Abstract
Study Objectives:
Whereas both insomnia and altered interoception are core symptoms in affective disorders, their neural mechanisms remain insufficiently understood and have not previously been linked. Insomnia Disorder (ID) is characterized by sensory hypersensitivity during wakefulness and sleep. Previous studies on sensory processing in ID addressed external stimuli only, but not interoception. Interoceptive sensitivity can be studied quantitatively by measuring the cerebral cortical response to one's heartbeat (heartbeat-evoked potential, HEP). We here investigated whether insomnia is associated with increased interoceptive sensitivity as indexed by the HEP amplitude.
Methods:
Sixty-four participants aged 21–70 years were recruited through www.sleepregistry.nl including 32 people suffering from ID and 32 age- and sex-matched controls without sleep complaints. HEPs were obtained from resting-state high-density electroencephalography (HD-EEG) recorded during evening wakeful rest in eyes-open (EO) and eyes-closed (EC) conditions of 5-minute duration each. Significance of group differences in HEP amplitude and their topographical distribution over the scalp were assessed by means of cluster-based permutation tests.
Results:
In particular during EC, and to a lesser extent during EO, people with ID had a larger amplitude late HEP component than controls at frontal electrodes 376–500 ms after the R-wave peak. Source localization suggested increased neural activity time-locked to heartbeats in people with ID mainly in anterior cingulate/medial frontal cortices.
Conclusions:
People with insomnia show insufficient adaptation of their brain responses to the ever-present heartbeats. Abnormalities in the neural circuits involved in interoceptive awareness including the salience network may be of key importance to the pathophysiology of insomnia.
Citation:
Wei Y, Ramautar JR, Colombo MA, Stoffers D, Gómez-Herrero G, van der Meijden WP, te Lindert BHW, van der Werf YD, Van Someren EJW. I keep a close watch on this heart of mine: increased interoception in insomnia. SLEEP 2016;39(12):2113–2124.
Keywords: insomnia disorder, interoception, heartbeat-evoked potential, event-related potential, high-density EEG, hyperarousal, resting state, salience network
Significance.
We here report novel results connecting, for the first time, the pathophysiology of insomnia and dysfunctional interoception—both have been key observations in affective disorders. We used high-density resting-state EEG in 32 patients with insomnia and 32 controls to objectively assess, at a fine-grained spatiotemporal resolution, a neural correlate of interoception as reflected in the heartbeat-evoked potential (HEP). Findings show that insomnia is associated with increased amplitude of a late frontal HEP component, indicating increased sensitivity to interoceptive signals. Our findings establish a link between two main characteristics of insomnia, physiological and cortical hyperarousal. Resolving the directionality underlying cortical and physiological hyperarousal is a key goal for future research regarding the pathophysiology of insomnia.
INTRODUCTION
Insomnia is one of the most common health complaints in general medical practice. Persistent complaint is part of the diagnosis of Insomnia Disorder (ID)1 which is not only the most prevalent of all sleep disorders,2 but also the second-most prevalent mental disorder.3 Despite its high prevalence, the underlying mechanisms of chronic insomnia remain elusive. A better understanding of the neural correlates of insomnia is highly desirable, not in the least because insomnia represents a primary risk factor for the development of depression4 and cardiovascular diseases.5,6 According to mechanistic models of ID,7–9 insomnia becomes chronic as a result of maladaptive cerebral cortical arousal around sleep onset or during sleep, such that increased levels of sensory and information processing interfere with the normal processes of sleep initiation or maintenance. Support for these models has accumulated from both psychological and neurobiological studies on ID. In a population study, self-reported hypersensitivity to sensory stimuli significantly correlated with poor quality of nocturnal sleep.10 Objective measures, such as event-related potentials (ERPs), have also been used to quantify sensitivity to external stimuli before, during, or after sleep, in association with insomnia. Several ERP components, including N1, P2, P300, and N350 of the auditory-evoked potential,11–21 as well as the recovery function of the somatosensory-evoked potential,22 have been studied in people suffering from ID. Results from these studies confirm increased sensory processing of external stimuli, reflecting enhanced cortical excitability,23 deficient inhibition,24 or both25 in ID.
The brain also responds to internal signals arising from one's own body, and their dysfunctional processing is a hallmark of the pathophysiology of depression and anxiety disorders.26–28 Senses of, and responses to, signals arising from one's own body are collectively known as “interoception.” The term traditionally refers to visceral sensations, but in a broad sense also encompasses (conscious and subconscious) sensations about one's physiological conditions such as hunger, thirst, pain, and temperature.29–31 In the current work, our use of the term “interoception” does not imply awareness of the physiological conditions, but refers to the continuous central nervous system (CNS) processing of such bodily signals which is essential to homeostatic control and integrated in higher-order cognitive functioning.32–35 Previous questionnaire studies have suggested abnormal interoceptive processes in people suffering from ID. One study36 reported significantly higher scores on the Somatic Sensation Inventory37 in a sample of people suffering from ID than in the general population. The authors interpreted the high scores as reflecting altered CNS processing of bodily information. A second questionnaire study38 showed an association between insomnia symptoms and scores on the Modified Somatic Perception Questionnaire39 in a non-clinical sample. These results thus suggest that ID may be characterized by heightened sensitivity to interoceptive signals, even during daytime. To our knowledge, however, no objective quantitative assessment of interoceptive sensitivity in ID has yet been reported. Given the importance of both insomnia and interoception in the pathophysiology of depression and anxiety, we here aimed to assess a neural correlate of interoceptive sensitivity in ID.
Interoceptive sensitivity can be studied quantitatively by means of the heartbeat-evoked potential (HEP).40–52 The HEP reflects the neuronal response to afferent cardiovascular signals and can be obtained by averaging the scalp potentials time-locked to heartbeats. Whereas cardiac electric field artifacts require careful preprocessing, intracranial recordings from the primary sensory and motor cortices in humans have confirmed a neural origin of the HEP.50,53 Early studies showed that the amplitude of the HEP correlated with one's accuracy in heartbeat detection.41,42,44–46 On the other hand, well-defined HEP waveforms could also be observed when people were not consciously paying attention to their heartbeats, such as in the resting state,47,52 during sleep,49 or when people were engaged in exteroceptive tasks in which they had to focus on external stimuli.42–44,47,48,51 In terms of scalp topology, most studies have reported a positive HEP component observed at fronto-central locations with latencies ranging from 200 to 600 ms relative to the electrocardiogram (ECG) R-wave peak.42,44–46,49 Others found a positive component at parieto-occipital sites.43 Discrepancies across studies might be explained by different EEG montages used, time windows examined, and behavioral states under which the HEP was measured. For instance, the positive frontal HEP component was mostly observed when people performed cardioception or tone perception tasks,42,44–46 while the positive parieto-occipital HEP component was reported when the participants perceived visual stimuli (silent movies).43 Source localization based on dipole modeling suggested that the HEP originates from four brain structures: anterior cingulate, medial frontal, insular, and somatosensory cortices,46 all of which had been shown by functional neuroimaging to be involved in a heartbeat discrimination task.54 The HEP thus represents an electrophysiological marker for the cortical processing of afferent cardiovascular information.
In the present study, we assessed the HEPs of people suffering from ID and age- and sex-matched controls using high-density electroencephalography (HD-EEG) recorded in eyes-open (EO) and eyes-closed (EC) resting states prior to bedtime, as indices of interoceptive sensitivity under the relatively natural resting-state conditions. Our objective was two-fold: (1) to compare the spatiotemporal patterns of the HEP during the two resting-state conditions, and (2) to investigate whether people suffering from ID show altered cortical responses to afferent cardiovascular information. We hypothesized that people suffering from ID would exhibit larger amplitude HEP components, reflecting excessive processing and/ or deficient inhibition of interoceptive signals. Additionally, since EC represents a natural behavioral state during which people progress from wakefulness to sleep, larger differences in this electrophysiological marker between people suffering from ID and controls were expected during EC than during EO, should it indeed relate to the mechanisms involved in disturbed sleep.
METHODS
The study was approved by the ethics committee of the VU University Medical Center, Amsterdam, The Netherlands. All participants provided written informed consent.
Participants
Participants for the current study were recruited through advertisement and the Sleep Registry.55 Participants were screened by telephone first, followed by face-to-face interviews. A total of 64 people including 32 suffering from ID (25 female, age range 21–67 y) and 32 controls (26 female, age range 22–70 y) contributed to the data for the present HEP assessment. There was no significant difference between participants with ID and controls in terms of age or sex distribution (Table 1).
Table 1.
Characteristics of the participants.
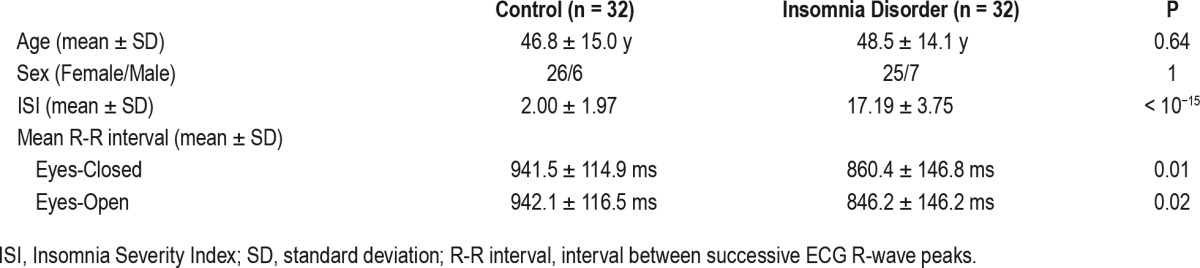
Participants were excluded in case of: (1) diagnosed sleep apnea, restless legs syndrome, narcolepsy, or other somatic, neurological, or psychiatric disorders; (2) use of sleep medications within the last 2 months; (3) overt circadian disorders or irregular sleep-wake rhythms, assessed using one week of actigraphy (Actiwatch AW4, Cambridge Neurotechnology Ltd., Cambridge, UK, or GENEActiv Sleep, Activinsights Ltd., Kimbolton, UK) supplemented by sleep diaries; or (4) scores above the minimal to mild range of anxiety or depression symptom severity, as evaluated by either the Hospital Anxiety and Depression Scale (HADS),56 or the Beck Anxiety Inventory (BAI)57 and Beck Depression Inventory (BDI-IA).58 The exclusion scores for each scale were; BAI: 19 or higher; BDIIA: 17 or higher; HADS: 11 or higher on either of the anxiety or the depression subscales; according to recommended clinical cutoffs.59,60 Scores within the mild range were allowed because scores in this range are more likely in people with ID even in the absence of any anxiety or depression.61,62 Smoking habits were assessed during the intake interview but were not part of the exclusion criteria for the current study. Two participants in the ID group and none in the control group were smokers (P = 0.49, Fisher exact test).
The inclusion criteria for the ID group were in line with DSM-5 diagnosis1 and the Research Diagnostic Criteria63 for Insomnia Disorder. Additional severity criteria required, for the ID group, self-reported sleep onset latency or wake after sleep onset greater than 30 min, and total sleep time less than 6.5 hours, for at least 6 months and for more than 3 nights per week at the time of intake. The Insomnia Severity Index (ISI)64 was administered during the intake interview. Following the cutoff with optimal classification accuracy as previously validated on a clinical sample,65 we only included people with ISI scores greater than 10. These additional quantitative criteria were applied to ensure objective supporting evidence for ID and to exclude possible equivocal cases. The quantitative criteria are commonly used in insomnia research and make our sample comparable to previous studies with either clinical or questionnaire-based criteria. The controls (CTRL) group included age- and sex-matched volunteers that reported to have no sleep difficulties, as confirmed by interviews and their ISI scores less than 8.
Protocol
HD-EEG recordings of people suffering from ID and matched controls were acquired in a laboratory setting. On the day of the recording session, participants were asked to refrain from alcohol and drugs, as well as to limit consumption of caffeinated beverages to a maximum of 2 cups, which were allowed only before noon. Intake of alcohol and caffeinated beverages within the week prior to recording was reported in the sleep diary, and the two groups did not differ in the average daily intake of either alcohol (mean ± standard deviation: ID = 0.95 ± 0.88, CTRL = 1.08 ± 0.99 glasses; P = 0.58) or caffeinated beverages (mean ± standard deviation: ID = 3.74 ± 2.06, CTRL = 4.42 ± 2.51 cups; P = 0.24). Participants underwent resting-state HD-EEG recording during evening wakeful rest (between 19:00 and habitual bedtime) while seated in EO and then EC conditions of 5-min duration each. The two conditions were not counterbalanced. During recording, participants were seated upright and instructed not to think about anything in particular or fall asleep. In addition, in the EO condition, they were requested to fixate at a plus sign on a monitor. Sleep was monitored in real-time during recording. In cases where signs of falling asleep were observed (e.g., slow eye movements, attenuation of alpha waves), the participant was alerted and recording of the 5-min assessment was restarted.
Resting-state HD-EEG was recorded using a 256-channel HydroCel EEG net (Electrical Geodesic Inc., Eugene, OR) connected to a Net Amps 300 amplifier (input impedance: 200 MΩ, A/D converter: 24 bits), with the ground electrode placed at the centro-parietal midline and reference at the vertex. ECG was recorded simultaneously from a Polygraphic Input Box (Electrical Geodesic Inc.), using Ag/AgCl electrodes placed in accordance with the standard lead II configuration.66 Electrode impedances were kept below 100 kΩ throughout the recording session. Signals were online band-pass filtered between 0.1– 100 Hz and digitized at 1000 Hz.
Data Preprocessing
All ECG and EEG analyses were carried out in MATLAB 8.3 (The Mathworks Inc., Natick, MA). R-waves were detected offline from the ECG time series with the Pan-Tompkins algorithm67 and verified visually. Preprocessing of EEG data was conducted separately for each participant using the MEEG-PIPE toolbox (https://github.com/meegpipe/meegpipe). Non-stereotyped artifacts (e.g., baseline drifts, movement artifacts) in each channel were estimated by local polynomial approximation with the LPA-ICI algorithm68 and subtracted from the continuous EEG data. The signals were then downsampled to 250 Hz, and band-pass filtered (0.5–62.5 Hz) with a Hamming-windowed sinc digital FIR filter.69 Noisy EEG channels and segments were automatically detected with the following statistical criteria. The continuous EEG data were first segmented into 2-s epochs, and 3 signal statistics were calculated for each channel for each epoch: range, range of the first derivative, and standard deviation. For each channel, the 3 statistics were averaged across epochs and then transformed into modified z-scores,70 thus obtaining robust measures of deviation from the median suitable for the detection of outliers. The channels with any of the modified z-scores greater than 2.7 were marked as noisy and were linearly interpolated from neighboring channels. Similarly, for each epoch, the three statistics were averaged across channels and transformed into modified z-scores; the epochs with any of the modified z-scores greater than 2.7 were marked as noisy and were excluded from subsequent analyses. An analysis of variance (ANOVA) revealed no significant group, condition, or group-by-condition differences with respect to the number of rejected epochs detected by this automatic procedure (all P > 0.24). The number of rejected channels exhibited large inter-individual variability, but the rejected electrodes were mostly around the neck or cheek regions, where electrodes were later excluded from HEP calculation for all participants. As channel interpolation and the subsequent independent component analysis both reduced dimensionality of the data, their effects on the scalp signals were assessed jointly in a separate ANOVA described below.
After noisy channels and epochs were rejected, the remaining signals were submitted to independent component analysis (ICA).71 Components of power-line noise, eye movement, pulse wave, and cardiac field artifacts were identified through visual inspection of their time courses and topographical distribution and regressed out. Importantly, the pulse wave and cardiac field artifacts are time-locked to heartbeats and thus likely to obscure the HEP or produce spurious group differences. Pulse wave artifacts are generated by movements due to pulsation, with largest amplitude around 200 ms after the ECG R-wave and spatially restricted to electrodes close to a pulsating vessel.53 Components with low-frequency waveforms time-locked to heartbeats and sparse spatial patterns were therefore identified as pulse wave artifacts and regressed out. Cardiac field artifacts, on the other hand, represent the cardiac electric field spread across the scalp due to volume conduction, and are especially prominent in the time windows of the ECG QRS-complex and T-wave.72 Such artifacts were removed by regressing out components that had clear ECG morphology and predominant back-projected activation at the neck region.
To evaluate whether there were group, condition, or group-by-condition differences in interpolation of excluded (noisy) channels or in ICA-based artifact removal, we performed an ANOVA on the dimensionality of the preprocessed data (i.e. rank of the continuous data matrix) over the 150 scalp electrodes that were included in the following HEP analyses. No significant group or group-by-condition interaction effect was observed (P > 0.16). The main effect of condition was significant (P < 0.001), indicating reduction of dimensionality in EO was greater than in EC. This difference was mainly attributed to the removal of more eye movement and blinking artifacts during EO. As these artifacts are asynchronous to heartbeats, we did not expect this difference in data modification would confound later comparisons. Moreover, an ANOVA that addressed the number of rejected ICA components associated with cardiovascular artifacts revealed no significant group, condition, or group-by-condition interaction effects (all P > 0.24).
Resting State HEP Analysis
The HEP was calculated for each of the 150 electrodes over-lying the scalp area. EEG signals were first re-referenced to the common average of these scalp channels. The HEP was subsequently obtained by averaging the EEG segments from −300 to 600 ms relative to the ECG R-wave peaks and then subtracting the mean over a 200-ms baseline (−300 to −100 ms), a period free from Q-wave and R-wave contamination.
A major factor that impedes the study of the HEP is its small amplitude, usually comparable to the background noise level. This poor signal-to-noise ratio on one hand renders detection of peak amplitude or latency, a common procedure in ERP research, rather imprecise, and on the other hand reduces the power of massive univariate testing involving the full spatiotemporal data matrix, especially for between-subjects comparisons where temporal jitters can be large. Indeed, in previous reports on the HEP, individual peak amplitude/latency detection has hardly been conducted, and between-group comparison was often done by first averaging the amplitude within arbitrary time windows selected to increase the signal-to-noise ratio.42,44–46,52 However, while the time window each study chose generally fell somewhere between 200–600 ms relative to the R-wave peak, the exact latency ranges over which the amplitude was averaged (and the corresponding topographical distribution) were not consistent across studies. A less arbitrary approach to make the choice of time window is to obtain a data-driven window from within-subjects comparison between two different conditions of interest.48,51 We here followed this time window selection approach since it is not only less arbitrary but also more physiologically motivated.
It has been known that the spontaneous activation patterns of the human brain are markedly different in the EC and EO resting states.73–76 The EC resting state has in particular been characterized as an “interoceptive state” and EO as an “exteroceptive state,”73,76 based on the finding that multiple sensory regions show activation during EC, whereas the attention and oculomotor systems are activated in EO. Since the HEP represents an electrophysiological marker of sensory processing, and since the signals originated from the attention and oculomotor systems during EO are likely to interfere with the HEP, we expected to see a larger amplitude HEP component in EC.
As explained in the Introduction section, the increased sensory processing during the EC period may be of particular relevance to the pathophysiology of ID and contribute to larger group differences, given that EC is the state wherein natural transition from wake to sleep takes place. A cluster-based non-parametric permutation test77 as implemented in FieldTrip78 was carried out to test the hypothesis that the HEP waveforms are more pronounced during EC, and to identify the time windows of interest for subsequent between-group analysis. Briefly, point-wise within-subjects t-statistic (EC vs. EO) was first computed at each electrode at each timeframe between 200–600 ms relative to the R-wave peak. The t-values above 1.998 or below −1.998 (thresholds corresponding to a two-tailed uncorrected significance level of P = 0.05 with 63 degrees of freedom) for at least 4 neighboring electrodes at each timeframe were then clustered according to spatiotemporal adjacency. Any resulting spatiotemporal cluster was deemed significant if the cluster mass (sum of t-values within the cluster) was above the 97.5 percentile or below the 2.5 percentile of a null randomization distribution, constructed by Monte Carlo simulation with 10,000 iterations,79 of the maximum cluster mass. The stringent criteria ensured that reasonably focal time windows of interest would be chosen.
Using this procedure, a single time window of interest was identified, spanning 376–500 ms relative to the R-wave peak and covering 2 supra-threshold spatiotemporal clusters, as detailed in the Results section. The mean HEP amplitude at each scalp channel over this time window was then submitted to the following between-group analysis. As the time window lies in the latency range of the late positive component (LPC) in the ERP literature, for brevity we hereafter refer to the frontal or parieto-occipital HEP within this time window as the “late HEP component” throughout the manuscript. We do not however imply that the functional role of the observed component is similar to that of the LPC.
Between-Group Statistical Analysis
Group differences in HEP amplitude between ID and CTRL were again assessed with cluster-based non-parametric permutation tests.77 The procedure was similar to the between-condition comparison above, but was done with respect to the mean HEP amplitude over the 376–500 ms time window rather than the full spatiotemporal data matrix.
To assess the main effect of ID, between-subjects t-statistic (ID vs. CTRL) was first evaluated for the mean HEP amplitude at each electrode, averaged over EC and EO. The t-values above 1.999 or below −1.999 (thresholds corresponding to a two-tailed uncorrected significance level of P = 0.05 with 62 degrees of freedom) were then clustered according to spatial adjacency, and the cluster mass was calculated for each spatial cluster by summing all supra-threshold t-values within it. The same procedure was repeated 10,000 times with the individuals' ID versus CTRL group membership labels randomly shuffled, to construct a null randomization distribution of the maximum cluster mass. A P value was obtained by comparing the real observed cluster masses against this null distribution. Note that this non-parametric method corrects for multiple comparisons since the null distribution was constructed using only the maximum cluster-level statistic in each iteration.77
To assess the group-by-condition interaction, we submitted the differences in mean HEP amplitude between conditions (EC − EO) to the same between-subjects cluster-based permutation procedure. Post-hoc tests were also conducted, by submitting the mean HEP amplitude during EC and EO to the between-subjects permutation procedure separately.
Exploratory Correlation Analyses
Exploratory correlation analyses were carried out to investigate the association between the mean amplitude of the late frontal HEP component, a consistent finding by the current and several previous studies,42,44–46,49 and overall insomnia severity as measured by ISI, as well as the associations between the mean frontal HEP late-component amplitude and different subjective sleep complaint as evaluated by the distinct ISI-items.
For all 64 participants, mean HEP amplitude within the 376–500 ms time window was averaged across 42 frontal and prefrontal electrodes where the HEP component was the most prominent, and then the Pearson correlation coefficients between the ISI-item scores and the average amplitude were calculated for both EC and EO conditions. Note that we did not include only the electrodes that showed significant group differences revealed by previous between-group analysis when calculating the average amplitude, so as to avoid finding spurious results due to circular inferences.80
Source Reconstruction of Between-Group HEP Differences
We investigated the cortical sources that underlie the observed group differences using the linearly constrained minimum variance (LCMV) beamforming method81 implemented in FieldTrip. A template head model contained in FieldTrip, constructed from the Montreal Neurological Institute (MNI) standard single-subject structural image (colin27), was used to compute the forward solution. Electrode positions were determined by applying an affine transformation to the template Geodesic Sensor Net coordinates based on four fiducial points: nasion, vertex, and left and right pre-auricular points. The LCMV beamforming algorithm estimates, for every source location, the time courses of electrical dipole strength along three orientations. To simplify later comparison, we computed (for each participant) the neural activity index (NAI)81 over the 376–500 ms time window of interest, a single score summarizing the source activity within this time period, at each source point on a three dimensional regular grid with 5 mm resolution. Subsequently, the NAIs were linearly interpolated to 1 mm3 voxels. The source reconstruction procedure was carried out for the EC condition, where statistically signifi-cant group differences were confirmed in scalp-level between-group analysis (for details see the Results section below). We visualized between-group differences in source activity by plotting the largest t-statistics comparing the log-transformed NAIs of the two groups, after applying a gray matter mask.
RESULTS
HEP Time Courses and Topographies
The grand average HEP time courses of all participants at each scalp electrode before and after ICA-based artifact removal71 are depicted in Figures S1 and S2 in the supplemental material, respectively. The associated topographic snapshots at every 100 ms for each group and condition are shown in Figures S3 and S4. Cardiac field artifacts are prominent and overwhelm cortical HEP components at all electrode sites before ICA-based artifact removal. The HEP waveforms after ICA correction appear similar to those observed in previous studies,42,45–47,49 although remnant cardiac field artifacts including the QRS-complex and T-wave are visible, especially at parietal and occipital regions. In the post T-wave time window (350–600 ms) where cardiac field artifacts have been previously shown to be minimal,43,72 a positive component at frontal and prefrontal electrodes with higher amplitude during EC (larger in people with ID), and a positive component at parietal and occipital electrodes with higher amplitude during EO (larger in controls) can be observed.
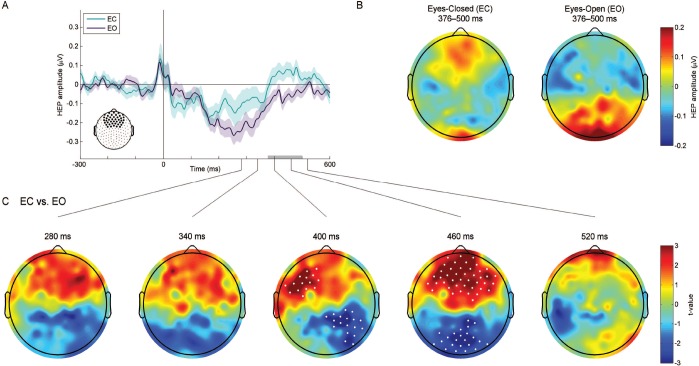
Within-subjects comparison confirmed these observed differences between the two resting-state conditions. A cluster-based permutation test revealed two spatiotemporal clusters indicating significant differences between EC and EO (Figure 1): a spatiotemporal cluster spanning 376–500 ms after the R-wave peak indicative of more positive late frontal activation during EC than during EO (P < 0.004 corrected for multiple comparisons), and a spatiotemporal cluster spanning almost the same time window (364–500 ms) indicative of more positive late parieto-occipital activation during EO than during EC (P < 0.008 corrected for multiple comparisons). Based on these results, we identified the latency range of 376–500 ms after the R-wave peak as the time window of interest and conducted the following between-group analysis on the mean HEP amplitude over this time window.
Figure 1.
Comparison of the heartbeat-evoked potential (HEP) between eyes-closed (EC) and eyes-open (EO) resting states. Data from all 64 participants are pooled. (A) Frontal HEP waveforms during EC and EO resting states. The average HEP time courses over the 42 frontal and prefrontal electrodes (large black dots) are depicted. Shaded areas indicate one standard error of the mean (SEM). The gray bar highlights the time window exhibiting significant differences between EC and EO (376–500 ms), as evaluated by cluster-based permutation testing. (B) Topographic maps of the mean HEP amplitude over the 376–500 ms time window during EC and EO. (C) Topographic maps of within-subjects t-statistics (EC vs. EO) at 5 different timeframes within the 400 ± 120 ms time range. Cluster-based permutation testing revealed two spatiotemporal clusters indicative of significant differences between EC and EO (white dots): A spatiotemporal cluster at the frontal region (EC > EO, P < 0.004 corrected for multiple comparisons) and a spatiotemporal cluster (EO > EC, P < 0.008 corrected for multiple comparisons) at the parieto-occipital region.
Between-Group HEP Differences
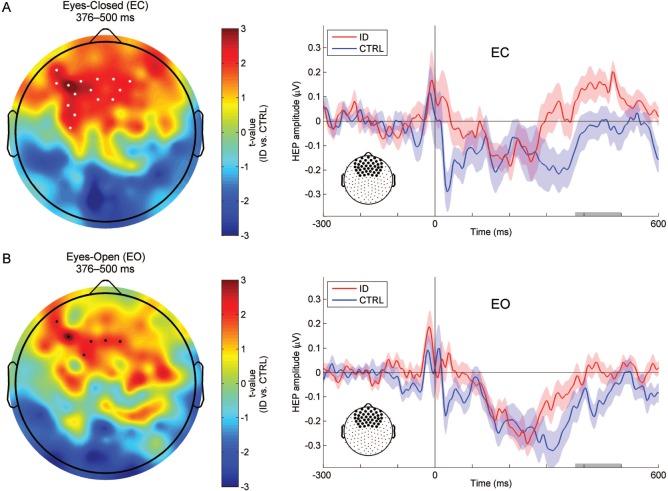
To investigate whether people with ID differed from controls with respect to the amplitude of the identified late frontal or parieto-occipital HEP component, we compared the groups using a cluster-based permutation test on the mean HEP amplitude within the 376–500 ms time window at each scalp electrode, averaged over the two resting-state conditions. A spatial cluster was found at frontal electrodes, indicating a significantly larger amplitude late frontal HEP component in ID as compared to CTRL (P < 0.02 corrected for multiple comparisons). Next, to investigate the group-by-condition interaction effect, we submitted the differences in mean HEP amplitude between conditions (EC − EO) to the same between-subjects permutation procedure. No significant interaction was found after correction for multiple comparisons. Nevertheless, in order to explore which resting-state condition best revealed the group differences, we conducted post-hoc tests by submitting the mean HEP amplitude for EC and EO to the between-subjects permutation procedure separately (Figure 2). During EC, ID had significantly larger late-component amplitude in a spatial cluster of frontal electrodes as compared to CTRL (P < 0.02 corrected for multiple comparisons). Similar differences in an overlapping spatial cluster were observed during EO, but did not survive correction for multiple comparisons.
Figure 2.
Comparison of the mean heartbeat-evoked potential (HEP) amplitude over the 376–500 ms time window between people with Insomnia Disorder (ID) and controls (CTRL), and waveforms illustrating the frontal dynamics of the HEP in the two groups. (A) Topographic map of between-subjects t-statistics (ID vs. CTRL) and frontal HEP waveforms of the two groups during the eyes-closed (EC) resting state. Significant group differences within the 376–500 ms time window as evaluated by cluster-based permutation testing are observed at a frontal spatial cluster (white dots, ID > CTRL, P < 0.02 corrected for multiple comparisons). (B) Topographic map of between-subjects t-statistics (ID vs. CTRL) and frontal HEP waveforms of the two groups during the eyes-open (EO) resting state. A supra-threshold spatial cluster (black dots, ID > CTRL, uncorrected P < 0.05) is observed at the frontal region but does not survive cluster-based correction for multiple comparisons. In the waveform plots, the average HEP time courses over the 42 frontal and prefrontal electrodes are depicted to allow for comparison with Figure 1A. The average amplitude over this predefined region was also used for exploratory correlation analyses (see text), to avoid circular inferences. Shaded areas indicate one standard error of the mean (SEM). Gray bars highlight the 376–500 ms time window of interest.
As the t-statistic maps suggest leftward lateralized between-group differences, separate repeated-measures ANOVAs were performed to test this asymmetry. However, no significant hemisphere main effect or group-by-hemisphere interaction was found in either resting-state condition (all P > 0.62).
Associations between HEP Amplitude and Sleep Complaint
The overall ISI exhibited a marginally significant correlation with the average amplitude of the late frontal HEP component during EC (r(62) = 0.24, P = 0.06). The weak correlation may be owing to the fact that insomnia is a heterogeneous disorder, and while the ISI is an overall score that weighs different facets of the disorder equally, the HEP amplitude might be differentially associated with these facets. We thus evaluated whether the mean frontal HEP late-component amplitude correlated with specific sleep complaint, as represented by scores on individual ISI-items. The average amplitude of the late frontal HEP component during EC correlated significantly with the score on the second ISI-item, Difficulty Maintaining Sleep (r(62) = 0.32, P < 0.01), and marginally significantly with the score on the fourth ISI-item, Dissatisfaction with Current Sleep Pattern (r(62) = 0.23, P = 0.06), but with none of the other items (0.11 < P < 0.30). The average amplitude of the late frontal component during EO did not correlate significantly with the overall ISI or any of the ISI-item scores (0.12 < P < 0.80).
Source Localization of Between-Group HEP Differences
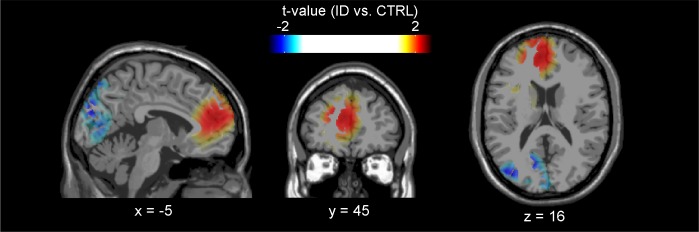
We quantified source activity during the 376–500 ms time window in the EC condition by means of the neural activity index estimated with the LCMV beamforming algorithm.81 The largest t-statistics (ID vs. CTRL) formed clusters in several cortical areas, showing spatial patterns paralleling previous neuroimaging or source localization results.46,54,82 Increased source activity in people suffering from ID compared to CTRL during the 376–500 ms time window was observed at bilateral anterior cingulate and medial frontal cortices (peak t-value = 2.21; Figure 3), and with less spatial extent at the right lateral parietal cortex (peak t-value = 2.86; not shown due to slice selection). In addition, we also observed decreased source activity at the left occipital region in people suffering from ID compared to CTRL (peak t-value = −2.31; Figure 3).
Figure 3.
Localization of between-group differences in source activity over the 376–500 ms time window after the ECG R-wave during the eyes-closed resting state. The t-statistics comparing the log-transformed neural activity indices (NAIs) between people with Insomnia Disorder (ID) and controls (CTRL) are displayed on top of the Montreal Neurological Institute (MNI) standard single-subject structural image, in accordance with the neurological convention (left is left). Increased source activity in people with ID is especially pronounced at bilateral anterior cingulate and medial frontal cortices. Decreased source activity in people with ID is observed at the left occipital cortex.
DISCUSSION
The current study is, to our knowledge, the first to quantify a neural correlate of interoceptive sensitivity in people suffering from Insomnia Disorder and compare it with healthy controls without sleep complaints. We assessed the amplitude of the resting-state heartbeat-evoked potential, a measure previously shown to reflect individual differences in interoceptive sensitivity without being confounded by active attentional manipulation.52 Our results show that during the wakeful resting state, people suffering from ID have a larger amplitude late HEP component at frontal electrodes. These findings suggest that ID is characterized by altered cerebral responses to afferent interoceptive signals, which could involve excessive cortical processing, deficient inhibition, or deficient adaptation. Specifically, while participants were not instructed to explicitly focus on the heartbeats, it is likely that the observed group differences can be partially attributed to unconscious attentional bias in ID towards sleep-related body sensations which has been posited to contribute to the persistence of insomnia.83 The differences in HEP amplitude between ID and CTRL are especially prominent during the eyes-closed condition. These results complement previous exteroceptive ERP findings by now demonstrating that people suffering from ID have altered brain responses not only to external stimuli, but also to internal ones.
Psychiatric conditions that are often comorbid with ID and known to influence the HEP, such as depression,48 are excluded through careful selection of the participants.61,62 Moreover, previous work has shown that interoceptive sensitivity and the HEP amplitude are actually decreased in depressed patients.48 Therefore, our findings cannot easily be attributed to unnoticed subclinical depressive symptomatology in people suffering from ID.
We addressed possible group differences owing to cardiovascular artifacts in EEG associated with different heart rates between the two groups with careful preprocessing of data, including ICA-based artifact removal. Furthermore, the pulse wave and cardiac field artifacts have been shown to be minimal within the time window of the late HEP component,43,53,72 and the topographical distribution of group differences exhibits distinct spatial patterns from those typically observed for pulse wave and cardiac field artifacts, suggesting the findings cannot be explained by differences in these cardiovascular artifacts. Possible contributions of age and sex differences were also minimized by matching. Additionally, it was verified that the two groups did not significantly differ with respect to the time of recording in terms of absolute clock time (P = 0.12). The time of recording relative to individual habitual bedtime showed a trend of group difference (P = 0.09), due to the fact that people suffering from ID tended to go to bed earlier. However, an ancillary analysis of covariance (ANCOVA) on the mean frontal HEP late-component amplitude that included age, sex, recording time, recording time relative to habitual bedtime, and heart rate as covariates ruled out that effects were secondary to possible confounding by these variables (all P > 0.30) and confirmed the finding of altered HEP amplitude in ID (P < 0.03 for the group main effect either with or without covariate adjustment).
A frontal positive component within similar time windows has been reported repeatedly in previous studies on the HEP during the resting state,52 during sleep,49 and during interoceptive or exteroceptive tasks,42,44–46 supporting the idea that it reflects the ongoing CNS processing of afferent cardiovascular information, even when one does not focus attention on the heartbeats.84 In the current study, within-subjects comparison revealed that the frontal positive HEP component is more prominent during the EC resting state than during EO. This result is consistent with previous characterization of EC as an “interoceptive state” and EO as an “exteroceptive state.”73,76 Notably, during the EO resting state, we find positive activity in the parieto-occipital region, a topographical distribution also observed in a previous study43 where the participants were instructed to fixate on the presented visual stimuli. We thus reason that the parieto-occipital positivity may represent an interaction between interoceptive processing and visual attention. That the amplitude of the late parieto-occipital component appears smaller in people suffering from ID (Figure S4) indicates such interaction might also be altered in ID, although the difference is not statistically significant. The interaction between interoceptive and exteroceptive processing has recently been proposed as a mechanism underlying the generation of perceptual experience.85 While future research is necessary to further investigate this hypothesis, our results suggest that measuring the HEP across conditions might provide a sensitive method to assess the interoception-exteroception interaction.
Source reconstruction suggests increased neural activity time-locked to heartbeats in bilateral anterior cingulate/me-dial frontal and the right lateral parietal cortices, as well as decreased activity in the left occipital cortex, in people suffering from ID. A similar activation pattern has been previously found in a neuroimaging study in which healthy participants performed a heartbeat discrimination task,54 supporting the idea that people suffering from ID may exhibit attentional bias towards interoceptive stimuli, especially during the pre-sleep period with eyes closed. Nevertheless, we note that the source reconstruction results should be interpreted with caution, due to their limited spatial resolution. Below, we briefly review previous neuroimaging findings on interoception and insomnia, aiming at further elucidating the possible links between the two.
The major brain structures mediating interoceptive information processing include the anterior cingulate, insular, and orbitofrontal cortices.29,30,54 The findings of the current study thus suggest that ID may involve changes in these brain structures, which is consistent with evidence from previous structural and functional neuroimaging studies. Specifically, we have previously shown that gray matter (GM) volume in part of the orbito-frontal cortex is reduced in people suffering from ID.86 Several other studies support possible involvement of reduced orbito-frontal GM in insomnia,87,88 and in the vulnerability to early morning awakening89,90 and sleep fragmentation,91 although one study could not find such association.92 In addition to these suggestions of deficient orbitofrontal processing, increased anterior cingulate cortex volume and insular coactivation with salience network activity have been reported in ID.87,93 In sum, there is a body of converging evidence suggesting ID is associated with structural and functional changes in the brain circuits involved in interoception. Hypersensitivity to interoceptive signals, as indexed by the increased amplitude of the late frontal HEP component, may reflect these changes and potentially contribute to the persistent complaints of people suffering from ID. The link between resting-state HEP amplitude and structural alterations is corroborated by a recent study,52 which reported positive correlations between the average late HEP amplitude during EC and GM volumes in the anterior cingulate and anterior insular cortices in a sample of patients with borderline personality disorder and healthy controls. Future research is needed to evaluate whether such association can be replicated in people suffering from ID, and whether previously reported high scores of people with insomnia disorder or symptoms on questionnaires about subjective body sensations36,38 are associated with the increased HEP amplitude we find in the present study.
The anterior cingulate, insular, and orbitofrontal cortices constitute the so-called “salience network.”94–97 This network, especially the anterior insular cortex, is hypothesized to integrate interoceptive and exteroceptive information, to detect salient sensory signals for additional higher-order processing, and to control the switching between activation of the default-mode and central-executive networks.97–100 In short, the salience network implements a mechanism by which irrelevant signals can be filtered out, allowing salient information (arising from the body or the environment) to access attentional or working memory resources.97 Malfunction of the salience network, which results in “noisy” afferent input, has been proposed as one important factor underlying anxiety symptoms and disorders,26,97 based on evidence that people with such symptoms or disorders also exhibit increased interoceptive sensitivity.27,54,101 Interestingly, not only are there many personality traits and symptoms commonly shared by people suffering from ID or anxiety disorders,62,102–104 but neuroimaging findings have also implicated aberrant activation of salience network-related structures in both types of disorders.93,105–107 These associations motivate us to propose that the pathophysiology of ID is mediated by similar salience network malfunctioning. Failure of the salience network to inhibit non-salient information processing and modulate the default-mode and central-executive networks in people suffering from ID can explain deficits in sensory gating of interoceptive and exteroceptive signals, as well as other dimensions of ID including excessive worry and thought intrusion at bedtime,108–110 and deficits in various cognitive domains (e.g., working memory and vigilance) that are not attributable to sleep deprivation.111
The symptomatology of ID is usually interpreted within the framework of physiological and cortical hyperarousal.8,103,112 Physiological hyperarousal refers to the elevated sympathetic tone often observed with cardiac, neuroendocrine, and metabolic measures in people suffering from ID.113,114 Cortical hyperarousal refers to enhanced information processing and cognitive activities, particularly at bedtime, as for instance reflected by increased high-frequency EEG power.25,115 Within this context, interoception can be regarded as the link between these two components of hyperarousal. As have been put forward by many, the “somatic marker” hypothesis,33 and its refined versions,29–31,34,35 hold that afferent interoceptive signals, by allowing representation of the internal body state within the CNS, provide essential feedback for proper physiological homeostatic control, and that such representation in turn sets the foundation for subjective sensory experience and shapes affective, emotional, and cognitive processes and behavior. It is thus not surprising that in people suffering from ID, autonomic dysregulation (physiological hyperarousal) is often accompanied by altered patterns in interoceptive and exteroceptive sensations, as well as abnormalities in the affective and cognitive domains (cortical hyperarousal). However, as most of the studies on ID to date have been cross-sectional or retrospective, a causal relationship between physiological and cortical hyperarousal has not yet been established. One possibility is that the heightened sympathetic tone is driven by altered body sensation feedback, while it is also possible that attentive processing of external and internal stimuli increases as a response to autonomic dysregulation. Resolving the causal relationship between physiological and cortical hyperarousal will be the key to better understanding the etiology of ID.
A limitation of the current study is the fact that the EO and EC resting-state conditions were not counterbalanced. Our findings may not be generalized to the transition from EC to EO. However, in spite of limitations on generalizability we believe that the EO to EC transition is most relevant, since it is the normal course in preparing for sleep. Future research is needed to clarify whether reverse differences are observable during the EC to EO transition that is representative for getting up after a period of sleep.
In conclusion, the current findings support increased interoceptive sensitivity in ID, as indexed by the amplitude of the late frontal HEP component. Integration of these findings with previous reports on ID suggests malfunction of the salience network as a neurobiological substrate of relevance to the pathophysiology of insomnia. HEP assessment provides a paradigm of value to bridge research on the pathophysiology of insomnia and interoception, both regarded of key importance to mood disorders.4,26,28
DISCLOSURE STATEMENT
This was not an industry supported study. This study was performed at the Netherlands Institute for Neuroscience (NIN), Meibergdreef 47, Amsterdam 1105 BA. Research leading to these results has received funding from the Bial Foundation grant 252/12, the Netherlands Organization of Scientific Research (NWO) grant VICI-453.07.001, and the European Research Council Advanced Grant 671084 INSOMNIA. This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Yvon Sweere for assisting in recruiting and interviewing the participants, and the collective efforts of many people who helped with data acquisition and assessment: Frank van Schalkwijk, Rick Wassing, Floor van Oosterhout, Jessica Bruyel, Marije Vermeulen, Kim Dekker, Arjan Miedema, Katerina Nikolakopoulou, Katerina Georgopoulou, Michelle de Haan, Bahar Adibi, Lina Vandermeulen, Josien Visser, Verena Sommer, Oti Kamal, Inger van Steenoven, Brit Giesbertz, Vincent Huson.
ABBREVIATIONS
- ANCOVA
analysis of covariance
- ANOVA
analysis of variance
- BAI
Beck Anxiety Inventory
- BDI-IA
(revised) Beck Depression Inventory
- CNS
central nervous system
- CTRL
controls
- EC
eyes-closed
- ECG
electrocardiogram
- EEG
electroencephalography
- EO
eyes-open
- ERP
event-related potential
- GM
gray matter
- HADS
Hospital Anxiety and Depression Scale
- HD-EEG
high-density electroencephalography
- HEP
heartbeat-evoked potential
- ICA
independent component analysis
- ID
Insomnia Disorder
- ISI
Insomnia Severity Index
- LCMV
linearly constrained minimum variance
- LPC
late positive component
- MNI
Montreal Neurological Institute
- NAI
neural activity index
- SD
standard deviation
- SEM
standard error of the mean
REFERENCES
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Publishing; 2013. [Google Scholar]
- 2.Bassetti CL, Ferini-Strambi L, Brown S, et al. Neurology and psychiatry: waking up to opportunities of sleep. State of the art and clinical/research priorities for the next decade. Eur J Neurol. 2015;22:1337–54. doi: 10.1111/ene.12781. [DOI] [PubMed] [Google Scholar]
- 3.Wittchen HU, Jacobi F, Rehm J, et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21:655–79. doi: 10.1016/j.euroneuro.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 4.Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J. Affect. Disord. 2011;135:10–9. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Laugsand LE, Vatten LJ, Platou C, Janszky I. Insomnia and the risk of acute myocardial infarction: a population study. Circulation. 2011;124:2073–81. doi: 10.1161/CIRCULATIONAHA.111.025858. [DOI] [PubMed] [Google Scholar]
- 6.Laugsand LE, Strand LB, Platou C, Vatten LJ, Janszky I. Insomnia and the risk of incident heart failure: a population study. Eur Heart J. 2014;35:1382–93. doi: 10.1093/eurheartj/eht019. [DOI] [PubMed] [Google Scholar]
- 7.Perlis ML, Giles DE, Mendelson WB, Bootzin RR, Wyatt JK. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. J Sleep Res. 1997;6:179–88. doi: 10.1046/j.1365-2869.1997.00045.x. [DOI] [PubMed] [Google Scholar]
- 8.Riemann D, Spiegelhalder K, Feige B, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14:19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Bastien CH. Insomnia: neurophysiological and neuropsychological approaches. Neuropsychol Rev. 2011;21:22–40. doi: 10.1007/s11065-011-9160-3. [DOI] [PubMed] [Google Scholar]
- 10.Engel-Yeger B, Shochat T. The relationship between sensory processing patterns and sleep quality in healthy adults. Can J Occup Ther. 2012;79:134–41. doi: 10.2182/cjot.2012.79.3.2. [DOI] [PubMed] [Google Scholar]
- 11.Devoto A, Violani C, Lucidi F, Lombardo C. P300 amplitude in subjects with primary insomnia is modulated by their sleep quality. J Psychosom Res. 2003;54:3–10. doi: 10.1016/s0022-3999(02)00579-2. [DOI] [PubMed] [Google Scholar]
- 12.Devoto A, Manganelli S, Lucidi F, Lombardo C, Russo PM, Violani C. Quality of sleep and P300 amplitude in primary insomnia: a preliminary study. Sleep. 2005;28:859–63. doi: 10.1093/sleep/28.7.859. [DOI] [PubMed] [Google Scholar]
- 13.Sforza E, Haba-Rubio J. Event-related potentials in patients with insomnia and sleep-related breathing disorders: evening-to-morning changes. Sleep. 2006;29:805–13. doi: 10.1093/sleep/29.6.805. [DOI] [PubMed] [Google Scholar]
- 14.Yang C-M, Lo H-S. ERP evidence of enhanced excitatory and reduced inhibitory processes of auditory stimuli during sleep in patients with primary insomnia. Sleep. 2007;30:585–92. doi: 10.1093/sleep/30.5.585. [DOI] [PubMed] [Google Scholar]
- 15.Bastien CH, St-Jean G, Morin CM, Turcotte I, Carrier J. Chronic psychophysiological insomnia: hyperarousal and/or inhibition deficits? An ERPs investigation. Sleep. 2008;31:887–98. doi: 10.1093/sleep/31.6.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turcotte I, Bastien CH. Is quality of sleep related to the N1 and P2 ERPs in chronic psychophysiological insomnia sufferers? Int J Psychophysiol. 2009;72:314–22. doi: 10.1016/j.ijpsycho.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Hairston IS, Talbot LS, Eidelman P, Gruber J, Harvey AG. Sensory gating in primary insomnia. Eur J Neurosci. 2010;31:2112–21. doi: 10.1111/j.1460-9568.2010.07237.x. [DOI] [PubMed] [Google Scholar]
- 18.Turcotte I, St-Jean G, Bastien CH. Are individuals with paradoxical insomnia more hyperaroused than individuals with psychophysiological insomnia? Event-related potentials measures at the peri-onset of sleep. Int J Psychophysiol. 2011;81:177–90. doi: 10.1016/j.ijpsycho.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Kertesz RS, Cote KA. Event-related potentials during the transition to sleep for individuals with sleep-onset insomnia. Behav Sleep Med. 2011;9:68–85. doi: 10.1080/15402002.2011.557989. [DOI] [PubMed] [Google Scholar]
- 20.Bastien CH, Turcotte I, St-Jean G, Morin CM, Carrier J. Information processing varies between insomnia types: measures of N1 and P2 during the night. Behav Sleep Med. 2013;11:56–72. doi: 10.1080/15402002.2012.660896. [DOI] [PubMed] [Google Scholar]
- 21.Cortoos A, De Valck E, Pattyn N, Mairesse O, Cluydts R. Excitatory versus inhibitory impairments in insomnia patients: an ERP study. Int J Psychophysiol. 2014;93:62–9. doi: 10.1016/j.ijpsycho.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Huang Z, Zhan S, Li N, Ding Y, Wang Y. Abnormal recovery function of somatosensory evoked potentials in patients with primary insomnia. Psychiatry Res. 2012;198:463–7. doi: 10.1016/j.psychres.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 23.van der Werf YD, Altena E, van Dijk KD, et al. Is disturbed intracortical excitability a stable trait of chronic insomnia? A study using transcranial magnetic stimulation before and after multimodal sleep therapy. Biol Psychiatry. 2010;68:950–5. doi: 10.1016/j.biopsych.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 24.Espie CA. Insomnia: conceptual issues in the development, persistence, and treatment of sleep disorder in adults. Annu Rev Psychol. 2002;53:215–43. doi: 10.1146/annurev.psych.53.100901.135243. [DOI] [PubMed] [Google Scholar]
- 25.Colombo MA, Ramautar JR, Wei Y, et al. Wake high-density electroencephalographic spatiospectral signatures of insomnia. Sleep. 2016;39:1015–27. doi: 10.5665/sleep.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paulus MP, Stein MB. Interoception in anxiety and depression. Brain Struct Funct. 2010;214:451–63. doi: 10.1007/s00429-010-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Domschke K, Stevens S, Pfleiderer B, Gerlach AL. Interoceptive sensitivity in anxiety and anxiety disorders: an overview and integration of neurobiological findings. Clin Psychol Rev. 2010;30:1–11. doi: 10.1016/j.cpr.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Harshaw C. Interoceptive dysfunction: toward an integrated framework for understanding somatic and affective disturbance in depression. Psychol Bull. 2015;141:311–63. doi: 10.1037/a0038101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 30.Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–5. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 31.Herbert BM, Pollatos O. The body in the mind: on the relationship between interoception and embodiment. Top Cogn Sci. 2012;4:692–704. doi: 10.1111/j.1756-8765.2012.01189.x. [DOI] [PubMed] [Google Scholar]
- 32.Wiens S. Interoception in emotional experience. Curr Opin Neurol. 2005;18:442–7. doi: 10.1097/01.wco.0000168079.92106.99. [DOI] [PubMed] [Google Scholar]
- 33.Damasio AR. New York, NY: Harcourt Brace; 1999. The Feeling of What Happens: Body and Emotion in the Making of Consciousness. [Google Scholar]
- 34.Critchley HD, Eccles J, Garfinkel SN. Interaction between cognition, emotion, and the autonomic nervous system. Handb Clin Neurol. 2013;117:59–77. doi: 10.1016/B978-0-444-53491-0.00006-7. [DOI] [PubMed] [Google Scholar]
- 35.Critchley HD, Harrison NA. Visceral influences on brain and behavior. Neuron. 2013;77:624–38. doi: 10.1016/j.neuron.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 36.Hammad MA, Barsky AJ, Regestein QR. Correlation between somatic sensation inventory scores and hyperarousal scale scores. Psychosomatics. 2001;42:29–34. doi: 10.1176/appi.psy.42.1.29. [DOI] [PubMed] [Google Scholar]
- 37.Barsky AJ, Wyshak G, Klerman GL. Hypochondriasis. An evaluation of the DSM-III criteria in medical outpatients. Arch Gen Psychiatry. 1986;43:493–500. doi: 10.1001/archpsyc.1986.01800050099013. [DOI] [PubMed] [Google Scholar]
- 38.Jansson M, Linton SJ. Psychological mechanisms in the maintenance of insomnia: arousal, distress, and sleep-related beliefs. Behav Res Ther. 2007;45:511–21. doi: 10.1016/j.brat.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 39.Main CJ. The Modified Somatic Perception Questionnaire (MSPQ) J Psychosom Res. 1983;27:503–14. doi: 10.1016/0022-3999(83)90040-5. [DOI] [PubMed] [Google Scholar]
- 40.Schandry R, Sparrer B, Weitkunat R. From the heart to the brain: a study of heartbeat contingent scalp potentials. Int J Neurosci. 1986;30:261–75. doi: 10.3109/00207458608985677. [DOI] [PubMed] [Google Scholar]
- 41.Katkin ES, Cestaro VL, Weitkunat R. Individual differences in cortical evoked potentials as a function of heartbeat detection ability. Int J Neurosci. 1991;61:269–76. doi: 10.3109/00207459108990745. [DOI] [PubMed] [Google Scholar]
- 42.Montoya P, Schandry R, Müller A. Heartbeat evoked potentials (HEP): topography and influence of cardiac awareness and focus of attention. Electroencephalogr Clin Neurophysiol. 1993;88:163–72. doi: 10.1016/0168-5597(93)90001-6. [DOI] [PubMed] [Google Scholar]
- 43.Dirlich G, Dietl T, Vogl L, Strian F. Topography and morphology of heart action-related EEG potentials. Electroencephalogr Clin Neurophysiol. 1998;108:299–305. doi: 10.1016/s0168-5597(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 44.Leopold C, Schandry R. The heartbeat-evoked brain potential in patients suffering from diabetic neuropathy and in healthy control persons. Clin Neurophysiol. 2001;112:674–82. doi: 10.1016/s1388-2457(01)00480-1. [DOI] [PubMed] [Google Scholar]
- 45.Pollatos O, Schandry R. Accuracy of heartbeat perception is reflected in the amplitude of the heartbeat-evoked brain potential. Psychophysiology. 2004;41:476–82. doi: 10.1111/1469-8986.2004.00170.x. [DOI] [PubMed] [Google Scholar]
- 46.Pollatos O, Kirsch W, Schandry R. Brain structures involved in interoceptive awareness and cardioafferent signal processing: a dipole source localization study. Hum Brain Mapp. 2005;26:54–64. doi: 10.1002/hbm.20121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shao S, Shen K, Wilder-Smith EP V, Li X. Effect of pain perception on the heartbeat evoked potential. Clin Neurophysiol. 2011;122:1838–45. doi: 10.1016/j.clinph.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 48.Terhaar J, Viola FC, Bär KJ, Debener S. Heartbeat evoked potentials mirror altered body perception in depressed patients. Clin Neurophysiol. 2012;123:1950–7. doi: 10.1016/j.clinph.2012.02.086. [DOI] [PubMed] [Google Scholar]
- 49.Lechinger J, Heib DPJ, Gruber W, Schabus M, Klimesch W. Heartbeat-related EEG amplitude and phase modulations from wakefulness to deep sleep: interactions with sleep spindles and slow oscillations. Psychophysiology. 2015;52:1441–50. doi: 10.1111/psyp.12508. [DOI] [PubMed] [Google Scholar]
- 50.Canales-Johnson A, Silva C, Huepe D, et al. Auditory feedback differentially modulates behavioral and neural markers of objective and subjective performance when tapping to your heartbeat. Cereb Cortex. 2015;25:4490–503. doi: 10.1093/cercor/bhv076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luft CDB, Bhattacharya J. Aroused with heart: modulation of heartbeat evoked potential by arousal induction and its oscillatory correlates. Sci Rep. 2015;5:15717. doi: 10.1038/srep15717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Müller LE, Schulz A, Andermann M, et al. Cortical representation of afferent bodily signals in borderline personality disorder. Neural correlates and relationship to emotional dysregulation. JAMA Psychiatry. 2015;72:1077–86. doi: 10.1001/jamapsychiatry.2015.1252. [DOI] [PubMed] [Google Scholar]
- 53.Kern M, Aertsen A, Schulze-Bonhage A, Ball T. Heart cycle-related effects on event-related potentials, spectral power changes, and connectivity patterns in the human ECoG. Neuroimage. 2013;81:178–90. doi: 10.1016/j.neuroimage.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 54.Critchley HD, Wiens S, Rotshtein P, Öhman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 55.Benjamins J, Migliorati F, Dekker K, et al. The Sleep Registry. An international online survey and cognitive test assessment tool and database for multivariate sleep and insomnia phenotyping. Sleep Med. 2013;14:e293–4. doi: 10.1016/j.sleep.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 56.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 57.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–7. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 58.Beck AT, Steer RA. San Antonio, TX: Psychological Corporation; 1993. Manual for the Beck Depression Inventory. [Google Scholar]
- 59.Smarr KL, Keefer AL. Measures of depression and depressive symptoms. Arthritis Care Res. 2011;63:S454–66. doi: 10.1002/acr.20556. [DOI] [PubMed] [Google Scholar]
- 60.Julian LJ. Measures of anxiety. Arthritis Care Res. 2011;63:S467–72. doi: 10.1002/acr.20561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carney CE, Ulmer C, Edinger JD, Krystal AD, Knauss F. Assessing depression symptoms in those with insomnia: an examination of the Beck Depression Inventory second edition (BDI-II) J Psychiatr Res. 2009;43:576–82. doi: 10.1016/j.jpsychires.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carney CE, Moss TG, Harris AL, Edinger JD, Krystal AD. Should we be anxious when assessing anxiety using the Beck Anxiety Inventory in clinical insomnia patients? J Psychiatr Res. 2011;45:1243–9. doi: 10.1016/j.jpsychires.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Edinger JD, Bonnet MH, Bootzin RR, et al. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine work group. Sleep. 2004;27:1567–96. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 64.Bastien CH, Vallières A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 65.Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601–8. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kligfield P, Gettes LS, Bailey JJ, et al. Recommendations for the standardization and interpretation of the electrocardiogram. Part I: the electrocardiogram and its technology. J Am Coll Cardiol. 2007;49:1109–27. doi: 10.1016/j.jacc.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 67.Pan J, Tompkins WJ. A real-time QRS detection algorithm. IEEE Trans Biomed Eng. 1985;32:230–6. doi: 10.1109/TBME.1985.325532. [DOI] [PubMed] [Google Scholar]
- 68.Katkovnik V, Egiazarian K, Astola J. Bellingham, WA: SPIE Press; 2006. Local Approximation Techniques in Signal and Image Processing. [DOI] [PubMed] [Google Scholar]
- 69.Widmann A, Schröger E. Filter effects and filter artifacts in the analysis of electrophysiological data. Front Psychol. 2012;3:233. doi: 10.3389/fpsyg.2012.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Iglewicz B, Hoaglin DC. Milwaukee, WI: ASQC Quality Press; 1993. How to Detect and Handle Outliers. [Google Scholar]
- 71.Jung TP, Makeig S, Humphries C, et al. Removing electroencephalographic artifacts by blind source separation. Psychophysiology. 2000;37:163–78. [PubMed] [Google Scholar]
- 72.Dirlich G, Vogl L, Plaschke M, Strian F. Cardiac field effects on the EEG. Electroencephalogr Clin Neurophysiol. 1997;102:307–15. doi: 10.1016/s0013-4694(96)96506-2. [DOI] [PubMed] [Google Scholar]
- 73.Marx E, Stephan T, Nolte A, et al. Eye closure in darkness animates sensory systems. Neuroimage. 2003;19:924–34. doi: 10.1016/s1053-8119(03)00150-2. [DOI] [PubMed] [Google Scholar]
- 74.McAvoy M, Larson-Prior L, Nolan TS, Vaishnavi SN, Raichle ME, D'Avossa G. Resting states affect spontaneous BOLD oscillations in sensory and paralimbic cortex. J Neurophysiol. 2008;100:922–31. doi: 10.1152/jn.90426.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jao T, Vértes PE, Alexander-Bloch AF, et al. Volitional eyes opening perturbs brain dynamics and functional connectivity regardless of light input. Neuroimage. 2013;69:21–34. doi: 10.1016/j.neuroimage.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu P, Huang R, Wang J, et al. Different topological organization of human brain functional networks with eyes open versus eyes closed. Neuroimage. 2014;90:246–55. doi: 10.1016/j.neuroimage.2013.12.060. [DOI] [PubMed] [Google Scholar]
- 77.Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164:177–90. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 78.Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011;2011:156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maris E. Randomization tests for ERP topographies and whole spatiotemporal data matrices. Psychophysiology. 2004;41:142–51. doi: 10.1111/j.1469-8986.2003.00139.x. [DOI] [PubMed] [Google Scholar]
- 80.Kriegeskorte N, Simmons WK, Bellgowan PSF, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12:535–40. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Van Veen BD, van Drongelen W, Yuchtman M, Suzuki A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. Biomed Eng IEEE Trans. 1997;44:867–80. doi: 10.1109/10.623056. [DOI] [PubMed] [Google Scholar]
- 82.Park H-D, Correia S, Ducorps A, Tallon-Baudry C. Spontaneous fluctuations in neural responses to heartbeats predict visual detection. Nat Neurosci. 2014;17:612–8. doi: 10.1038/nn.3671. [DOI] [PubMed] [Google Scholar]
- 83.Harvey AG. A cognitive model of insomnia. Behav Res Ther. 2002;40:869–93. doi: 10.1016/s0005-7967(01)00061-4. [DOI] [PubMed] [Google Scholar]
- 84.Schandry R, Montoya P. Event-related brain potentials and the processing of cardiac activity. Biol Psychol. 1996;42:75–85. doi: 10.1016/0301-0511(95)05147-3. [DOI] [PubMed] [Google Scholar]
- 85.Park H-D, Tallon-Baudry C. The neural subjective frame: from bodily signals to perceptual consciousness. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130208. doi: 10.1098/rstb.2013.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Altena E, Vrenken H, van der Werf YD, van den Heuvel OA, Van Someren EJW. Reduced orbitofrontal and parietal gray matter in chronic insomnia: a voxel-based morphometric study. Biol Psychiatry. 2010;67:182–5. doi: 10.1016/j.biopsych.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 87.Winkelman JW, Plante DT, Schoerning L, et al. Increased rostral anterior cingulate cortex volume in chronic primary insomnia. Sleep. 2013;36:991–8. doi: 10.5665/sleep.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Joo EY, Noh HJ, Kim J-S, et al. Brain gray matter deficits in patients with chronic primary insomnia. Sleep. 2013;36:999–1007. doi: 10.5665/sleep.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stoffers D, Moens S, Benjamins J, et al. Orbitofrontal gray matter relates to early morning awakening: a neural correlate of insomnia complaints? Front Neurol. 2012;3:105. doi: 10.3389/fneur.2012.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Weber M, Webb CA, Deldonno SR, et al. Habitual “sleep credit” is associated with greater grey matter volume of the medial prefrontal cortex, higher emotional intelligence and better mental health. J Sleep Res. 2013;22:527–34. doi: 10.1111/jsr.12056. [DOI] [PubMed] [Google Scholar]
- 91.Lim ASP, Fleischman DA, Dawe RJ, et al. Regional neocortical gray matter structure and sleep fragmentation in older adults. Sleep. 2015;39:227–35. doi: 10.5665/sleep.5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Spiegelhalder K, Regen W, Baglioni C, et al. Insomnia does not appear to be associated with substantial structural brain changes. Sleep. 2013;36:731–7. doi: 10.5665/sleep.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen MC, Chang C, Glover GH, Gotlib IH. Increased insula coactivation with salience networks in insomnia. Biol Psychol. 2014;97:1–8. doi: 10.1016/j.biopsycho.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Downar J, Crawley AP, Mikulis DJ, Davis KD. A cortical network sensitive to stimulus salience in a neutral behavioral context across multiple sensory modalities. J Neurophysiol. 2002;87:615–20. doi: 10.1152/jn.00636.2001. [DOI] [PubMed] [Google Scholar]
- 95.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Taylor KS, Seminowicz DA, Davis KD. Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp. 2009;30:2731–45. doi: 10.1002/hbm.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105:12569–74. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Craig AD. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 100.Simmons WK, Avery JA, Barcalow JC, Bodurka J, Drevets WC, Bellgowan P. Keeping the body in mind: insula functional organization and functional connectivity integrate interoceptive, exteroceptive, and emotional awareness. Hum Brain Mapp. 2013;34:2944–58. doi: 10.1002/hbm.22113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pollatos O, Traut-Mattausch E, Schroeder H, Schandry R. Interoceptive awareness mediates the relationship between anxiety and the intensity of unpleasant feelings. J Anxiety Disord. 2007;21:931–43. doi: 10.1016/j.janxdis.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 102.LeBlanc M, Beaulieu-Bonneau S, Mérette C, Savard J, Ivers H, Morin CM. Psychological and health-related quality of life factors associated with insomnia in a population-based sample. J Psychosom Res. 2007;63:157–66. doi: 10.1016/j.jpsychores.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 103.Harvey AG, Tang NKY. (Mis)perception of sleep in insomnia: a puzzle and a resolution. Psychol Bull. 2012;138:77–101. doi: 10.1037/a0025730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Calkins AW, Hearon BA, Capozzoli MC, Otto MW. Psychosocial predictors of sleep dysfunction: the role of anxiety sensitivity, dysfunctional beliefs, and neuroticism. Behav Sleep Med. 2013;11:133–43. doi: 10.1080/15402002.2011.643968. [DOI] [PubMed] [Google Scholar]
- 105.Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161:2126–9. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]
- 106.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Damsa C, Kosel M, Moussally J. Current status of brain imaging in anxiety disorders. Curr Opin Psychiatry. 2009;22:96–110. doi: 10.1097/YCO.0b013e328319bd10. [DOI] [PubMed] [Google Scholar]
- 108.Wicklow A, Espie CA. Intrusive thoughts and their relationship to actigraphic measurement of sleep: towards a cognitive model of insomnia. Behav Res Ther. 2000;38:679–93. doi: 10.1016/s0005-7967(99)00136-9. [DOI] [PubMed] [Google Scholar]
- 109.Fichten CS, Libman E, Creti L, et al. Role of thoughts during nocturnal awake times in the insomnia experience of older adults. Cognit Ther Res. 2001;25:665–92. [Google Scholar]
- 110.Harvey AG. Trouble in bed: the role of pre-sleep worry and intrusions in the maintenance of insomnia. J Cogn Psychother. 2002;16:161–77. [Google Scholar]
- 111.Shekleton JA, Rogers NL, Rajaratnam SMW. Searching for the daytime impairments of primary insomnia. Sleep Med Rev. 2010;14:47–60. doi: 10.1016/j.smrv.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 112.Bonnet MH, Arand DL. Hyperarousal and insomnia. Sleep Med Rev. 1997;1:97–108. doi: 10.1016/s1087-0792(97)90012-5. [DOI] [PubMed] [Google Scholar]
- 113.Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14:9–15. doi: 10.1016/j.smrv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 114.Spiegelhalder K, Riemann D. Hyperarousal and insomnia. Sleep Med Clin. 2013;8:299–307. [Google Scholar]
- 115.Perlis ML, Merica H, Smith MT, Giles DE. Beta EEG activity and insomnia. Sleep Med Rev. 2001;5:363–74. doi: 10.1053/smrv.2001.0151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.