Abstract
Study Objectives:
A number of subjective and objective studies provide compelling evidence of chronic post-concussion changes in sleep, yet very little is known about the acute effects of concussion on sleep quality and quantity. Therefore, the purpose of this prospective pilot study was to use actigraphy to examine the changes in sleep quality and quantity acutely following concussion at home rather than in a hospital or sleep laboratory.
Methods:
Seventeen young adults (7 with acute concussion, 10 controls) were recruited for this study. All participants completed two 5-day testing sessions separated by 30 days from intake (controls) or day of injury (concussion). Participants wore actigraphs and kept a sleep journal. Sleep parameter outcomes included nighttime total sleep time (nTST), 24-h total sleep time (TST), wake after sleep onset (WASO), and sleep efficiency (SE). The coefficient of variation (CV) for each sleep parameter was computed for each session.
Results:
nTST and TST CV was significantly greater in the concussion group. There is the additional indication that individuals with a concussion may require and obtain more sleep shortly after injury and subsequently have a shorter duration of sleep at 1 mo post-injury. This pattern was not seen in the measures of sleep quality (WASO, SE).
Conclusions:
Individuals with a concussion demonstrated increased nighttime sleep duration variability. This increase persisted at 1 mo post-injury and may be associated with previously documented self-reports of poor sleep quality lasting months and years after a concussion. Additionally, this increase may predispose individuals to numerous negative health outcomes if left untreated.
Citation:
Raikes AC, Schaefer SY. Sleep quantity and quality during acute concussion: a pilot study. SLEEP 2016;39(12):2141–2147.
Keywords: actigraph, concussion, sleep duration, sleep quality
Significance.
This is one of the first prospective studies of the effect of concussions on sleep in the acute stage with at-home actigraphic monitoring. Furthermore, this is one of the first to identify increased variability in sleep duration as an acute consequence of concussion. Further research is needed to identify whether objective sleep parameters (duration, quality) differ between individuals with and without a concussion and to identify the mechanisms by which sleep-wake disturbances occurs with concussion.
INTRODUCTION
Sleep is essential to normal day-to-day functioning. Deficits in both sleep quality and quantity due to sleep-wake disturbances can impair cognitive, psychological, and physical function.1–4 Although a number of conditions or situations can disrupt sleep, sleep-wake disturbances have been reported following traumatic brain injury (TBI) regardless of diagnosed injury severity (mild to severe)5–8 and age.5,7,9,10 Even in cases of mild TBI, 30% to 80% of individuals with a diagnosis of concussion are estimated to have sleep-wake disturbances including insomnia,11–16 hypersomnia,17,18 and pleiosomnia.19–21 Consequently, sleep-wake disturbances have been added to recent concussion diagnosis and management position statements as a symptom domain.22–24
In addition, individuals often experience cognitive, psychological, and/or physical symptoms immediately following concussion and during the acute (< 2 w post-injury) stage.25–29 For example, individuals often experience impaired memory,27,28 postural instability,30,31 and headaches,32 among other symptoms. If they also experience sleep-wake disturbances, the associated deficits in sleep quality and quantity could significantly exacerbate symptoms and delay recovery. A number of subjective6,9–11,13,15,16,33,34 and objective studies6,19–21,35–39 provide compelling evidence of chronic post-concussion changes in sleep, yet very little is known about the acute effects of concussion on sleep quality and quantity. Moreover, preliminary findings in the acute stage have been conflicting.5,8,40 One study demonstrated no differences between individuals with a concussion and control patients in total sleep time (TST), sleep efficiency (SE), or wake after sleep onset (WASO),40 whereas another study showed significantly lower SE and WASO compared with normative data.5 Further, significant differences in daytime and 24-h TST between individuals with varying severities of TBI have been reported, with individuals with both moderate and severe TBI getting more daytime and total sleep than individuals with mild TBI.8 Thus, more research in how sleep quality and quantity change early after concussion is needed.
Despite the observed chronic effects of concussions on sleep, most of these studies have been retrospective. There have been very few prospective studies examining the relationship between concussion and sleep,5,8,19,20,38 even fewer using individuals with acute concussion,5,8 and fewer still objectively monitoring sleep at home rather than during a period of hospitalization. When done, such at-home monitoring is commonly accomplished through actigraphy, which utilizes activity counts from wrist-worn accelerometers to distinguish periods of sleep from wake. This is a valid and reliable method when compared with polysomnography (the gold standard for studying sleep), and has the advantage of allowing easier multiple-night monitoring to determine patterns in sleep and wake.41,42 Although several studies have used actigraphy to examine sleep-wake disturbances in individuals with concussion symptoms lasting more than 3 mo,9,10,19–21,43 only one has investigated the acute phase.5 In that study, average sleep quality over 1 w in individuals with mild TBI was poorer compared to reported normative data. In addition, higher quantities of sleep were seen in severe TBI cases, compared to mild cases, and both tended to decrease over the course of the week. In this earlier study, participants were tested on-site at their hospitalization location.5
Given the prevalence of poor sleep outcomes chronically following TBI (including concussion),6,9–16,19–21,34,36–39,44 the limited number of prospective studies in the acute phase, and few published studies on sleep in the acute phase of concussion, additional research is warranted to more completely understand the effect of concussions on sleep. Therefore, the purpose of this prospective pilot study was to use actigraphy to measure acute post-concussion changes in sleep quality and quantity at home rather than in a hospital or sleep laboratory.
METHODS
Participants
Seventeen young adults (7 with acute concussion, 10 controls) were recruited for this study. Concussions were diagnosed by a team physician or athletic trainer for Utah State University according to the Zurich guidelines.22 Under these guidelines, a concussion is suspected following a direct (head, face, neck) or indirect (elsewhere on the body, with impulse transferred to the head) force and (1) one or more symptoms, including somatic symptoms (e.g., headache, photosensitivity or phono-sensitivity), cognitive symptoms (e.g., fogginess), and changes in emotional lability; (2) physical signs, including loss of consciousness or amnesia; (3) behavioral changes, including irritability or depression; (4) cognitive impairment, including impaired memory or attention, or increased reaction time; and (5) sleep disturbance, including insomnia.22
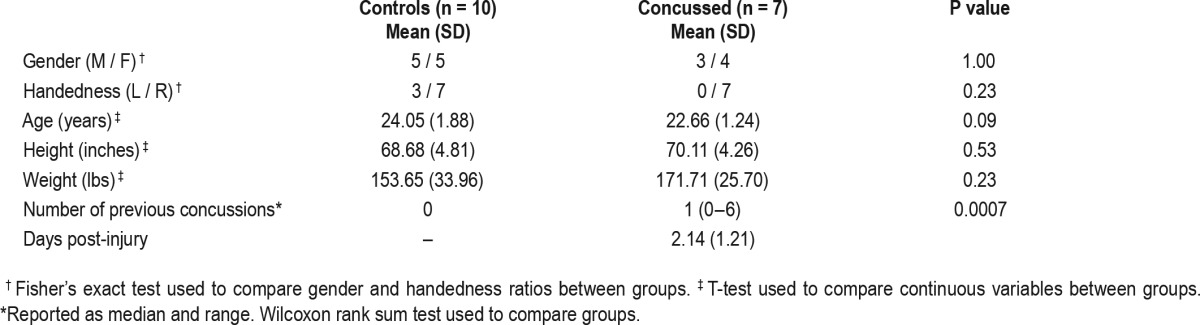
Participants were enrolled within the first 4 days following injury. Participants with a concussion were excluded if they reported having crossed more than two time zones in the 2 mo prior to participation; a self-reported history of chronic pain; and/or a history of diagnosed depression, anxiety, sleep disorder, or other psychiatric condition. Age-matched controls were recruited through Utah State University's Research Participation portal. Exclusion criteria for the control participants was the same as for participants with concussion and also included any self-reported history of physician-diagnosed concussion or more serious traumatic brain injury. The Institutional Review Board at Utah State University approved all procedures and all participants provided written consent prior to participation. Table 1 contains demographic comparisons between the participant groups.
Table 1.
Demographic characteristics of participants.
Materials
Sleep-related data were collected using an actigraph (wActiSleep-BT Monitor, ActiGraph LLC, Pensacola, FL). This actigraph is a wrist-worn triaxial accelerometer specifically designed to discriminate between sleep and wake periods. Previous validation studies of actigraphy have demonstrated high accuracy (> 80%) and sensitivity (> 90%) in determining sleep and wake cycles compared to polysomnography in healthy and sleep-disordered patients.41,42,45–47 With this device, data can be collected continuously for up to 25 days on a single battery charge and then stored on an internal 2 GB hard drive. Data were sampled continuously at 30 Hz, 24 h per day for this study.
Procedures
Testing consisted of two 5-day testing sessions that were 30 days apart. For control subjects, session 2 began 30 days after the first day of session 1; for subjects with a concussion, session 2 began 30 days after the date of injury. Participants wore the actigraph continuously on the dominant wrist for the entire testing period, except when bathing or showering. Additionally, participants maintained a sleep log of bed and wake times. For purposes of this study, bedtime was defined as the time when the participant laid down with the intent to sleep, including naps. Wake time was defined as the time when the participant rose without intention to return to sleep.
Following each testing period, data were uploaded via USB cable and imported into the ActiLife v6.0 software (ActiGraph LLC). Sleep log data were used to align the actigraphic data with self-reported bedtimes and wake times. Data were then automatically scored in the software using the Cole-Kripke algorithm.45 The following sleep parameters were extracted to assess sleep quality (sleep onset latency; WASO) and quantity (TST; SE). WASO was the amount of time in minutes spent awake after sleep onset but before waking time. Total nighttime sleep time (nTST) was the amount of time in minutes spent sleeping between bed and wake time. SE was the percentage of time in bed spent sleeping:
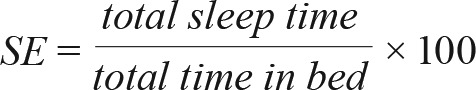 |
Twenty-four-hour TST was defined as nTST plus the duration of daytime naps in minutes. Daytime naps were defined as any short period of sleep initiated after the morning wake time and prior to 22:00 without restriction on duration. These periods were identified from the sleep logs and these periods were manually added to the automatic scoring. Data from the 5 nights were then averaged per person per session for each parameter.
In addition to these parameters we also explored day-to-day variation in sleep duration. To test this, the coefficient of variation (CV) for each individual was calculated as:
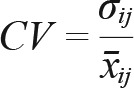 |
where σij is the standard deviation for the ith participant in session j and x̄ij is the mean value for the ith participant in session j. In this way, intraindividual variability is standardized and can be compared between individuals. Conventionally, individuals with a low CV value exhibit low variability around their mean.
Statistical Analyses
For all statistical tests, the a priori significance level was set at P ≤ 0.05. All plots were constructed in R using the ggplot2 package.48 Group differences (control vs. concussion) in height, weight, and age were analyzed with two-sample t-tests and by sex with a chi-square test in R (Table 1). Each of the 5-day averaged sleep variables were initially analyzed with two-way group (control vs. concussion) × session (time 1 vs. time 2) repeated-measures analyses of variance (ANOVAs) in SAS software v9.4 (SAS Institute Inc., Cary, NC, USA). However, the small and unequal sample sizes resulted in large deviations from normality and significant heteroscedasticity. Due to the small sample size, particularly for the concussion group, data transformations were not successful in reducing the nonnormality or heteroscedasticity. Additionally, we computed twoway group × session repeated-measure ANOVAs for the CV values. These values also exhibited significant nonnormality and heteroscedasticity for CVWASO and CVSE.
RESULTS
The groups did not differ from each other in height, weight, or age (Table 1). One follow-up session from the control group was removed from analyses due to equipment failure. Of 33 total recorded 5-day sessions, 27 sessions (controls: n = 15; concussion: n = 12) included both weeknights (Monday night through Friday night) and at least 1 weekend night (Saturday or Sunday night). Additionally, while participants with a concussion were restricted from sport participation for part of, or all, of the initial monitoring session per return-to-play protocols, all participants reported continuing to engage in academic, team-related (meetings, travel), and religious activities. Control participants were unrestricted from school, work, sport, or social activities.
Group data per session are presented in Table 2. With respect to 5-day averaged sleep outcomes (nTST, TST, WASO, SE), the variance in the concussion group was greater than that of the control group (Figure 1A–1D). Asterisks in these figures indicate single outlier values. Therefore, these data did not meet the assumptions for a repeated-measures ANOVA. However, the data shown in Figure 1A suggest that individuals with a concussion had the same duration of nighttime sleep (nTST) shortly after injury (session 1), but then slept less on average at 1-mo follow-up (session 2). Additionally, individuals with a concussion may get more total sleep (TST, Figure 1D) on average shortly after injury as compared to controls, again sleeping less on average at 1-mo follow-up. However, WASO and SE do not appear to differ between the two groups (Figure 1B and 1C).
Table 2.
Sleep parameter and coefficient of variation values by group and session.
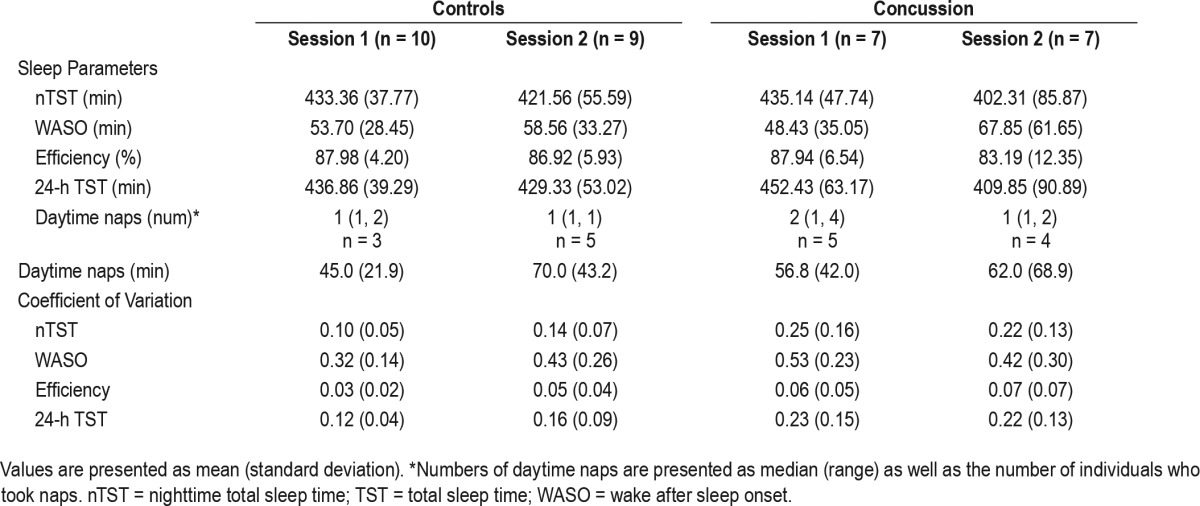
Figure 1.
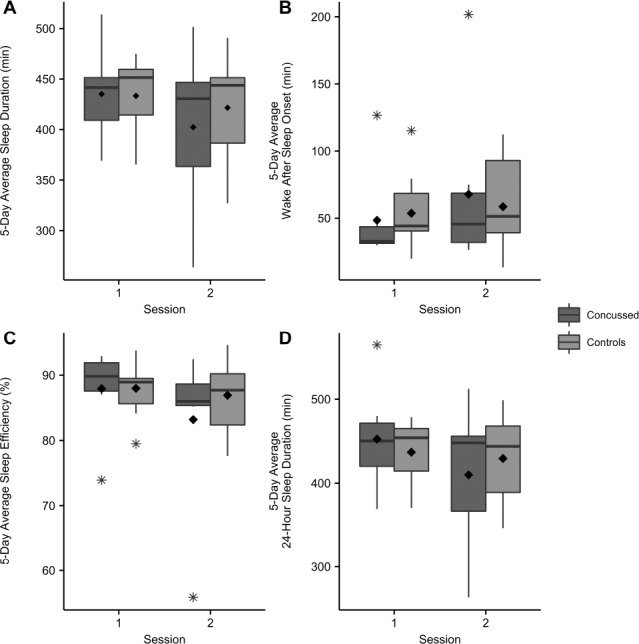
A. 5-day average sleep duration (min) per group per session. B. 5-day average wake after sleep onset (min) per group per session. C. 5-day average sleep efficiency (%) per group per session. D. 5-day average 24-hour total sleep (min) per group per session. Boxplots show medians and whiskers represent 1.5× the interquartile range. No statistically significant differences were observed. Outliers indicated with asterisk. Group means indicated with diamond symbol.
Individuals in the concussion group did exhibit significantly greater day-to-day variability in total nighttime sleep compared to the control participants (Figure 2), measured as CV for nTST (Figure 3A; F1,15 = 5.07, P = 0.04). Additionally, the concussion group exhibited a trend toward increased day-to-day 24-h total sleep variability (Figure 3D; F1,15 = 3.63, P = 0.08). Although the CV for WASO and SE did not meet the assumptions for parametric analyses, individuals in the concussion group tended to also be more variable in these measures shortly after injury (WASO; Figure 3B) or overall (SE; Figure 3C).
Figure 2.
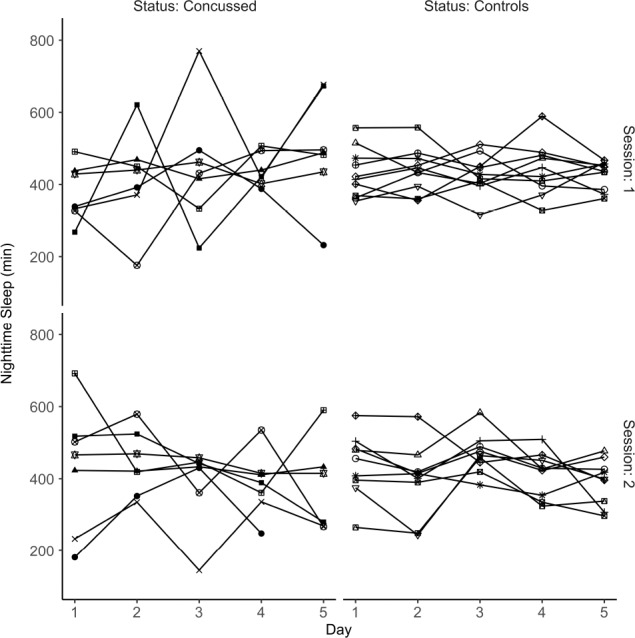
Nighttime sleep duration in minutes. Individual participants are differentiated by shape. Individuals in the concussion group demonstrated increased night-to-night variability in sleep duration.
Figure 3.
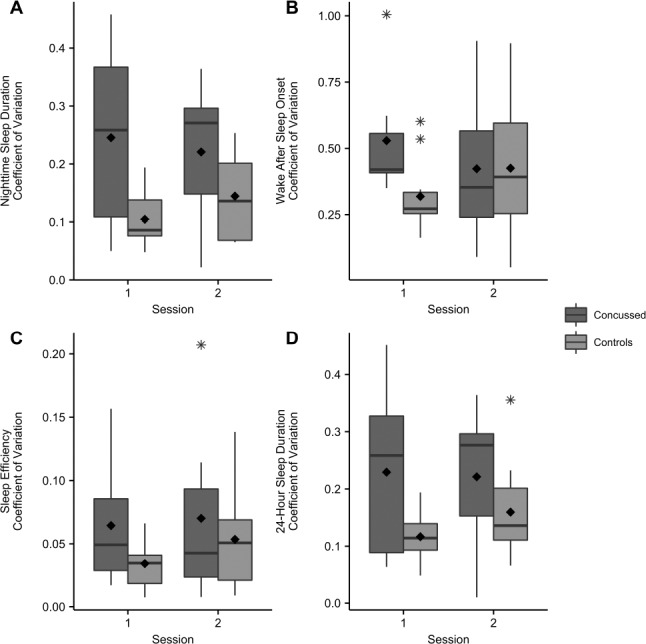
A. Coefficient of variation for nighttime sleep duration per group per session. B. Coefficient of variation for wake after sleep onset per group per session. C. Coefficient of variation for average sleep efficiency (%) per group per session. D. Coefficient of variation for 24-hour total sleep (min) per group per session. Boxplots show medians and whiskers represent 1.5× the interquartile range. Statistically significant differences were observed between groups for nighttime sleep duration (P = 0.03). Twenty-four-hour total sleep duration coefficient of variation demonstrated a trend toward greater variability in the concussed group (P = 0.08). Outliers indicated with asterisks. Group means indicated with diamond symbol.
DISCUSSION
The purpose of this prospective pilot study was to measure sleep quantity and quality in individuals with a concussion shortly after injury and at 1 mo post-injury, relative to that of a control group of individuals without a concussion. We observed more day-to-day variability in nighttime and total sleep quantity (duration in minutes) in the concussion group as compared to the control group. We were unable to detect differences between the two groups for 5-day average nighttime sleep duration, WASO, or SE, as well as 5-day average total sleep duration. However, the nighttime and total sleep duration data suggest that there may be a trend toward shorter sleep duration by 1 mo post-injury in the concussion group.
Broadly speaking, these findings in individuals with acute concussion are consistent with previous work in individuals with a concussion who have chronic sleep complaints. For example, several studies have identified no significant differences in sleep duration, WASO, or SE based on polysomnography or other objective measures, despite participants' perceptions of poor sleep quality6,9,10,13,40 or reports of insomnia.11,15,16,33,34,39 In many of these studies, participants were first observed/interviewed months or even years after the date of head injury rather than days or weeks after (i.e., in a more acute stage). Our findings for average 24-h sleep duration suggest that shortly after injury, individuals may demonstrate an increased need for sleep. However, by the time individuals are 1 mo post-injury, the average sleep duration was lower than that in controls, albeit not significantly in this small sample.
Additionally, we observed that individuals with a concussion in our sample experienced greater day-to-day variability in nighttime sleep, which may have substantial negative health consequences. Even in typical adults without a concussion, research has linked such variability to poor subjective sleep quality and lower subjective well-being,49,50 weight gain,51 depressive symptoms,52,53 and stress.54 In older adults, increased sleep duration variability has been associated with higher rates of diabetes,55,56 cardiovascular disease,56 and obesity56; increased inflammatory markers (interleukin-6, tumor necrosis factor-α)57; poorer dietary habits58; and antidepressant use.56 In adolescents, increased caloric consumption,59 obesity,60 alterations in normal brain development on diffusion tensor imaging,61 lower heart rate variability,62 negative cognitive and academic outcomes,63,64 and increases in anxiety, fatigue, and depression65 have been linked to increased sleep duration variability. There is, therefore, substantive evidence indicating a myriad of negative physical, psychological, and emotional outcomes related to increased sleep duration variability. Moreover, perceptions or self-reports of poor sleep quality are known to negatively affect concussion-sensitive neurocognitive and postural test outcomes.44,66,67 Interestingly, previous studies suggest that subjective reports of poor sleep quality may be related more to increased variability in sleep duration, rather than true sleep quality, over several days,49,50 which may explain the prevalence of self-reported poor sleep quality following concussion.6,34,44,66,67 For example, a concussed individual may sleep well but very little one night, then sleep a lot the next night to “catch up”, and report poor sleep quality despite having high quality as measured by actigraphy or polysomnography. Although we did not gather participants' self-reported sleep quality, we did observe greater sleep duration variability shortly after injury and at 1 mo after the injury, providing insight into why perceptions of poor sleep quality are linked to poor performance on neurocognitive or postural tasks post-concussion.44,66,67 If the increase in duration variability persists beyond this time frame, as suggested by those studies citing chronically poor sleep after concussion,5,6,8–10,13–16,34,37 then the individual who had a concussion is at increased risk for long-term negative outcomes across multiple cognitive and physiological systems. Therefore, sleep- and health-related outcomes following concussion may be improved by teaching and encouraging consistency in sleep habits (consistent bedtimes and wake times) both prior to and after a concussion event.
The mechanisms of sleep-wake disturbances following concussion are not clearly understood. Proposed explanations include disruption of the overall brain function68 and deficiencies in sleep-wake regulating neurotransmitters.19,69 In particular, hypocretin-I, a wake-promoting neurotransmitter, has been observed to be lower in the cerebrospinal fluid immediately after TBI, particularly moderate and severe TBI.19,69 Additionally, this diminished level persists in individuals with excessive daytime sleepiness across the spectrum of severity, and may contribute to altered sleeping.19 Furthermore, examination of the hypothalamus examination at autopsy has shown fewer hypocretin and wake-promoting histaminergic neurons in individuals with severe TBI relative to no TBI.70,71 However, these observations have not been confirmed in concussions or mild TBI.72 Despite the lack of clarity, it is likely that there are multiple interrelationships between the proposed mechanisms. Further research is needed to more completely explain not only the individual roles of these factors but their interrelationships as related to sleep and overall outcomes following concussion.
The results of this study, previous reports of both no changes in sleep duration, SE, or WASO40 and decreased SE and WASO5 during the acute phase following a concussion, as well as the varied range of sleep-wake disturbances (insomnia,11–16 hypersomnia,17,18 pleiosomnia19,20) chronically following concussion, suggest that there are multiple post-concussion trajectories that sleep-wake disturbances may take. These outcomes, like those related to cognitive function, are likely related not only to the concussion mechanism, but also personal, familial, and social factors.28,73,74 For this reason, there is the need for more comprehensive sleep evaluation after concussion, including self-reported nighttime sleep duration and daytime sleepiness coupled with actigraphy. The combination of these methods could provide a comprehensive view of the sleep-wake disturbances for the individual and result in a tailored treatment plan.
One major limitation in this study was the small sample size, particularly for the concussion group. However, given the limited number of prospective studies measuring quality and quantity of sleep in the acute stage following a concussion, this study contributes potentially valuable data on the patterns of change in sleep duration by 1 mo post-injury. Additionally, this study is among the first to report objective measures of sleep quantity and quality in the acute stage through at-home monitoring, rather than retrospective self-report or in-hospital monitoring, identifying increased day-to-day variability in sleep duration as a consequence of concussion.
Furthermore, we acknowledge that there are additional personal characteristics that may have influenced the sleep patterns of our participants, including menstrual cycles in female participants, as well as pain, anxiety, and mood disorders specifically resulting from the concussion. Given the small sample size and the pilot nature of this study, we were unable to control for these variables. However, these personal characteristics are important considerations and merit investigation in future studies with larger samples.
Finally, it is possible that the observed increase in sleep variability was the result of the participants' rearranging their schedules due to activity restriction (sport, work, school) after sustaining a concussion. However, all of our participants reported continuing to engage in academic and social activities with a concussion and had returned to full participation in school and sport by the 1-mo follow-up. Thus, the persistent increase in variability is better explained by the occurrence of the concussion than transient alterations in typical day-to-day activities. However, future studies should address the extent to which changes in daily activities influence, or are influenced by, changes in sleeping habits in the context of concussion.
In conclusion, individuals with a concussion in this prospective pilot study demonstrated increased day-to-day variability in nighttime sleep duration acutely following concussion. This increase persisted at 1 mo post-injury and may be associated with previously documented self-reports of poor sleep quality lasting months and years after a concussion. Additionally, this increase may predispose individuals to numerous negative health outcomes if left untreated. Although there was no statistically significant evidence of differences between individuals with a concussion and controls for average sleep duration, WASO, or SE, there is the limited suggestion that individuals with a concussion may be more likely to get less sleep on average at 1 mo post-injury without decreases in objective sleep quality (WASO and SE).
DISCLOSURE STATEMENT
This was not an industry supported study. This study was partially funded by the National Athletic Trainers' Association Research and Education Foundation. The authors have indicated no financial conflicts of interest. All research was conducted at Utah State University. This was not a clinical trial.
REFERENCES
- 1.Dijk D-J, Duffy JF, Czeisler CA. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J Sleep Res. 1992;1:112–7. doi: 10.1111/j.1365-2869.1992.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 2.Pilcher JJ, Walters AS. How sleep deprivation affects psychological variables related to college students' cognitive performance. J Am Coll Health. 1997;46:121–6. doi: 10.1080/07448489709595597. [DOI] [PubMed] [Google Scholar]
- 3.Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9:335–52. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 4.Reilly T, Edwards B. Altered sleep-wake cycles and physical performance in athletes. Physiol Behav. 2007;90:274–84. doi: 10.1016/j.physbeh.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 5.Chiu H-Y, Chen P-Y, Chen N-H, Chuang L-P, Tsai P-S. Trajectories of sleep changes during the acute phase of traumatic brain injury: a 7-day actigraphy study. J Formos Med Assoc. 2013;112:545–53. doi: 10.1016/j.jfma.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Gosselin N, Lassonde M, Petit D, et al. Sleep following sport-related concussions. Sleep Med. 2009;10:35–46. doi: 10.1016/j.sleep.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 7.Sumpter RE, Dorris L, Kelly T, McMillan TM. Pediatric sleep difficulties after moderate-severe traumatic brain injury. J Int Neuropsychol Soc. 2013;19:829–34. doi: 10.1017/S1355617713000465. [DOI] [PubMed] [Google Scholar]
- 8.Chiu H-Y, Lo W-C, Chiang Y-H, Tsai P-S. The effects of sleep on the relationship between brain injury severity and recovery of cognitive function: a prospective study. Int J Nurs Stud. 2014;51:892–9. doi: 10.1016/j.ijnurstu.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 9.Kaufman Y, Tzischinsky O, Epstein R, Etzioni A, Lavie P, Pillar G. Long-term sleep disturbances in adolescents after minor head injury. Pediatr Neurol. 2001;24:129–34. doi: 10.1016/s0887-8994(00)00254-x. [DOI] [PubMed] [Google Scholar]
- 10.Milroy G, Dorris L, McMillan T. Brief report: sleep disturbances following mild traumatic brain injury in childhood. J Pediatr Psychol. 2008;33:242–7. doi: 10.1093/jpepsy/jsm099. [DOI] [PubMed] [Google Scholar]
- 11.Fichtenberg NL, Zafonte RD, Putnam S, Mann NR, Millard AE. Insomnia in a post-acute brain injury sample. Brain Inj. 2002;16:197–206. doi: 10.1080/02699050110103940. [DOI] [PubMed] [Google Scholar]
- 12.Verma A, Anand V, Verma NP. Sleep disorders in chronic traumatic brain injury. J Clin Sleep Med. 2007;3:357–62. [PMC free article] [PubMed] [Google Scholar]
- 13.Ouellet M-C, Morin CM. Subjective and objective measures of insomnia in the context of traumatic brain injury: a preliminary study. Sleep Med. 2006;7:486–97. doi: 10.1016/j.sleep.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 14.Castriotta RJ, Wilde MC, Lai JM, Atanasov S, Masel BE, Kuna ST. Prevalence and consequences of sleep disorders in traumatic brain injury. J Clin Sleep Med. 2007;3:349–56. [PMC free article] [PubMed] [Google Scholar]
- 15.Kempf J, Werth E, Kaiser PR, Bassetti CL, Baumann CR. Sleep-wake disturbances 3 years after traumatic brain injury. J Neurol Neurosurg Psychiatry. 2010;81:1402–5. doi: 10.1136/jnnp.2009.201913. [DOI] [PubMed] [Google Scholar]
- 16.Ouellet M-C, Beaulieu-Bonneau S, Morin CM. Insomnia in patients with traumatic brain injury: frequency, characteristics, and risk factors. J Head Trauma Rehabil. 2006;21:199–212. doi: 10.1097/00001199-200605000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Billiard M, Podesta C. Recurrent hypersomnia following traumatic brain injury. Sleep Med. 2013;14:462–5. doi: 10.1016/j.sleep.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Watson NF, Dikmen S, Machamer J, Doherty M, Temkin N. Hypersomnia following traumatic brain injury. J Clin Sleep Med. 2007;3:363–8. [PMC free article] [PubMed] [Google Scholar]
- 19.Baumann CR, Werth E, Stocker R, Ludwig S, Bassetti CL. Sleep-wake disturbances 6 months after traumatic brain injury: a prospective study. Brain. 2007;130:1873–83. doi: 10.1093/brain/awm109. [DOI] [PubMed] [Google Scholar]
- 20.Imbach LL, Valko PO, Li T, Maric A, Symeonidou E-R, Stover JF, et al. Increased sleep need and daytime sleepiness 6 months after traumatic brain injury: a prospective controlled clinical trial. Brain. 2015;138:726–35. doi: 10.1093/brain/awu391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sommerauer M, Valko PO, Werth E, Baumann CR. Excessive sleep need following traumatic brain injury: a case-control study of 36 patients. J Sleep Res. 2013;22:634–9. doi: 10.1111/jsr.12068. [DOI] [PubMed] [Google Scholar]
- 22.McCrory P, Meeuwisse WH, Aubry M, Cantu RC, Dvořák J, Echemendia RJ, et al. Consensus statement on concussion in sport: the 4th international conference on concussion in sport held in Zurich, November 2012. Br J Sports Med. 2013;47:1–12. doi: 10.1136/bjsports-2013-092313. [DOI] [PubMed] [Google Scholar]
- 23.Harmon KG, Drezner JA, Gammons M, et al. American Medical Society for Sports Medicine position statement: concussion in sport. Br J Sports Med. 2013;47:15–26. doi: 10.1136/bjsports-2012-091941. [DOI] [PubMed] [Google Scholar]
- 24.Giza CC, Kutcher JS, Ashwal S, et al. Summary of evidence-based guideline update: evaluation and management of concussion in sports: report of the guideline development subcommittee of the American Academy of Neurology. Neurology. 2013;80:2250–7. doi: 10.1212/WNL.0b013e31828d57dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Max JE, Schachar RJ, Levin HS, et al. Predictors of attention-deficit/hyperactivity disorder within 6 months after pediatric traumatic brain injury. J Am Acad Child Adolesc Psychiatry. 2005;44:1032–40. doi: 10.1097/01.chi.0000173293.05817.b1. [DOI] [PubMed] [Google Scholar]
- 26.Kirkwood M, Janusz J, Yeates KO, et al. Prevalence and correlates of depressive symptoms following traumatic brain injuries in children. Child Neuropsychol. 2000;6:195–208. doi: 10.1076/chin.6.3.195.3157. [DOI] [PubMed] [Google Scholar]
- 27.McCrea M, Kelly JP, Randolph C, Cisler R, Berger L. Immediate neurocognitive effects of concussion. Neurosurgery. 2002;50:1032–42. doi: 10.1097/00006123-200205000-00017. [DOI] [PubMed] [Google Scholar]
- 28.McCrea M, Guskiewicz KM, Marshall SW, et al. Acute effects and recovery time following concussion in collegiate football players: the NCAA concussion study. J Am Med Assoc. 2003;290:2556–63. doi: 10.1001/jama.290.19.2556. [DOI] [PubMed] [Google Scholar]
- 29.Villemure R, Nolin P, Le Sage N. Self-reported symptoms during post-mild traumatic brain injury in acute phase: influence of interviewing method. Brain Inj. 2011;25:53–64. doi: 10.3109/02699052.2010.531881. [DOI] [PubMed] [Google Scholar]
- 30.Guskiewicz KM, Ross SE, Marshall SW. Postural stability and neuropsychological deficits after concussion in collegiate athletes. J Athl Train. 2001;36:263–73. [PMC free article] [PubMed] [Google Scholar]
- 31.Cavanaugh JT, Guskiewicz KM, Giuliani C, Marshall S, Mercer VS, Stergiou N. Detecting altered postural control after cerebral concussion in athletes with normal postural stability. Br J Sports Med. 2005;39:805–11. doi: 10.1136/bjsm.2004.015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faux S, Sheedy J. A prospective controlled study in the prevalence of posttraumatic headache following mild traumatic brain injury. Pain Med. 2008;9:1001–11. doi: 10.1111/j.1526-4637.2007.00404.x. [DOI] [PubMed] [Google Scholar]
- 33.Clinchot DM, Bogner J, Mysiw WJ, Fugate L, Corrigan J. Defining sleep disturbance after brain injury. Am J Phys Med Rehabil. 1998;77:291–5. doi: 10.1097/00002060-199807000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Chaput G, Giguère J-F, Chauny J-M, Denis R, Lavigne G. Relationship among subjective sleep complaints, headaches, and mood alterations following a mild traumatic brain injury. Sleep Med. 2009;10:713–6. doi: 10.1016/j.sleep.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 35.Mysliwiec V, McGraw L, Pierce R, Smith P, Trapp B, Roth BJ. Sleep disorders and associated medical comorbidities in active duty military personnel. Sleep. 2013;36:167–74. doi: 10.5665/sleep.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wallace D, Shafazand S, Ramos A, et al. Insomnia characteristics and clinical correlates in Operation Enduring Freedom/Operation Iraqi Freedom veterans with post-traumatic stress disorder and mild traumatic brain injury: an exploratory study. Sleep Med. 2011;12:850–9. doi: 10.1016/j.sleep.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Williams BR, Lazic SE, Ogilvie RD. Polysomnographic and quantitative EEG analysis of subjects with long-term insomnia complaints associated with mild traumatic brain injury. Clin Neurophysiol. 2008;119:429–38. doi: 10.1016/j.clinph.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Imbach LL, Büchele F, Valko PO, Li T, Maric A, Stover JF, et al. Sleep-wake disorders persist 18 months after traumatic brain injury but remain underrecognized. Neurology. 2016 Apr 27; doi: 10.1212/WNL.0000000000002697. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 39.Rao V, Spiro J, Vaishnavi S, et al. Prevalence and types of sleep disturbances acutely after traumatic brain injury. Brain Inj. 2008;22:381–6. doi: 10.1080/02699050801935260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rao V, Bergey A, Hill H, Efron D, McCann U. Sleep disturbance after mild traumatic brain injury: indicator of injury? J Neuropsychiatry Clin Neurosci. 2011;23:201–5. doi: 10.1176/jnp.23.2.jnp201. [DOI] [PubMed] [Google Scholar]
- 41.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–92. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 42.Littner M, Kushida CA, Anderson WM, et al. Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: an update for 2002. Sleep. 2003;26:337–41. doi: 10.1093/sleep/26.3.337. [DOI] [PubMed] [Google Scholar]
- 43.Sinclair KL, Ponsford J, Rajaratnam SM. Actigraphic assessment of sleep disturbances following traumatic brain injury. Behav Sleep Med. 2014;12:13–27. doi: 10.1080/15402002.2012.726203. [DOI] [PubMed] [Google Scholar]
- 44.Kostyun RO, Milewski MD, Hafeez I. Sleep disturbance and neurocognitive function during the recovery from a sport-related concussion in adolescents. Am J Sports Med. 2015;43:633–40. doi: 10.1177/0363546514560727. [DOI] [PubMed] [Google Scholar]
- 45.Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15:461–9. doi: 10.1093/sleep/15.5.461. [DOI] [PubMed] [Google Scholar]
- 46.de Souza L, Benedito-Silva AA, Pires MLN, Poyares D, Tufik S, Calil HM. Further validation of actigraphy for sleep studies. Sleep. 2003;26:81–5. doi: 10.1093/sleep/26.1.81. [DOI] [PubMed] [Google Scholar]
- 47.Sadeh A. The role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev. 2011;15:259–67. doi: 10.1016/j.smrv.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 48.Wickham H. New York, NY: Springer-Verlag; 2009. ggplot2: elegant graphics for data analysis. [Google Scholar]
- 49.Monk TH, Reynolds CF, III, Buysse DJ, DeGrazia JM, Kupfer DJ. The relationship between lifestyle regularity and subjective sleep quality. Chronobiol Int. 2003;20:97–107. doi: 10.1081/cbi-120017812. [DOI] [PubMed] [Google Scholar]
- 50.Lemola S, Ledermann T, Friedman EM. Variability of sleep duration is related to subjective sleep quality and subjective well-being: an actigraphy study. Plos One. 2013;8:e71292. doi: 10.1371/journal.pone.0071292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roane BM, Seifer R, Sharkey KM, et al. What role does sleep play in weight gain in the first semester of university? Behav Sleep Med. 2015;13:491–505. doi: 10.1080/15402002.2014.940109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dillon HR, Lichstein KL, Dautovich ND, Taylor DJ, Riedel BW, Bush AJ. Variability in self-reported normal sleep across the adult age span. J Gerontol B Psychol Sci Soc Sci. 2014:gbu035. doi: 10.1093/geronb/gbu035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vanderlind WM, Beevers CG, Sherman SM, et al. Sleep and sadness: exploring the relation among sleep, cognitive control, and depressive symptoms in young adults. Sleep Med. 2014;15:144–9. doi: 10.1016/j.sleep.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mezick EJ, Matthews KA, Hall M, et al. Intra-individual variability in sleep duration and fragmentation: associations with stress. Psychoneuroendocrinology. 2009;34:1346–54. doi: 10.1016/j.psyneuen.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baron KG, Reid KJ, Malkani RG, Kang J, Zee PC. Sleep variability among older adults with insomnia: associations with sleep quality and cardiometabolic disease risk. Behav Sleep Med. 2016:1–14. doi: 10.1080/15402002.2015.1120200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patel SR, Hayes AL, Blackwell T, et al. The association between sleep patterns and obesity in older adults. Int J Obes. 2014;38:1159–64. doi: 10.1038/ijo.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Okun ML, Reynolds CF, III, Buysse DJ, et al. Sleep variability, health-related practices and inflammatory markers in a community dwelling sample of older adults. Psychosom Med. 2011;73:142. doi: 10.1097/PSY.0b013e3182020d08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duncan MJ, Kline CE, Rebar AL, Vandelanotte C, Short CE. Greater bed-and wake-time variability is associated with less healthy lifestyle behaviors: a cross-sectional study. J Public Health. 2015:1–10. doi: 10.1007/s10389-015-0693-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.He F, Bixler EO, Berg A, et al. Habitual sleep variability, not sleep duration, is associated with caloric intake in adolescents. Sleep Med. 2015;16:856–61. doi: 10.1016/j.sleep.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.He F, Bixler EO, Liao J, et al. Habitual sleep variability, mediated by nutrition intake, is associated with abdominal obesity in adolescents. Sleep Med. 2015;16:1489–94. doi: 10.1016/j.sleep.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Telzer EH, Goldenberg D, Fuligni AJ, Lieberman MD, Gálvan A. Sleep variability in adolescence is associated with altered brain development. Dev Cogn Neurosci. 2015;14:16–22. doi: 10.1016/j.dcn.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodríguez-Colón SM, He F, Bixler EO, et al. Sleep variability and cardiac autonomic modulation in adolescents-Penn State Child Cohort (PSCC) study. Sleep Med. 2015;16:67–72. doi: 10.1016/j.sleep.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matos MG, Gaspar T, Tomé G, Paiva T. Sleep variability and fatigue in adolescents: associations with school-related features. Int J Psychol. 2016;51:323–31. doi: 10.1002/ijop.12167. [DOI] [PubMed] [Google Scholar]
- 64.Anderson B, Storfer-Isser A, Taylor HG, Rosen CL, Redline S. Associations of executive function with sleepiness and sleep duration in adolescents. Pediatrics. 2009;123:e701–7. doi: 10.1542/peds.2008-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fuligni AJ, Hardway C. Daily variation in adolescents' sleep, activities, and psychological well-being. J Res Adolesc. 2006;16:353–78. [Google Scholar]
- 66.Mihalik JP, Lengas E, Register-Mihalik JK, Oyama S, Begalle RL, Guskiewicz KM. The effects of sleep quality and sleep quantity on concussion baseline assessment. Clin J Sport Med. 2013;23:343–8. doi: 10.1097/JSM.0b013e318295a834. [DOI] [PubMed] [Google Scholar]
- 67.McClure DJ, Zuckerman SL, Kutscher SJ, Gregory AJ, Solomon GS. Baseline neurocognitive testing in sports-related concussions: the importance of a prior night's sleep. Am J Sports Med. 2014;42:472–8. doi: 10.1177/0363546513510389. [DOI] [PubMed] [Google Scholar]
- 68.Dean PJA, Sato JR, Vieira G, McNamara A, Sterr A. Long-term structural changes after mTBI and their relation to post-concussion symptoms. Brain Inj. 2015;29:1211–8. doi: 10.3109/02699052.2015.1035334. [DOI] [PubMed] [Google Scholar]
- 69.Baumann CR, Stocker R, Imhof H, et al. Hypocretin-1 (orexin A) deficiency in acute traumatic brain injury. Neurology. 2005;65:147–9. doi: 10.1212/01.wnl.0000167605.02541.f2. [DOI] [PubMed] [Google Scholar]
- 70.Baumann CR, Bassetti CL, Valko PO, et al. Loss of hypocretin (orexin) neurons with traumatic brain injury. Ann Neurol. 2009;66:555–9. doi: 10.1002/ana.21836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Valko PO, Gavrilov YV, Yamamoto M, et al. Damage to histaminergic tuberomammillary neurons and other hypothalamic neurons with traumatic brain injury. Ann Neurol. 2015;77:177–182. doi: 10.1002/ana.24298. [DOI] [PubMed] [Google Scholar]
- 72.Wickwire EM, Williams SG, Roth T, et al. Sleep, sleep disorders, and mild traumatic brain injury: what we know and what we need to know: findings from a national working group. Neurotherapeutics. 2016;13:403–17. doi: 10.1007/s13311-016-0429-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Trenchard S, Rust S, Bunton P. A systematic review of psychosocial outcomes within 2 years of paediatric traumatic brain injury in a school-aged population. Brain Inj. 2013;27:1217–37. doi: 10.3109/02699052.2013.812240. [DOI] [PubMed] [Google Scholar]
- 74.McCrory P, Collie A, Anderson V, Davis G. Can we manage sport related concussion in children the same as in adults? Br J Sports Med. 2004;38:516–9. doi: 10.1136/bjsm.2004.014811. [DOI] [PMC free article] [PubMed] [Google Scholar]


