-objectives/topic/sleep-health.
- 4.Buysse DJ. Insomnia. JAMA. 2013;309:706–16. doi: 10.1001/jama.2013.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–43. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parthasarathy S, Vasquez MM, Halonen M, et al. Persistent insomnia is associated with mortality risk. Am J Med. 2015;128:268–75. e262. doi: 10.1016/j.amjmed.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kripke DF, Garfinkel L, Wingard DL, et al. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–6. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 9.Althuis MD, Fredman L, Langenberg PW, et al. The relationship between insomnia and mortality among community-dwelling older women. J Am Geriatr Soc. 1998;46:1270–3. doi: 10.1111/j.1532-5415.1998.tb04544.x. [DOI] [PubMed] [Google Scholar]
- 10.Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 11.Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 12.Gami AS, Howard DE, Olson EJ, et al. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352:1206–14. doi: 10.1056/NEJMoa041832. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Zhang X, Winkelman JW, et al. The association between insomnia symptoms and mortality: a prospective study of US men. Circulation. 2014;129:737–46. doi: 10.1161/CIRCULATIONAHA.113.004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foley DJ, Monjan A, Simonsick EM, et al. Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6,800 persons over three years. Sleep. 1999;22(Suppl 2):S366–72. [PubMed] [Google Scholar]
- 15.U.S. Department of Health and Human Services. Office of Disease Prevention and Health Promotion. Healthy People 2020. http://www.healthypeople.gov/2020/topics-objectives/topic/sleep-health.
- 16.Clinkinbeard SS, Simi P, Evans MK, et al. Sleep and delinquency: Does the amount of sleep matter? J Youth Adolesc. 2011;40:916–30. doi: 10.1007/s10964-010-9594-6. [DOI] [PubMed] [Google Scholar]
- 17.McGlinchey EL, Harvey AG. Risk behaviors and negative health outcomes for adolescents with late bedtimes. J Youth Adolesc. 2015;44:478–88. doi: 10.1007/s10964-014-0110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKnight-Eily LR, Eaton DK, Lowry R, et al. Relationships between hours of sleep and health-risk behaviors in US adolescent students. Prev Med. 2011;53:271–3. doi: 10.1016/j.ypmed.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 19.O'Brien EM, Mindell JA. Sleep and risk-taking behavior in adolescents. Behav Sleep Med. 2005;3:113–33. doi: 10.1207/s15402010bsm0303_1. [DOI] [PubMed] [Google Scholar]
- 20.Pasch KE, Laska MN, Lytle LA, et al. Adolescent sleep, risk behaviors, and depressive symptoms: are they linked? Am J Health Behav. 2010;34:237–48. doi: 10.5993/ajhb.34.2.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peach HD, Gaultney JF. Sleep, impulse control, and sensation-seeking predict delinquent behavior in adolescents, emerging adults, and adults. J Adolesc Health. 2013;53:293–9. doi: 10.1016/j.jadohealth.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Troxel WM, Ewing B, D'Amico EJ. Examining racial/ethnic disparities in the association between adolescent sleep and alcohol or marijuana use. Sleep Health. 2015;1:104–8. doi: 10.1016/j.sleh.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richards J, Gumz ML. Advances in understanding the peripheral circadian clocks. FASEB J. 2012;26:3602–13. doi: 10.1096/fj.12-203554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sunderram J, Sofou S, Kamisoglu K, et al. Time-restricted feeding and the realignment of biological rhythms: translational opportunities and challenges. J Transl Med. 2014;12:79. doi: 10.1186/1479-5876-12-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–7. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hatori M, Vollmers C, Zarrinpar A, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848–60. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaix A, Zarrinpar A, Miu P, et al. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014;20:991–1005. doi: 10.1016/j.cmet.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stringari C, Wang H, Geyfman M, et al. In vivo single-cell detection of metabolic oscillations in stem cells. Cell Rep. 2015;10:1–7. doi: 10.1016/j.celrep.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colten HR, Altevogt BM. Sleep disorders and sleep deprivation: an unmet public health problem. National Academies Press. 2006 [PubMed] [Google Scholar]
- 30.Horak BJ, Welton W, Shortell S. Crossing the quality chasm: implications for health services administration education. J Health Adm Educ. 2004;21:15–38. [PubMed] [Google Scholar]
- 31.Morris ZS, Wooding S, Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. J R Soc Med. 2011;104:510–20. doi: 10.1258/jrsm.2011.110180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balas E, Boren S. Managing clinical knowledge for health care improvement. In: van Bemmel JH, M A, editors. Yearbook of Medical Informatics. Stuttgart: Schattauer Verlagsgesellschaft mbH; 2000. pp. 65–70. [PubMed] [Google Scholar]
- 33.McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348:2635–45. doi: 10.1056/NEJMsa022615. [DOI] [PubMed] [Google Scholar]
- 34.Collins FS. Research agenda. Opportunities for research and NIH. Science. 2010;327:36–7. doi: 10.1126/science.1185055. [DOI] [PubMed] [Google Scholar]
- 35.Selby JV, Beal AC, Frank L. The Patient-Centered Outcomes Research Institute (PCORI) national priorities for research and initial research agenda. JAMA. 2012;307:1583–4. doi: 10.1001/jama.2012.500. [DOI] [PubMed] [Google Scholar]
- 36.Mensah GA, Engelgau M, Stoney C, et al. News from NIH: a center for translation research and implementation science. Transl Behav Med. 2015;5:127–30. doi: 10.1007/s13142-015-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Forsythe LP, Szydlowski V, Murad MH, et al. A systematic review of approaches for engaging patients for research on rare diseases. J Gen Intern Med. 2014;29(Suppl 3):S788–800. doi: 10.1007/s11606-014-2895-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.American Academy of Sleep Medicine. International classification of sleep disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 39.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 40.Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders, Second Edition criteria: results from the America Insomnia Survey. Biol Psychiatry. 2011;69:592–600. doi: 10.1016/j.biopsych.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 41.Morin CM, Jarrin DC. Epidemiology of insomnia : prevalence, course, risk factors, and public health burden. Sleep Med Clin. 2013;8:281–97. doi: 10.1016/j.jsmc.2022.03.003. [DOI] [PubMed] [Google Scholar]
- 42.Daley M, Morin CM, LeBlanc M, et al. The economic burden of insomnia: direct and indirect costs for individuals with insomnia syndrome, insomnia symptoms, and good sleepers. Sleep. 2009;32:55–64. [PMC free article] [PubMed] [Google Scholar]
- 43.Kyle SD, Morgan K, Espie CA. Insomnia and health-related quality of life. Sleep Med Rev. 2010;14:69–82. doi: 10.1016/j.smrv.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 44.Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135:10–19. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 45.Vgontzas AN, Liao D, Bixler EO, et al. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32:491–7. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Foley DJ, Monjan AA, Brown SL, et al. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18:425–32. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 47.Newman AB, Spiekerman CF, Enright P, et al. Daytime sleepiness predicts mortality and cardiovascular disease in older adults. The Cardiovascular Health Study Research Group. J Am Geriatr Soc. 2000;48:115–23. doi: 10.1111/j.1532-5415.2000.tb03901.x. [DOI] [PubMed] [Google Scholar]
- 48.Rockwood K, Davis HS, Merry HR, et al. Sleep disturbances and mortality: results from the Canadian Study of Health and Aging. J Am Geriatr Soc. 2001;49:639–41. doi: 10.1046/j.1532-5415.2001.49125.x. [DOI] [PubMed] [Google Scholar]
- 49.Nilsson PM, Nilsson JA, Hedblad B, et al. Sleep disturbance in association with elevated pulse rate for prediction of mortality--consequences of mental strain? J Intern Med. 2001;250:521–9. doi: 10.1046/j.1365-2796.2001.00913.x. [DOI] [PubMed] [Google Scholar]
- 50.Mallon L, Broman JE, Hetta J. Sleep complaints predict coronary artery disease mortality in males: a 12-year follow-up study of a middle-aged Swedish population. J Intern Med. 2002;251:207–16. doi: 10.1046/j.1365-2796.2002.00941.x. [DOI] [PubMed] [Google Scholar]
- 51.Suzuki E, Yorifuji T, Ueshima K, et al. Sleep duration, sleep quality and cardiovascular disease mortality among the elderly: a population-based cohort study. Prev Med. 2009;49:135–41. doi: 10.1016/j.ypmed.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 52.Rod NH, Vahtera J, Westerlund H, et al. Sleep disturbances and cause-specific mortality: Results from the GAZEL cohort study. Am J Epidemiol. 2011;173:300–9. doi: 10.1093/aje/kwq371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Almeida OP, Alfonso H, Yeap BB, et al. Complaints of difficulty to fall asleep increase the risk of depression in later life: the health in men study. J Affect Disord. 2011;134:208–16. doi: 10.1016/j.jad.2011.05.045. [DOI] [PubMed] [Google Scholar]
- 54.Eaker ED, Pinsky J, Castelli WP. Myocardial infarction and coronary death among women: psychosocial predictors from a 20-year follow-up of women in the Framingham Study. Am J Epidemiol. 1992;135:854–64. doi: 10.1093/oxfordjournals.aje.a116381. [DOI] [PubMed] [Google Scholar]
- 55.Vgontzas AN, Liao D, Pejovic S, et al. Insomnia with short sleep duration and mortality: the Penn State cohort. Sleep. 2010;33:1159–64. doi: 10.1093/sleep/33.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murtagh DR, Greenwood KM. Identifying effective psychological treatments for insomnia: a meta-analysis. J Consult Clin Psychol. 1995;63:79–89. doi: 10.1037//0022-006x.63.1.79. [DOI] [PubMed] [Google Scholar]
- 57.Okajima I, Komada Y, Inoue Y. A meta-analysis on the treatment effectiveness of cognitive behavioral therapy for primary insomnia. Sleep Biol Rhythms. 2011;9:24–34. [Google Scholar]
- 58.Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy. Am J Psychiatry. 1994;151:1172–80. doi: 10.1176/ajp.151.8.1172. [DOI] [PubMed] [Google Scholar]
- 59.Irwin MR, Cole JC, Nicassio PM. Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychol. 2006;25:3–14. doi: 10.1037/0278-6133.25.1.3. [DOI] [PubMed] [Google Scholar]
- 60.Pallesen S, Nordhus IH, Kvale G. Nonpharmacological interventions for insomnia in older adults: A meta-analysis of treatment efficacy. Psychotherapy (Chic) 1998;35:472–82. [Google Scholar]
- 61.Geiger-Brown JM, Rogers VE, Liu W, et al. Cognitive behavioral therapy in persons with comorbid insomnia: A meta-analysis. Sleep Med Rev. 2014;23C:54–67. doi: 10.1016/j.smrv.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 62.Taylor DJ, Pruiksma KE. Cognitive and behavioural therapy for insomnia (CBT-I) in psychiatric populations: a systematic review. Int Rev Psychiatry. 2014;26:205–13. doi: 10.3109/09540261.2014.902808. [DOI] [PubMed] [Google Scholar]
- 63.Smith MT, Huang MI, Manber R. Cognitive behavior therapy for chronic insomnia occurring within the context of medical and psychiatric disorders. Clin Psychol Rev. 2005;25:559–92. doi: 10.1016/j.cpr.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 64.Smith MT, Perlis ML, Park A, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159:5–11. doi: 10.1176/appi.ajp.159.1.5. [DOI] [PubMed] [Google Scholar]
- 65.Mitchell MD, Gehrman P, Perlis M, et al. Comparative effectiveness of cognitive behavioral therapy for insomnia: a systematic review. BMC Fam Pract. 2012;13:40. doi: 10.1186/1471-2296-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vitiello MV, McCurry SM, Rybarczyk BD. The future of cognitive behavioral therapy for insomnia: what important research remains to be done? J Clin Psychol. 2013;69:1013–21. doi: 10.1002/jclp.21948. [DOI] [PubMed] [Google Scholar]
- 67.Koffel EA, Koffel JB, Gehrman PR. A meta-analysis of group cognitive behavioral therapy for insomnia. Sleep Med Rev. 2015;19:6–16. doi: 10.1016/j.smrv.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ho FY, Chung KF, Yeung WF, et al. Self-help cognitive-behavioral therapy for insomnia: a meta-analysis of randomized controlled trials. Sleep Med Rev. 2015;19:17–28. doi: 10.1016/j.smrv.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 69.Cheng SK, Dizon J. Computerised cognitive behavioural therapy for insomnia: a systematic review and meta-analysis. Psychother Psychosom. 2012;81:206–16. doi: 10.1159/000335379. [DOI] [PubMed] [Google Scholar]
- 70.Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: A Clinical Practice Guideline From the American College of Physicians Management of Chronic Insomnia Disorder in Adults. Ann Intern Med. 2016;165:125–33. doi: 10.7326/M15-2175. [DOI] [PubMed] [Google Scholar]
- 71.Buscemi N, Vandermeer B, Hooton N, et al. The efficacy and safety of exogenous melatonin for primary sleep disorders. A meta-analysis. J Gen Intern Med. 2005;20:1151–8. doi: 10.1111/j.1525-1497.2005.0243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Krystal AD, Erman M, Zammit GK, et al. Long-term efficacy and safety of zolpidem extended-release 12.5 mg, administered 3 to 7 nights per week for 24 weeks, in patients with chronic primary insomnia: a 6-month, randomized, double-blind, placebo-controlled, parallel-group, multicenter study. Sleep. 2008;31:79–90. doi: 10.1093/sleep/31.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Walsh JK, Krystal AD, Amato DA, et al. Nightly treatment of primary insomnia with eszopiclone for six months: effect on sleep, quality of life, and work limitations. Sleep. 2007;30:959–68. doi: 10.1093/sleep/30.8.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Glass J, Lanctot KL, Herrmann N, et al. Sedative hypnotics in older people with insomnia: meta-analysis of risks and benefits. BMJ. 2005;331:1169. doi: 10.1136/bmj.38623.768588.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Licata SC, Rowlett JK. Abuse and dependence liability of benzodiazepine-type drugs: GABA(A) receptor modulation and beyond. Pharmacol Biochem Behav. 2008;90:74–89. doi: 10.1016/j.pbb.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kripke DF, Langer RD, Kline LE. Hypnotics' association with mortality or cancer: a matched cohort study. BMJ Open. 2012;2:e000850. doi: 10.1136/bmjopen-2012-000850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bertisch SM, Herzig SJ, Winkelman JW, et al. National use of prescription medications for insomnia: NHANES 1999-2010. Sleep. 2014;37:343–9. doi: 10.5665/sleep.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yeung WF, Chung KF, Yung KP, et al. Doxepin for insomnia: a systematic review of randomized placebo-controlled trials. Sleep Med Rev. 2015;19:75–83. doi: 10.1016/j.smrv.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 79.Buysse DJ, Ancoli-Israel S, Edinger JD, et al. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155–73. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 80.Carney CE, Buysse DJ, Ancoli-Israel S, et al. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35:287–302. doi: 10.5665/sleep.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Morin CM, Belleville G, Belanger L, et al. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601–8. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ritchey MD, Wall HK, Gillespie C, et al. Million hearts: prevalence of leading cardiovascular disease risk factors--United States, 2005-2012. MMWR Morb Mortal Wkly Rep. 2014;63:462–7. [PMC free article] [PubMed] [Google Scholar]
- 83.Buysse DJ, Reynolds CF, 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 84.Hays RD, Martin SA, Sesti AM, et al. Psychometric properties of the Medical Outcomes Study Sleep measure. Sleep Med. 2005;6:41–44. doi: 10.1016/j.sleep.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 85.Yu L, Buysse DJ, Germain A, et al. Development of short forms from the PROMIS sleep disturbance and Sleep-Related Impairment item banks. Behav Sleep Med. 2011;10:6–24. doi: 10.1080/15402002.2012.636266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morin CM, Vallieres A, Ivers H. Dysfunctional beliefs and attitudes about sleep (DBAS): validation of a brief version (DBAS-16) Sleep. 2007;30:1547–54. doi: 10.1093/sleep/30.11.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Edinger JD, Buysse DJ, Deriy L, et al. Quality measures for the care of patients with insomnia. J Clin Sleep Med. 2015;11:311–34. doi: 10.5664/jcsm.4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chaudoir SR, Dugan AG, Barr CH. Measuring factors affecting implementation of health innovations: a systematic review of structural, organizational, provider, patient, and innovation level measures. Implement Sci. 2013;8:22. doi: 10.1186/1748-5908-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lamberts M, Nielsen O, Lip G, et al. Cardiovascular risk in patients with sleep apnoea with or without continuous positive airway pressure therapy: follow-up of 4.5 million Danish adults. J Intern Med. 2014;276:659–66. doi: 10.1111/joim.12302. [DOI] [PubMed] [Google Scholar]
- 90.Barbé F, Durán-Cantolla J, Capote F, et al. Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;181:718–26. doi: 10.1164/rccm.200901-0050OC. [DOI] [PubMed] [Google Scholar]
- 91.Phillips CL, Grunstein RR, Darendeliler MA, et al. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2013;187:879–87. doi: 10.1164/rccm.201212-2223OC. [DOI] [PubMed] [Google Scholar]
- 92.Ballard RD, Gay PC, Strollo PJ. Interventions to improve compliance in sleep apnea patients previously non-compliant with continuous positive airway pressure. J Clin Sleep Med. 2007;3:706. [PMC free article] [PubMed] [Google Scholar]
- 93.Smith I, Lasserson TJ. Pressure modification for improving usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea. Cochrane Database Syst Rev. 2009:CD003531. doi: 10.1002/14651858.CD003531.pub3. [DOI] [PubMed] [Google Scholar]
- 94.Wozniak DR, Lasserson TJ, Smith I. Educational, supportive and behavioural interventions to improve usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea. Cochrane Database Syst Rev. 2014;1:CD007736. doi: 10.1002/14651858.CD007736.pub2. [DOI] [PubMed] [Google Scholar]
- 95.Sharma SK, Agrawal S, Damodaran D, et al. CPAP for the metabolic syndrome in patients with obstructive sleep apnea. N Engl J Med. 2011;365:2277–86. doi: 10.1056/NEJMoa1103944. [DOI] [PubMed] [Google Scholar]
- 96.Permut I, Diaz-Abad M, Chatila W, et al. Comparison of positional therapy to CPAP in patients with positional obstructive sleep apnea. J Clin Sleep Med. 2010;6:238. [PMC free article] [PubMed] [Google Scholar]
- 97.Martínez-García MA, Capote F, Campos-Rodríguez F, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA. 2013;310:2407–15. doi: 10.1001/jama.2013.281250. [DOI] [PubMed] [Google Scholar]
- 98.Barbé F, Durán-Cantolla J, Sánchez-de-la-Torre M, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307:2161–8. doi: 10.1001/jama.2012.4366. [DOI] [PubMed] [Google Scholar]
- 99.Barnes M, McEvoy RD, Banks S, et al. Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:656–64. doi: 10.1164/rccm.200311-1571OC. [DOI] [PubMed] [Google Scholar]
- 100.Doff M, Hoekema A, Wijkstra PJ, et al. Oral appliance versus continuous positive airway pressure in obstructive sleep apnea syndrome: a 2-year follow-up. Sleep. 2013;36:1289–96. doi: 10.5665/sleep.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Marklund M, Verbraecken J, Randerath W. Non-CPAP therapies in obstructive sleep apnoea: mandibular advancement device therapy. Eur Respir J. 2012;39:1241–7. doi: 10.1183/09031936.00144711. [DOI] [PubMed] [Google Scholar]
- 102.Kohler M, Stoewhas A-C, Ayers L, et al. Effects of continuous positive airway pressure therapy withdrawal in patients with obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2011;184:1192–9. doi: 10.1164/rccm.201106-0964OC. [DOI] [PubMed] [Google Scholar]
- 103.Parra O, Sánchez-Armengol Á, Capote F, et al. Efficacy of continuous positive airway pressure treatment on 5-year survival in patients with ischaemic stroke and obstructive sleep apnea: a randomized controlled trial. J Sleep Res. 2015;24:47–53. doi: 10.1111/jsr.12181. [DOI] [PubMed] [Google Scholar]
- 104.Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 105.Peker Y, Glantz H, Eulenburg C, et al. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with non-sleepy obstructive sleep apnea: The RICCADSA Randomized Controlled Trial. Am J Respir Crit Care Med. 2016;194:613–20. doi: 10.1164/rccm.201601-0088OC. [DOI] [PubMed] [Google Scholar]
- 106.McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919–31. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 107.Kezirian EJ, Goding GS, Malhotra A, et al. Hypoglossal nerve stimulation improves obstructive sleep apnea: 12-month outcomes. J Sleep Res. 2014;23:77–83. doi: 10.1111/jsr.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–43. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Olafiranye O, Akinboboye O, Mitchell JE, et al. Obstructive sleep apnea and cardiovascular disease in blacks: a call to action from the Association of Black Cardiologists. Am Heart J. 2013;165:468–76. doi: 10.1016/j.ahj.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Garg N, Rolle AJ, Lee TA, et al. Home-based diagnosis of obstructive sleep apnea in an urban population. J Clin Sleep Med. 2013;10:879–85. doi: 10.5664/jcsm.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Parthasarathy S. The positive and negative about positive airway pressure therapy. Am J Respir Crit Care Med. 2016;194:535–6. doi: 10.1164/rccm.201603-0484ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Billings ME, Auckley D, Benca R, et al. Race and residential socioeconomics as predictors of CPAP adherence. Sleep. 2011;34:1653–8. doi: 10.5665/sleep.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Platt AB, Field SH, Asch DA, et al. Neighborhood of residence is associated with daily adherence to CPAP therapy. Sleep. 2009;32:799–806. doi: 10.1093/sleep/32.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wallace DM, Shafazand S, Aloia MS, et al. The association of age, insomnia, and self-efficacy with continuous positive airway pressure adherence in black, white, and Hispanic U.S. Veterans. J Clin Sleep Med. 2013;9:885–95. doi: 10.5664/jcsm.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Billings ME, Auckley D, Benca R, et al. Race and residential socioeconomics as predictors of CPAP adherence. Sleep. 2011;34:1653–8. doi: 10.5665/sleep.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.El-Solh AA, Ayyar L, Akinnusi M, et al. Positive airway pressure adherence in veterans with posttraumatic stress disorder. Sleep. 2010;33:1495–500. doi: 10.1093/sleep/33.11.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Law M, Naughton M, Ho S, et al. Depression may reduce adherence during CPAP titration trial. J Clin Sleep Med. 2014;10:163–9. doi: 10.5664/jcsm.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fox N, Hirsch-Allen AJ, Goodfellow E, et al. The impact of a telemedicine monitoring system on positive airway pressure adherence in patients with obstructive sleep apnea: a randomized controlled trial. Sleep. 2012;35:477–81. doi: 10.5665/sleep.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kushida CA, Nichols DA, Holmes TH, et al. SMART DOCS: a new patient-centered outcomes and coordinated-care management approach for the future practice of sleep medicine. Sleep. 2015;38:315–26. doi: 10.5665/sleep.4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Parthasarathy S, Wendel C, Haynes PL, et al. A pilot study of CPAP adherence promotion by peer buddies with sleep apnea. J Clin Sleep Med. 2013;9:543–50. doi: 10.5664/jcsm.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kuna ST, Gurubhagavatula I, Maislin G, et al. Noninferiority of functional outcome in ambulatory management of obstructive sleep apnea. Am J Respir Crit Care Med. 2011;183:1238–44. doi: 10.1164/rccm.201011-1770OC. [DOI] [PubMed] [Google Scholar]
- 122.Cross MD, Vennelle M, Engleman HM, et al. Comparison of CPAP titration at home or the sleep laboratory in the sleep apnea hypopnea syndrome. Sleep. 2006;29:1451–5. doi: 10.1093/sleep/29.11.1451. [DOI] [PubMed] [Google Scholar]
- 123.Rosen CL, Auckley D, Benca R, et al. A multisite randomized trial of portable sleep studies and positive airway pressure autotitration versus laboratory-based polysomnography for the diagnosis and treatment of obstructive sleep apnea: the HomePAP study. Sleep. 2012;35:757–67. doi: 10.5665/sleep.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Deutsch P, Simmons M, Wallace J. Cost-effectiveness of split-night polysomnography and home studies in the evaluation of obstructive sleep apnea syndrome. J Clin Sleep Med. 2006;2:145–53. [PubMed] [Google Scholar]
- 125.Ayas NT, Pack A, Marra C. The demise of portable monitoring to diagnose OSA? Not so fast! Sleep. 2011;34:691–2. doi: 10.5665/SLEEP.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pietzsch JB, Garner A, Cipriano LE, et al. An integrated health-economic analysis of diagnostic and therapeutic strategies in the treatment of moderate-to-severe obstructive sleep apnea. Sleep. 2011;34:695–709. doi: 10.5665/SLEEP.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Skomro RP, Gjevre J, Reid J, et al. Outcomes of home-based diagnosis and treatment of obstructive sleep apnea. Chest. 2010;138:257–63. doi: 10.1378/chest.09-0577. [DOI] [PubMed] [Google Scholar]
- 128.Safadi A, Etzioni T, Fliss D, et al. The effect of the transition to home monitoring for the diagnosis of OSAS on test availability, waiting time, patients' satisfaction, and outcome in a large health provider system. Sleep Disord. 2014;2014:418246. doi: 10.1155/2014/418246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Brownson RC, Colditz GA, Proctor EK. Dissemination and implementation research in health: translating science to practice. Oxford University Press. 2012:3–22. [Google Scholar]
- 130.Parthasarathy S. CON: thoughtful steps informed by more comparative effectiveness research is needed in home testing. J Clin Sleep Med. 2013;9:9–12. doi: 10.5664/jcsm.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.American Academy of Sleep Medicine. AASM Preauthorization Survey National Data Report. 2013.
- 132.Parthasarathy S, Subramanian S, Quan SF. A multicenter prospective comparative effectiveness study of the effect of physician certification and center accreditation on patient-centered outcomes in obstructive sleep apnea. J Clin Sleep Med. 2014;10:243–9. doi: 10.5664/jcsm.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Brofenbrenner U. International Encyclopedia of Education. 2nd ed. Vol. 3. New York, NY: Pergamon; 1993. Ecological models of human development; pp. 37–43. [Google Scholar]
- 134.Mueller M, Purnell TS, Mensah GA, et al. Reducing racial and ethnic disparities in hypertension prevention and control: what will it take to translate research into practice and policy? Am J Hypertens. 2015;28:699–716. doi: 10.1093/ajh/hpu233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shaw R, McKenzie S, Taylor T, et al. Beliefs and attitudes toward obstructive sleep apnea evaluation and treatment among blacks. J Natl Med Assoc. 2012;104:510–9. doi: 10.1016/s0027-9684(15)30217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.U.S. Department of Health and Human Services. Office of Disease Prevention and Health Promotion. Healthy People 2020. https://www.healthypeople.gov/
- 137.Ghiassi R, Murphy K, Cummin AR, et al. Developing a pictorial Epworth Sleepiness Scale. Thorax. 2011;66:97–100. doi: 10.1136/thx.2010.136879. [DOI] [PubMed] [Google Scholar]
- 138.Ghiassi R, Partridge MR. Health literacy and sleep apnoea. Thorax. 2011;66:180. doi: 10.1136/thx.2010.149070. [DOI] [PubMed] [Google Scholar]
- 139.Sorscher AJ. How is your sleep: a neglected topic for health care screening. J Am Board Fam Med. 2008;21:141–8. doi: 10.3122/jabfm.2008.02.070167. [DOI] [PubMed] [Google Scholar]
- 140.Haponik EF, Frye AW, Richards B, et al. Sleep history is neglected diagnostic information - challenges for primary care physicians. J Gen Intern Med. 1996;11:759–61. doi: 10.1007/BF02598994. [DOI] [PubMed] [Google Scholar]
- 141.Parthasarathy S, Haynes PL, Budhiraja R, et al. A national survey of the effect of sleep medicine specialists and American Academy of Sleep Medicine Accreditation on management of obstructive sleep apnea. J Clin Sleep Med. 2006;2:133–42. [PubMed] [Google Scholar]
- 142.Kramer NR, Cook TE, Carlisle CC, et al. The role of the primary care physician in recognizing obstructive sleep apnea. Arch Intern Med. 1999;159:965–8. doi: 10.1001/archinte.159.9.965. [DOI] [PubMed] [Google Scholar]
- 143.Tran D, Wallace J. Obstructive sleep apnea syndrome in a publicly funded healthcare system. J Natl Med Assoc. 2005;97:370–4. [PMC free article] [PubMed] [Google Scholar]
- 144.Hayley AC, Williams LJ, Kennedy GA, et al. Excessive daytime sleepiness and metabolic syndrome: a cross-sectional study. Metabolism-Clinical and Experimental. 2015;64:244–52. doi: 10.1016/j.metabol.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 145.Ford ES, Wheaton AG, Cunningham TJ, et al. Trends in outpatient visits for insomnia, sleep apnea, and prescriptions for sleep medications among US Adults: Findings from the National Ambulatory Medical Care Survey 1999-2010. Sleep. 2014;37:1283–93. doi: 10.5665/sleep.3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Colten H, Altevogt A, editors. Sleep disorders and sleep deprivation: an unmet public health problem. Washington, DC: National Academies Press; 2006. [PubMed] [Google Scholar]
- 147.Weaver TE, Sawyer AM. Adherence to continuous positive airway pressure treatment for obstructive sleep apnoea: Implications for future interventions. Indian J Med Res. 2010;131:245–58. [PMC free article] [PubMed] [Google Scholar]
- 148.Victor RG, Ravenell JE, Freeman A, et al. Effectiveness of a barber-based intervention for improving hypertension control in black men: the BARBER-1 study: a cluster randomized trial. Arch Intern Med. 2011;171:342–50. doi: 10.1001/archinternmed.2010.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Clinicaltrials.gov. 2014.
- 150.American Academy of Sleep Medicine. AASM partners with CDC to address chronic sleep loss epidemic: American Academy of Sleep Medicine, 2013. [Accessed June 24, 2016]. http://www.aasmnet.org/articles.aspx?id=4320.
- 151.Blum K, Febo M, Smith DE, et al. Neurogenetic and epigenetic correlates of adolescent predisposition to and risk for addictive behaviors as a function of prefrontal cortex dysregulation. J Child Adolesc Psychopharmacol. 2015;25:286–92. doi: 10.1089/cap.2014.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gruber R, Cassoff J, Frenette S, et al. Impact of sleep extension and restriction on children's emotional lability and impulsivity. Pediatrics. 2012;130:e1155–61. doi: 10.1542/peds.2012-0564. [DOI] [PubMed] [Google Scholar]
- 153.Saxbe D, Del Piero L, Immordino-Yang MH, et al. Neural correlates of adolescents' viewing of parents' and peers' emotions: Associations with risk-taking behavior and risky peer affiliations. Soc Neurosci. 2015;10:592–604. doi: 10.1080/17470919.2015.1022216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Grunbaum JA, Kann L, Kinchen S, et al. Youth risk behavior surveillance--United States, 2003. MMWR: Surveillance summaries. 2004;53:1–96. [PubMed] [Google Scholar]
- 155.Womack SD, Hook JN, Reyna SH, et al. Sleep loss and risk-taking behavior: a review of the literature. Behav Sleep Med. 2013;11:343–59. doi: 10.1080/15402002.2012.703628. [DOI] [PubMed] [Google Scholar]
- 156.CDC National Center for Injury Prevention and Control. WISQARS Leading Causes of Death Reports, 1999 - 2007. 2010. [Last accessed June 24, 2016]. http://webappa.cdc.gov/sasweb/ncipc/leadcaus10.html.
- 157.Winsler A, Deutsch A, Vorona RD, et al. Sleepless in Fairfax: the difference one more hour of sleep can make for teen hopelessness, suicidal ideation, and substance use. J Youth Adolesc. 2015;44:362–78. doi: 10.1007/s10964-014-0170-3. [DOI] [PubMed] [Google Scholar]
- 158.Hasler BP, Martin CS, Wood DS, et al. A longitudinal study of insomnia and other sleep complaints in adolescents with and without alcohol use disorders. Alcohol Clin Exp Res. 2014;38:2225–33. doi: 10.1111/acer.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pasch KE, Latimer LA, Cance JD, et al. Longitudinal bi-directional relationships between sleep and youth substance use. Journal of Youth and Adolescence. 2012;41:1184–96. doi: 10.1007/s10964-012-9784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wong MM, Robertson GC, Dyson RB. Prospective relationship between poor sleep and substance-related problems in a national sample of adolescents. Alcohol Clin Exp Res. 2015;39:355–62. doi: 10.1111/acer.12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Waloszek JM, Schwartz O, Simmons JG, et al. The SENSE Study (Sleep and Education: learning New Skills Early): a community cognitive-behavioural therapy and mindfulness-based sleep intervention to prevent depression and improve cardiac health in adolescence. BMC Psychol. 2015;3:39. doi: 10.1186/s40359-015-0096-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Beebe DW, Rose D, Amin R. Attention, learning, and arousal of experimentally sleep-restricted adolescents in a simulated classroom. J Adolesc Health. 2010;47:523–5. doi: 10.1016/j.jadohealth.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Vorona RD, Szklo-Coxe M, Lamichhane R, et al. Adolescent crash rates and school start times in two central Virginia counties, 2009-2011: a follow-up study to a southeastern Virginia study, 2007-2008. J Clin Sleep Med. 2014;10:1169–77. doi: 10.5664/jcsm.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Vorona RD, Szklo-Coxe M, Wu A, et al. Dissimilar teen crash rates in two neighboring southeastern Virginia cities with different high school start times. J Clin Sleep Med. 2011;7:145–51. [PMC free article] [PubMed] [Google Scholar]
- 165.Danner F, Phillips B. Adolescent sleep, school start times, and teen motor vehicle crashes. J Clin Sleep Med. 2008;4:533–5. [PMC free article] [PubMed] [Google Scholar]
- 166.Wahlstrom K, Dretzke B, Gordon M, et al. Center for Applied Research and Educational Improvement. St Paul, MN: University of Minnesota; 2014. Examining the impact of later high school start times on the health and academic performance of high school students: a multi-site study. [Google Scholar]
- 167.School start times for adolescents. Pediatrics. 2014;134:642–9. doi: 10.1542/peds.2014-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Countryman AJ, Saab PG, Llabre MM, et al. Cardiometabolic risk in adolescents: associations with physical activity, fitness, and sleep. Ann Behav Med. 2013;45:121–31. doi: 10.1007/s12160-012-9428-8. [DOI] [PubMed] [Google Scholar]
- 169.Cappuccio FP, Taggart FM, Kandala NB, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–26. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hart CN, Cairns A, Jelalian E. Sleep and obesity in children and adolescents. Pediatr Clin North Am. 2011;58:715–33. doi: 10.1016/j.pcl.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Arora T, Taheri S. Associations among late chronotype, body mass index and dietary behaviors in young adolescents. Int J Obes (Lond) 2015;39:39–44. doi: 10.1038/ijo.2014.157. [DOI] [PubMed] [Google Scholar]
- 172.Golley RK, Maher CA, Matricciani L, et al. Sleep duration or bedtime? Exploring the association between sleep timing behaviour, diet and BMI in children and adolescents. Int J Obes (Lond) 2013;37:546–51. doi: 10.1038/ijo.2012.212. [DOI] [PubMed] [Google Scholar]
- 173.Roenneberg T, Allebrandt KV, Merrow M, et al. Social jetlag and obesity. Curr Biol. 2012;22:939–43. doi: 10.1016/j.cub.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 174.Dalen J, Brody JL, Staples JK, et al. A conceptual framework for the expansion of behavioral interventions for youth obesity: a family-based mindful eating approach. Child Obes. 2015;11:577–84. doi: 10.1089/chi.2014.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Beebe DW, Simon S, Summer S, et al. Dietary intake following experimentally restricted sleep in adolescents. Sleep. 2013;36:827–34. doi: 10.5665/sleep.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Klingenberg L, Chaput JP, Holmback U, et al. Acute sleep restriction reduces insulin sensitivity in adolescent boys. Sleep. 2013;36:1085–90. doi: 10.5665/sleep.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Simon SL, Field J, Miller LE, et al. Sweet/dessert foods are more appealing to adolescents after sleep restriction. PLoS One. 2015;10:e0115434. doi: 10.1371/journal.pone.0115434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nugent R, Althouse A, Yaqub Y, et al. Modeling the relation between obesity and sleep parameters in children referred for dietary weight reduction intervention. South Med J. 2014;107:473–80. doi: 10.14423/SMJ.0000000000000145. [DOI] [PubMed] [Google Scholar]
- 179.Strasburger VC. Children, adolescents, obesity, and the media. Pediatrics. 2011;128:201–8. doi: 10.1542/peds.2011-1066. [DOI] [PubMed] [Google Scholar]
- 180.Foehr UG, Roberts DF. Menlo Park, CA: Henry J. Kaiser Family Foundation; 2010. Generation M2: media in the lives of 8- to 18-year-olds. [Google Scholar]
- 181.Cain N, Gradisar M. Electronic media use and sleep in school-aged children and adolescents: A review. Sleep Med. 2010;11:735–42. doi: 10.1016/j.sleep.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 182.Hale L, Guan S. Screen time and sleep among school-aged children and adolescents: a systematic literature review. Sleep Med Rev. 2015;21:50–8. doi: 10.1016/j.smrv.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.King DL, Gradisar M, Drummond A, et al. The impact of prolonged violent video-gaming on adolescent sleep: an experimental study. J Sleep Res. 2013;22:137–43. doi: 10.1111/j.1365-2869.2012.01060.x. [DOI] [PubMed] [Google Scholar]
- 184.Weaver E, Gradisar M, Dohnt H, et al. The effect of presleep video-game playing on adolescent sleep. J Clin Sleep Med. 2010;6:184–9. [PMC free article] [PubMed] [Google Scholar]
- 185.Tavernier R, Willoughby T. Sleep problems: predictor or outcome of media use among emerging adults at university? J Sleep Res. 2014;23:389–96. doi: 10.1111/jsr.12132. [DOI] [PubMed] [Google Scholar]
- 186.Eggermont S, Van den Bulck J. Nodding off or switching off? The use of popular media as a sleep aid in secondary-school children. J Paediatr Child Health. 2006;42:428–33. doi: 10.1111/j.1440-1754.2006.00892.x. [DOI] [PubMed] [Google Scholar]
- 187.Wahi G, Parkin PC, Beyene J, et al. Effectiveness of interventions aimed at reducing screen time in children: a systematic review and meta-analysis of randomized controlled trials. Arch Pediatr Adolesc Med. 2011;165:979–86. doi: 10.1001/archpediatrics.2011.122. [DOI] [PubMed] [Google Scholar]
- 188.Blunden S, Rigney G. Lessons learned from sleep education in schools: a review of dos and don'ts. J Clin Sleep Med. 2015;11:671–80. doi: 10.5664/jcsm.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Klein JD, Wilson KM, McNulty M, et al. Access to medical care for adolescents: results from the 1997 Commonwealth Fund Survey of the Health of Adolescent Girls. J Adolesc Health. 1999;25:120–30. doi: 10.1016/s1054-139x(98)00146-3. [DOI] [PubMed] [Google Scholar]
- 190.Yu SM, Bellamy HA, Kogan MD, et al. Factors that influence receipt of recommended preventive pediatric health and dental care. Pediatrics. 2002;110:e73. doi: 10.1542/peds.110.6.e73. [DOI] [PubMed] [Google Scholar]
- 191.Owens J. Classification and epidemiology of childhood sleep disorders. Prim Care. 2008;35:533–46. vii. doi: 10.1016/j.pop.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 192.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:e714–55. doi: 10.1542/peds.2012-1672. [DOI] [PubMed] [Google Scholar]
- 193.Honaker SM, Meltzer LJ. Sleep in pediatric primary care: a review of the literature. Sleep Med Rev. 2016;25:31–9. doi: 10.1016/j.smrv.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 194.Meltzer LJ, Johnson C, Crosette J, et al. Prevalence of diagnosed sleep disorders in pediatric primary care practices. Pediatrics. 2010;125:e1410–8. doi: 10.1542/peds.2009-2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:576–84. doi: 10.1542/peds.2012-1671. [DOI] [PubMed] [Google Scholar]
- 196.Flygare J, Parthasarathy S. Narcolepsy: let the patient's voice awaken us! Am J Med. 2014;128:10–3. doi: 10.1016/j.amjmed.2014.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Combs D, Goodwin J, Quan SF, et al. Insomnia, health-related quality of life and health outcomes in children: a seven year longitudinal cohort. Sci Rep. 2016:6. doi: 10.1038/srep27921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Belamarich PF, Gandica R, Stein RE, et al. Drowning in a sea of advice: pediatricians and American Academy of Pediatrics policy statements. Pediatrics. 2006;118:e964–78. doi: 10.1542/peds.2006-0652. [DOI] [PubMed] [Google Scholar]
- 199.Cassoff J, Knauper B, Michaelsen S, et al. School-based sleep promotion programs: effectiveness, feasibility and insights for future research. Sleep Med Rev. 2013;17:207–14. doi: 10.1016/j.smrv.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 200.Spilsbury JC, Storfer-Isser A, Drotar D, et al. Effects of the home environment on school-aged children's sleep. Sleep. 2005;28:1419–27. doi: 10.1093/sleep/28.11.1419. [DOI] [PubMed] [Google Scholar]
- 201.Spilsbury JC, Storfer-Isser A, Drotar D, et al. Sleep behavior in an urban US sample of school-aged children. Arch Pediatr Adolesc Med. 2004;158:988–94. doi: 10.1001/archpedi.158.10.988. [DOI] [PubMed] [Google Scholar]
- 202.Owens JA, Jones C. Parental knowledge of healthy sleep in young children: results of a primary care clinic survey. J Dev Behav Pediatr. 2011;32:447–53. doi: 10.1097/DBP.0b013e31821bd20b. [DOI] [PubMed] [Google Scholar]
- 203.Combs D, Goodwin JL, Quan SF, Morgan WJ, Parthasarathy S. Longitudinal differences in sleep duration in Hispanic and Caucasian children. Sleep Med. 2016;18:61–6. doi: 10.1016/j.sleep.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Owens JA. The practice of pediatric sleep medicine: results of a community survey. Pediatrics. 2001;108:E51. doi: 10.1542/peds.108.3.e51. [DOI] [PubMed] [Google Scholar]
- 205.Faruqui F, Khubchandani J, Price JH, et al. Sleep disorders in children: a national assessment of primary care pediatrician practices and perceptions. Pediatrics. 2011;128:539–46. doi: 10.1542/peds.2011-0344. [DOI] [PubMed] [Google Scholar]
- 206.Boerner KE, Coulombe JA, Corkum P. Core competencies for health professionals' training in pediatric behavioral sleep care: a Delphi study. Behav Sleep Med. 2015;13:265–84. doi: 10.1080/15402002.2013.874348. [DOI] [PubMed] [Google Scholar]