Abstract
Study Objectives:
To compare polysomnographic parameters in high altitude (HA) native Andean children with low altitude (LA) native peers in order to explain the nocturnal oxyhemoglobin saturation (SpO2) instability reported in HA native children and to study the effect on sleep quality.
Methods:
Ninety-eight healthy children aged 7–10 y and 13–16 y were recruited at LA (500 m) or HA (3,650 m) above sea level. Physical examination was undertaken and genetic ancestry determined from salivary DNA to determine proportion of European ancestry, a risk factor for poor HA adaptation. Attended polysomnography was carried out over 1 night for 58 children at their resident location.
Results:
Of 98 children recruited, 85 met inclusion criteria, 58 of 85 (68.2%) completed polysomnography, of which 56 were adequate for analysis: 30 at LA (17 male) and 26 at HA (16 male). There were no altitude differences in genetic ancestry, but a high proportion of European admixture (median 50.6% LA; 44.0% HA). SpO2 was less stable at HA with mean 3% and 4% oxygen desaturation indices greater (both P < 0.001) than at LA. This was not explained by periodic breathing. However, more obstructive hypopnea was observed at HA (P < 0.001), along with a trend toward more central apnea (P = 0.053); neither was explained by clinical findings. There was no difference in sleep quality between altitudes.
Conclusions:
HA native Andean children have more respiratory events when scoring relies on SpO2 desaturation due to inherent SpO2 instability. Use of American Academy of Sleep Medicine scoring criteria may yield false-positive results for obstructive sleep-disordered breathing at HA.
Citation:
Hill CM, Carroll A, Dimitriou D, Gavlak J, Heathcote K, L'Esperance V, Baya A, Webster R, Pushpanathan M, Bucks RS. Polysomnography in Bolivian children native to high altitude compared to children native to low altitude. SLEEP 2016;39(12):2149–2155.
Keywords: adaptation, apnea, hypopnea, high altitude, hypoxia, polysomnography, sleep-disordered breathing
Significance.
This is the first published study of polysomnographic sleep quality and respiratory parameters in children and adolescents native to high altitude that includes a low altitude control group. Although sleep architecture does not differ between high altitude and low altitude peers, children living at high altitude have significantly more hypopnea. Increased hypopneas are likely to reflect oxyhemoglobin kinetics in the low oxygen tension state rather than absolute differences in extent of airflow limitation. Standard respiratory event scoring criteria need to be adjusted for children living in situations of hypobaric hypoxia, or who are hypoxic by virtue of chronic ventilation perfusion mismatch, in order to avoid false diagnosis of airway obstruction.
INTRODUCTION
Over 140 million people live at high altitude (HA), that is, at greater than 2,500 m above sea level. At these altitudes, low barometric pressure results in a fall in the partial pressure of atmospheric oxygen such that populations living above 4,000 m breathe air containing only ∼60% of the oxygen found at sea level (Figure 1). HA native populations have evolved ethnically unique responses to this hypoxic challenge. Andean adults increase oxygen carriage through erythrocytosis and increased oxygen uptake, through increased pulmonary artery pressure and increased pulmonary diffusion capacity. In contrast, Himalayan HA natives increase oxygen uptake through higher resting ventilation and increase tissue oxygen delivery through denser capillarization, but have lower hemoglobin concentrations than altitude-equivalent Andean HA residents.1
Figure 1.
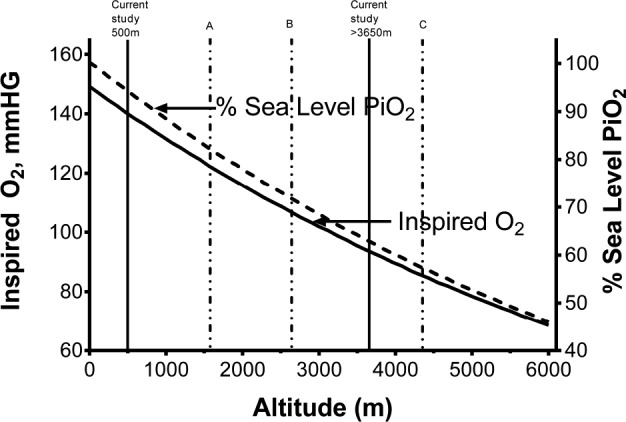
Partial pressure of oxygen as a percent of sea-level values at increasing altitudes. Vertical solid lines indicate altitudes at which participants were recruited in this study (500 m and 3,650 m) and dashed lines altitudes reported in previous studies (A: Burg et al.7, at 1,600 m; B: Duenas-Meza et al.6 at 2,640 m, C: Coote et al.4, Spicuzza et al.,5 at 4,380 m). Adapted from Beall et al. Percent of oxygen saturation of arterial hemoglobin among Bolivian Aymara at 3,900-4,000 m. Am J Phys Anthropol 1999;108(1):41–51, copyright (2007) National Academy of Sciences, U.S.A.
The sleep state further compromises respiratory adaptation at HA, due to decreased minute ventilation, circadian small airway constriction, and vulnerability to upper airway obstruction.2 Most research into sleep physiology at HA has been conducted in healthy, adult mountaineers in whom periodic breathing in sleep3 is commonly reported. Hypoxia at altitude stimulates hyperventilation which generates alkalotic hypocapnia. In sleep the eucapnic threshold is low and hyperventilation readily triggers apnea, thus generating periodic breathing. Limited studies of native HA resident adults have, intriguingly, also reported respiratory instability in sleep. An early polysomnographic study of 8 healthy, young, native, Peruvian adult males residing at 4,380 m reported episodes of periodic breathing resulting in marked oxygen desaturation.4 This was later confirmed in 20 native, adult males at the same location.5 There is only one published, polysomnographic study in HA resident children. This was limited to infants aged 1–18 mo born and living at 2,640 m in Bogotá, Colombia.6 As there was no ancestry similar, sea-level comparison group in this study, values were compared to published normative data. Results indicated preservation of sleep architecture, but higher numbers of respiratory events (both obstructive and central apnea) as well as a higher oxygen desaturation index, both associated with, and independent of, respiratory events in HA native children. Improvements in these parameters were noted across infancy. A polysomnographic study of 45 children aged 3 to 5 y, who resided in Colorado at 1,600 m for at least 1 y, an elevation technically below the 2,500 m threshold for HA, nonetheless, reported findings consistent with the infant study: specifically, higher central and obstructive apnea indices compared to published, sea-level data.7 Importantly, these children were mostly White, non-Hispanic (88.9%) and unlikely to have the advantage of genetic adaptation to HA residence conferred by Amerindian inheritance. We have recently reported significant differences in oxyhemoglobin saturation (SpO2) stability in Andean native children resident at 500 m, 2,500 m, and 3,650 m who were matched for socioeconomic status and genetic ancestry.8 In line with the Colombian infant study, we found improvements in these parameters from late infancy to childhood, suggesting developmental adaptation in sleep respiratory physiology at HA.
In summary, convergent literature indicates that children residing at HA are likely to be vulnerable to sleep-related breathing abnormalities. This is important, as children spend half of their lives asleep, a critical period for brain plasticity and maturation.9 Early exposure to intermittent, nocturnal hypoxia may compromise neurocognitive development10 both directly through hypoxia and indirectly through sleep fragmentation. In this study, we aimed to compare polysomno-graphic variables between carefully characterized samples of healthy children living at HA and children with similar ancestry who lived at 500 m.
METHODS
Design and Subjects
This was a cross-sectional study of 98 healthy children aged 7 to 10 y and 13 to 16 y across two altitude settings in Bolivia: a low altitude (LA) city—Santa Cruz, 500 m above sea-level, and a high altitude (HA) city—La Paz, at 3,650 m. All children were studied in their native altitude setting, that is HA-native children were studied in La Paz and LA altitude-native children were studied in Santa Cruz. Children were recruited through advertisement in the universities of each town. Inclusion criteria specified that children were native to their resident altitude. Native status required children to have been born at and have continuously resided at their resident altitude, other than visits of less than 6 mo duration to other altitudes, but not within the past year. All participants were from families where Spanish was spoken as the first language. Children were excluded if they had an established cardiorespiratory disease (other than mild asthma or snoring), neurological or neurode-generative condition, epilepsy, or were smokers. Approval for the study was obtained from the Institutional Ethics committees of the Universidad Privada Abierta Latinoamericana, de Santa Cruz de la Sierra, Bolivia and the University of Western Australia (reference RA/4/1/2553).
Procedures
All participants were provided with information sheets about the study and parents signed consent forms. Data collection took place within university premises at Universidad Privada de Santa Cruz de la Sierra, Santa Cruz (500 m) and Universidad de La Salle, La Paz (3,650 m) in October and November when the climate was temperate at HA and warm at LA. Parents provided information on maternal education, parental smoking in the household and their child's medical and developmental history, including whether the child was a regular snorer (defined by a positive response to the question does your child snore “usually” or “all the time”) and their history of wheezing. In addition, the Chronic Mountain Sickness Score was completed based on neurological, cardiovascular, and haematological variables, where a score of 12 or less is considered normal.11
Physical Examination
All children underwent a detailed physical examination supervised by a consultant physician or otolaryngologist (CMH/ KH) including cardiorespiratory examination, resting blood pressure (Microlife, Zurich), Brodsky grading of tonsillar size, Mallampati score, and height and weight. Body mass index centiles were derived from standard Centers for Disease Control and Prevention growth charts. Sex and height referenced systolic blood pressure centiles were computed.12
Genetics
DNA was extracted from saliva samples (Western Australia DNA Bank, University of Western Australia) and whole gene amplified (K BioSciences, Hoddesdon, UK). Individual European, Amerindian, and African admixture proportions were estimated using a panel of 28 ancestry informative markers previously noted to demonstrate high frequency differences in allele frequency between these different ancestry groups.13,14 The admixture modelling program admixmap15 was used to model the distribution of admixture in the cohort (http://homepages.ed.ac.uk/pmckeigu/admixmap/index.html) and to generate individual ancestry estimates. Ancestry informative marker-specific allele frequencies were estimated from their reported counts in modern European, African, and Amerindian populations.13,14
Polysomnography
Attended polysomnography was carried out in an established sleep laboratory setting at LA (Santa Cruz) and temporary, adapted facility at HA (La Paz), in both settings using computerized ambulatory systems (Compumedics PS2 system, Melbourne, Australia) according to accepted guidelines.16 All studies were performed by an experienced polysomnographic technologist (AC). Sleep montage included electroencephalography (C3/A2, C4/A1, O1/A2, O2/A1) with electrode placement according to the international 10–20 system,17 electromyography at submentalis, bipolar electrooculography, electrocardiography, and oxyhemoglobin saturation (SpO2) monitoring (Nonin, Plymouth, MN) with 1 Hz sampling rate and data averaged over 4 successive pulse beats. Respiratory inductance plethysmography bands were used to measure abdominal and thoracic excursions and nasal thermistors (Protech, Mukilteo, WA) provided a constant flow monitor. Polysomnographs were scored by a single technologist (AC), based on the established sleep staging18 and respiratory19 criteria for pediatrics, and all studies were peer reviewed by a certified somnologist (CMH). Obstructive apnea was defined as chest or abdominal wall movement in the absence or decrease of airflow by more than 90% of the preceding breath, for two or more breaths. Hypopneas were classified as for apneas, but where the reduction in flow was 50% to 90% of the previous breath and only if accompanied by either oxyhemoglobin (SpO2) desaturation of 3% or greater or arousal within 2 breaths of event termination. Central apneas were scored if there was a reduction in airflow amplitude by more than 90%, in the absence of respiratory effort, associated with either an arousal, an awakening or a more than 3% oxyhemoglobin desaturation. Periodic breathing was scored if there were greater than 3 episodes of absent respiratory effort of at least 3 sec duration separated by no more than 20 sec of normal breathing. Percentage of time in periodic breathing was calculated as time in periodic breathing / total sleep time × 100. The obstructive apnea-hypopnea index (OAHI) was defined as the number of obstructive apneas, hypopneas, and mixed apneas per hour of total sleep time.
Analysis
Data were analyzed in SPSS version 22 (Armonk, NY: IBM Corp). Simple age-group or altitude differences were explored using Mann-Whitney U tests, given the non-normality of some variables. For analyses exploring interaction terms, for which there is no nonparametric alternative, analyses of variance (ANOVAs) were conducted and then nonparametric comparisons were run to confirm significant effects, for the sake of parsimony only significant effects are reported. Given the exploratory nature of these analyses, adjustments to P values for post hoc analyses were not made to reduce the risk of a type 2 error.20,21 Where the ANOVA and nonparametric follow-up tests did not agree, to reduce the risk of a type 1 error, results are taken as nonsignificant. Partial eta-squared effect sizes (η2p) were computed for all ANOVAs. Categorical group differences were explored using χ2 (Fisher exact) test.
RESULTS
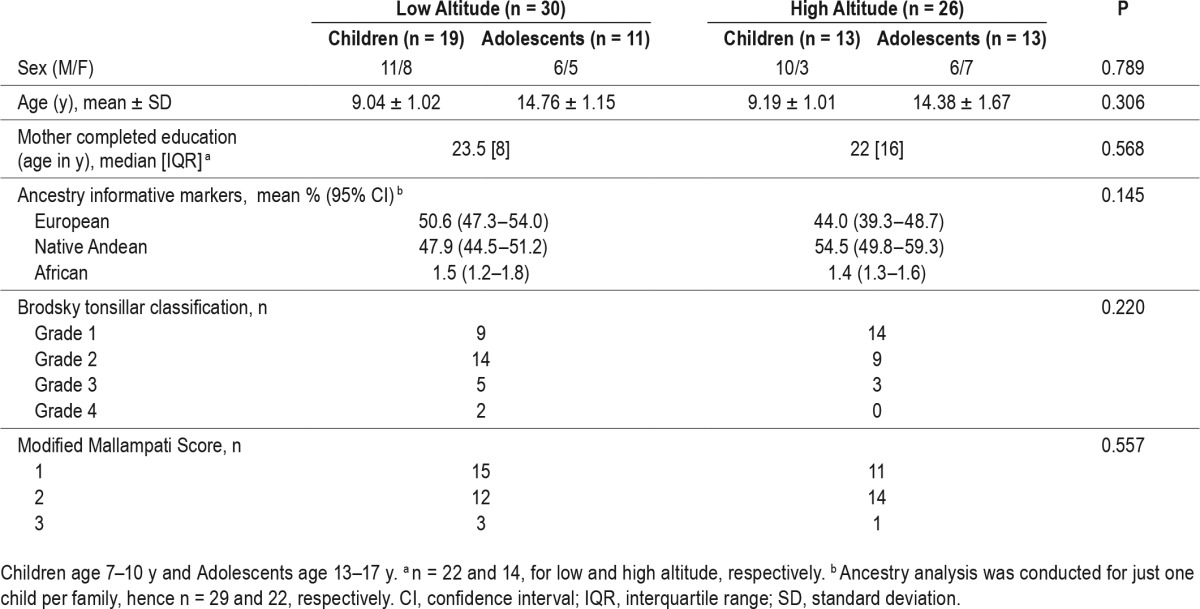
Of 98 children recruited, 13 were excluded because there was no confirmation that they met our criteria as native to the relevant altitude. Of the remaining 85 children, 58 (68.2%) completed overnight polysomnography. Of these, 2 studies recorded insufficient total sleep time to be included (< 234 min), leaving 56 children: 30 at low altitude (17 male) and 26 at high altitude (16 male), with no difference between altitudes in sex distribution, mean age, the number of years of education completed by the mother, nor in the proportion of children where either or both parents smoked in the home (Table 1).
Table 1.
Demographic and clinical data by altitude.
Clinical Measures
Medical history: Regular snoring was reported in a total of 16 children (28% of participants), 11 of 30 at LA and 5 of 26 at HA, although this did not differ between HA and LA children. Similarly, there were no differences between the number of LA and HA children with a history of wheezing (13, 43.3% children at LA and 5, 19.2% at HA, P = 0.218) or wheeze in the 12 months prior to study (7, 23.3% at LA and 3, 11.5% at HA, P = 0.507). All children had normal Chronic Mountain Sickness Scale scores.
Clinical examination: Cardiorespiratory examination was normal in all participants. There were no significant differences between HA and LA children in Brodsky tonsillar classification, or in modified Mallampati scores. Six children at LA and 3 at HA were obese (16% overall) with no altitude differences in the distribution of children categorized as obese, overweight, or of normal weight (no child was categorized as underweight), χ2 < 1. Six children were hypertensive (age, sex, and height referenced greater than the 95th centile): 5 at HA and 1 at LA, but this did not differ by altitude, P = 0.086.
Multivariate ANOVA of admixture (European, African, Native Andean) by Altitude (high, low) revealed no differences between altitudes in genetic admixture, [Pillai's Trace = 0.10, F(3, 47) = 1.88, P = 0.145, η2p = 0.11].
Polysomnography
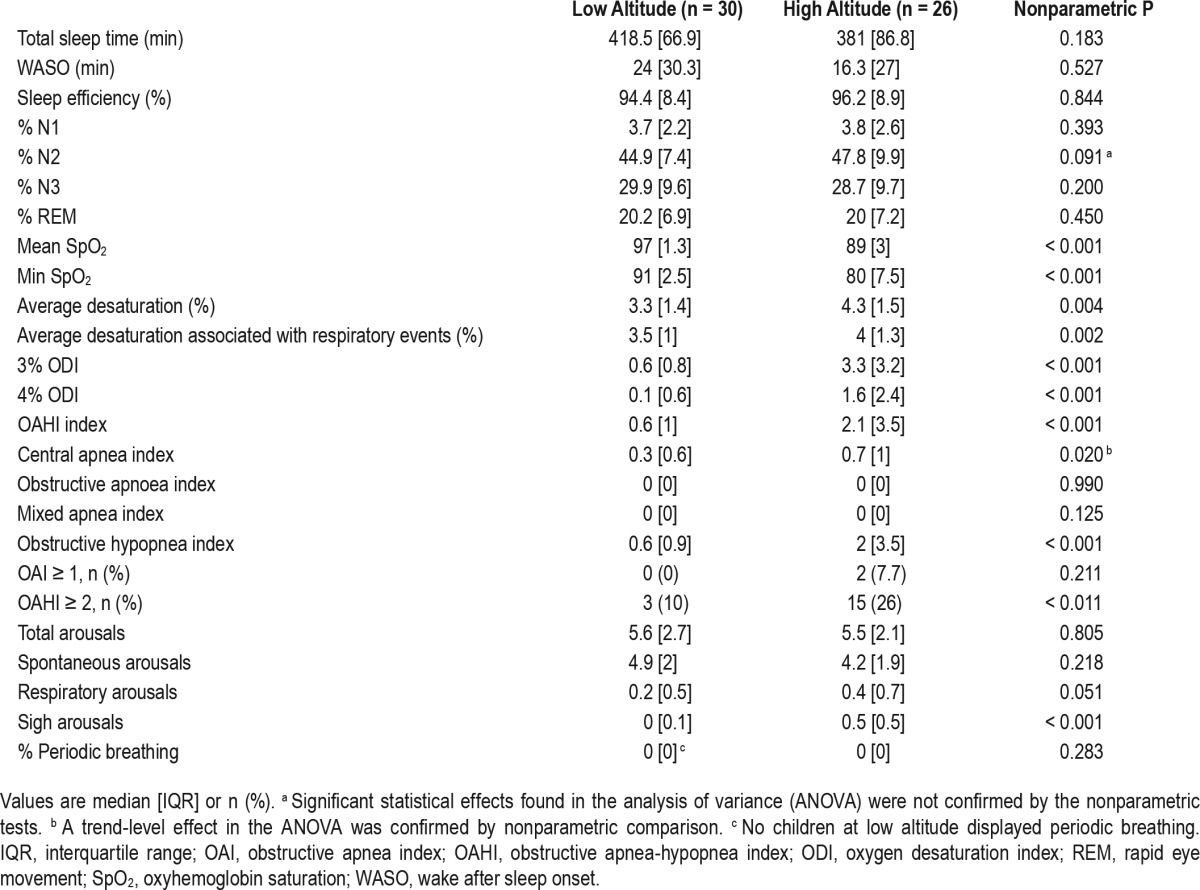
Sleep architecture and sleep quality: Age group by altitude ANOVA, confirmed by nonparametric comparisons, revealed a significant effect of age group on % N3, [F(1,52) = 9.78, P = 0.003, η2p = 0.16], where younger children had more slow wave sleep (median 29.9%; interquartile range 8.5) compared to adolescents (median 25.7%, interquartile range 9.1) but no significant effects of altitude, and no interactions (see Table 2 for altitude comparisons).
Table 2.
Polysomnography data.
Oxyhemoglobin Saturation
Mean overnight SpO2 and minimum oxygen saturation were much lower in HA children (Table 2), [F(1,50) = 494.03, P < 0.001, η 2p = 0.91 and F(1,52) = 102.80, P < 0.001, η2p = 0.67], respectively. Likewise, mean percentage desaturations, [F(1,52) = 5.67, P = 0.021, η2p = 0.10], and mean desaturations associated with respiratory events, [F(1,52) = 7.39, P = 0.009, η2p = 0.12] were greater in HA, confirmed by a significantly higher proportion of 3%, [F(1,52) = 27.21, P < 0.001, η2p = 0.34], and 4% desaturations, [F(1,52) = 19.03, P < 0.001, η2p = 0.27].
Respiratory Events
Although the OAHI was higher at HA, [F(1,52) = 15.96, P < 0.001, η2p = 0.25], this was driven by differences in obstructive hypopnea, [F(1,52) = 16.84, P < 0.001, η2 p = 0.25], with no differences in obstructive or mixed apneas by altitude. A trend-level altitude difference in central apneas by altitude, [F(1,52) = 3.93, P = 0.053, η2p = 0.07], was confirmed by nonparametric follow-up testing (see Table 2). Finally, there were no differences in the number of spontaneous or respiratory arousals, but sigh arousals were more common at HA, [F(1,52) = 27.7, P < 0.001, η2p = 0.35]. One HA child spent 5% of total sleep time in periodic breathing; no other children at either LA or HA had periodic breathing. Excluding either the 2 children at HA who had an obstructive apnea index ≥ 1 and < 2 events/h or the 18 with an OAHI ≥ 2 events/h (see Table 2), the altitude differences were maintained in all oxyhaemoglobin, respiratory event parameters, and in sigh arousals.
Characteristics of Children Meeting Standard Criteria for Diagnosis of Obstructive Sleep Apnea
There were no relationships between polysomnographic indices of obstructive sleep apnea and history of snoring, obesity, or Mallampati/Brodsky scores. Of the 18 children (3 LA, 15 HA) with OAHI ≥ 2, five (all 3 LA and 2 HA) were reported as snorers, by their parents, and three were obese (2 LA, 1 HA). Of the 2 children with OAI ≥ 1 (both HA, both also with OAHI ≥ 2), neither was a snorer or obese. Furthermore, restricting the analysis to the 40 children with no parent report of snoring did not change the effects reported.
DISCUSSION
Amerindian peoples settled on the Andean HA plains around 11,000 years ago22 and developed unique phenotypic adaptation to hypoxia. Spanish colonization, 500 years ago, diluted the original native Amerindian gene pool, potentially threatening this adaptation. Children in our study had roughly equal European and Amerindian ancestry. Adaptation to HA survival may, therefore, be imperfect in these children. This is the first published study, of which the authors are aware, that describes the polysomnographic features of sleep architecture alongside respiratory and oximetry parameters in a healthy sample of non-infant children, native to HA. Importantly, we compare our data to a control group of children living at 500 m who share a similar, mixed, European and Amerindian genetic ancestry and sociodemographic background.
Our initial motivation for performing sleep studies at altitude was to further explore the respiratory physiology underlying the increased oxyhaemoglobin saturation variability we have previously reported in children at HA.8 We hypothesized that the periodic breathing, observed in adult Andeans, was a potential cause of this instability. Children and adolescents in this study, however, did not display periodic breathing; rather, they had a higher prevalence of obstructive sleep- disordered breathing, characterized principally by hypopnea. To put this in context, using diagnostic thresholds from a recent multicenter study of adenotonsillectomy for obstructive sleep apnea in children23 (OAHI of ≥ 2 events/h or apnea index of ≥ 1 event/h), 57.7% of children at HA in this study would, potentially, be classified as having obstructive sleep apnea compared to 10.0% of a comparable group at LA. Whether or not this represents genuine alterations in upper airway function is questionable. Clinical data did not indicate a higher prevalence of snoring at HA, although overall prevalence was high in this population, at 28%. Habitual snoring has previously been reported in 18% of healthy children aged 7 to 17 y in Chile, suggesting that South American children may be phenotypically more vulnerable to mild, upper airway obstruction.24
A more plausible explanation for the higher prevalence of hypopnea at HA relates to our scoring criteria. Alongside a decrease of 50% to 90% in the amplitude of the airflow, scoring required either an electroencephalography arousal or a 3% SpO2 desaturation. American Academy of Sleep Medicine scoring criteria are based on normative data derived from healthy populations living below 2,500 m.18 At HA, where oxyhemoglobin saturation is low, small perturbations in arterial partial pressure of oxygen, due to sleep-related fluctuations in ventilation, will be associated with larger decreases in SpO2. This is reflected in significantly higher 3% and 4% desaturation indices at HA in this study. In further support of this theory, the mean central apnea index, also scored when events are associated with SpO2 desaturation, showed a trend level increase at HA. Some caution should be exercised in interpreting the central apnea index data, however, which did not produce consistent altitude differences across parametric and nonparametric follow-up tests. Given that the effect size of 0.07 suggests that altitude explains just 7% of the variance in the central apnea index as a function of variance in each of the effects and the associated error that is accounted for by that effect,25 differences in central apnea may not be a marked feature of altitude dwelling in Andean children. Higher hypopnea and central apnea indices were also reported by Burg and colleagues7 in 3- to 5-y-old asymptomatic healthy children in Colorado, living at intermediate altitude, namely 1,600 m, lending support to the need for adapted scoring criteria in conditions of low oxygen tension.
In interpreting our findings, technical limitations in polysomnographic data acquisition should be considered. Our ambulatory monitoring equipment lacked constant CO2 measures, or more sophisticated calibrated plethysmography or oesophageal pressure monitoring, all of which could have provided a more confident classification of respiratory events. This was a pragmatic decision, based on the study setting and a tradeoff between cost and the risk of impaired sleep quality with extended monitoring. Furthermore, the use of a thermistor alone, rather than alongside a nasal pressure gauge, may have resulted in underestimation of obstructive respiratory events.26 Studies were limited to a single night with the potential for “first night effect”, although night-to-night variability in respiratory events is unlikely to be significant given the adequate total sleep times reported. Importantly, however, these technical limitations applied equally across the entire study, allowing valid comparison between the altitude locations. Finally, the sleep technologist was not blinded to the altitude location of the child, risking bias in reporting. However, given that our a priori hypothesis was that children may exhibit periodic breathing, the fact that our findings did not support this suggests no systematic reporting bias.
Little is known about the effect of HA residence on sleep quality in childhood. This is a relevant question as sleep quality is associated with academic performance27 and neurobehavioral health in children,28 and we have demonstrated that HA-dwelling Andean children are susceptible to subtle, neurocognitive impairments.29,30 Certainly, sleep quality is impaired in adult lowlanders who ascend to altitude, generally as a consequence of sleep disordered breathing.31 There are no prior data, to our knowledge, reporting sleep quality in matched groups of children at high and low altitude settings. Our data indicate that macroscopic sleep architecture and sleep quality are preserved in children at HA with no differences in total sleep time, wake after sleep onset, sleep efficiency, or sleep stage distribution. Differences were noted, however, in respiratory event-related arousals and sigh arousals, albeit subtle ones, with no differences in total arousal indices between the altitude settings. It would be of interest to study associations between sleep quality, respiratory, and SpO2 variables and cognitive performance in this HA population.
We report samples of Andean children with unique, mixed European and Amerindian ancestry and findings may not translate to other populations of children living at HA. However, data from the Colorado study, representing a predominantly White population, albeit at intermediate altitude, show similar findings7. Future studies in children of different genetic ancestry will confirm this. Selection bias is always a risk in resource-poor settings where participants may be attracted to what is perceived as a free health check. This was offset by the fact that children were from middle-high income families and there were no differences between altitudes in socioeconomic status.
In summary, Andean children aged between 7 and 17 y living at HA demonstrated a higher hypopnea index and greater SpO2 desaturation compared to ancestry- and socioeconomically matched peers living at LA. This apparent difference is likely to reflect a lower threshold for scoring hypopnea in low oxygen tension settings and indicates that scoring rules should accommodate such differences at both HA settings and in medical conditions where ventilation perfusion mismatch means that a child's PaO2 is chronically low. Although most pediatric populations living at HA are unlikely to be subject to unnecessary surgery based on a false diagnosis, due largely to inequalities of health access in these settings, the same may not be true in children in first-world settings with chronic hypoxia, whose very survival indicates privileged access to healthcare and a lower threshold for intervention. Future studies should seek to replicate these findings in pediatric populations living at HA and children with chronic hypoxia due to ventilation perfusion mismatch.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was made possible by grants from the Gerald Kerkut Trust and the London Law Trust. Study planning was possible due to a grant from the Worldwide Universities network. Dr Hill has received lecture fees from Janssen Pharmaceuticals in the past 3 years. Dr. Bucks has received royalties from Hogrete Publishers. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors are indebted to Universidad Privada de Santa Cruz de la Sierra (Santa Cruz) and Universidad de La Salle (La Paz), and to their student volunteers who supported these studies. We would also like to thank Dr Mario Camargo Villarreal and his team at the NeuroCenter, Santa Cruz for loan of their sleep laboratory facilities. The authors also gratefully acknowledge the assistance of the Australian Medical Bioinformatics Resource, a National Health and Medical Research Council of Australia Medical Bioinformatics Genomics Proteomics Program. Author contributions: Dr. Hill is the guarantor of the content of the manuscript, including the data and analysis. She was joint principal investigator and co-authored the present manuscript with Dr. Bucks, who had joint full access to the study data and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Baya managed study recruitment and assisted with data collection. Dr. Gavlak, Ms. Carroll, Ms. Heathcote, Dr. Dimitriou, and Dr. L'Esperance were involved in study design, data collection and have contributed to the manuscript. Dr. Webster advised on methodology for genetic analyses and undertook this element of data analysis. Dr. Pushpanathan assisted with PSG data management. All co-authors have reviewed and contributed to the manuscript preparation.
ABBREVIATIONS
- ANOVA
analysis of variance
- DNA
deoxyribonucleic acid
- HA
high altitude
- LA
low altitude
- OAHI
obstructive apnea-hypopnea index
- SpO2
oxyhemoglobin saturation
REFERENCES
- 1.Beall CM. Two routes to functional adaptation: Tibetan and Andean high-altitude natives. Proc Natl Acad Sci U S A. 2007;104(Suppl 1):8655–60. doi: 10.1073/pnas.0701985104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newton K, Malik V, Lee-Chiong T. Sleep and breathing. Clin Chest Med. 2014;35:451–6. doi: 10.1016/j.ccm.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Wickramasinghe H, Anholm JD. Sleep and breathing at high altitude. Sleep Breath. 1999;3:89–102. doi: 10.1007/s11325-999-0089-1. [DOI] [PubMed] [Google Scholar]
- 4.Coote JH, Tsang G, Baker A, Stone B. Respiratory changes and structure of sleep in young high-altitude dwellers in the Andes of Peru. Eur J Appl Physiol Occup Physiol. 1993;66:249–53. doi: 10.1007/BF00235102. [DOI] [PubMed] [Google Scholar]
- 5.Spicuzza L, Casiraghi N, Gamboa A, et al. Sleep-related hypoxaemia and excessive erythrocytosis in Andean high-altitude natives. Eur Respir J. 2004;23:41–6. doi: 10.1183/09031936.03.00000703. [DOI] [PubMed] [Google Scholar]
- 6.Duenas-Meza E, Bazurto-Zapata MA, Gozal D, González-García M, Durán-Cantolla J, Torres-Duque CA. Overnight polysomnographic characteristics and oxygen saturation of healthy infants, 1 to 18 months of age, born and residing at high altitude (2,640 meters) Chest. 2015;148:120–7. doi: 10.1378/chest.14-3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burg CJ, Montgomery-Downs HE, Mettler P, Gozal D, Halbower AC. Respiratory and polysomnographic values in 3- to 5-year-old normal children at higher altitude. Sleep. 2013;36:1707–14. doi: 10.5665/sleep.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill CM, Baya A, Gavlak J, et al. Adaptation to life in the high Andes: nocturnal oxyhaemoglobin saturation in early development. Sleep. 2016;39:1001–8. doi: 10.5665/sleep.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang G, Grone B, Colas D, Appelbaum L, Mourrain P. Synaptic plasticity in sleep: learning, homeostasis, and disease. Trends Neurosci. 2011;34:452–63. doi: 10.1016/j.tins.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bass JL, Corwin M, Gozal D, et al. The effect of chronic or intermittent hypoxia on cognition in childhood: a review of the evidence. Pediatrics. 2004;114:805–16. doi: 10.1542/peds.2004-0227. [DOI] [PubMed] [Google Scholar]
- 11.León-Velarde F, McCullough RG, McCullough RE, Reeves JT CMS Consensus Working Group. Proposal for scoring severity in chronic mountain sickness (CMS). Background and conclusions of the CMS Working Group. Adv Exp Med Biol. 2003;543:339–54. doi: 10.1007/978-1-4419-8997-0_24. [DOI] [PubMed] [Google Scholar]
- 12.National Heart, Lung and Blood Institute 4th Report of the Diagnosis, Evaluation and Treatment of High Blood Pressure in Children and Adolescents. Blood Pressure Tables for Children and Adolescents. 2004. [Accessed March 3, 2015]. http://www.nhlbi.nih.gov/health-pro/guidelines/current/hypertension-pediatric-jnc-4/blood-pressure-tables.htm.
- 13.Tsai HJ, Kho JY, Shaikh N, et al. Admixture matched case-control study: a practical approach for genetic association studies in admixed populations. Hum Genet. 2006;118:626–39. doi: 10.1007/s00439-005-0080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brutsaert TD, Parra E, Shriver M, et al. Effects of birthplace and individual genetic admixture on lung volume and exercise phenotypes of Peruvian Quechua. Am J Phys Anthropol. 2004;123:390–8. doi: 10.1002/ajpa.10319. [DOI] [PubMed] [Google Scholar]
- 15.Hoggart CJ, Shriver MD, Kittles RA, Clayton DG, McKeigue PM. Design and analysis of admixture mapping studies. Am J Hum Genet. 2004;74:965–78. doi: 10.1086/420855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Standards and indications for cardiopulmonary sleep studies in children. American Thoracic Society. Am J Respir Crit Care Med. 1996;153:866–78. doi: 10.1164/ajrccm.153.2.8564147. [DOI] [PubMed] [Google Scholar]
- 17.Klem GH, Luders HO, Jasper HH, Elger C. The ten-twenty electrode system of the International Federation. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:3–6. [PubMed] [Google Scholar]
- 18.Rechtschaffen A, Kales A. Los Angeles: Brain Information Service/Brain Research Institute, University of California; 1968. A manual of standardized terminology, techniques and scoring system of sleep stages in human subjects. [Google Scholar]
- 19.Iber C, Ancoli-Israel S, Chesson A, Quan SF. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specification. [Google Scholar]
- 20.Perneger TV. What's wrong with Bonferroni adjustments. BMJ. 1998;316:1236–8. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–6. [PubMed] [Google Scholar]
- 22.Aldenderfer MS. Moving up in the world: archaeologists seek to understand how and when people came to occupy the Andean and Tibetan plateaus. Am Sci. 2003;91:542–49. [Google Scholar]
- 23.Marcus CL, Moore RH, Rosen CL, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea Childhood Adenotonsillectomy Trial (CHAT) N Engl J Med. 2013;368:2366–76. doi: 10.1056/NEJMoa1215881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brockmann PE, Bertrand P, Pardo T, Cerda J, Reyes B, Holmgren NL. Prevalence of habitual snoring and associated neurocognitive consequences among Chilean school aged children. Int J Pediatr Otorhinolaryngol. 2012;76:1327–31. doi: 10.1016/j.ijporl.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 25.Tabachnick BG, Fidell LS. Using Multivariate Statistics. 6th ed. Boston, MA: Allyn & Bacon; 2013. [Google Scholar]
- 26.Budhiraja R, Goodwin JL, Parthasarathy S, Quan SF. Comparison of nasal pressure transducer and thermistor for detection of respiratory events during polysomnography in children. Sleep. 2005;28:1117–21. doi: 10.1093/sleep/28.9.1117. [DOI] [PubMed] [Google Scholar]
- 27.Dewald JF, Meijer AM, Oort FJ, Kerkhof GA, Bögels SM. The influence of sleep quality, sleep duration and sleepiness on school performance in children and adolescents: a meta-analytic review. Sleep Med Rev. 2010;14:179–89. doi: 10.1016/j.smrv.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Astill RG, Van der Heijden KB, Van Ijzendoorn MH, et al. Sleep, cognition, and behavioral problems in school-age children: a century of research meta-analyzed. Psychol Bull. 2012;138:1109–38. doi: 10.1037/a0028204. [DOI] [PubMed] [Google Scholar]
- 29.Hogan AM, Virues-Ortega JV, Baya Botti A, et al. Development of aptitude at altitude. Devel Sci. 2010;13:533–44. doi: 10.1111/j.1467-7687.2009.00909.x. [DOI] [PubMed] [Google Scholar]
- 30.Hill CM, Annaz D, Baya A, et al. Cognitive performance in high altitude Andean residents compared to low altitude populations: from childhood to older age. Neuropsychology. 2014;28:752–60. doi: 10.1037/neu0000065. [DOI] [PubMed] [Google Scholar]
- 31.Ainslie PN, Samuel J.E., Lucas SJE, Burgess KR. Breathing and sleep at high altitude. Resp Physiol Neurobiol. 2013;188:233–56. doi: 10.1016/j.resp.2013.05.020. [DOI] [PubMed] [Google Scholar]


