Abstract
Study Objectives:
Insufficient sleep in individuals appears increasingly common due to the demands of modern work schedules and technology use. Consequently, there is a growing need to understand the interactions between sleep deprivation and memory. The current study determined the effects of acute sleep deprivation on short and long-term associative memory using the marine mollusk Aplysia californica, a relatively simple model system well known for studies of learning and memory.
Methods:
Aplysia were sleep deprived for 9 hours using context changes and tactile stimulation either prior to or after training for the operant learning paradigm, learning that food is inedible (LFI). The effects of sleep deprivation on short-term (STM) and long-term memory (LTM) were assessed.
Results:
Acute sleep deprivation prior to LFI training impaired the induction of STM and LTM with persistent effects lasting at least 24 h. Sleep deprivation immediately after training blocked the consolidation of LTM. However, sleep deprivation following the period of molecular consolidation did not affect memory recall. Memory impairments were independent of handling-induced stress, as daytime handled control animals demonstrated no memory deficits. Additional training immediately after sleep deprivation failed to rescue the induction of memory, but additional training alleviated the persistent impairment in memory induction when training occurred 24 h following sleep deprivation.
Conclusions:
Acute sleep deprivation inhibited the induction and consolidation, but not the recall of memory. These behavioral studies establish Aplysia as an effective model system for studying the interactions between sleep and memory formation.
Citation:
Krishnan HC, Gandour CE, Ramos JL, Wrinkle MC, Sanchez-Pacheco JJ, Lyons LC. Acute sleep deprivation blocks short- and long-term operant memory in Aplysia. SLEEP 2016;39(12):2161–2171.
Keywords: Aplysia, sleep deprivation, learning and memory
Significance.
Our studies established that the interactions of sleep with memory formation are phylogenetically conserved in an invertebrate model system, Aplysia californica, ideally suited for studies of learning and memory. Acute sleep deprivation inhibited the induction of short- and long-term operant memory with persistent effects lasting more than 24 h. Acute sleep deprivation also inhibited the molecular consolidation of long-term memory. Sleep deprivation is a growing problem in children and adult populations with potentially severe effects on learning and memory. By identifying a relatively simple model system in which the effects of sleep deprivation could be investigated, our findings provide a foundation for future studies delineating the molecular mechanisms through which sleep deprivation affects memory formation.
INTRODUCTION
Increasingly, longer working hours for individuals are becoming more prevalent due to the demands of modern society and the availability of technology.1 Research as early as 18962 demonstrated the link between sleep deprivation and cognitive performance. Acute sleep deprivation and chronic sleep restriction cause significant decrements in short-term and long-term memories in humans and in animal models.3–9 In mammals, the consolidation of hippocampal dependent spatial memory is particularly susceptible to the effects of sleep deprivation.10–12 In Drosophila and mouse models, genetic mutants exhibiting reduced sleep also show impairments in memory.13–15 However, increasing sleep, either pharmacologically or genetically, can enhance learning and memory in suboptimal situations.16,17 Neither the effects of sleep deprivation nor the role of sleep in the induction and consolidation of memories are well understood at the neuronal or molecular level. The complexity of the interactions between sleep and memory create a need for a simple model system to unravel the connections and underlying molecular intersections between sleep and memory formation.
In the past 30 years, sleep has been demonstrated in numerous invertebrate species including insects,6,18–21 nematodes,22 and mollusks.18,23 Sleep in invertebrates can be defined using behavioral characteristics including defined periods of inactivity, characteristic body posture during rest, preferred resting location, elevated sensory arousal thresholds during rest, and rebound sleep following sleep deprivation.24–26 Recently, sleep has been characterized in the marine mollusk Aplysia californica, a model system frequently used for studies of learning and memory. Sleep in Aplysia occurs during the night in long bouts, with virtually no daytime sleep evident, and is characterized by preferred body position, preferred rest location, and decreased responsiveness to appetitive and aversive sensory stimuli.27 Following a single night of sleep deprivation, Aplysia exhibit rebound sleep, demonstrating homeostatic as well as circadian regulation of sleep.27 Aplysia has been an excellent model for dissecting the molecular mechanisms involved in long-term and short-term associative and nonassociative memory formation.28–33 The underlying mechanisms of memory formation identified in Aplysia extrapolate to more complex systems as they are highly conserved between vertebrates and invertebrates species.34–36 Thus, the marine mollusk A. californica, with its relatively simple neural circuitry, provides an ideal model for studying the interactions between sleep and memory formation.
In the current study, we investigated the effects of acute sleep deprivation on the formation of short-term and long-term associative memory using the operant learning paradigm, learning that food is inedible (LFI) paradigm. During LFI training, the animal associates specific seaweed with the inability to swallow the seaweed,37 forming memory that the food is inedible. A single LFI training session induces temporally different forms of memory (short-term, intermediate-term, long-term) that are mechanistically distinct.38 We found that acute sleep deprivation for 9 h inhibited the induction of short-term and long-term LFI memory. Moreover, sleep deprivation impaired the consolidation but not the recall of long-term memory (LTM). Handling alone had no adverse effects as daytime-handled animals demonstrated robust short-term memory (STM) and LTM. Interestingly, for both STM and LTM the memory impairments following 9 h acute sleep deprivation persisted for at least 24 h, preventing the effectiveness of training for the induction of memory. The prohibitions to the formation of memory induced by sleep deprivation were ameliorated approximately 48 h after acute sleep deprivation. These studies demonstrate that sleep deprivation inhibits short-and long-term LFI memory induction and consolidation in Aplysia and that the effects of acute sleep deprivation persist for at least 24 h.
METHODS
Animal Maintenance
A. californica (100-200 g; South Coast Bio-Marine, San Pedro, CA) were housed on a 12-h light/12-h dark (LD) cycle in individual boxes within chilled 110-gallon tanks maintained at 15°C with circulating artificial seawater (Instant Ocean, United Pet Group, Blacksburg, VA). Animals were fed romaine lettuce (∼5 g) every 1-2 days with varied feeding times. Zeitgeber time (ZT) 0 is defined as lights on and ZT 12 reflects the time of lights off. Sleep deprivation experiments in the dark were performed using dim red light.
Sleep Deprivation
Sleep deprivation was performed using context changes every 30 min and tactile stimulation similar to previous studies27 with slight modifications. Briefly, animals were transferred to individual, chilled, and aerated plastic containers (40 cm × 40 cm) filled with artificial seawater. Each container was equipped with a different context (big stones, small pebbles mixed with coral sand, filter, terrarium liner). Animals were observed once per minute to assess movement and handled dependent upon whether the animals were immobile for three consecutive observations. In general, animals were irregularly handled two to five times per half hour. As a control for the effects of physical handling of the animals on subsequent training responses and memory formation, similar procedures (context changes and irregular handling) were performed for daytime-handled controls animals during the light period beginning at ZT 1 and continuing for 9 h. The number of times that handling of daytime animals occurred was determined for each half-hour bin based upon the mean number of times handling occurred in analogous half-hour segments for sleep deprived animals in corresponding experiments.
Behavioral Training and Testing
Aplysia were fed to satiation with laver seaweed and removed from appetitive stimuli for 6 days prior to training. LFI training was performed as previously described using a 25-min training protocol.38,39 Briefly, animals were presented with laver seaweed in a tulle-mesh bag that could not be swallowed. Animals responded with head-waving, orientation to the seaweed, the in-take of the seaweed bag into the mouth, and repeated swallowing attempts during training as in previous studies.37,38 Testing occurred using duplicate procedures 30 min after the end of training for STM or 24 h later for LTM. Testing proceeded until the animal failed to take the netted seaweed into the mouth for 3 consecutive min. Memory was represented by significant decreases in total response time and the time the seaweed was retained in the mouth compared to naïve animals. For all behavior experiments including sleep deprivation, LFI training and LFI testing, a single experiment involved multiple individuals.
Statistical Analysis
Statistical analysis of the data was by analysis of variance using Bonferroni post hoc analyses. P values less than 0.05 were considered significant.
RESULTS
Sleep Deprivation Blocks the Induction of LTM
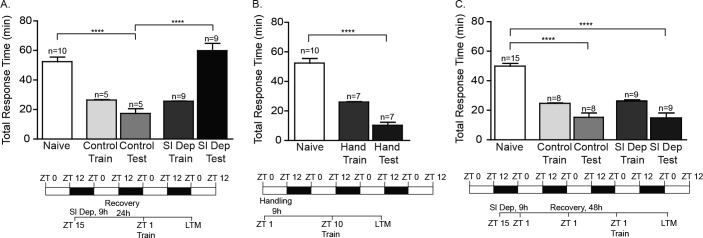
LTM formation requires multiple stages including the perception of stimuli and the acquisition of the new behavior, the early induction of cell signaling pathways following training, and the molecular consolidation of the memory that necessitates protein synthesis and gene expression.40,41 As a first step in establishing a simple model system suitable for investigating interactions between sleep deprivation and memory formation, we investigated whether sleep deprivation adversely affected the induction of LTM in Aplysia. Animals were sleep deprived using periodic context changes and tactile stimulation for the last 9 h of the night (ZT 15–ZT 24), trained using the LFI paradigm for 25 min at ZT 1 (1 h after lights on) and tested for LTM 24 h after training. Sleep deprivation did not affect the baseline responses of the animals, as there was no significant difference in the time the animals retained the seaweed in the mouth during training between sleep deprived and control animals (Figure S1A in the supplemental material). Sleep deprivation prior to training blocked the induction of LTM as sleep deprived animals had total response times similar to naïve animals during testing (Figure 1A). Control animals that were not sleep deprived displayed robust LTM with significantly decreased total response time and the time the seaweed was retained in the mouth compared to naïve animals (Figure S1A).
Figure 1.
Sleep deprivation blocks induction of long-term memory (LTM). (A) To investigate the effect of sleep deprivation on the induction of LTM, animals were sleep deprived for the last 9 h of the night, trained at ZT 1 and tested for LTM 24 h after training. All animals were trained using a 25 min abbreviated training protocol. The mean duration of training is shown for each group. Minor variance occurs in training times, as the seaweed bag has to be gently extracted from the animal's mouth. No significant differences were observed between the training times for non-sleep deprived animals (Control Train) and sleep deprived animals (Sl Dep Train). Trained sleep deprived animals (Sl Dep Test) did not exhibit LTM with significantly longer total response time compared to trained non-sleep deprived animals (Control Test) (one-way analysis of variance F(4,33) = 18.55, P < 0.0001). Numbers of animals in each group are shown above the columns. Asterisks represent Bonferroni post hoc analyses ****P < 0.0001. (B) Daytime handling did not inhibit the induction of LTM. Handling did not affect the duration of training as no significant differences were observed in training between nonhandled (Control Train) and handled animals (Hand Train). Trained daytime-handled control animals exhibited robust LTM with significantly decreased total response time during testing (Hand Test) compared to naïve animals with times similar to trained nonhandled animals (Control Test) (one-way analysis of variance F(4,35) = 39.95, P < 0.0001) Asterisks represent Bonferroni post hoc analyses ****P < 0.0001.
Sleep deprivation protocols using gentle handling in rats may induce memory deficits and retrograde amnesia independent of the impact of sleep deprivation.42 Handling can also produce unwanted side effects, including increased levels of glucocorticoids or changes in neurotransmitter receptors, that could conceivably mask the expression of memory.43,44 To rule out potential stress-induced memory deficits caused by the physical handling of the animals during the sleep deprivation procedure, animals were handled with context changes for 9 h during the day similar to the degree of handling in corresponding sleep deprivation experiments, received LFI training at the end of the day at ZT 11 and then tested for LTM 24 h later. Daytime-handled animals demonstrated robust LTM with significantly shorter total response times (Figure 1B) and mouth times (Figure S1B) than naïve animals. These results suggest that sleep deprivation was responsible for impairing the induction of LTM independent of the handling protocol itself or the context changes.
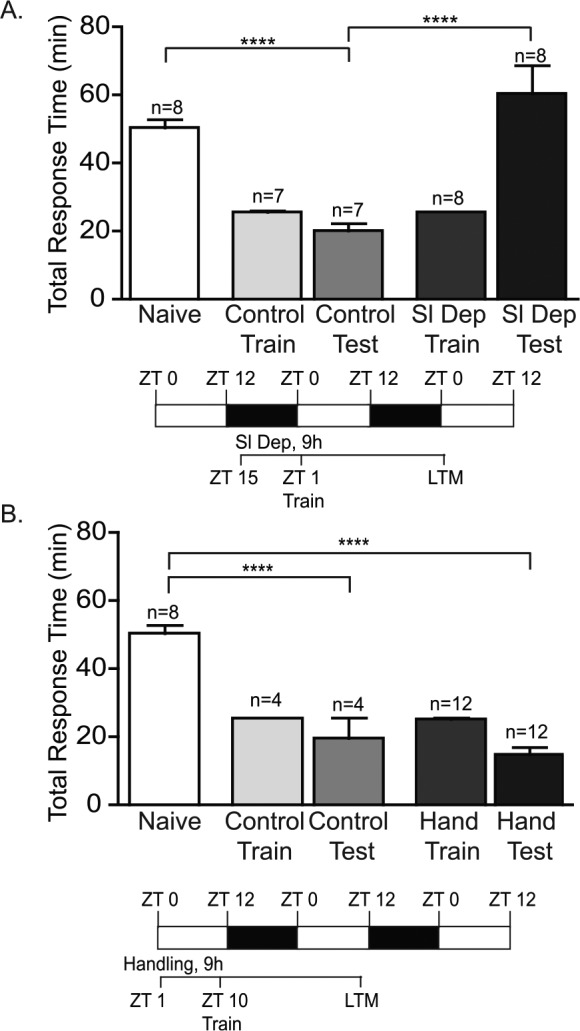
Sleep Deprivation Blocks the Consolidation of LTM
During the molecular consolidation of memory involving macromolecular synthesis and synaptic strengthening following training, memory is labile and can be disrupted by sleep deprivation.45–48 In Aplysia, gene expression and protein synthesis after training are necessary for long-term LFI memory.32,49,50 To determine whether the effect of sleep deprivation on the consolidation of memory is phylogenetically conserved, animals were trained at ZT 11 during the late day, sleep deprived for the first 9 h of the night (ZT 12-ZT 21), and then tested for LTM 24 h after training. Sleep deprivation after training impaired LTM as sleep deprived animals had response times significantly greater than non-sleep deprived control animals (Figure 2A; Figure S1C). It should be noted that one sleep deprived animal exhibited LTM. Although this animal was a statistical outlier, this animal was included in the data and analysis for Figure 2A and Figure S1C as in other species, including humans, individual variation may be observed in response to sleep deprivation.51,52 Non-sleep deprived control animals demonstrated robust LTM with significantly decreased response times compared to naïve animals.
Figure 2.
Sleep deprivation blocks the consolidation of long-term memory (LTM). (A) To investigate the effects of sleep deprivation on consolidation of memory, animals were trained at ZT 11 and then sleep deprived for first 9 h of the night, during the period of molecular consolidation. No differences existed in training duration between groups. Although all groups were trained prior to sleep deprivation or handling, the training columns on the figure are labeled to correspond with subsequent procedures. Animals were tested for LTM 24 h after training. Sleep deprived trained animals (Sl Dep Test) did not exhibit LTM with long response times significantly different from the non-sleep deprived trained animals (Control Test; one-way analysis of variance F(4,34) = 31.74, P < 0.0001). Numbers of animals in each group are shown above the columns. Asterisks represent Bonferroni post hoc analyses ****P < 0.0001 for testing between naïve and non-sleep deprived animals and between non-sleep deprived and sleep deprived animals and *P < 0.05 for testing between naive and sleep deprived animals. (B) Daytime handling did not affect memory consolidation. Daytime, handled-trained animals exhibited robust LTM with short response times (Hand Test) similar to nonhandled trained animals (Control Test) that were significantly different than naïve animals (one-way analysis of variance F(4,25) = 32.63, P < 0.0001). Asterisks represent Bonferroni post hoc analyses ****P < 0.0001.
Potentially, the handling and context changes used during sleep deprivation could interfere with the molecular consolidation of the LFI memory independent of the effects of sleep loss. To test for the possibility that handling after training resulted in the disruption of LTM, animals were trained at ZT 1 early in the day and then handled for 9 h following training with context changes every 30 min. The degree of handling of daytime animals was based on the amount of handling needed to achieve sleep deprivation in previous experiments. Animals that were trained and handled during the day showed robust LTM similar to nonhandled trained control animals (Figure 2B, Figure S1D). Thus, the adverse effects of sleep deprivation on memory consolidation appear due to the loss of sleep and not the handling procedures used for sleep deprivation.
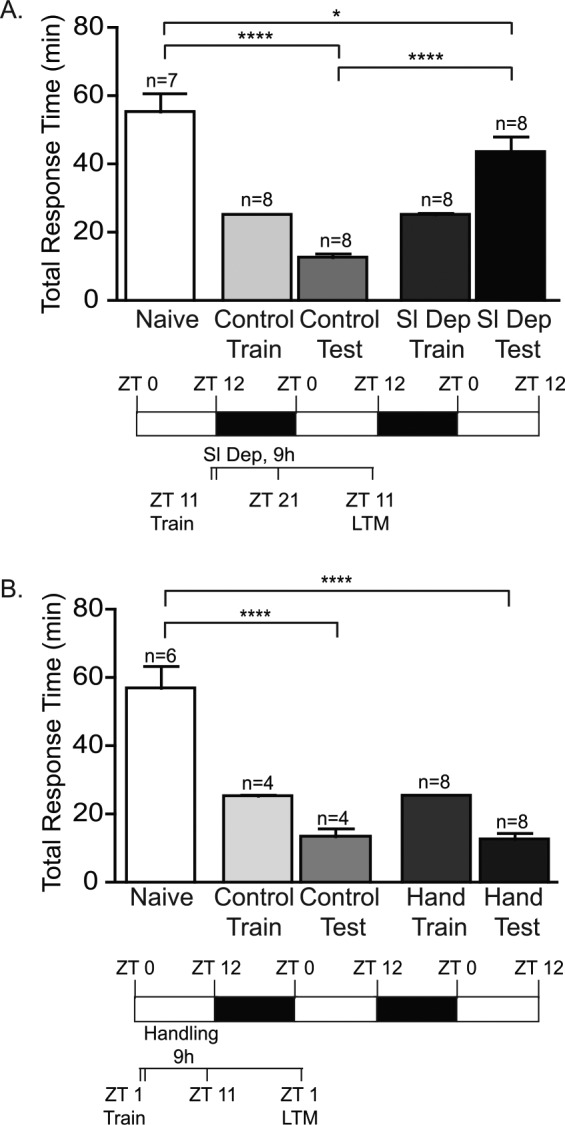
Sleep Deprivation Does Not Affect Memory Recall
LTM is generally more stable following the period of macro-molecular synthesis and molecular consolidation.40,53 However, recall of memory can be affected by external factors. To determine whether sleep deprivation affected the recall of memory independently of interference with molecular consolidation of LFI memory, animals were trained early in the day at ZT 1, sleep deprived for the last 9 h of the night (ZT 15–ZT 24), and then tested 1 h following sleep deprivation at ZT 1 the next day. Sleep deprived animals showed a significant decrease in total response time when compared to naïve animals, reflecting the expression of LTM (Figure 3A; Figure S1E). Thus, sleep deprivation did not affect recall of memory when sleep deprivation occurred after the period of molecular consolidation. Non-sleep deprived trained control animals also showed robust LTM, with response times significantly decreased compared to naïve animals. In rats, gentle handling 12 h posttraining interfered with contextual fear memory when rats were handled during either wake or sleep phases.42 Although handling immediately before or after training did not obstruct LTM formation in our previous experiments, we tested whether handling specifically interfered with memory recall or the expression of memory. Similar to the protocol design used by Cai and colleagues to independently assess the effects of handling on associative memory,42 animals were trained at ZT 11 and then remained undisturbed throughout the sleep phase. Animals were then handled for 9 h beginning 14 h after training. Trained daytime-handled animals demonstrated robust LTM with significantly decreased response time compared to naïve animals (Figure 3B; Figure S1F). These results demonstrate that sleep deprivation using handling and context changes did not affect LTM formation or recall when it occurred after the period of molecular consolidation of the memory.
Figure 3.
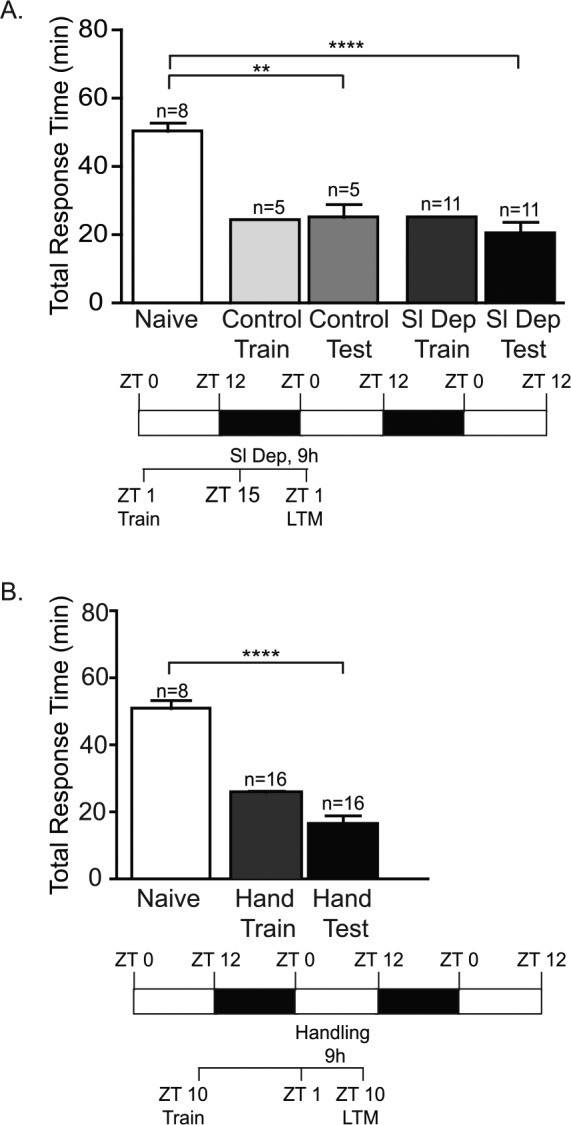
Sleep deprivation does not affect recall of long-term memory (LTM). (A) To investigate the effects of sleep deprivation on memory recall, animals were trained early in the day at ZT 1 and then sleep deprived for the last 9 h of the night (ZT 15–ZT 24) after the window of molecular consolidation. No significant differences were observed between the training times for non-sleep deprived animals (Control Train) and sleep deprived animals (Sl Dep Train). Animals were tested for LTM 24 h after training. Trained sleep deprived animals showed a significant decrease in total response time compared to naive animals (one-way analysis of variance A F(4,35) = 25.55, P < 0.0001). Numbers of animals in each group are shown above the columns. Asterisks represent Bonferroni post hoc analyses ****P < 0.0001 for testing between naïve and sleep deprived animals and **P < 0.01 for testing between naive and non-sleep deprived animals. (B) Daytime handling did not affect recall of memory. Trained daytime-handled control animals (Hand Test) showed robust LTM with significantly shorter response compared to naïve animals (one-way analysis of variance F(2,37) = 69.79, P < 0.0001) Asterisks represent Bonferroni post hoc analyses ****P < 0.0001.
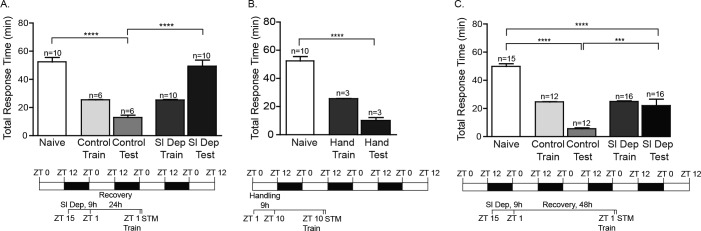
Sleep Deprivation Persistently Inhibits the Induction of LTM
To determine the extent to which a single night of acute sleep deprivation affected the induction of long-term LFI memory, animals were sleep deprived from ZT 15–ZT 24 and then allowed to recover undisturbed for 24 h. Animals were trained at ZT 1, 25 h after the end of the sleep deprivation protocol, and tested for LTM 24 h after training (∼ 48 h after sleep deprivation). Sleep deprived animals failed to show LTM with response times similar to naïve animals whereas control animals had significantly decreased response times (Figure 4A; Figure S1G). The deleterious effects of sleep deprivation on attention, performance, and memory can be ameliorated with recovery sleep, although the amount of recovery sleep needed varies dependent on the type of learning and the extent of sleep deprivation. As 24 h was insufficient for recovery from acute sleep deprivation, animals were sleep deprived and then allowed to recover for 48 h prior to LFI training. We found that sleep deprived animals allowed to recover for 48 h prior to training exhibited significant LTM when tested 24 h later with total response times significantly different than naïve animals (Figure 4C; Figure S1I). Thus, acute sleep deprivation for 9 h persistently inhibited the induction of LTM for 24 h with the impairments in induction ameliorated after 48 h. Analogous daytime handling experiments were performed to verify that handling alone had no lasting effects on the induction of memory. Daytime-handled animals displayed robust LTM significantly different than naïve animals (Figure 4B; Figure S1H).
Figure 4.
Sleep deprivation impairs long-term memory (LTM) for at least 24 h. To determine the persistence of sleep deprivation on LTM, animals were sleep deprived for 9 h and allowed to recover for 24 or 48 h prior to learning that food is inedible (LFI) training. No significant differences were observed between the training times for non-sleep deprived animals (Control Train) and sleep deprived animals (Sl Dep Train). (A) Animals were sleep deprived from ZT 15–ZT 24, allowed to recover for 24 h, and trained at ZT 1 the following day. Acute sleep deprivation inhibited LTM when animals were trained 24 hours after sleep deprivation. Trained sleep deprived animals (Sl Dep Test) had significantly longer total response times compared to trained non-sleep deprived animals (Control Test; one-way analysis of variance F(4,33) = 28.48, P < 0.0001). Numbers of animals in each group are shown above the columns. Asterisks represent Bonferroni post hoc analyses ****P < 0.0001. (B) Trained daytime-handled control animals (Hand Test) had shorter total response times during testing significantly different than naïve animals (one-way analysis of variance F(2,21) = 70.99, P < 0.0001). Asterisks represents Bonferroni post hoc analyses ****P < 0.0001 for testing between naïve and handled animals. (C) Forty-eight hours recovery time attenuated the effects of sleep deprivation as trained sleep deprived animals (Sl Dep Test) demonstrated LTM similar to trained non-sleep deprived animals (Control Test). Sleep deprived animals showed a significant decrease in response time when compared to naïve animals (one-way analysis of variance F(4,44) = 48.20, P < 0.0001). Asterisks represent Bonferroni post hoc analyses ****P < 0.0001.
Sleep Deprivation Blocks STM
The effect of sleep deprivation on the induction of short- and LTM may vary due to differences in the molecular mechanisms necessary for the induction of these two forms of memory. In LFI memory, the induction of LTM requires the activation of multiple kinase signaling pathways, including protein kinase A, protein kinase C and mitogen-activated protein kinase (MAPK) signaling, and macromolecular synthesis.32,38,39 In contrast, STM does not require protein synthesis and appears to rely primarily on MAPK activation,32,38 even though the training protocol for STM and LTM is identical. To determine whether 9 h of sleep deprivation was sufficient to disrupt the induction of STM, animals were sleep deprived from ZT 15–ZT 24 with LFI training performed at ZT 1 and animals tested 30 min after the end of training for STM. Sleep deprivation inhibited the induction of STM with animals actually demonstrating greater response times than naïve animals (Figure 5A; Figure S2A in the supplemental material). Control animals exhibited significantly reduced response times, indicating robust STM (Figure 5A). Although gentle handling of the animals did not affect the formation of LTM in our experiments, handling could interfere with the expression of STM because testing for STM occurs approximately 2 h after the end of the handling period. In control experiments, daytime-handled animals showed robust STM upon testing with significantly decreased response times compared to naïve animals for both total response time and the time the food was retained in the mouth. These response times were similar to those observed during testing of nonhandled animals (Figure 5B; Figure S2B), suggesting that the handling procedure itself did not affect STM. These results indicate that 9 h of acute sleep deprivation inhibited the induction of STM.
Figure 5.
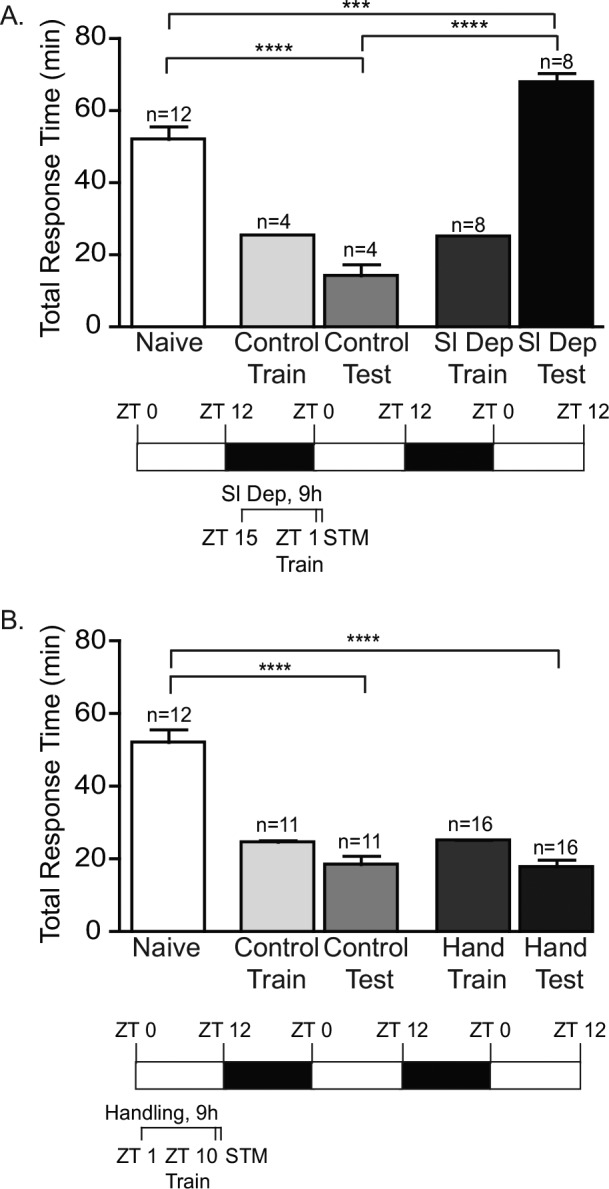
Sleep deprivation blocks short-term memory (STM). (A) To investigate the effects of sleep deprivation on the induction of STM, animals were sleep deprived for last 9 h of the night, trained at ZT 1 and tested for STM 30 min after training. No significant differences were observed between the training times for non-sleep deprived animals (Control Train) and sleep deprived animals (Sl Dep Train). Trained sleep deprived animals did not exhibit STM with significantly longer response (Sl Dep Test) compared to trained non-sleep deprived animals (Control Test; one-way analysis of variance F(4,31) = 49.26, P < 0.0001). Numbers of animals in each group are shown above the columns. Asterisks represent Bonferroni post hoc analyses ****P < 0.0001 for testing between naïve and non-sleep deprived animals and between non-sleep deprived and sleep deprived animals. ***P < 0.001 for testing between naïve and sleep deprived animals. (B) Daytime handling had no effect on the training of animals after handling with training times similar between groups (Control Train compared to Hand Train). Daytime handling did not block STM. Trained daytime handled control animals exhibited STM with total response times (Hand Test) similar to trained nonhandled animals (Control Test) that were significantly different than naïve animals (one-way analysis of variance F(4,61) = 35.50, P < 0.0001) Asterisks represent Bonferroni post hoc analyses ****P < 0.0001.
Sleep Deprivation Persistently Inhibits STM
As the training and testing procedures for LFI memory are identical, LFI testing acts as an additional training session. In the experiments performed to test the effects of sleep deprivation on the induction of LTM shown in Figure 1A, the testing session for LTM acted as a second training session (mean session length = 60.45 min ± 2.40) occurring 24 h after sleep deprivation. To determine how long the adverse consequences of sleep deprivation on STM persisted, as an extension of these experiments, animals that showed no LTM were re-tested 30 min later for STM (Figure S3 in the supplemental material). Retrained animals did not show STM when compared to the handled STM retest controls with significantly greater response times. Although these experiments suggested that sleep deprivation interfered with the induction of STM for 24 h, it is possible that the initial training immediately after the period of sleep deprivation either aggravated the stress of sleep deprivation or disrupted the recovery from sleep deprivation, thereby inhibiting the efficacy of training 24 h later.
Consequently, independent experiments were performed to determine the persistence effects of sleep deprivation on STM. Animals were sleep deprived from ZT 15–ZT 24 and allowed to recover for 24 h prior to training at ZT 1 (24 h after sleep deprivation). Sleep deprived animals did not show STM when tested 30 min after training, with response times similar to naïve animals (Figure 6A; Figure S2C). Non-sleep deprived control animals exhibited robust STM with response times significantly decreased compared to naïve and sleep deprived animals. Daytime-handled controls were also trained and tested for STM to rule out any detrimental effects of handling stress on memory formation with animals demonstrating significant STM (Figure 6B; Figure S2D). To determine the period of recovery necessary following sleep deprivation on STM, animals were sleep deprived from ZT 15–ZT 24 and allowed to recover for 48 h before training. Forty-eight hours of recovery following sleep deprivation was sufficient to permit the induction of STM with experimental animals exhibiting decreased response times compared to naïve animals. However, sleep deprivation still appeared to take a toll on STM as sleep deprived animals exhibited response times significantly greater than trained non-sleep deprived control animals, suggesting that the memory was less robust in the sleep deprived animals (Figure 6C; Figure S2E).
Figure 6.
Sleep deprivation impairs the induction of short-term memory (STM) for at least 24 h. To determine the persistence of sleep deprivation on short-term memory, animals were sleep deprived for 9 h and allowed to recover for 24 or 48 h prior to learning that food is inedible (LFI) training. (A) Animals were sleep deprived from ZT 15–ZT 24, allowed to recover for 24 h, and trained at ZT 1 the following day. Acute sleep deprivation inhibited STM when animals were trained 24 h after sleep deprivation. Trained sleep deprived animals (Sl Dep Test) did not exhibit STM with significantly longer total response times compared to trained non-sleep deprived animals (Control Test; one-way analysis of variance [ANOVA] F(4,37) = 31.87, P < 0.0001). Numbers of animals in each group are shown above the columns. Asterisks represent Bonferroni post hoc analyses ****P < 0.0001. (B) Trained daytime handled animals (Hand Test) had significantly shorter total response times compared to naïve animals (one-way ANOVA F(2,13) = 32.06, P < 0.0001). Asterisks represent Bonferroni post hoc analyses ****P < 0.0001. (C) Forty-eight hours recovery time ameliorated the effects of sleep deprivation on STM as trained sleep deprived animals (Sl Dep Test) demonstrated STM compared to naïve animals, although the memory was not as robust as in trained non-sleep deprived animals (Control Test; one-way ANOVA F(4,66) = 36, P < 0.0001). Asterisks represent Bonferroni post hoc analyses. ****P < 0.0001 for testing between naive and non-sleep deprived animals and between naïve and sleep deprived animals. ***P < 0.001 for testing between non-sleep deprived and sleep deprived animals
Additional Training Does Not Rescue the Induction of LTM Immediately after Sleep Deprivation but Attenuates the Persistent Effects of Sleep Deprivation
For many forms of learning, spaced training sessions result in stronger and longer lasting memory.54 However, with LFI memory a single training session is sufficient to induce LTM lasting 48 hours.55 In other learning paradigms for which one-trial memory is formed, additional training sessions can compensate for deficits that would normally prevent LTM.56 To determine whether additional training ameliorates the effects of sleep deprivation, the animals trained and tested for STM in the experiments shown in Figure 5A were retested after 24 h for LTM. As the testing session is procedurally identical to training, the STM test acted as a second training session with a mean length of 68.08 min ± 2.45. Two training sessions immediately after sleep deprivation were insufficient to induce LTM with sleep deprived animals showing significantly greater response times during testing than control animals (Figure 7A). To determine if additional training was sufficient to overcome the persistent effects of sleep deprivation, animals that were trained and tested for STM 24 h after sleep deprivation in the experiments shown in Figure 6A were tested again 24 h later for LTM. The second training session had a mean length of 49.46 min ± 4.33. Upon testing 24 h later, these animals demonstrated significantly decreased response times indicative of robust LTM when compared to naïve animals (Figure 7B). Thus, additional training appears sufficient to mitigate the persistent effects of sleep deprivation, although it is not adequate to overcome the acute effects of sleep deprivation on memory formation.
Figure 7.
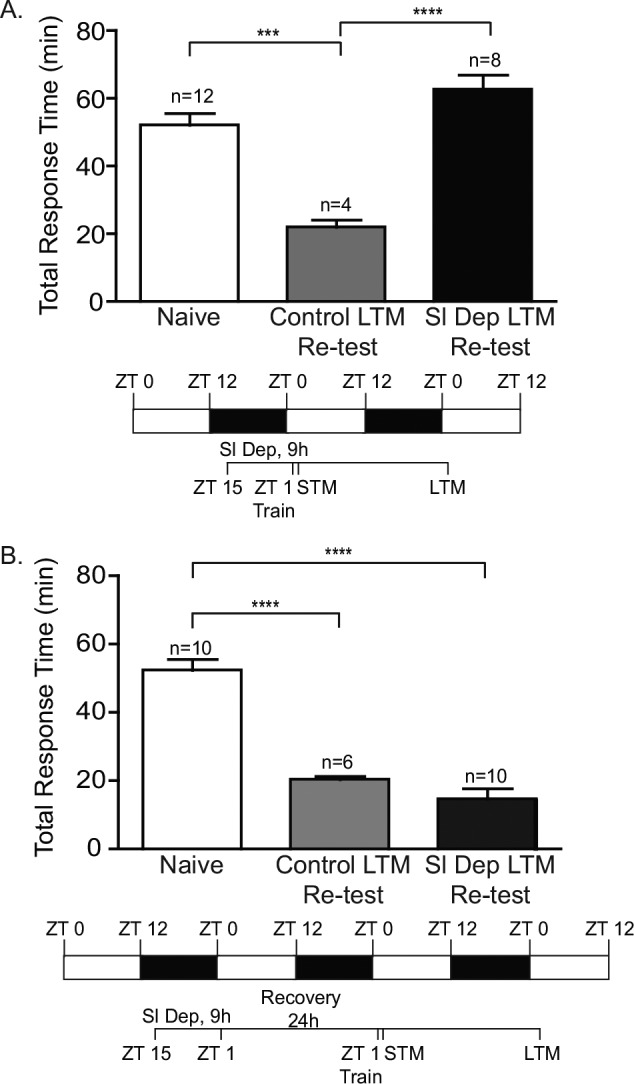
Overtraining does not rescue long-term memory (LTM) immediately after sleep deprivation, but attenuates the persistent effects of sleep deprivation on LTM 24 h after sleep deprivation. (A) To determine whether additional training mitigated the effects of sleep deprivation, animals were retested 24 h for LTM after the initial short-term memory (STM) test. As the testing session is procedurally identical to training, the STM test acted as a second training session. Control animals demonstrated robust LTM following the two training sessions (Control LTM retest). Sleep deprived animals that failed to exhibit STM (Figure 4) were retested for LTM 24 h later (Sl Dep LTM retest). Two training sessions did not induce LTM in sleep deprived animals (one-way analysis of variance F(2,21) = 17.54, P < 0.0001). Numbers of animals in each group are shown above the columns. Asterisks represent Bonferroni post hoc analyses. ***P < 0.001 for testing between naïve and control trained animals and ****P < 0.0001 for testing between control trained and sleep deprived animals. (B) To determine if additional training was sufficient to overcome the persistent effects of sleep deprivation, sleep deprived animals that did not show STM when trained 24 h after sleep deprivation (Figure 5) were retested for LTM 24 h later (Sl Dep LTM retest). STM testing acted as a second training session. Upon testing 24 h later, sleep deprived animals demonstrated significantly decreased response times when compared to naïve animals, indicating that these animals formed LTM after two training sessions (one-way analysis of variance F(2,23) = 37.39, P < 0.0001). Non-sleep deprived animals also demonstrated robust LTM following the two training sessions (Control LTM retest). Asterisks represent Bonferroni post hoc analyses ****P < 0.0001.
DISCUSSION
Almost all animals, vertebrates and invertebrates, exhibit sleep and rest behavior.57 Although the functions of sleep are not clearly defined, sleep has important contributions in a wide array of physiological functions including cell repair, energy restoration, metabolic regulation, and immune functions.58–62 Sleep also appears necessary for memory, with sleep deprivation leading to decrements in cognitive performance and memory impairments.4,63–65 Additionally, sleep following learning can enhance memory strength, particularly for hippocampal-dependent learning in humans and animal models.66–69 Invertebrates, with their comparatively simple nervous system, have proven invaluable for elucidating the molecular mechanisms underlying complex behavior. The processes underlying memory formation are evolutionarily conserved; however, investigations of sleep and memory in invertebrates have been primarily limited to studies in insects, namely Drosophila and bees. The biphasic sleep patterns of these models can potentially complicate their use for studying sleep and memory. In Drosophila, although the majority of sleep occurs during the night, flies sleep a substantial amount during the day with males exhibiting more daytime sleep than females.19,21,70–72 Similarly, forager bees sleep primarily during the night73–75 but also nap during the day.76 In all other castes, sleep is distributed with approximately equal sleep occurring during the day and the night.76 Recently, the nematode Caenorhabditis elegans has been identified as a simple model for investigating the functions of sleep.77 Sleep in C. elegans shares many conserved features with Drosophila sleep or vertebrate sleep; however, the sleep-like states present in C. elegans are distinctive in occurrence. Sleep, through discrete mechanisms, occurs either developmentally during the quiescent periods between larval stages or following the induction of cellular stress with the amount of sleep correlated to the degree of the stressful stimulus.22,77–80 Thus, the timing of developmental sleep and the stress-induced sleep during adulthood in C. elegans make it more difficult to study the interactions of sleep with memory.
A. californica represents a novel invertebrate model system to study sleep and the interactions of sleep with learning and memory. Aplysia sleep in consolidated bouts with longer bouts occurring in the first half of the night, demonstrate rebound sleep after sleep deprivation, and exhibit longer latencies to appetitive and aversive stimuli during sleep.27 The almost complete absence of daytime rest and the lack of fragmentation in the sleep pattern at night,27 reminiscent of the human sleep pattern, facilitates the study of acute sleep deprivation on the induction of STM and LTM. Aplysia as a model system provides the future potential to study the interactions between sleep and memory at the level of the neuronal circuit as associative and nonassociative learning paradigms have been well characterized. The associative LFI paradigm permits direct comparisons between the effects of sleep deprivation on STM and LTM as the same training protocol induces temporally and mechanistically distinct forms of memory.
Identifying an animal model that demonstrates strong behavioral effects on learning and memory after a single night of sleep deprivation is important for our eventual understanding of the effects of extended wakefulness in humans at the molecular and neuronal levels. We found that 9 h of acute sleep deprivation in Aplysia blocked the effectiveness of LFI training inhibiting the induction of STM and LTM. Moreover, sleep deprivation inhibited the induction of both STM and LTM for at least 24 h. In comparison, studies of sleep deprivation in Drosophila for associative olfactory memory indicate that the induction of STM, but not LTM, is inhibited by 9 h or even 24 h of prior sleep deprivation.6 Short-term learning was also shown to be affected by prior sleep deprivation in Drosophila using an aversive photoaxis assay.81,82 In contrast, sleep deprivation in bees does not affect the acquisition of memory but rather its consolidation.18,74 Based on our behavioral studies, Aplysia presents advantages as a simple model system for understanding the interactions between sleep deprivation and the induction of STM and LTM.
Acute total sleep deprivation in humans results in decrements in short-term performance and memory as well as multiple types of long-term memory.63,83 Researchers analyzing occupational performance found that 1 night of sleep deprivation and prolonged wakefulness significantly decreased performance.84 However, the effects of sleep deprivation can vary greatly on performance and memory between individuals and cognitive tasks,51,52 making it difficult to identify the mechanisms through which sleep deprivation affects cognitive performance. Although differences have been reported across studies, some studies suggest that the amount of recovery sleep needed may be underestimated and that 2 nights of recovery sleep following 1 night of sleep loss is necessary for full recovery85,86 In contrast, in Drosophila, performance can be restored by recovery sleep as short as 2 h82 or 4 h for associative olfactory conditioning.6 Thus, Aplysia may be a useful model for insights into the mechanisms through which the effects of sleep deprivation affect performance and memory in higher species as both immediate and persistent effects of sleep deprivation on STM and LTM can be observed.
Potentially, sleep deprivation affected memory formation in our studies via changes in the salience of the appetitive stimulus used in training. We find this explanation unlikely as sleep deprivation does not appear to decrease the perception of strong stimuli. In Drosophila, sleep deprivation does not have an effect on baseline olfactory responses or response to electric shock6 and in rats, rapid eye movement (REM) sleep deprivation was found to have little effect on perception of electric shocks.87 As all animals in our studies were removed from appetitive stimuli 6 days prior to training, the presentation of the netted seaweed represented a strong appetitive stimulus. Analysis of the latency for the animals to respond to the seaweed during training (summated for all experiments in which acute sleep deprivation occurred prior to training) determined that the latency to respond for sleep deprived animals was similar to the latencies seen in previous studies using the LFI paradigm (data not shown).
The detrimental effect of sleep disruption during the molecular consolidation of memory appears to be a conserved feature across species. We found that sleep deprivation following training during the late day impaired the consolidation of LTM. This is consistent with previous research in rodents demonstrating that posttraining sleep deprivation interferes with LTM7,45,88–92 and studies in Drosophila.6,93 In contrast, when sleep deprivation occurred after the period of macromolecular synthesis necessary for 24 h memory, we found that long-term LFI memory was unaffected by sleep deprivation. Similarly in other animal models, LTM is not affected when sleep deprivation occurs post consolidation.45
The question arises as to whether sleep is necessary for memory formation or whether the role of sleep deprivation in memory depends on the timing of sleep deprivation in relationship to the molecular processes involved in memory formation. Unlike rodent models, Aplysia do not exhibit any sleep during their active period, maintaining a strong diurnal pattern of activity.27 Although long-term memory formation in Aplysia is strongly regulated by the circadian clock with training-induced kinase activation and gene expression dependent on the time of training,94,95 there appear to be no differences in the molecular pathways required for 24 h memory when animals are trained early in the day compared to later in the day, as long as the training still occurs during the animal's active period. Presumably, sleep deprivation interferes with molecular signaling processes affecting the induction of gene expression and protein synthesis necessary for LTM. Thus, when sleep deprivation follows late-day training, the molecular consolidation of memory is disrupted. We hypothesize that it is the disruption of normally timed sleep in relation to molecular consolidation that affects memory formation. Potentially, if longer forms of LFI memory, e.g., 48 h or 72 h memory, were examined that require additional later phases of molecular consolidation,55 then sleep deprivation overlapping with these subsequent periods of protein synthesis would interfere with memory.
In some cases, long-term memory can be state dependent such that memory is enhanced when conditions or brain states are similar during training and testing of the behavior. State dependence of memory has been demonstrated for aversive fear memory, emotional memory, and drug-related behaviors.96,97 In the desert locust, decision making in an odor-associated food choice test occurs based on a state-dependent valuation of the choices98 It is possible that the effects of sleep deprivation that we observed on long-term memory in Aplysia were state dependent, with the neuronal effects of sleep deprivation incorporated as part of the learning context. In mice, memory impairments due to sleep deprivation of REM sleep can be state dependent. For avoidance tasks, decrements in LTM are observed with either pretraining or pretesting REM sleep deprivation; however, the effects on memory are mitigated when animals receive sleep deprivation prior to training and testing.99 Although we could not test whether the impairments in LTM were state dependent as 2 consecutive nights of 9 h of sleep deprivation rendered the animals unresponsive to the stimulus during testing (data not shown), we think it is unlikely that state dependence played a role in the long-term memory impairments as the induction of STM was similarly affected by sleep deprivation. In mice, previous research has shown that the effects of total sleep deprivation on memory were not state dependent for avoidance tasks.99
Understanding the temporal and molecular aspects of the recovery of cognitive function and performance following sleep deprivation is critical in light of the increasing occupational demands on individuals in modern society. Recent research in mice highlighted the role of cyclic adenosine monophosphate signaling pathway48,100 and the suppression of protein synthesis as a probable mechanism through which sleep deprivation affects the consolidation of long-term memory.47 However, the mechanisms through which sleep deprivation affects STM or the induction of LTM remain unclear. The current study demonstrated the phylogenetically conserved nature of the necessity of sleep for memory formation outlining the behavioral parameters of the interactions between acute sleep deprivation and STM and LTM in Aplysia. These behavioral studies have established Aplysia, with its relatively simple neural circuitry, as a valid model system for studying the interactions between sleep and memory formation and provide a foundation for future studies delineating the underlying mechanisms through which sleep affects memory. Understanding these mechanisms is necessary for the potential identification of methods to alleviate cognitive deficits incurred due to restricted sleep and to mitigate the toll that the technological and occupational demands of a modern society exacts upon individuals.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported by NIH grant R21NS088835 to Dr. Lyons. The authors have indicated no financial conflicts of interest. The work was performed at Florida State University, Tallahassee Florida.
REFERENCES
- 1.Bixler E. Sleep and society: an epidemiological perspective. Sleep Med. 2009;10(Suppl 1):S3–6. doi: 10.1016/j.sleep.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Patrick GT, Gilbert JA. Studies from the psychological laboratory of the University of Iowa. On the effects of loss of sleep. Psychol Rev. 1896;3:469–83. [Google Scholar]
- 3.Alvarenga TA, Patti CL, Andersen ML, et al. Paradoxical sleep deprivation impairs acquisition, consolidation, and retrieval of a discriminative avoidance task in rats. Neurobiol Learn Mem. 2008;90:624–32. doi: 10.1016/j.nlm.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Havekes R, Meerlo P, Abel T. Animal studies on the role of sleep in memory: from behavioral performance to molecular mechanisms. Curr Top Behav Neurosci. 2015;25:183–206. doi: 10.1007/7854_2015_369. [DOI] [PubMed] [Google Scholar]
- 5.Kreutzmann JC, Havekes R, Abel T, Meerlo P. Sleep deprivation and hippocampal vulnerability: changes in neuronal plasticity, neurogenesis and cognitive function. Neuroscience. 2015;309:173–90. doi: 10.1016/j.neuroscience.2015.04.053. [DOI] [PubMed] [Google Scholar]
- 6.Li X, Yu F, Guo A. Sleep deprivation specifically impairs short-term olfactory memory in Drosophila. Sleep. 2009;32:1417–24. doi: 10.1093/sleep/32.11.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prince TM, Wimmer M, Choi J, Havekes R, Aton S, Abel T. Sleep deprivation during a specific 3-hour time window post-training impairs hippocampal synaptic plasticity and memory. Neurobiol Learn Mem. 2014;109:122–30. doi: 10.1016/j.nlm.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–8. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- 9.Vecsey CG, Park AJ, Khatib N, Abel T. Effects of sleep deprivation and aging on long-term and remote memory in mice. Learn Mem. 2015;22:197–202. doi: 10.1101/lm.036590.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan Z, Peng X, Fang J. Sleep deprivation impairs spatial memory and decreases extracellular signal-regulated kinase phosphorylation in the hippocampus. Brain Res. 2004;1018:38–47. doi: 10.1016/j.brainres.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 11.Hagewoud R, Havekes R, Novati A, Keijser JN, Van der Zee EA, Meerlo P. Sleep deprivation impairs spatial working memory and reduces hippocampal AMPA receptor phosphorylation. J Sleep Res. 2010;19:280–8. doi: 10.1111/j.1365-2869.2009.00799.x. [DOI] [PubMed] [Google Scholar]
- 12.Smith C, Rose GM. Posttraining paradoxical sleep in rats is increased after spatial learning in the Morris water maze. Behav Neurosci. 1997;111:1197–204. doi: 10.1037//0735-7044.111.6.1197. [DOI] [PubMed] [Google Scholar]
- 13.Bushey D, Huber R, Tononi G, Cirelli C. Drosophila hyperkinetic mutants have reduced sleep and impaired memory. J Neurosci. 2007;27:5384–93. doi: 10.1523/JNEUROSCI.0108-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cirelli C. The genetic and molecular regulation of sleep: from fruit flies to humans. Nat Rev Neurosci. 2009;10:549–60. doi: 10.1038/nrn2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feil R, Hölter SM, Weindl K, et al. cGMP-dependent protein kinase I, the circadian clock, sleep and learning. Commun Integr Biol. 2009;2:298–301. doi: 10.4161/cib.2.4.8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dissel S, Melnattur K, Shaw PJ. Sleep, performance, and memory in flies. Curr Sleep Med Rep. 2015;1:47–54. doi: 10.1007/s40675-014-0006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donlea JM, Thimgan MS, Suzuki Y, Gottschalk L, Shaw PJ. Inducing sleep by remote control facilitates memory consolidation in Drosophila. Science. 2011;332:1571–6. doi: 10.1126/science.1202249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beyaert L, Greggers U, Menzel R. Honeybees consolidate navigation memory during sleep. J Exp Biol. 2012;215:3981–8. doi: 10.1242/jeb.075499. [DOI] [PubMed] [Google Scholar]
- 19.Hendricks JC, Finn SM, Panckeri KA, et al. Rest in Drosophila is a sleep-like state. Neuron. 2000;25:129–38. doi: 10.1016/s0896-6273(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 20.Le Glou E, Seugnet L, Shaw PJ, Preat T, Goguel V. Circadian modulation of consolidated memory retrieval following sleep deprivation in Drosophila. Sleep. 2012;35:1377–84. doi: 10.5665/sleep.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–7. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- 22.Raizen DM, Zimmerman JE, Maycock MH, et al. Lethargus is a Caenorhabditis elegans sleep-like state. Nature. 2008;451:569–72. doi: 10.1038/nature06535. [DOI] [PubMed] [Google Scholar]
- 23.Frank MG, Waldrop RH, Dumoulin M, Aton S, Boal JG. A preliminary analysis of sleep-like states in the cuttlefish Sepia officinalis. PLoS One. 2012;7:e38125. doi: 10.1371/journal.pone.0038125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cirelli C, Tononi G. Is sleep essential? PLoS Biol. 2008;6:e216. doi: 10.1371/journal.pbio.0060216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tobler I. Effect of forced locomotion on the rest-activity cycle of the cockroach. Behav Bran Res. 1983;8:351–60. doi: 10.1016/0166-4328(83)90180-8. [DOI] [PubMed] [Google Scholar]
- 26.Zimmerman JE, Naidoo N, Raizen DM, Pack AI. Conservation of sleep: insights from non-mammalian model systems. Trends Neurosci. 2008;31:371–6. doi: 10.1016/j.tins.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vorster AP, Krishnan HC, Cirelli C, Lyons LC. Characterization of sleep in Aplysia californica. Sleep. 2014;37:1453–63. doi: 10.5665/sleep.3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baxter DA, Byrne JH. Feeding behavior of Aplysia: a model system for comparing cellular mechanisms of classical and operant conditioning. Learn Mem. 2006;13:669–80. doi: 10.1101/lm.339206. [DOI] [PubMed] [Google Scholar]
- 29.Byrne JH, Hawkins RD. Nonassociative learning in invertebrates. Cold Spring Harb Perspect Biol. 2015:7. doi: 10.1101/cshperspect.a021675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castellucci VF, Carew TJ, Kandel ER. Cellular analysis of long-term habituation of the gill-withdrawal reflex of Aplysia californica. Science. 1978;202:1306–8. doi: 10.1126/science.214854. [DOI] [PubMed] [Google Scholar]
- 31.Kandel ER, Schwartz JH. Molecular biology of learning: modulation of transmitter release. Science. 1982;218:433–43. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- 32.Michel M, Green CL, Lyons LC. PKA and PKC are required for long-term but not short-term in vivo operant memory in Aplysia. Learn Mem. 2011;18:19–23. doi: 10.1101/lm.2026311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michel M, Lyons LC. Unraveling the complexities of circadian and sleep interactions with memory formation through invertebrate research. Front Syst Neurosci. 2014;8:133. doi: 10.3389/fnsys.2014.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Esdin J, Pearce K, Glanzman DL. Long-term habituation of the gill-withdrawal reflex in aplysia requires gene transcription, calcineurin and L-type voltage-gated calcium channels. Front Behav Neurosci. 2010;4:181. doi: 10.3389/fnbeh.2010.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hawkins RD, Kandel ER, Bailey CH. Molecular mechanisms of memory storage in Aplysia. Biol Bull. 2006;210:174–91. doi: 10.2307/4134556. [DOI] [PubMed] [Google Scholar]
- 36.Kandel ER, Dudai Y, Mayford MR. The molecular and systems biology of memory. Cell. 2014;157:163–86. doi: 10.1016/j.cell.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Susswein AJ, Schwarz M, Feldman E. Learned changes of feeding behavior in Aplysia in response to edible and inedible foods. J Neurosci. 1986;6:1513–27. doi: 10.1523/JNEUROSCI.06-05-01513.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michel M, Green CL, Gardner JS, Organ CL, Lyons LC. Massed training-induced intermediate-term operant memory in Aplysia requires protein synthesis and multiple persistent kinase cascades. J Neurosci. 2012;32:4581–91. doi: 10.1523/JNEUROSCI.6264-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michel M, Gardner JS, Green CL, Organ CL, Lyons LC. Protein phosphatase-dependent circadian regulation of intermediate-term associative memory. J Neurosci. 2013;33:4605–13. doi: 10.1523/JNEUROSCI.4534-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dudai Y, Karni A, Born J. The consolidation and transformation of memory. Neuron. 2015;88:20–32. doi: 10.1016/j.neuron.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Sweatt JD. Mechanisms of memory. second edition. San Diego, CA: Academic Press. Elsevier; 2010. [Google Scholar]
- 42.Cai DJ, Shuman T, Harrison EM, Sage JR, Anagnostaras SG. Sleep deprivation and Pavlovian fear conditioning. Learn Mem. 2009;16:595–9. doi: 10.1101/lm.1515609. [DOI] [PubMed] [Google Scholar]
- 43.Campbell IG, Guinan MJ, Horowitz JM. Sleep deprivation impairs long-term potentiation in rat hippocampal slices. J Neurophysiol. 2002;88:1073–6. doi: 10.1152/jn.2002.88.2.1073. [DOI] [PubMed] [Google Scholar]
- 44.Longordo F, Fan J, Steimer T, Kopp C, Lüthi A. Do mice habituate to “gentle handling?” A comparison of resting behavior, corticosterone levels and synaptic function in handled and undisturbed C57BL/6J mice. Sleep. 2011;34:679–81. doi: 10.1093/sleep/34.5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Graves LA, Heller EA, Pack AI, Abel T. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn Mem. 2003;10:168–76. doi: 10.1101/lm.48803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ravassard P, Hamieh AM, Joseph MA, et al. REM sleep-dependent bidirectional regulation of hippocampal-based emotional memory and LTP. Cereb Cortex. 2016;26:1488–500. doi: 10.1093/cercor/bhu310. [DOI] [PubMed] [Google Scholar]
- 47.Tudor JC, Davis EJ, Peixoto L, et al. Sleep deprivation impairs memory by attenuating mTORC1-dependent protein synthesis. Sci Signal. 2016;9:ra41. doi: 10.1126/scisignal.aad4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vecsey CG, Baillie GS, Jaganath D, et al. Sleep deprivation impairs cAMP signalling in the hippocampus. Nature. 2009;461:1122–5. doi: 10.1038/nature08488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levitan D, Lyons LC, Perelman A, et al. Training with inedible food in Aplysia causes expression of C/EBP in the buccal but not cerebral ganglion. Learn Mem. 2008;15:412–6. doi: 10.1101/lm.970408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Michel M, Green CL, Eskin A, Lyons LC. PKG-mediated MAPK signaling is necessary for long-term operant memory in Aplysia. Learn Mem. 2011;18:108–17. doi: 10.1101/lm.2063611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Dongen HP, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. 2004;27:423–33. [PubMed] [Google Scholar]
- 52.Van Dongen HP, Bender AM, Dinges DF. Systematic individual differences in sleep homeostatic and circadian rhythm contributions to neurobehavioral impairment during sleep deprivation. Accid Anal Prev. 2012;45(Suppl):11–6. doi: 10.1016/j.aap.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McGaugh JL. Memory--a century of consolidation. Science. 2000;287:248–51. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 54.Sutton MA, Ide J, Masters SE, Carew TJ. Interaction between amount and pattern of training in the induction of intermediate- and long-term memory for sensitization in Aplysia. Learn Mem. 2002;9:29–40. doi: 10.1101/lm.44802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levitan D, Twitto R, Levy R, Lyons LC, Susswein AJ. A brief retraining regulates the persistence and lability of a long-term memory. Learn Mem. 2010;17:402–6. doi: 10.1101/lm.1820010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Irvine EE, von Hertzen LS, Plattner F, Giese KP. alphaCaMKII autophosphorylation: a fast track to memory. Trends Neurosci. 2006;29:459–65. doi: 10.1016/j.tins.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 57.Hartse KM. The phylogeny of sleep. Handb Clin Neurol. 2011;98:97–109. doi: 10.1016/B978-0-444-52006-7.00007-1. [DOI] [PubMed] [Google Scholar]
- 58.Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3:519–28. [PMC free article] [PubMed] [Google Scholar]
- 59.Davies SK, Ang JE, Revell VL, et al. Effect of sleep deprivation on the human metabolome. Proc Natl Acad Sci U S A. 2014;111:10761–6. doi: 10.1073/pnas.1402663111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Everson CA, Henchen CJ, Szabo A, Hogg N. Cell injury and repair resulting from sleep loss and sleep recovery in laboratory rats. Sleep. 2014;37:1929–40. doi: 10.5665/sleep.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krueger JM, Frank MG, Wisor JP, Roy S. Sleep function: toward elucidating an enigma. Sleep Med Rev. 2015;28:42–50. doi: 10.1016/j.smrv.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Besedovsky L, Lange T, Born J. Sleep and immune function. Pflugers Arch. 2012;463:121–37. doi: 10.1007/s00424-011-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vorster AP, Born J. Sleep and memory in mammals, birds and invertebrates. Neurosci Biobehav Rev. 2015;50:103–19. doi: 10.1016/j.neubiorev.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 64.Diekelmann S. Sleep for cognitive enhancement. Front Syst Neurosci. 2014;8:46. doi: 10.3389/fnsys.2014.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rasch B, Born J. About sleep's role in memory. Physiol Rev. 2013;93:681–766. doi: 10.1152/physrev.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Binder S, Baier PC, Mölle M, Inostroza M, Born J, Marshall L. Sleep enhances memory consolidation in the hippocampus-dependent object-place recognition task in rats. Neurobiol Learn Mem. 2012;97:213–9. doi: 10.1016/j.nlm.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 67.Deliens G, Neu D, Peigneux P. Rapid eye movement sleep does not seem to unbind memories from their emotional context. J Sleep Res. 2013;22:656–62. doi: 10.1111/jsr.12065. [DOI] [PubMed] [Google Scholar]
- 68.Ferrara M, Iaria G, Tempesta D, et al. Sleep to find your way: the role of sleep in the consolidation of memory for navigation in humans. Hippocampus. 2008;18:844–51. doi: 10.1002/hipo.20444. [DOI] [PubMed] [Google Scholar]
- 69.Gais S, Lucas B, Born J. Sleep after learning aids memory recall. Learn Mem. 2006;13:259–62. doi: 10.1101/lm.132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Andretic R, Shaw PJ. Essentials of sleep recordings in Drosophila: moving beyond sleep time. Methods Enzymol. 2005;393:759–72. doi: 10.1016/S0076-6879(05)93040-1. [DOI] [PubMed] [Google Scholar]
- 71.Huber R, Hill SL, Holladay C, Biesiadecki M, Tononi G, Cirelli C. Sleep homeostasis in Drosophila melanogaster. Sleep. 2004;27:628–39. doi: 10.1093/sleep/27.4.628. [DOI] [PubMed] [Google Scholar]
- 72.van Alphen B, Yap MH, Kirszenblat L, Kottler B, van Swinderen B. A dynamic deep sleep stage in Drosophila. J Neurosci. 2013;33:6917–27. doi: 10.1523/JNEUROSCI.0061-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eban-Rothschild AD, Bloch G. Differences in the sleep architecture of forager and young honeybees (Apis mellifera) J Exp Biol. 2008;211:2408–16. doi: 10.1242/jeb.016915. [DOI] [PubMed] [Google Scholar]
- 74.Hussaini SA, Bogusch L, Landgraf T, Menzel R. Sleep deprivation affects extinction but not acquisition memory in honeybees. Learn Mem. 2009;16:698–705. doi: 10.1101/lm.1578409. [DOI] [PubMed] [Google Scholar]
- 75.Kaiser W, Steiner-Kaiser J. Neuronal correlates of sleep, wakefulness and arousal in a diurnal insect. Nature. 1983;301:707–9. doi: 10.1038/301707a0. [DOI] [PubMed] [Google Scholar]
- 76.Klein BA, Stiegler M, Klein A, Tautz J. Mapping sleeping bees within their nest: spatial and temporal analysis of worker honey bee sleep. PLoS One. 2014;9:e102316. doi: 10.1371/journal.pone.0102316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Trojanowski NF, Raizen DM. Call it worm sleep. Trends Neurosci. 2016;39:54–62. doi: 10.1016/j.tins.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fry AL, Laboy JT, Huang H, Hart AC, Norman KR. A conserved GEF for rho-family GTPases acts in an EGF signaling pathway to promote sleep-like quiescence in Caenorhabditis elegans. Genetics. 2016;202:1153–66. doi: 10.1534/genetics.115.183038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hill AJ, Mansfield R, Lopez JM, Raizen DM, Van Buskirk C. Cellular stress induces a protective sleep-like state in C. elegans. Curr Biol. 2014;24:2399–405. doi: 10.1016/j.cub.2014.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Trojanowski NF, Nelson MD, Flavell SW, Fang-Yen C, Raizen DM. Distinct Mechanisms Underlie Quiescence during Two Caenorhabditis elegans Sleep-Like States. J Neurosci. 2015;35:14571–84. doi: 10.1523/JNEUROSCI.1369-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Seugnet L, Galvin JE, Suzuki Y, Gottschalk L, Shaw PJ. Persistent short-term memory defects following sleep deprivation in a Drosophila model of Parkinson disease. Sleep. 2009;32:984–92. doi: 10.1093/sleep/32.8.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Seugnet L, Suzuki Y, Vine L, Gottschalk L, Shaw PJ. D1 receptor activation in the mushroom bodies rescues sleep-loss-induced learning impairments in Drosophila. Curr Biol. 2008;18:1110–7. doi: 10.1016/j.cub.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alhola P, Polo-Kantola P. Sleep deprivation: impact on cognitive performance. Neuropsychiatr Dis Treat. 2007;3:553–67. [PMC free article] [PubMed] [Google Scholar]
- 84.Philibert I. Sleep loss and performance in residents and nonphysicians: a meta-analytic examination. Sleep. 2005;28:1392–402. doi: 10.1093/sleep/28.11.1392. [DOI] [PubMed] [Google Scholar]
- 85.Ikegami K, Ogyu S, Arakomo Y, et al. Recovery of cognitive performance and fatigue after one night of sleep deprivation. J Occup Health. 2009;51:412–22. doi: 10.1539/joh.l8127. [DOI] [PubMed] [Google Scholar]
- 86.Lamond N, Jay SM, Dorrian J, Ferguson SA, Jones C, Dawson D. The dynamics of neurobehavioural recovery following sleep loss. J Sleep Res. 2007;16:33–41. doi: 10.1111/j.1365-2869.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 87.Hicks RA, Paulus MJ, Johnson JC. Effect of REM sleep deprivation on electric shock threshold in rats. Psychol Rep. 1973;32:1242. doi: 10.2466/pr0.1973.32.3c.1242. [DOI] [PubMed] [Google Scholar]
- 88.Hagewoud R, Havekes R, Tiba PA, et al. Coping with sleep deprivation: shifts in regional brain activity and learning strategy. Sleep. 2010;33:1465–73. doi: 10.1093/sleep/33.11.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kumar T, Jha SK. Sleep deprivation impairs consolidation of cued fear memory in rats. PLoS One. 2012;7:e47042. doi: 10.1371/journal.pone.0047042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Smith C, Rose GM. Evidence for a paradoxical sleep window for place learning in the Morris water maze. Physiol Behav. 1996;59:93–7. doi: 10.1016/0031-9384(95)02054-3. [DOI] [PubMed] [Google Scholar]
- 91.Smith CT, Conway JM, Rose GM. Brief paradoxical sleep deprivation impairs reference, but not working, memory in the radial arm maze task. Neurobiol Learn Mem. 1998;69:211–7. doi: 10.1006/nlme.1997.3809. [DOI] [PubMed] [Google Scholar]
- 92.Varga AW, Kang M, Ramesh PV, Klann E. Effects of acute sleep deprivation on motor and reversal learning in mice. Neurobiol Learn Mem. 2014;114:217–22. doi: 10.1016/j.nlm.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ganguly-Fitzgerald I, Donlea J, Shaw PJ. Waking experience affects sleep need in Drosophila. Science. 2006;313:1775–81. doi: 10.1126/science.1130408. [DOI] [PubMed] [Google Scholar]
- 94.Lyons LC, Collado MS, Khabour O, Green CL, Eskin A. The circadian clock modulates core steps in long-term memory formation in Aplysia. J Neurosci. 2006;26:8662–71. doi: 10.1523/JNEUROSCI.2307-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lyons LC, Rawashdeh O, Katzoff A, Susswein AJ, Eskin A. Circadian modulation of complex learning in diurnal and nocturnal Aplysia. Proc Natl Acad Sci U S A. 2005;102:12589–94. doi: 10.1073/pnas.0503847102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jovasevic V, Corcoran KA, Leaderbrand K, et al. GABAergic mechanisms regulated by miR-33 encode state-dependent fear. Nat Neurosci. 2015;18:1265–71. doi: 10.1038/nn.4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Overton DA. Historical context of state dependent learning and discriminative drug effects. Behav Pharmacol. 1991;2:253–64. [PubMed] [Google Scholar]
- 98.Pompilio L, Kacelnik A, Behmer ST. State-dependent learned valuation drives choice in an invertebrate. Science. 2006;311:1613–5. doi: 10.1126/science.1123924. [DOI] [PubMed] [Google Scholar]
- 99.Patti CL, Zanin KA, Sanday L, et al. Effects of sleep deprivation on memory in mice: role of state-dependent learning. Sleep. 2010;33:1669–79. doi: 10.1093/sleep/33.12.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Havekes R, Bruinenberg VM, Tudor JC, et al. Transiently increasing cAMP levels selectively in hippocampal excitatory neurons during sleep deprivation prevents memory deficits caused by sleep loss. doi: 10.1523/JNEUROSCI.2403-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.