Abstract
Study Objectives:
We estimated rates of cardiometabolic disease, pain conditions, and psychiatric illness associated with Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) insomnia disorder (current and in remission) and habitual short sleep (fewer than 6 h), and examined the roles of insomnia and short sleep in racial disparities in disease burden between black and non-Hispanic white Americans.
Methods:
This epidemiological survey study was cross-sectional. The community-based sample consisted of 3,911 subjects (46.0 y ± 13.3; 65.4% female; 25.0% black) across six sleep groups based on DSM-5 insomnia classification (never vs. remitted vs. current) and self-reported habitual sleep duration (normal vs. short). Vascular events, cardiometabolic disease, pain conditions, and psychiatric symptoms were self-reported.
Results:
Short sleeping insomniacs were at elevated risk for myocardial infarction, stroke, treated hypertension, diabetes, chronic pain, back pain, depression, and anxiety, independent of sex, age, and obesity. Morbidity profiles for insomniacs with normal sleep duration and former insomniacs, irrespective of sleep duration, were similar with elevations in treated hypertension, chronic pain, depression, and anxiety. Regarding racial disparities, cardiometabolic and psychiatric illness burden was greater for blacks, who were more likely to have short sleep and the short sleep insomnia phenotype. Evidence suggested that health disparities may be attributable in part to race-related differences in sleep.
Conclusions:
Insomnia disorder with short sleep is the most severe phenotype of insomnia and comorbid with many cardiometabolic and psychiatric illnesses, whereas morbidity profiles are highly similar between insomniacs with normal sleep duration and former insomniacs. Short sleep endemic to black Americans increases risk for the short sleep insomnia phenotype and likely contributes to racial disparities in cardiometabolic disease and psychiatric illness.
Citation:
Kalmbach DA, Pillai V, Arnedt JT, Drake CL. DSM-5 insomnia and short sleep: comorbidity landscape and racial disparities. SLEEP 2016;39(12):2101–2111.
Keywords: anxiety, cardiometabolic disease, depression, DSM-5, insomnia, pain, short sleep
Significance.
Insomnia disorder with habitual short sleep duration represents a critical health crisis in the United States. In our study, DSM-5 insomnia with short sleep was strongly associated with risk for cardiometabolic disease and psychiatric illness. Critically, black Americans endorsed high rates of short sleep and insomnia disorder with short sleep, as well as poorer cardiometabolic and mental health. Important evidence suggested that these racial differences in sleep may contribute to the disproportionate disease burden for black Americans. Future research on disease morbidity associated with short sleep insomnia should be directed toward identifying shared risk factors giving rise to comorbidity with cardiometabolic and psychiatric illnesses, as well as explore further the potential role of short sleep insomnia in racial health disparities.
INTRODUCTION
This report aims to present representative estimates of cardiometabolic disease (CMD), pain conditions, and psychiatric illness associated with Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5)1 insomnia disorder and habitual short sleep duration (i.e., fewer than 6 h) assessed in the Evolution of Pathways to Insomnia Cohort (EPIC) study. In addition, we examine the role of sleep in racial disparities in disease burden between black and white Americans.
While elevated rates of medical and psychiatric morbidities have been demonstrated in insomniacs and short sleepers,2–6 these estimates are based on either prior conceptualizations of insomnia (e.g., DSM-IV7) or study specific operationalizations (e.g., moderate to severe difficulty initiating/maintaining sleep). More recently, diagnostic criteria for insomnia disorder have been standardized per empirical findings on clinically significant thresholds for the chronicity and frequency of symptoms.8 There is now nearly perfect overlap between major diagnostic systems such as the DSM-5 and the ICSD-39; discrepancies between earlier iterations of these systems yielded highly variable prevalence estimates of insomnia and associated morbidity.10 Consistent with the ICSD-3, DSM-5 insomnia stipulates that symptoms occur for at least three nights a week for three months, whereas the DSM-IV required only one month of symptoms. Morbidity estimates based on older diagnostic systems may therefore be misleading. As such, there is a critical need to establish rates of medical and psychiatric illnesses associated with insomnia consistent with current diagnostic guidelines and practices. In addition, recent research suggests that a subset of insomniacs stop meeting diagnostic criteria despite continued sleep disturbance.11 “Remission” in such cases is attributable to reduced daytime distress/sleep dissatisfaction. What remains unclear, however, is whether morbidity rates are elevated among former insomniacs similar to current insomniacs.
Short or insufficient sleep has been linked to poor medical and mental health outcomes in the general population and in insomniacs.2,5,6,12–16 The synergistic effects of sleep disturbance and insufficient sleep on cardiometabolic dysfunction have received growing attention. Insomnia with short sleep duration has been characterized as the most biologically severe insomnia phenotype, owing to evidence that this insomnia subtype confers the greatest risk for CMD and early mortality.2,13,17 Recent studies have classified insomniacs as having short sleep based on a single night of polysomnography (PSG) defined sleep duration of fewer than five or six hours (see Vgontzas et al. for review2). Though this research has benefited from gold-standard objective sleep measurement, the reliability of single-night PSG-defined sleep duration is unsubstantiated and may not represent habitual sleep duration outside the laboratory. This concern is particularly relevant to individuals vulnerable to sleep disturbance while sleeping in laboratory environments.18 Therefore, replicating prior findings using estimates of habitual nightly sleep duration under normal conditions would provide ecologically valid support for the inimical relationship between short sleep insomnia and CMD, and would serve to rule out potential laboratory confounds such as adverse sleep response to PSG or sleeping away from home among individuals sensitive to such challenges.
Research on race-related disparities in sleep have reliably shown that Black or African American (henceforth, “black”) individuals have shorter objective and self-reported habitual sleep duration than White or Caucasian (henceforth, “white”) individuals.19 Though social and environmental disadvantages and higher obesity rates influence disparities in health outcomes, short sleep endemic to black Americans has been suggested as a possible contributor to elevated rates of disease burden in this population.19–22 Blacks with sleep disturbances endorse greater health problems than poorly sleeping whites.23 Moreover, by virtue of having shorter habitual sleep duration, black individuals with insomnia are likely at greater risk for the short sleep insomnia phenotype. However, this hypothesis has not been tested. Given racial differences in obesity, sleep duration, and burden of illness, it is crucial to explore the potential roles of insomnia and short sleep in racial disparities in health outcomes between black and white Americans.
The primary aim of this study was to evaluate rates of CMD, pain conditions, and psychiatric illness associated with DSM-5-based insomnia disorder and habitual short sleep duration (fewer than 6 h nightly). Because of sleep disturbance that often persists following remission of insomnia disorder,11 we included individuals with remitted insomnia in our comparison of never versus current insomniacs. Specifically, we compared morbidity rates across the following groups: 1, subjects with no lifetime history of insomnia and normal sleep duration (i.e., good sleeping controls); 2, subjects with short sleep duration but no history of insomnia; 3, former insomniacs with normal sleep duration; 4, former insomniacs with short sleep duration; 5, insomniacs with normal sleep duration; and 6, short sleeping insomniacs. Consistent with prior research,2,24 we predicted that rates of medical and psychiatric conditions would be greater across all poorly sleeping groups compared to good sleeping controls, and that short sleeping insomniacs would be at highest risk for medical and psychiatric morbidities. In exploration of racial health disparities, we compared rates of insomnia, short sleep, and medical and psychiatric illnesses between non-Hispanic white vs black subjects, i.e., the two most well-represented racial groups in our study comprising over 90% of the total sample.
METHODS
Participants
All subjects were recruited from a major statewide Health Maintenance Organization (HMO) database. The current sample derives from two highly similar protocols as part of the EPIC study, a large National Institute of Mental Health-funded investigation examining the etiology of and health morbidities associated with insomnia in a large community-based sample from southeast Michigan. The two protocols differed in sample characteristics such that subjects without a lifetime history of insomnia were recruited for the insomnia etiology arm of the EPIC study, whereas individuals with current and former insomnia were recruited for a hypertension-focused protocol. In the current study, we had data from 4,277 subjects enrolled in the EPIC study. Recruitment procedures and broader demographic statistics have been reported in detail elsewhere for both protocols.25,26 Data from a total of 363 individuals were excluded from analyses due to being identified as having high risk for sleep apnea, two individuals were excluded for missing data on age, and one individual was excluded from analyses as an age-related outlier (more than 3 standard deviations above the sample mean). These exclusionary criteria resulted in a sample size of 3,911 subjects.
Procedure
The current study was cross-sectional and involved the analysis of web-based survey data. All study procedures were approved by the Institutional Review Board at Henry Ford Hospital.
Assessments
Demographics
Subjects reported age, sex, race, and height and weight (used to calculate body mass index [BMI]). Subjects with a BMI ≥ 30 were classified as obese.
Insomnia
Insomnia classification was based on DSM-5 criteria.1 Subjects were classified as having insomnia if they reported experiencing one or more sleep conditions (e.g., “have you experienced difficulty falling asleep?”; “have you experienced difficulty staying asleep?”) for at least 3 nights a week for a period of 3 mo or longer. Further, positive insomnia classification required endorsement of sleep-related daytime distress or impairment as measured by the following question: “to what extent do you consider your sleep problems to interfere with your daily functioning (e.g., daytime fatigue, ability to function at work/daily chores, concentration, memory, mood etc.)?” Responses to nocturnal and daytime items were coded on a Likert type scale ranging from “0” (“not at all”) to “4” (“very much interfering”). Subjects who reported a score of “2” (“somewhat”) or higher met that criterion. This technique has been validated against clinician-administered diagnostic interviews.27,28 Subjects who met diagnostic criteria at the time of assessment were classified as current insomniacs, whereas subjects who met diagnostic criteria for a past history of DSM-5 insomnia but no longer met diagnostic criteria were classified as remitted/former insomniacs. Good sleeping controls with no history of insomnia were defined by not meeting diagnostic criteria for history of insomnia.
Habitual sleep duration
Total sleep time (TST) was assessed over an average weekday (“thinking about your average weekday, how long did you actually sleep each night?”), given the tendency to engage in compensatory sleep (“sleeping in”) on the weekends. Subjects were classified as short sleepers if they reported fewer than 6 h of average nightly sleep duration, whereas subjects who obtained 6 h or more of sleep were classified as having normal sleep duration.
Other sleep parameters
Sleep onset latency: “on average (including weekends and weekdays), how long did it take you to fall asleep?”; Wake-time after sleep onset: “on average (including weekdays and weekends), how long are you awake during the night?” Risk for obstructive sleep apnea (OSA) was assessed using a self-report version of the STOP-BANG questionnaire,29 though one item was omitted (neck circumference). A traditional cut-point of 5 or greater indicates high risk of OSA; therefore, we used a cutoff of 4 to account for the omitted item in our study. Subjects screening positive for sleep apnea risk were excluded from analyses.
CMD and pain conditions
On a survey of medical conditions, subjects indicated “Yes” or “No” in response to items inquiring about past cerebrovascular and cardiovascular events (myocardial infarction [MCI], cerebrovascular accidents, transient ischemic attacks), current cardiometabolic illnesses (high blood pressure/hypertension, antihypertensive medication use, high cholesterol/hyperlipidemia, diabetes), and pain conditions (chronic pain, back pain).
Depression
Subjects completed the 16-item version of the Quick Inventory of Depressive Symptomatology (QIDS-16).30 The QIDS-16 has a total score range from 0 to 27, with higher scores indicating greater depression severity. To reduce collinearity with insomnia and short sleep, the sleep disturbance items of the QIDS-16 were excluded. Total scores of 11 or higher indicate moderate depression symptoms. To account for the elimination of the sleep subscale, we used a prorated cutoff of QIDS ≥ 10 in the current study (see Endnote A).
Anxiety
Subjects self-reported anxiety levels using the Beck Anxiety Inventory,31 a 21-item questionnaire which measures the severity of common anxiety symptoms. Responses are rated on a 4-point (0–3) Likert-type scale with higher scores indicating greater anxiety severity. Total scores of 22 or higher indicate moderate severity of anxiety.
Data Analysis
All statistical analyses were conducted using IBM SPSS Statistics for Windows – Version 20 (Armonk, NY). Contingency tables and chi-square analyses were used to compare rates of CMD, pain, and psychiatric illness across groups defined by sleep classification and race. For evaluating health disparities between races, we compared subjects who identified as non-Hispanic White (n = 2,556, 65.4% of total sample) and Black or African American (n = 977, 25.0%) as representation among other racial groups was modest (each group < 5% of sample). Dummy coded logistic regression was used to compare associations across the problematic sleeping groups against good sleeping controls as the reference group, while controlling for demographic characteristics.
RESULTS
Sample Characteristics
Subject ages ranged from 18–67 y, with most in middle adulthood (46.0 y ± 13.3). Women comprised 65.3% (n = 2,557) of the sample. More than 90% of subjects identified as white (n = 2,557, 65.3%) or black (n = 979, 25.0%). Differences across sleep classifications were found in age, sex, and obesity rates, and thus were used as covariates in the logistic regression models to follow (see supplemental materials for group comparisons of demographic characteristics). See Table 1 for full sample characteristics.
Table 1.
Sample characteristics for each sleep classification.
Disease Rates by Sleep Classification
Cardiometabolic disease
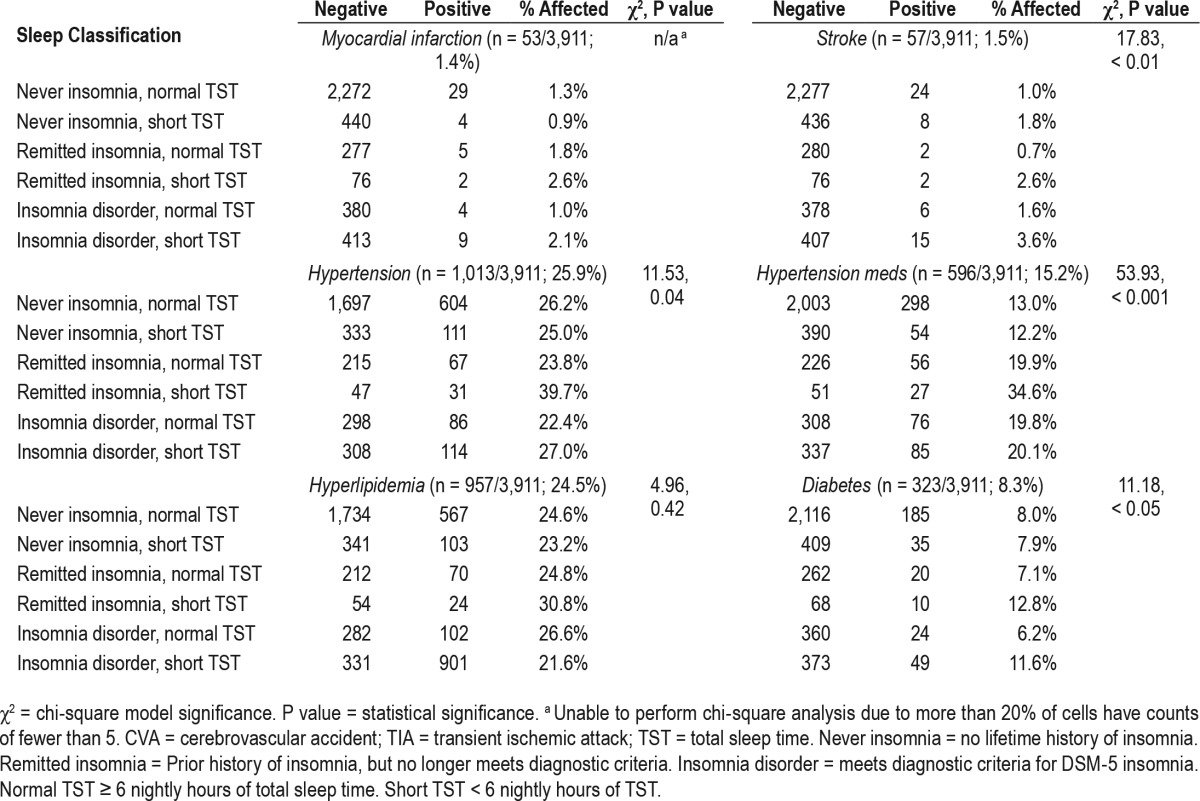
Rates of MCI, stroke, and CMD are reported in Table 2 for all sleep groups. The most prevalent cardiometabolic illnesses in the full sample were hypertension (25.9% per self-report, 15.2% based on antihypertensive medication), hyperlipidemia (24.5%), and diabetes (8.3%), whereas rates of MCI (1.4%) and stroke (1.5%) were comparatively uncommon. Chi-square analyses revealed significant differences in disease rates across sleep groups for stroke, hypertension, and diabetes (Table 2), whereas there were no significant group differences for hyperlipidemia; chi-square analysis could not be performed for MCI due to insufficient representation of positive cases (i.e., fewer than five cases) in three of the six sleep groups. After evaluating CMD rates across groups, we used dummy-coded logistic regression to predict odds of having CMD for groups of problematic sleepers in comparison to the never insomnia, normal sleep duration controls (i.e., as the reference group), while controlling for age, sex, and obesity.
Table 2.
Prevalence of cardiovascular events and cardiometabolic illness by sleep classification.
Overall, short sleep insomnia was most strongly and robustly associated with CMD (see Table 3 for full results), independent of any age-, sex-, and obesity-related effects. Short sleeping insomniacs were at more than threefold odds of endorsing MCI (odds ratio [OR] = 3.23, P < 0.01) and stroke (OR = 3.79, P < 0.001) than good sleeping controls, and were approximately twice as likely to report antihypertensive medication use (OR = 2.13, P < 0.001) and diabetes (OR = 1.83, P < 0.01). Notably, all current and remitted insomnia groups, irrespective of sleep duration, were more likely to report hypertension treatment than controls (ORs = 1.65–2.13, P < 0.01), though short sleepers without history of insomnia did not differ (P = 0.52). Insomniacs with normal sleep duration were at elevated odds of endorsing hyperlipidemia (OR = 1.40, P < 0.01), though no other problematic sleep group differed from controls.
Table 3.
Dummy-coded logistic regression comparing problematic sleep groups against good sleeping controls (as reference group) on risk for cardiovascular events and cardiometabolic disease.

Pain conditions
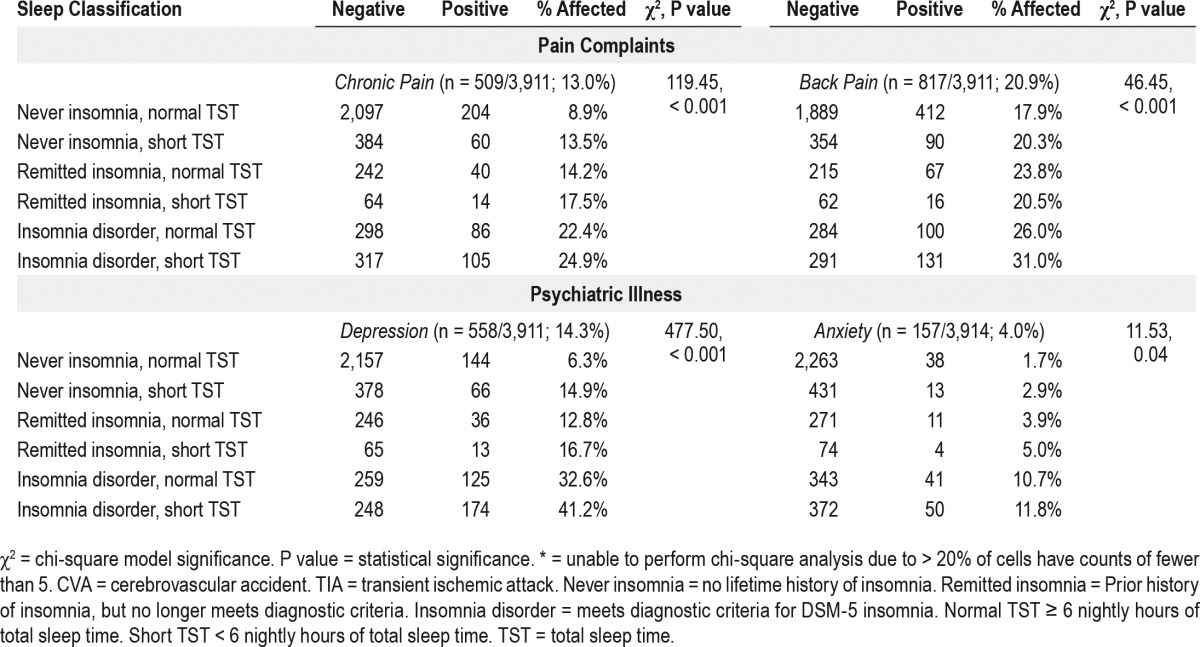
Overall rates of chronic pain and back pain were 13.0% and 20.9% in the sample, respectively (full rates are reported in Table 4 for all sleep groups). Chi-square analyses revealed significant group differences in rates of chronic pain and back pain, with the highest rates of pain conditions observed for insomniacs with normal sleep duration (chronic pain: 22%, back pain: 26%) and short sleeping insomniacs (chronic pain: 25%, back pain: 31%), whereas the lowest rates were observed among individuals with normal sleep duration and no insomnia history, i.e., good sleeping controls (chronic pain: 9%, back pain: 18%).
Table 4.
Prevalence of pain complaints and psychiatric illness by sleep classification.
When controlling for age, gender, and obesity, dummy coded logistic regression showed that nearly all problematic sleep groups were more likely to endorse chronic pain than controls (see Table 5 for full results). Current insomniacs with normal sleep duration were at nearly three-fold odds of chronic pain than controls (OR = 2.95, P < 0.001), and risk was even higher for insomniacs with short sleep duration (OR = 3.61, P < 0.001). Remitted insomniacs with normal sleep duration were more likely than controls to report chronic pain (OR = 1.56, P = 0.02), as were short sleepers with no history of insomnia (OR = 1.58, P < 0.01). A relationship between short sleeping remitters and chronic pain approached significance (OR = 1.78, P = 0.06). In comparison, only short sleeping insomniacs were at elevated risk for back pain (OR = 3.79, P < 0.001) after accounting for the effects of age, gender, and obesity, whereas no other sleep group differed from controls.
Table 5.
Dummy coded logistic regression comparing problematic sleep groups against good sleeping controls (as reference group) on risk for pain conditions and psychiatric illness.
Psychiatric illness
Rates of depression (14%) were notably higher than anxiety prevalence (4%; full rates are reported in Table 4 for all sleep groups). Chi-square analyses revealed significant group differences in rates of depression and anxiety, with short sleeping insomniacs screening positive for depression (41%) and anxiety (12%) at the highest rates, whereas the lowest rates of psychiatric illness were observed among individuals with normal sleep duration and no history of insomnia (depression: 6%, anxiety: 2%). Logistic regression showed that, even after controlling for age, gender, and obesity, all groups of poor sleepers were more likely to screen positive for depression than never insomniacs with normal sleep duration. Current insomniacs were at greatest likelihood of a positive depression screen such that the odds for short sleeping insomniacs meeting threshold for depression were nearly 10 times higher than controls (OR = 9.73, P < 0.001), whereas insomniacs with normal sleep duration were at seven times the odds for depression than good sleepers (OR = 6.97, P < 0.001). Follow-up post-hoc comparison of short sleeping insomniacs and insomniacs with normal sleep duration revealed short sleeping insomniacs to be at greater risk for depression (OR = 1.39, 95% CI = 1.03– 1.86, P = 0.03). The odds for depression were two- to three-fold odds greater than controls for short sleepers without insomnia (OR = 2.55, P < 0.001) and remitted insomniacs with normal (OR = 2.14, P < 0.001) or short sleep duration (OR = 3.16, P < 0.001). A similar trend was observed for anxiety. Insomniacs with short sleep (OR = 7.54, P < 0.001) and normal sleep duration (OR = 6.71, P < 0.001) were at the greatest odds of screening positive for anxiety compared to controls. Notably, remitted insomniacs, irrespective of sleep duration, were more likely to screen positive for anxiety, though the effect was larger among short sleeping remitters (OR = 3.84, P = 0.01) than remitters with normal sleep duration (OR = 2.14, P = 0.01). An association between anxiety and short sleep with no insomnia approached significance (OR = 1.83, P = 0.07).
Race-Related Health Disparities
We first compared demographic characteristics between subjects who racially identified as white versus black. We observed a greater proportion of women among black subjects (74.5%) than white subjects (62.4%), χ2 = 45.77, P < 0.001). Blacks in our sample were also modestly younger than whites (44.20 y ± 12.88 vs. 47.26 y ± 13.12, t = −6.23, P < 0.001, Cohen d = 0.23) and had higher rates of BMI-defined obesity (46.7% vs. 27.4%), χ2 = 118.50, P < 0.001, relative risk [RR] = 1.70.
Next, we ran a 2 × 6 chi-square analysis to evaluate sleep classification proportions between blacks and whites (χ2 = 104.34, P < 0.001). Comparisons of column proportions revealed disparities in proportions of blacks versus whites across all sleep classifications (Table 6). Overall, blacks were more likely than whites to be classified as short sleepers, irrespective of insomnia status (36.2% vs. 20.1%, RR = 1.80, collapsed across all insomnia groups). Importantly, the elevated rates of short sleep among blacks were not attributable to the higher rates of obesity in these subjects (see Endnote B). Notably, comparison of current insomnia rates, irrespective of sleep duration, did not reveal any race-related differences. We then evaluated racial disparities in CMD, pain conditions, and psychiatric illness, which revealed overall greater disease burden for blacks as compared to whites in our sample (Table 7). After controlling for age effects, sex, and obesity, blacks were at greater risk for stroke (OR = 1.84, P = 0.03), hypertension (OR = 1.57–1.90, P < 0.001), diabetes (OR = 1.80, P < 0.001), and depression (OR = 1.28, P = 0.02), whereas whites were at greater risk for chronic pain (OR = 1.30, P = 0.03; Table S1 in the supplemental material).
Table 6.
Sleep classification differences between white and black subjects.

Table 7.
Race-related health disparities between white and black subjects.
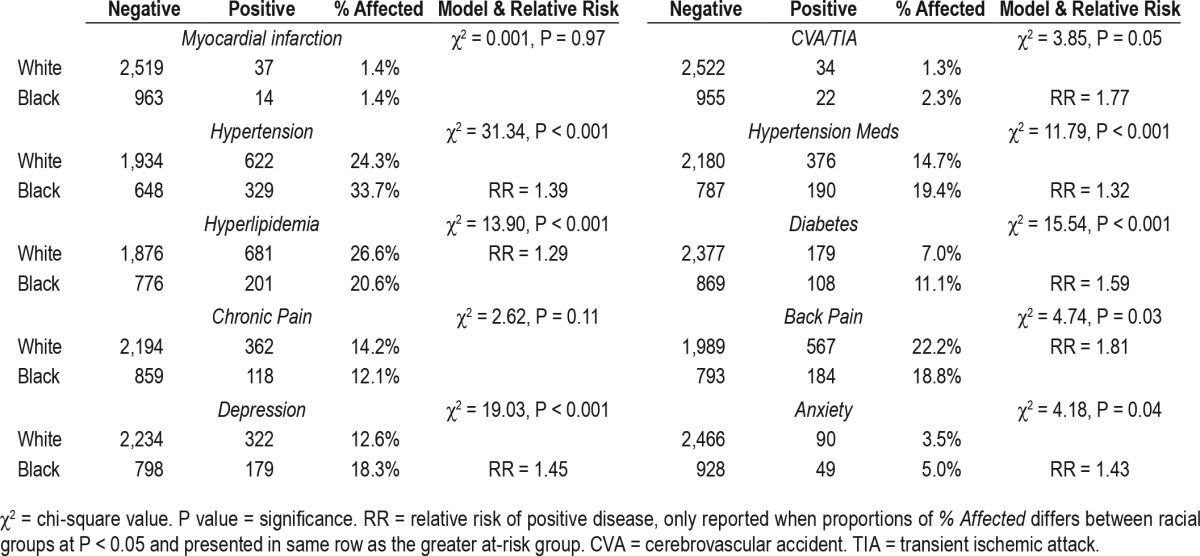
Last, we examined how accounting for the effects of problematic sleep may change the relationship between race and negative health outcomes. To do so, we ran logistic regression estimating risk for CMD, pain, and psychiatric illness as predicted by race (black vs. white) while controlling sleep classification and demographic information (Table S2 in the supplemental material). Despite striking health disparities across medical and psychiatric conditions, racial differences in stroke, hypertension, and chronic pain became nonsignificant. Moreover, though black subjects remained at greater odds of endorsing diabetes (OR = 1.16, P < 0.01) and depression (OR = 1.08, P = 0.03), the effect sizes greatly diminished after controlling for problematic sleep.
DISCUSSION
Findings of the current study support prior description2 of insomnia with short sleep duration as the most biologically severe phenotype of the disorder. Risk for CMD was markedly elevated among subjects with DSM-5 based insomnia with habitual sleep duration of fewer than 6 h per night. In addition, this study revealed that both former and current insomnia are robustly associated with pain conditions and psychiatric illness, but that the highest risk for chronic pain, back pain, depression, and anxiety was observed among short sleeping insomniacs. That remitted insomnia and insomnia with normal sleep duration were largely unassociated with CMD is consistent with prior evidence showing that persistent insomnia, without episodes of remission, is associated with shorter objective sleep duration and higher rates of CMD.2 In addition, striking racial disparities were observed between non-Hispanic white and black subjects in regard to sleep classification and health outcomes. Though no race-related differences in insomnia rates were found, blacks reported higher rates of short sleep, obesity, CMD, and psychiatric illness than whites, thus highlighting the devastating disease burden for this disadvantaged population in the United States.
Insomnia with Short Sleep: The Most Severe Insomnia Phenotype
Prevalence rates of vascular events and CMD suggested that disruptions to the cardiovascular and metabolic systems may be associated with insomnia and short sleep. Even so, likely owing to etiological complexity, no clear pattern emerged from disease rates alone. It was not until the effects of age, gender, and obesity were accounted for that short sleeping insomniacs were revealed to be at highest risk for MCI, stroke, treated hypertension, and diabetes in comparison to good sleeping controls with normal sleep duration and no insomnia history. In comparison, insomniacs with normal sleep duration were only at greater risk for hyperlipidemia and treated hypertension than controls. These results are highly consistent with prior research by Vgontzas et al. highlighting the increased risk for CMD and related mortality for short sleeping insomniacs compared to good sleepers, insomniacs with normal sleep duration, and short sleepers without insomnia.2 Importantly, results from the present study in which sleep duration was self-reported lends ecologically valid support to findings of studies that based sleep duration on one night of in-lab PSG monitoring,2 and are consistent with a prior investigation showing increased rates of mortality in insomniacs with short self-rated sleep duration.13
Alarmingly high rates for pain conditions (chronic: 25%, back: 31%) and psychiatric illness (depression: 41%, anxiety: 12%) were also observed among short sleeping insomniacs. Insomnia with short sleep was the only sleep group to be at greater risk for back pain than controls. Moreover, though insomniacs, irrespective of sleep duration, were much more likely to be depressed than good sleeping controls, short sleeping insomniacs were at greater depression-risk than insomniacs with normal sleep duration. This finding is consistent with recent evidence showing short sleepers with insomnia to be at highest risk for incident depression.32 Risk for chronic pain and anxiety did not differ between normal versus short sleeping insomniacs. Even so, given the stronger associations of back pain and depression with short-sleep insomnia, our data support short sleep insomnia as the most severe phenotype of insomnia disorder.
Remitted Insomnia: Evidence for Elevated Rates of Pain and Psychiatric Illness
Former insomniacs, largely irrespective of habitual sleep duration, were more likely than good sleepers to endorse treated hypertension, chronic pain, depression, and anxiety. Notably, the morbidity landscape for remitted insomnia was similar to that of current insomnia with normal sleep duration, though the relationships between insomnia and psychiatric illness tended to be less strong among remitters. These data are consistent with prior investigations highlighting elevated risk for mood pathology even after insomnia remission.24 Recent research has shown that insomnia remission does not typically reflect normalization of nocturnal sleep parameters.11 Thus, it is possible that insomnia has a scarring effect evident after remission such that insomnia-related nocturnal flotsam and elevated rates of pain and psychiatric symptoms are reflective of unameliorated perturbations to shared systems modulating mood, pain, and sleep.
Short Sleepers without Insomnia History
Even without any lifetime history of insomnia, short sleepers were more likely to endorse chronic pain and depression than never insomniacs with normal sleep length, independent of demographic differences and obesity effects. These results emphasize the relationship between insufficient sleep and psychological wellbeing even in the absence of subjective sleep complaints. Important to note is that it is unclear to what extent insufficient opportunity versus biological inability to sleep longer contributed to short sleep in this group. Though obesity rates were elevated among short sleepers, other influences including biological and environmental factors (e.g., work schedules, family obligations) may contribute to short sleep in this group and confer risk for depression and chronic pain.
Racial Health Disparities between Blacks and Whites
Higher rates of obesity, habitual short sleep duration, CMD, and psychiatric illness were observed in black subjects compared to whites. Reflecting prior findings, blacks were nearly twice as likely as whites to suffer from obesity and short sleep.22,33 It is notable, however, that the higher rate of short sleep among blacks was not entirely attributable to differences in obesity between races, which is also consistent with prior research.22 Despite the higher rates of a wide variety of cardiometabolic illnesses, depression, and anxiety among black subjects, many race-related health disparities became nonsignificant after accounting for racial differences in problematic sleep. Interestingly, the only illness disparities to remain after controlling for these factors showed blacks to be at greater risk for diabetes and depression, though the observed effect sizes for race were very modest. Consistent with prior research,19 these results suggest that disparities in CMD and psychiatric illness between whites and blacks are at least partly attributable to racial differences in sleep and obesity.
Limitations and Future Directions
The current study should be interpreted in light of certain limitations. First, we must acknowledge the potential threats to validity due to the use of self-report instruments. Future investigations on short sleep insomnia may consider utilizing both sleep diaries and actigraphy over a 1- to 2-w periods to capture objectively and subjectively defined habitual sleep duration, particularly when PSG administration is not feasible. It is noteworthy, however, that our results for insomnia with self-reported short sleep and CMD are consistent with studies assessing sleep duration with PSG,2 and that self-reported short sleep among poor-quality sleepers has been shown to be associated with increased mortality13 and depression risk.34 Second, the STOP-BANG screener for OSA was modified in our study (neck circumference item not administered), which may have affected the predictive validity of the measure. Third, the QIDS is often used as a diagnostic tool in clinical samples and may be less sensitive to capture subclinical symptoms in the general population. Additionally, our group of short sleeping remitted insomniacs consisted of just 78 subjects owing to inclusion/exclusion criteria of the parent study, which may limit the generalizability of findings and morbidity rates for this group. Last, because the sample for the current study was the product of two recruitment efforts with specific and separate insomnia eligibility criteria, the total sample may not represent population proportions for insomnia prevalence and should not be interpreted as such.
Owing to high risk for CMD, pain conditions, and psychiatric illness among short sleeping insomniacs, our findings support short sleep insomnia as the most severe phenotype of the disorder. This study had many strengths: Though prior research has shown that risk for CMD development and mortality is elevated for short sleeping insomniacs,2,13 this study was the first to evaluate morbidity rates and associations using DSM-5 insomnia diagnostic criteria coupled with habitual short sleep. Further, this study was the first to report morbidity rates for former insomniacs with short versus normal sleep. Critically, we thoroughly examined racial disparities across a wide range of morbidities associated with insomnia and short sleep. Although blacks were at overall higher risk for medical and psychiatric illnesses, these health disparities were attributable in large part to race-related differences in sleep, such as higher rates of sleep deprivation and short sleep insomnia among black Americans. Thus, short sleep insomnia represents an especially burdensome health crisis for the black population in the US. Future prospective investigations should test whether the development of short sleep and insomnia mediates racial differences in risk for cardiometabolic and psychiatric illnesses.
Although relationships among sleep and medical and mental health have received growing attention, much of what is known centers on inter-illness transactions such as the effects of insomnia on psychiatric illness and the cardiometabolic system. However, given risk factor mutuality,2,15,35–37 efforts should be directed toward better understanding the transdiagnostic roles of shared biological vulnerabilities underlying these conditions. Perturbations to neurobiological systems modulating sleep, mood, and cardiometabolic function may give rise to disease co-occurrence; ergo highly vulnerable individuals may be marred by diathetic fragility of these systems. A key component of the risk relationships among short sleep insomnia, mood pathology, pain, and CMD may involve persistent over-activation or heightened reactivity of physiological arousal systems governing stress response (e.g., hypothalamic-pituitary-adrenal axis hyperactivity, parasympathetic-deficient autonomic imbalance, cortical arousal). Indeed, insomnia has been characterized as a sleep disorder marked by 24-h hyper-arousal with deficient downregulation of wake-promoting processes,35,38 and hyperarousal, rather than sleep loss, is complicit in the development of CMD2 and mood pathology.39,40 Thus, co-occurring disease expressions may reflect destabilized or corroded neurobiological systems in potentiated manifest. Advancement of nosological frameworks and comorbidity research would benefit from directed exploration and characterization of these transdiagnostic risk factors.
ENDNOTES
The range of scores on the QIDS-16 is 0 to 27. Thus, a cutoff of ≥ 11 corresponds to the 40.74th %ile of the scale's total 27 points. After removing the sleep subscale, the maximum score possible is 24. The 40.74th %ile then corresponds to a score of 9.78, which we rounded to ≥ 10 to create our adjusted cutoff for moderate depression.
As obesity rates were higher in blacks than whites, we ran a 2 × 2 chi-square analysis comparing rates of normal vs. short sleepers between whites and blacks among nonobese subjects (BMI < 30). Chi-square analysis revealed significant group differences (χ2 = 66.99, P < 0.001) such that nonobese blacks had higher rates of short sleep (n = 181/520, 34.8%) than nonobese whites (n = 335/1,855, 18.1%), RR = 1.92.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was supported by a National Institute of Mental Health (NIMH) Grant (R01 MH082785) and an investigator-initiated research award from Merck & Co, both to Dr. Drake. Dr. Kalmbach's effort was supported by a National Heart, Lung, and Blood Institute (NHLBI) Grant (T32 HL110952, PI: Pack). The NIMH, NHLBI, and Merck & Co. had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; nor preparation, review, or approval of the manuscript. Dr. Drake has received research support from Merck & Co., Interclinic, Aladdin Dreamer, and Teva; and has served on a speakers' bureau for Teva and Merck. Dr. Pillai has received research support from Merck & Co. The other authors indicated no financial conflicts of interest.
REFERENCES
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association Publishing; 2013. [Google Scholar]
- 2.Vgontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev. 2013;17:241–54. doi: 10.1016/j.smrv.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor DJ, Lichstein KL, Durrence HH, Reidel BW, Bush AJ. Epidemiology of insomnia, depression, and anxiety. Sleep. 2005;28:1457–64. doi: 10.1093/sleep/28.11.1457. [DOI] [PubMed] [Google Scholar]
- 4.Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Dis. 2011;135:10–9. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11:163–78. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51:294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed text revision. Arlington, VA: American Psychiatric Association; 2000. [Google Scholar]
- 8.Morin CM, Leblanc M, Ivers H, et al. Monthly fluctuations of insomnia symptoms in a population-based sample. Sleep. 2014;37:319–26. doi: 10.5665/sleep.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Academy of Sleep Medicine. International classification of sleep disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 10.Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; international statistical classification of diseases and related health problems, tenth revision; and research diagnostic criteria/international classification of sleep disorders, criteria: results from the America insomnia survey. Biol Psychiatry. 2011;69:592–600. doi: 10.1016/j.biopsych.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 11.Pillai V, Roth T, Drake CL. Towards quantitative cut-offs for insomnia: how current diagnostic criteria mischaracterize remission. Sleep Med. 2016 Feb 13; doi: 10.1016/j.sleep.2016.01.013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalmbach DA, Arnedt JT, Swanson LM, Rapier JL, Ciesla JA. Reciprocal dynamics between self-rated sleep and symptoms of depression and anxiety in young adult women: a 14-day diary study. Sleep Med. 2016 May 12; doi: 10.1016/j.sleep.2016.03.014. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Sivertsen B, Pallesen S, Glozier N, et al. Midlife insomnia and subsequent mortality: the Hordaland health study. BMC Public Health. 2014;14:1. doi: 10.1186/1471-2458-14-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altman NG, Izci-Balserak B, Schopfer E, et al. Sleep duration versus sleep insufficiency as predictors of cardiometabolic health outcomes. Sleep Med. 2012;13:1261–70. doi: 10.1016/j.sleep.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grandner MA, Sands-Lincoln MR, Pak VM, Garland SN. Sleep duration, cardiovascular disease, and proinflammatory biomarkers. Nat Sci Sleep. 2013;5:93–107. doi: 10.2147/NSS.S31063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bathgate CJ, Edinger JD, Wyatt JK, Krystal AD. Objective but Not Subjective Short Sleep Duration Associated with Increased Risk for Hypertension in Individuals with Insomnia. Sleep. 2015;39:1037–45. doi: 10.5665/sleep.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vgontzas AN, Liao D, Pejovic S, et al. Insomnia with short sleep duration and mortality: the Penn State cohort. Sleep. 2010;33:1159–64. doi: 10.1093/sleep/33.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonnet MH, Arand DL. Situational insomnia: consistency, predictors, and outcomes. Sleep. 2003;26:1029–37. doi: 10.1093/sleep/26.8.1029. [DOI] [PubMed] [Google Scholar]
- 19.Grandner MA, Petrov M, Rattanaumpawan P, Jackson N, Platt A, Patel NP. Sleep symptoms, race/ethnicity, and socioeconomic position. J Clin Sleep Med. 2013;9:897–905. doi: 10.5664/jcsm.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mensah GA, Brown DW. An overview of cardiovascular disease burden in the United States. Health Affairs. 2007;26:38–48. doi: 10.1377/hlthaff.26.1.38. [DOI] [PubMed] [Google Scholar]
- 21.Boyle JP, Honeycutt AA, Narayan KV, et al. Projection of diabetes burden through 2050 impact of changing demography and disease prevalence in the US. Diabetes Care. 2001;24:1936–40. doi: 10.2337/diacare.24.11.1936. [DOI] [PubMed] [Google Scholar]
- 22.Petrov ME, Lichstein KL. Differences in sleep between black and white adults: an update and future directions. Sleep Med. 2016;18:74–81. doi: 10.1016/j.sleep.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Baldwin CM, Ervin A-M, Mays MZ, et al. Sleep disturbances, quality of life, and ethnicity: the Sleep Heart Health Study. J Clin Sleep Med. 2010;6:176–83. [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandez-Mendoza J, Vgontzas AN, Bixler EO, et al. Clinical and polysomnographic predictors of the natural history of poor sleep in the general population. Sleep. 2012;35:689–97. doi: 10.5665/sleep.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drake CL, Pillai V, Roth T. Stress and sleep reactivity: a prospective investigation of the stress-diathesis model of insomnia. Sleep. 2013;37:1295–304. doi: 10.5665/sleep.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pillai V, Roth T, Drake C. The nature of stable insomnia phenotypes. Sleep. 2015;38:127–38. doi: 10.5665/sleep.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Espie CA, Kyle SD, Hames P, Cyhlarova E, Benzeval M. The daytime impact of DSM-5 insomnia disorder: comparative analysis of insomnia subtypes from the Great British Sleep Survey. J Clin Psychiatry. 2012;73:1478–84. doi: 10.4088/JCP.12m07954. [DOI] [PubMed] [Google Scholar]
- 28.Kessler RC, Coulouvrat C, Hajak G, et al. Reliability and validity of the brief insomnia questionnaire in the America insomnia survey. Sleep. 2010;33:1539–49. [PMC free article] [PubMed] [Google Scholar]
- 29.Chung F, Subramanyam R, Liao P, Sasaki E, Shapiro C, Sun Y. High STOP-Bang score indicates a high probability of obstructive sleep apnoea. Br J Anaesth. 2012;108:768–75. doi: 10.1093/bja/aes022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–83. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 31.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychology. 1988;56:893. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 32.Fernandez-Mendoza J, Shea S, Vgontzas AN, Calhoun SL, Liao D, Bixler EO. Insomnia and incident depression: role of objective sleep duration and natural history. J Sleep Res. 2015;24:390–8. doi: 10.1111/jsr.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Beydoun MA. The obesity epidemic in the United States—gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 34.Fernández-Mendoza J, Vela-Bueno A, Vgontzas AN, et al. Cognitive-emotional hyperarousal as a premorbid characteristic of individuals vulnerable to insomnia. Psychosom Med. 2010;72:397–403. doi: 10.1097/PSY.0b013e3181d75319. [DOI] [PubMed] [Google Scholar]
- 35.Fernandez-Mendoza J, Shaffer ML, Olavarrieta-Bernardino S, et al. Cognitive-emotional hyperarousal in the offspring of parents vulnerable to insomnia: a nuclear family study. J Sleep Res. 2014;23:489–98. doi: 10.1111/jsr.12168. [DOI] [PubMed] [Google Scholar]
- 36.Kalmbach DA, Pillai V, Arnedt JT, Anderson JR, Drake C. Sleep system sensitization: evidence for changing roles of etiological factors in insomnia. Sleep Med. 2016;21:63–9. doi: 10.1016/j.sleep.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161:2126–8. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]
- 38.Fernandez-Mendoza J, Li Y, Vgontzas A, et al. Insomnia is associated with cortical hyperarousal as early as adolescence. Sleep. 2016;39:1029–36. doi: 10.5665/sleep.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roth T, Roehrs T, Pies R. Insomnia: pathophysiology and implications for treatment. Sleep Med Rev. 2007;11:71–9. doi: 10.1016/j.smrv.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Benca RM, Peterson MJ. Insomnia and depression. Sleep Med. 2008;9:S3–9. doi: 10.1016/S1389-9457(08)70010-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.