Abstract
Study Objectives:
Investigators assign sleep-waking states using brain activity collected from a single site, with the assumption that states occur at the same time throughout the brain. We sought to determine if sleep-waking states differ between two separate structures: the hippocampus and neocortex.
Methods:
We measured electrical signals (electroencephalograms and electromyograms) during sleep from the hippocampus and neocortex of five freely behaving adult male rats. We assigned sleep-waking states in 10-sec epochs based on standard scoring criteria across a 4-h recording, then analyzed and compared states and signals from simultaneous epochs between sites.
Results:
We found that the total amount of each state, assigned independently using the hippocampal and neocortical signals, was similar between the hippocampus and neocortex. However, states at simultaneous epochs were different as often as they were the same (P = 0.82). Furthermore, we found that the progression of states often flowed through asynchronous state-pairs led by the hippocampus. For example, the hippocampus progressed from transition-to-rapid eye movement sleep to rapid eye movement sleep before the neocortex more often than in synchrony with the neocortex (38.7 ± 16.2% versus 15.8 ± 5.6% mean ± standard error of the mean).
Conclusions:
We demonstrate that hippocampal and neocortical sleep-waking states often differ in the same epoch. Consequently, electrode location affects estimates of sleep architecture, state transition timing, and perhaps even percentage of time in sleep states. Therefore, under normal conditions, models assuming brain state homogeneity should not be applied to the sleeping or waking brain.
Citation:
Emrick JJ, Gross BA, Riley BT, Poe GR. Different simultaneous sleep states in the hippocampus and neocortex. SLEEP 2016;39(12):2201–2209.
Keywords: dissociated states, EEG, hippocampus, homeostasis, local sleep, neocortex, REM, sleep, slow wave sleep, transition-to-REM
Significance.
We found that the hippocampus and neocortex independently progress through sleep-waking states, challenging the utility of single EEG electrode and purely behavioral characterizations of states as well as canonical views of sleep as a global phenomenon. We suggest that electrode location affects the scoring of states, with implications for prior sleep and sleep deprivation studies. We propose that future sleep studies should measure electrical activity and score sleep-waking states directly from the brain structures of interest rather than inferring states from neocortical electrical activity. Our findings may shed new light on the functions of sleep and raise new questions regarding how sleep may be independently generated in different brain areas.
INTRODUCTION
Researchers assess sleep using electroencephalographic (EEG) signals from cortical sites1 as well as other corporeal signals (e.g., eye movements, respiratory effort, muscle tonus) without examining EEG signals from subcortical structures, e.g., the hippocampus. Studies have shown that sleep intensity is locally variant2–13 and have found unihemispheric sleep in sea mammals and birds,14–17 but the possibility that an entire brain structure such as the hippocampus could be in another stage of sleep different than the neocortex—and that this could be a normal physiological occurrence—has not been reported.
Hippocampal studies in humans and other animals have reported a dichotomy of EEG states between the hippocampus and neocortex, with the hippocampus showing slow wave activity typical of slow wave sleep (SWS) while the cortex shows the low-voltage, fast EEG signals of waking.18,19 Indeed, one study showed that the medial pulvinar nucleus of the thalamus—a structure that relays to and from structures in the temporal lobe such as the hippocampus in humans20—is deactivated minutes before the neocortex registers sleep onset.21 While recording from the hippocampus and neocortex simultaneously during sleep in freely behaving rats, we observed another dichotomy: signs of rapid eye movement (REM) sleep surfaced in the hippocampal electrode leads long before signatures of the same state appeared in the neocortex. Furthermore, we noted that the hippocampus seemed to transition between sleep states independent of the neocortex.
From these initial observations, we hypothesized that these two distinct brain regions could simultaneously exist in different sleep states, suggesting that the behavioral or even electrophysiological characterizations of sleep could be inaccurate for large, functionally significant portions of the brain. We asked whether such state dichotomies arise during other sleep states such as the transition-to-REM (akin to the spindle-rich human stage 2 sleep), SWS, and REM sleep. Importantly, if sleep is not a unitary, homogeneous state engaged in by the entire organism at once, studies could overlook important sleep-waking state changes in the hippocampus or other sub-cortical brain areas where sleep may serve a critical function.
METHODS
Surgical Procedures
Adult male rats (n = 5; mean weight = 360.5 ± 44.5 g; mean age = 5.8 ± 1.9 mo) were anesthetized with sodium pento-barbital (60 mg/kg, intraperitoneally) then fitted with a hyperdrive22 over the hippocampus with stereotaxic guidance. Implanted devices housed 12 to 16 tetrodes, each comprised of four 12-μm diameter gold-plated nichrome wires. Tetrodes were post-surgically lowered deep in the dorsal hippocampus along with a reference tetrode placed 0.5 mm dorsal to the hippocampal cell layer in the neocortical deep white matter. In addition, three jeweler's screw electrodes were placed in the skull over the left and right frontal lobe and the left parietal lobe for cortical EEG recordings. Two wires were threaded through the nuchal muscles for electromyographic (EMG) electrodes. Following surgery, animals were given an intramuscular injection of 1 mL Pro-Pen-G to prevent infection. Animals were then placed on a heating blanket and monitored until they regained licking and swallowing reflexes. Subsequently, acetaminophen oral suspension (80 mg/0.8 mL) was administered orally, and animals were observed until consciousness was regained.
Experimental Protocol
After recovery from surgery (> 7 d), each rat was habituated to recording conditions, including daily running on a spatial maze.23 The hippocampal local field potential (LFP) was zero-gain current amplified and obtained by referencing one of the twelve deep hippocampal tetrodes to the reference tetrode. The cortical EEG was obtained by cross-referencing one frontal screw electrode to another cortical screw electrode. The two EMG wires were differentially recorded to form one channel of EMG signal. Following more than 3 days of training (> 10 d post-surgery), 4-h recordings of sleep-waking state activity were obtained after rats ran spatial maze tasks, roughly 60 to 90 min into the light period.
Sleep-Waking State Scoring
Each recording was analyzed for state separately in the cortically and hippocampally derived electrodes with the scorer blind to the state scored in the other site. The hippocampal or neocortical EEG signal and the EMG signal from each rat were read into Sleep Scorer, a custom MATLAB-based (Math-Works, Natick, MA) software program for sleep-waking state characterization.24 Each 10-sec epoch was manually scored as one of five sleep-waking states according to standard power spectral density (PSD) value parameters and the same power and frequency criteria were used to score states at both sites25,26:
Active waking (AW) = theta activity with high EMG activity
Quiet waking (QW) = low amplitude, desynchronized EEG (neocortex) or LFP (hippocampus) and relatively little EMG activity
SWS = high amplitude synchronized EEG/LFP with low EMG activity
Transition-to-REM sleep (TR) = high-amplitude spindle activity with low EMG activity
REM sleep = clear, sustained theta activity and phasic muscle twitches on a background of low EMG activity
In our laboratory, interscorer agreement for the same signals from the same record is 83% across the five sleep-waking states.24 To eliminate the error induced by inter-scorer variability, two experts came to a consensus on the state of each 10-sec epoch, blind to the state scored at the other site.
Power Spectral Analysis
To validate that state scoring between sites conformed to the same EEG scoring criteria, power spectra were calculated for each 10-sec epoch for frequencies 0–30 Hz in 0.244 Hz steps in each rat's 4-h recording. To arrive at normalized power, the PSD value for each frequency step was divided by the mean across the 4-h period and averaged across animals. These averaged spectral values were log-transformed into decibels for each state separately for the hippocampus and neocortex.
State Occurrence Rate
To assess the frequency of states occurring at each site, we quantified the relative proportion of epochs scored as each sleep-waking state per recording period, per site and compared them using a paired t-test.
State-Pair Characterization
When characterizing state-pairs, the state scored in the hippo-campus is listed first and the state scored from the neocortical site, second. For example, REM/TR indicates that the hippo-campus was scored in REM sleep whereas the cortical site was scored as TR sleep.
Analysis of REM Sleep and TR Heterogeneous State Pairs
To test for the rate of occurrence of heterogeneous REM sleep and TR state pairs (Figure 4), Excel (Microsoft, Redmond, WA) was used for Student t-test analyses of differences in the incidence of dissimilar and similar state-pair epochs. The mean normalized power (described under Power Spectral Analysis) was calculated for delta, theta, and sigma frequency bands in hippocampal and cortical sites for relevant state-pair categories. Using GraphPad Prism 6 (GraphPad Software Inc., La Jolla, CA), statistical analyses were performed to determine if significant differences existed between any state pairs within each frequency band at each recording site. Holm-Sidak multiple comparisons test was used to calculate corrected P values with significance considered at P < 0.05.
Figure 4.
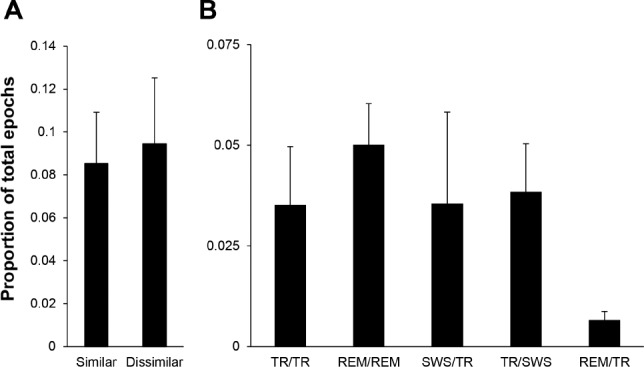
The hippocampus and neocortex demonstrate independent simultaneous sleep states. (A) Proportion (mean ± standard error of the mean) of epochs where the hippocampus and neocortex were scored as having the same (Similar) or different (Dissimilar) sleep states when either site was in rapid eye movement (REM) or transition-to-REM sleep (TR) (n = 5 rats). The groups were not significantly different (Student's t-test; two-tailed; P = 0.82). (B) Proportion (Mean ± standard error of the mean) of states scored as TR or REM from hippocampal and neocortical sites at simultaneous epochs resulted in five distinct state-pair categories. State-pair categories follow the notation “hippocampal state/neocortical state” (n = 5 rats). SWS = slow wave sleep.
Band Power of State Pairs
PSD values in the delta (0.4–4 Hz), theta (5–9 Hz), sigma (10–14 Hz), and beta (15–20 Hz) frequency bands were calculated for each 10-sec epoch and averaged for each band in either the cortical EEG or hippocampal LFP separately over the 4-h recording. The PSD value for each frequency band per 10-sec epoch was normalized to the mean power in that band over the recording period. The mean normalized band power for each state for each rat was then calculated for each recording site. Average values were calculated across all animals.
Category Progression Analysis
Under the model of state homogeneity, progressions between state-pair categories would be predicted to occur from one similar state-pair to another, i.e. from SWS/SWS to TR/TR and from TR/TR to REM/REM. We conducted a state-pair category progression analysis for all defined categories for each rat. For each instance of a state-pair category, the subsequent state-pair category was determined and tallied. The χ2 test was used to determine which state-pair category progressions were significant, occurring more often than would be expected due to chance. The χ2 test was performed for progressions both to (Pull) and from (Push) each state pair. The χ2 values were calculated using the formula (O-E)2/E where O is the observed and E is the expected count for each prior or subsequent state-pair category. Expected counts were calculated as the product of the total counts of the final (Pull) or initial (Push) state-pair category and the proportion of total epochs of the prior or subsequent state-pair category. The P values were calculated for the χ2 distribution with two degrees of freedom. A value of P < 0.05 was considered significant.
RESULTS
Sleep-Waking States From Individual Sites Follow Standard State Scoring Criteria
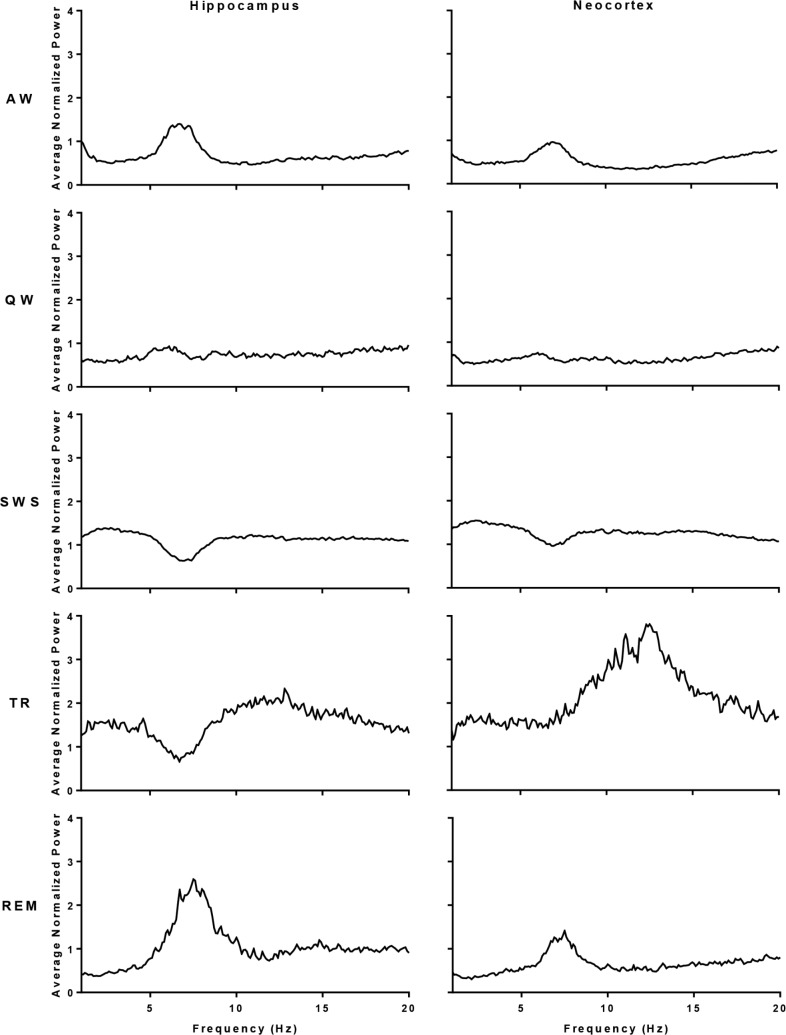
We analyzed power spectral values for each sleep-waking state by frequency and site. Hippocampal and neocortical power spectral values confirmed that our state assignments followed standard scoring criteria.24,26 Sleep-waking states from both the hippocampus and neocortex demonstrated canonical changes in power, i.e., REM sleep in the hippocampus and neocortex was accompanied by a relative increase in the theta frequency range and SWS in the hippocampus and neocortex showed the highest spectral power in the delta range (Figure 1).
Figure 1.
Mean power spectral values by state and brain site. Mean of the normalized power spectra across frequencies (1 to 30 Hz) for epochs scored as active wake (AW), quiet wake (QW), slow wave sleep (SWS), REM sleep (REM), or transition-to-REM sleep (TR) from hippocampal LFP and neocortical EEG (n = 5).
The Hippocampus and Neocortex Spend the Same Percentage of Time in Each State
There were no significant differences in the overall percentage of time the hippocampus and neocortex spent in each sleep and waking state (Figure 2; AW, P = 0.50; QW, P = 0.15; SWS, P = 0.12; TR, P = 0.94; REM, P = 0.44).
Figure 2.
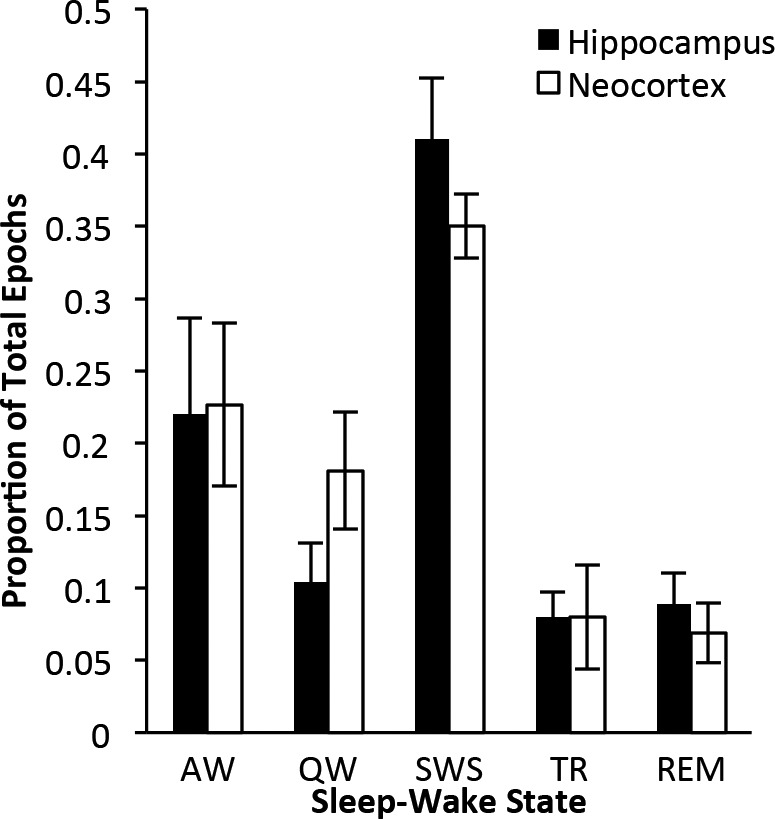
Neocortical and hippocampal sleep-waking state distribution. Proportion (mean ± standard error of the mean) of epochs scored as active wake (AW), quiet wake (QW), slow wave sleep (SWS), transition-to-REM sleep (TR), or REM sleep (REM) from hippocampal and neocortical sites (n = 5 rats). No significant differences were detected in proportions of each state between the hippocampus and neocortex (Student's paired t-tests; all P > 0.05).
The Hippocampus and Neocortex Can Simultaneously Exist in Different Sleep States
We found that the hippocampus and neocortex enter different sleep states at different times. Figure 3 shows an example of a progression to REM sleep from SWS where the hippocampus enters REM sleep 30 sec before the neocortex. The hippocampal local field potential (H LFP) registered a REM-characteristic increase in theta and decrease in delta at the same time that the onset of atonia appeared on the neck EMG (N EMG, Figure 3B). Concurrently, high sigma power remained in the cortical EEG (C EEG), indicative of TR. The enlarged time scale (Figure 3C, inset b) shows the discrepancy in the two states: theta dominated the H LFP whereas the high sigma and beta characterizing the TR state continued to be prominent features in the C EEG. Subsequently, as seen in inset c of Figure 3C, both the hippocampus and neocortex eventually showed the characteristics typical of REM sleep. Figure S1 in the supplemental material has additional examples of similar and dissimilar state-pair epochs from each rat.
Figure 3.
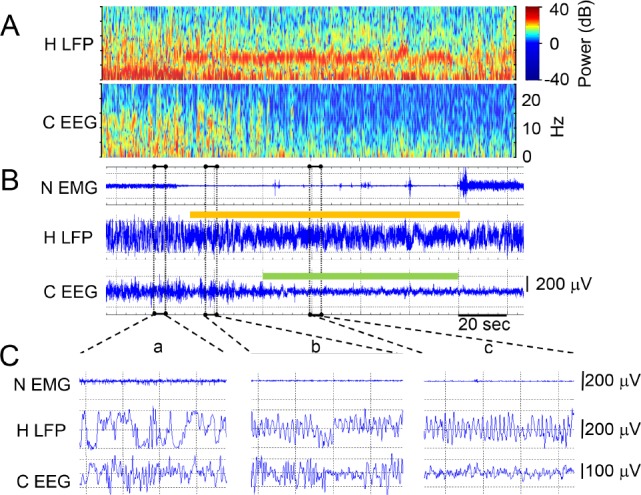
Example transition from slow wave sleep (SWS) through transition-to-REM sleep (TR) to rapid eye movement (REM) sleep in a rat recording during sleep after running the 8-box maze. An arousal terminates the REM period in the last 26 sec shown in panels A and B. (A) Power (color scale) and frequency (y axis) over time (x axis) of the hippocampal local field potential (H LFP) signal and the neocortical EEG (C EEG) signal from the same signals shown as traces in panel B and using the timescale bar from B. (B) Neck EMG (N EMG), H LFP, and C EEG signal traces over time. Epochs (10 sec) scored as REM are shown in the color bar separately over the hippocampal and neocortical traces just as they were independently scored. Three time periods outlined in dashed rectangles are expanded in panel C. (C) N EMG, H LFP, and C EEG traces from B expanded from 4 sec of simultaneous SWS (inset a), 4 sec of TR in the neocortex and REM in the hippocampus (inset b), and simultaneous REM (inset c).
Dissimilar States are as Common as Similar States Between Sites at Single Epochs
We identified epochs scored as REM sleep or TR in either the hippocampal or neocortical recording site (on average, between them, 18.0 ± 5.3% of the entire recording) and asked how often the identical epoch was scored as the same state at the alternate recording site by the consensus scoring method, i.e. two experts agreeing on the state of each epoch from each record. We found that the proportion of epochs scored as different states was not significantly different than the proportion scored as the same state between sites (Figure 4A; 9.4 ± 3.1% versus 8.5 ± 2.4%, P = 0.82). Indeed, instead of finding asynchronous states to be infrequent, we found that particular combinations of asynchronous states (e.g., TR sleep in the hippocampus while SWS is in the neocortex: TR/SWS) occurred as often as the expected simultaneous states (e.g., TR sleep simultaneously at both sites: TR/TR) (Figure 4B). In contrast, agreement rates for TR and REM sleep at the same site between different expert scorers is much higher, at approximately 80%.24
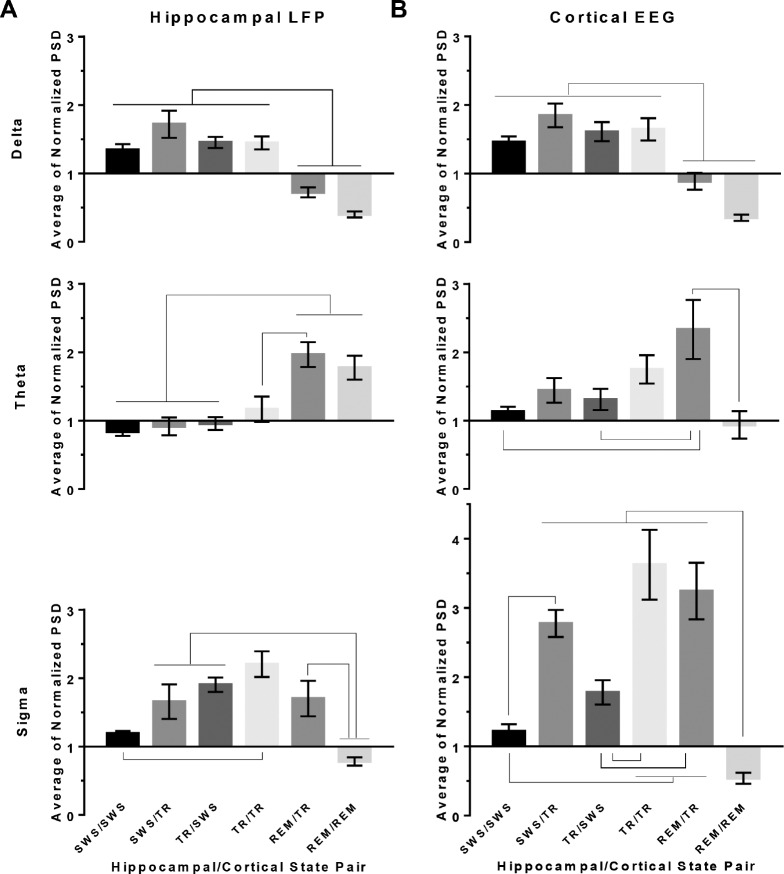
Differences in Band Powers Among State-Pair Categories
Although the PSD values for each state conformed to standard state-scoring criteria at each site when considered individually across the recording (Figure 1), we asked whether individual state-pair categories might reveal unique PSD values at the hippocampal or neocortical site in one or more bands—delta, theta, or sigma (Figure 5). For the following results, Figure S2 in the supplemental material provides the full power spectra comparisons between the two sites for each state-pair.
Figure 5.
Power spectral density by state-pair categories and band. Normalized power spectral density values (PSD) (mean ± standard error of the mean; n = 5 rats) are shown in the hippocampus (A) and neocortex (B) for delta, theta, and sigma bandwidths. Each 10-sec epoch PSD in each frequency band was normalized by the respective mean PSD across the entire recording period for each rat. State-pair categories are denoted as “hippocampal state/neocortical state”. Connectors between state-pair indicate statistically significant differences (P < 0.05, Holm-Sidak multiple comparisons test). REM = rapid eye movement sleep; SWS = slow wave sleep; TR = transition-to-REM sleep.
As expected, either site's mean normalized delta PSD was significantly higher when that site was in SWS or TR than when in REM sleep (Figure 5). In addition, theta power in the hippocampus during REM sleep was significantly higher than during SWS whether the neocortex was concurrently in REM sleep or TR sleep. However, neocortical theta power was highest when the neocortex was in TR and the hippocampus was concurrently in REM sleep (REM/TR). Interestingly, when both the hippocampus and neocortex were simultaneously in REM sleep, theta power in the neocortex was relatively low, and not significantly different from theta power in the neocortex in SWS.
More detailed analyses of the neocortical power revealed that peak theta frequency (7.5 Hz) remained the same whether the neocortex joined the hippocampus in REM or remained in TR (Figure S2), but theta power was threefold higher in the asynchronous state pair (REM/TR) (Figure 5).
Unlike delta and theta power, hippocampal and cortical spindle-frequency sigma power (characteristic of TR sleep) appeared to be additive when the hippocampus and neocortex were simultaneously in transition-to-REM sleep. Interestingly, TR in the neocortex exhibited a twofold to threefold increase in the sigma band PSD over SWS (Figure 5B), whereas the hippo-campus showed a smaller (though significant) sigma power increase from SWS to TR (SWS/SWS versus TR/TR, Figure 5A, rightmost panel).
State-Pair Category Progressions Reveal Site-Independent Activity
We have thus far described new combinations of sleep-waking states, called state-pair categories, in which dissimilar states can occur simultaneously in the hippocampus and neocortex. We next asked how the canonical progression of categories (SWS/SWS to TR/TR to REM/REM) integrates these dissimilar states, and whether similar state-pair categories are reached via dissimilar state intermediates.
Figure 6 shows state-pair progressions that had significant χ2 results (P < 0.05) for at least three of the five rats. For ease of illustration we combined active waking (AW) and quiet waking (QW) into a single waking (W) state. We found that there was not a significant progression from W/W to SWS/ SWS. Instead, the hippocampus transitioned to SWS before the neocortex (i.e. SWS/W). While in SWS/SWS, we found significant progressions in which either the hippocampus or neocortex transitioned to TR while the converse site remained in SWS. However, there were no significant progressions to TR/TR if the neocortex transitioned to TR first. That is, simultaneous TR sleep was entered through either simultaneous SWS or via entry of the hippocampus into TR first. The percentage of progressions to TR/TR was similar between SWS/ SWS or TR/SWS. Similarly, the hippocampus entered REM sleep before the neocortex. However, we found that the hippo-campus led the progression to simultaneous REM sleep threefold more often than the sites progressed together. Table S1 in the supplemental material shows the percentages of transitions from similar and dissimilar state-pairs illustrated in Figure 7.
Figure 6.
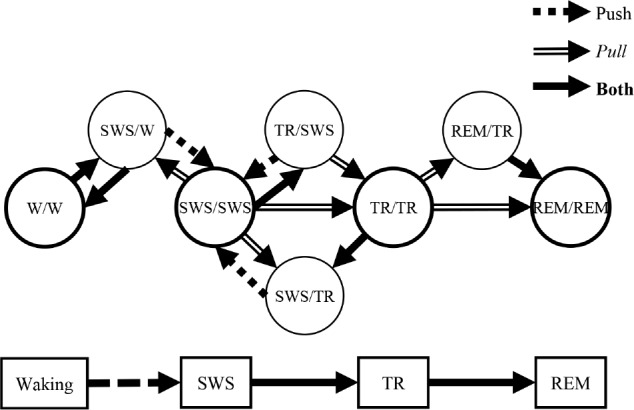
Statistically significant state-pair category progressions. Statistically significant state-pair category progressions are shown with arrows signifying direction of each progression. Expected counts were calculated as the product of the total counts of the final state-pair category and the proportion of total epochs of the prior or subsequent state-pair category. Push lines indicate where a state-pair goes next with statistical significance, and Pull lines represent from whence a state-pair originates. The Push and Pull percentages of these transitions are presented in Tables S1 and S2 in the supplemental material, respectively. Individual circles correspond to separate state-pair categories with simultaneous states denoted as “hippocampal state/neocortical state.” (P < 0.05, χ2 Goodness-of-fit test, degrees of freedom = 1). REM = rapid eye movement sleep; SWS = slow wave sleep; TR = transition-to-REM sleep; W = waking.
Figure 7.
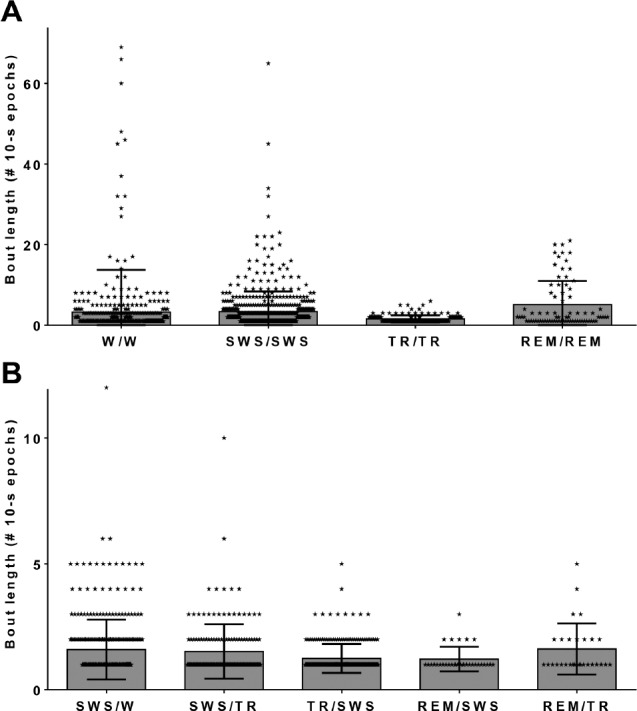
Bout durations for each state-pair category. Bout lengths in number of 10-sec epochs for significant state-pair categories when the hippocampus and neocortex are in the same state (A) or different states (B). The mean bout length is indicated by the height of each column. The standard error of the mean is shown by the length of the whiskers. Individual state-pairs are labeled by symbols overlying the bars. Category pairs follow the notation “hippocampal state/neocortical state”. REM = rapid eye movement sleep; SWS = slow wave sleep; TR = transition-to-REM sleep; W = waking.
Figure 7 shows the length of time spent in each state-pair in 10-sec epochs. The hippocampus leads the neocortex into SWS (as shown in Figure 6), preceding the neocortex into SWS by anywhere from 10 sec to over 1 min (Figure 7B; SWS/W). The REM/TR state-pair category, when the hippocampus was in the theta mode of REM sleep and the neocortex remained in the spindling mode of TR sleep (as in Figure 3), lasted from 10 to 50 sec; most of those asynchronous REM/TR bouts (76.5 ± 10.4%) transitioned to simultaneous REM sleep (REM/REM), as shown in Table S1.
DISCUSSION
Our findings represent the first direct neurophysiological evidence for sleep state heterogeneity between the hippocampus and neocortex. The existence of dissimilar state-pairs and progression through dissimilar state-pairs does not support an assumption of sleep-waking state homogeneity. Noncanonical, asynchronous progressions are as numerous as or more numerous than canonical, synchronous progressions. Interestingly, in cases of asynchronous progressions, the hippocampus leads the transition both to SWS, leaving the cortex in quiet waking for an epoch or two, and to TR and REM sleep, leaving the cortex in SWS for a time.
Given the care taken to mitigate scoring variability via expert consensus (see Methods), the proportion of dissimilarly scored states between sites cannot be attributed to interscorer variations. These same experts show much higher agreement using the same signals at the same site (approximately 80% for REM and TR24) than agreement found in this report in REM and TR scores between the hippocampal and neocortical sites (approximately 50%). Like the neocortex, LFPs in the hippocampus have been scored for sleep-waking states together with other signals, such as video movement, position, neck muscle activity, etc.27,28 The hippocampus and neocortical areas are connected independently to areas of the brain contributing to sleep state generation, i.e. the basal forebrain, the thalamus, and the hypothalamus.29,30 These independent functional inputs could allow for the observed state asynchrony by independently influencing each structure's timing and ability to participate in each sleep state.
Other studies have described local sleep within areas of the neocortex depending on the involvement of each cortical region in waking behaviors.3,13,31,32 Intrahemispheric cortical EEG power differences have also been described in mammals during SWS.5–13 Our findings go beyond cortical measures to find hippocampal sleep state differences and extend SWS/ waking intracortical discrepancies to include TR and REM sleep heterogeneities. Hints of REM heterogeneities have been found in monotremes33 and ostriches2,34: the neocortex may show EEG slow waves whereas other parameters controlled by subcortical brain structures may show aspects of REM sleep (rapid eye movements and muscle atonia). Future studies could assess other subcortical areas and physiological signals other than EEG that are known to change with sleep state, such as respiratory and heart rate, to determine whether these variables more closely align with the hippocampal, cortical, or other subcortical EEG states.
The finding that theta power is higher at both sites when REM sleep was scored in the hippocampus and TR was scored in the neocortex was unexpected because theta is not generated in the neocortex. One possibility is that cortical neurons are theta phase locked with limbic structures.35 It was not unexpected that sigma frequency power was additive: both the hippocampus and neocortex participate in local spindle generation, and spindle phase locking between the hippocampus and neocortex has been shown during sleep.36 Through volume conduction, a physical property that decreases with increased frequency, both sites may independently contribute to both theta and spindle signal power measured by an electrode in the other structure.
Broader Implications of the Findings
Sleep is found to be universal in all animals studied,37 essential to life,38,39 and important to development40,41; yet there are reports of constant locomotion behaviors in dolphins,42 whale calves,43 and migrating birds.44 Although these studies do not show electrophysiological evidence of sleep, some authors posit that unihemispheric sleep is likely45,46 and that migratory birds must somehow sleep in flight.44 Given our findings, sub-cortical structures of the brain could be in SWS, TR, or REM sleep while overt behavior indicates wakefulness.
Breaking away from the assumption of state homogeneity may prove critical to understanding the functions of sleep. It is common for investigations of the role of sleep in learning to rely on EEG-based or behavioral sleep deprivation without knowledge of the sleep status of the learning-critical hippocampus.47,48 Our data suggest that studies could be underestimating or over-estimating sleep states, occurring in a subcortical structure such as the hippocampus when such states are assigned using only cortical EEG and corporeal signals. For example, when air puffs were used to induce total sleep deprivation (REM, SWS, and TR) based solely on neo-cortical EEG and EMG, we found that sleep was totally eliminated from the neocortical signals, but that the hippocampus still entered non-REM sleep (SWS and TR) 10% to 30% of the time.49
With regard to learning, significant increases in TR sleep are detected in rats when the recording electrodes are placed within range to detect hippocampal signals,50 but such increases in the analogous spindle-rich non-REM stage 2 sleep after learning are not found in some human studies.51 However, aside from studies using subcortical electrodes in patients with epilepsy undergoing evaluation for surgical resection,52,53 human studies are limited to analyses of surface electrodes that are unable to detect subcortical LFPs. Our study suggests that because sleep–waking states cannot be reliably measured from the hippocampus in humans using external (scalp) electrodes, increased expression of TR (or REM or SWS) expressly from the hippocampus would be missed.
It is possible that the learning effects of these asynchronous states could be profound and unique. For instance, the finding that theta and spindle power is especially strong during REM/ TR may have functional relevance as both states and frequencies are important to memory consolidation.54 It would be worthwhile for future studies to explore the possibility of a functional role for this asynchrony in memory consolidation.
Taking the state of the hippocampus or alternative subcortical structures into consideration may reconcile controversial reports.55,56 For example, covert REM57 and externally measured apparent intermediate stages of sleep58–60 may be additive signals of distinct states simultaneously seen in the neocortex and subcortical structures. Furthermore, the increase in intermediate stages of sleep under some psychiatric conditions61,62 and following some pharmacological manipulations63 may reflect an increase in subcortical-neocortical asynchronous states.
Dissociated sleep states57,64 have been presented as an explanation for parasomnias such as sleepwalking and night terrors, which feature a mix of signals normally seen in different sleep stages. Our findings suggest that these dissociated states may be related to extended periods of normal asynchrony between neocortical and subcortical structures.
Our findings support a new model of regional sleep occurring under normal conditions. The discovery of normal, hidden hippocampal sleep compels a reexamination of past sleep studies where sleep was assessed outside the brain structure under investigation. As sleep is often independently expressed in the two different brain areas, it may also be homeostatically regulated in these areas independently. In future studies, therefore, it will be critical to characterize sleep within the brain region of interest in order to understand its function.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest. Research was supported by MH60670 and the Department of Anesthesiology, University of Michigan. The work was performed at the University of Michigan, Ann Arbor, MI.
ACKNOWLEDGMENTS
The authors thank David Bauer for excellent technical support; Elliott Cropp, MD and Chi Zheng, MD for their assistance in sleep scoring; and Anna Sergeeva, MD and Theresa Bjorness, PhD for support in surgery and data acquisition.
REFERENCES
- 1.Benington JH, Frank MG. Cellular and molecular connections between sleep and synaptic plasticity. Prog Neurobiol. 2003;69:71–101. doi: 10.1016/s0301-0082(03)00018-2. [DOI] [PubMed] [Google Scholar]
- 2.Lesku JA, Vyssotski AL, Martinez-Gonzalez D, Wilzeck C, Rattenborg NC. Local sleep homeostasis in the avian brain: convergence of sleep function in mammals and birds? Proc Biol Sci Royal Soc. 2011;278:2419–28. doi: 10.1098/rspb.2010.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rector DM, Topchiy IA, Carter KM, Rojas MJ. Local functional state differences between rat cortical columns. Brain Res. 2005;1047:45–55. doi: 10.1016/j.brainres.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Krueger JM, Obal F. A neuronal group theory of sleep function. J Sleep Res. 1993;2:63–9. doi: 10.1111/j.1365-2869.1993.tb00064.x. [DOI] [PubMed] [Google Scholar]
- 5.Cottone LA, Adamo D, Squires NK. The effect of unilateral somatosensory stimulation on hemispheric asymmetries during slow wave sleep. Sleep. 2004;27:63–8. doi: 10.1093/sleep/27.1.63. [DOI] [PubMed] [Google Scholar]
- 6.Ferrara M, De Gennaro L, Curcio G, Cristiani R, Bertini M. Interhemispheric asymmetry of human sleep EEG in response to selective slow-wave sleep deprivation. Behav Neurosci. 2002;116:976–81. doi: 10.1037//0735-7044.116.6.976. [DOI] [PubMed] [Google Scholar]
- 7.Huber R, Ghilardi MF, Massimini M, et al. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat Neurosci. 2006;9:1169–76. doi: 10.1038/nn1758. [DOI] [PubMed] [Google Scholar]
- 8.Iwasaki N, Karashima A, Tamakawa Y, Katayama N, Nakao M. Sleep EEG dynamics in rat barrel cortex associated with sensory deprivation. Neuroreport. 2004;15:2681–4. doi: 10.1097/00001756-200412030-00026. [DOI] [PubMed] [Google Scholar]
- 9.Kattler H, Dijk DJ, Borbely AA. Effect of unilateral somatosensory stimulation prior to sleep on the sleep EEG in humans. J Sleep Res. 1994;3:159–64. doi: 10.1111/j.1365-2869.1994.tb00123.x. [DOI] [PubMed] [Google Scholar]
- 10.Miyamoto H, Katagiri H, Hensch T. Experience-dependent slow-wave sleep development. Nat Neurosci. 2003;6:553–4. doi: 10.1038/nn1064. [DOI] [PubMed] [Google Scholar]
- 11.Yasuda T, Yasuda K, Brown RA, Krueger JM. State-dependent effects of light-dark cycle on somatosensory and visual cortex EEG in rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1083–9. doi: 10.1152/ajpregu.00112.2005. [DOI] [PubMed] [Google Scholar]
- 12.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 13.Vyazovskiy V, Borbely AA, Tobler I. Unilateral vibrissae stimulation during waking induces interhemispheric EEG asymmetry during subsequent sleep in the rat. J Sleep Res. 2000;9:367–71. doi: 10.1046/j.1365-2869.2000.00230.x. [DOI] [PubMed] [Google Scholar]
- 14.Goley PD. Behavioral aspects of sleep in Pacific White-sided Dolphins (Lagenorhynchus obliquidens, Gill 1865) Marine Mammal Science. 1999;15:1054–64. [Google Scholar]
- 15.Lyamin OI, Mukhametov LM, Siegel JM. Relationship between sleep and eye state in Cetaceans and Pinnipeds. Arch Ital Biol. 2004;142:557–68. [PMC free article] [PubMed] [Google Scholar]
- 16.Rattenborg NC, Amlaner CJ, Lima SL. Unilateral eye closure and interhemispheric EEG asymmetry during sleep in the pigeon (Columba livia) Brain Behav Evol. 2001;58:323–32. doi: 10.1159/000057573. [DOI] [PubMed] [Google Scholar]
- 17.Rattenborg NC, Lima SL, Amlaner CJ. Facultative control of avian unihemispheric sleep under the risk of predation. Behav Brain Res. 1999;105:163–72. doi: 10.1016/s0166-4328(99)00070-4. [DOI] [PubMed] [Google Scholar]
- 18.Vanderwolf CH, Kramis R, Robinson TE. Hippocampal electrical activity during waking behaviour and sleep: analyses using centrally acting drugs. Ciba Found Symp. 1977:199–226. doi: 10.1002/9780470720394.ch10. [DOI] [PubMed] [Google Scholar]
- 19.Sarasso S, Proserpio P, Pigorini A, et al. Hippocampal sleep spindles preceding neocortical sleep onset in humans. NeuroImage. 2014;86:425–32. doi: 10.1016/j.neuroimage.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg DS, Mauguiere F, Catenoix H, Faillenot I, Magnin M. Reciprocal thalamocortical connectivity of the medial pulvinar: a depth stimulation and evoked potential study in human brain. Cereb Cortex. 2009;19:1462–73. doi: 10.1093/cercor/bhn185. [DOI] [PubMed] [Google Scholar]
- 21.Magnin M, Rey M, Bastuji H, Guillemant P, Mauguière F, Garcia-Larrea L. Thalamic deactivation at sleep onset precedes that of the cerebral cortex in humans. Proc Natl Acad Sci. 2010;107:3829–33. doi: 10.1073/pnas.0909710107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venkatachalam S, Fee MS, Kleinfeld D. Ultra-miniature headstage with 6-channel drive and vacuum-assisted micro-wire implantation for chronic recording from the neocortex. J Neurosci Methods. 1999;90:37–46. doi: 10.1016/s0165-0270(99)00065-5. [DOI] [PubMed] [Google Scholar]
- 23.Poe GR, Nitz DA, McNaughton BL, Barnes CA. Experience-dependent phase-reversal of hippocampal neuron firing during REM sleep. Brain Res. 2000;855:176–80. doi: 10.1016/s0006-8993(99)02310-0. [DOI] [PubMed] [Google Scholar]
- 24.Gross BA, Walsh CM, Turakhia AA, Booth V, Mashour GA, Poe GR. Open-source logic-based automated sleep scoring software using electrophysiological recordings in rats. J Neurosci Methods. 2009;184:10–8. doi: 10.1016/j.jneumeth.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bjorness TE. Theta power decreases within REM sleep bouts. Effects of REM sleep manipulations on spatial learning in the rat. Dissertation. University of Michigan. 2008:93–4. 22 Aug. 2016. [Google Scholar]
- 26.Benington JH, Kodali SK, Heller HC. Scoring transitions to REM sleep in rats based on the EEG phenomena of pre-REM sleep: an improved analysis of sleep structure. Sleep. 1994;17:28–36. doi: 10.1093/sleep/17.1.28. [DOI] [PubMed] [Google Scholar]
- 27.Wilson M, McNaughton B. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 28.Louie K, Wilson MA. Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron. 2001;29:145–56. doi: 10.1016/s0896-6273(01)00186-6. [DOI] [PubMed] [Google Scholar]
- 29.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68:1023–42. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gvilia I. Underlying brain mechanisms that regulate sleep- wakefulness cycles. Int Rev Neurobiol. 2010;93:1–21. doi: 10.1016/S0074-7742(10)93001-8. [DOI] [PubMed] [Google Scholar]
- 31.Vyazovskiy VV, Borbély AA, Tobler I. Interhemispheric sleep EEG asymmetry in the rat is enhanced by sleep deprivation. J Neurophysiol. 2002;88:2280–6. doi: 10.1152/jn.00304.2002. [DOI] [PubMed] [Google Scholar]
- 32.Vyazovskiy VV, Olcese U, Hanlon EC, Nir Y, Cirelli C, Tononi G. Local sleep in awake rats. Nature. 2011;472:443–7. doi: 10.1038/nature10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siegel JM, Manger PR, Nienhuis R, Fahringer HM, Shalita T, Pettigrew JD. Sleep in the platypus. Neuroscience. 1999;91:391–400. doi: 10.1016/s0306-4522(98)00588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lesku JA, Meyer LC, Fuller A, et al. Ostriches sleep like platypuses. PloS Oone. 2011;6:e23203. doi: 10.1371/journal.pone.0023203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46:141–51. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 36.Wierzynski CM, Lubenov EV, Gu M, Siapas AG. State-dependent spike-timing relationships between hippocampal and prefrontal circuits during sleep. Neuron. 2009;61:587–96. doi: 10.1016/j.neuron.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shein-Idelson M, Ondracek JM, Liaw HP, Reiter S, Laurent G. Slow waves, sharp waves, ripples, and REM in sleeping dragons. Science. 2016;352:590–5. doi: 10.1126/science.aaf3621. [DOI] [PubMed] [Google Scholar]
- 38.Rechtschaffen A, Bergmann BM. Sleep deprivation in the rat by the disk-over-water method. Behav Brain Res. 1995;69:55–63. doi: 10.1016/0166-4328(95)00020-t. [DOI] [PubMed] [Google Scholar]
- 39.Lugaresi E, Medori R, Montagna P, et al. Fatal familial insomnia and dysautonomia with selective degeneration of thalamic nuclei. N Engl J Med. 1986;315:997–1003. doi: 10.1056/NEJM198610163151605. [DOI] [PubMed] [Google Scholar]
- 40.Cirelli C, Tononi G. Is sleep essential? PLoS Biol. 2008;6:e216. doi: 10.1371/journal.pbio.0060216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roffwarg HP, Muzio JN, Dement WC. Ontogenetic development of the human sleep-dream cycle. Science. 1966;152:604–19. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
- 42.Lyamin O, Pryaslova J, Kosenko P, Siegel J. Behavioral aspects of sleep in bottlenose dolphin mothers and their calves. Physiol Behav. 2007;92:725–33. doi: 10.1016/j.physbeh.2007.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lyamin O, Pryaslova J, Lance V, Siegel J. Animal behaviour: continuous activity in cetaceans after birth. Nature. 2005;435:1177. doi: 10.1038/4351177a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liechti F, Witvliet W, Weber R, Bachler E. First evidence of a 200-day non-stop flight in a bird. Nat Commun. 2013;4:2554. doi: 10.1038/ncomms3554. [DOI] [PubMed] [Google Scholar]
- 45.Sekiguchi Y, Arai K, Kohshima S. Sleep behaviour: sleep in continuously active dolphins. Nature. 2006;441:E9–10. doi: 10.1038/nature04898. discussion E1. [DOI] [PubMed] [Google Scholar]
- 46.Gnone G, Moriconi T, Gambini G. Sleep behaviour: activity and sleep in dolphins. Nature. 2006;441:E10–1. doi: 10.1038/nature04899. discussion E1. [DOI] [PubMed] [Google Scholar]
- 47.Karni A, Tanne D., Rubenstein B.S., Askenasy J.J.M, Sagi D. Dependence on rem sleep of overnight improvement of a perceptual skill. Science. 1994;265:679–82. doi: 10.1126/science.8036518. [DOI] [PubMed] [Google Scholar]
- 48.Gross BA, Vanderheyden WM, Urpa LM, et al. Stress-free automatic sleep deprivation using air puffs. J Neurosci Methods. 2015;251:83–91. doi: 10.1016/j.jneumeth.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gross B, Davis D, Prabhu K, et al. Society for Neuroscience. San Diego, CA: Society for Neuroscience; 2013. Sleep can occur in the hippocampus despite full sleep deprivation in the cortex in freely-behaving rats. [Google Scholar]
- 50.Datta S. Avoidance task training potentiates phasic pontine-wave density in the rat: a mechanism for sleep-dependent plasticity. J Neurosci. 2000;20:8607–13. doi: 10.1523/JNEUROSCI.20-22-08607.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peters KR, Smith V, Smith CT. Changes in sleep architecture following motor learning depend on initial skill level. J Cogn Neurosci. 2007;19:817–29. doi: 10.1162/jocn.2007.19.5.817. [DOI] [PubMed] [Google Scholar]
- 52.Clemens Z, Molle M, Eross L, Barsi P, Halasz P, Born J. Temporal coupling of parahippocampal ripples, sleep spindles and slow oscillations in humans. Brain. 2007;130:2868–78. doi: 10.1093/brain/awm146. [DOI] [PubMed] [Google Scholar]
- 53.Clemens Z, Molle M, Eross L, et al. Fine-tuned coupling between human parahippocampal ripples and sleep spindles. Eur J Neurosci. 2011;33:511–20. doi: 10.1111/j.1460-9568.2010.07505.x. [DOI] [PubMed] [Google Scholar]
- 54.Poe GR, Walsh CM, Bjorness TE. Cognitive neuroscience of sleep. Prog Brain Res. 2010;185:1–19. doi: 10.1016/B978-0-444-53702-7.00001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vertes RP, Eastman KE. The case against memory consolidation in REM sleep. Behav Brain Sci. 2000;23:867–76. doi: 10.1017/s0140525x00004003. discussion 904-1121. [DOI] [PubMed] [Google Scholar]
- 56.Fogel SM, Smith CT, Cote KA. Dissociable learning-dependent changes in REM and non-REM sleep in declarative and procedural memory systems. Behav Brain Res. 2007;180:48–61. doi: 10.1016/j.bbr.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 57.Nielsen TA. A review of mentation in REM and NREM sleep: “covert” REM sleep as a possible reconciliation of two opposing models. Behav Brain Sci. 2000;23:851–66. doi: 10.1017/s0140525x0000399x. discussion 904-1121. [DOI] [PubMed] [Google Scholar]
- 58.Arnaud C, Gauthier P, Gottesmann C. Atropine effects on the intermediate stage and paradoxical sleep in rats. Psychopharmacology (Berl) 1994;116:304–8. doi: 10.1007/BF02245333. [DOI] [PubMed] [Google Scholar]
- 59.Gottesmann C. The transition from slow-wave sleep to paradoxical sleep: evolving facts and concepts of the neurophysiological processes underlying the intermediate stage of sleep. Neurosci Biobehav Rev. 1996;20:367–87. doi: 10.1016/0149-7634(95)00055-0. [DOI] [PubMed] [Google Scholar]
- 60.Gottesmann C, Kirkham PA, LaCoste G, Rodrigues L, Arnaud C. Automatic analysis of the sleep-waking cycle in the rat recorded by miniature telemetry. Brain Res. 1977;132:562–8. doi: 10.1016/0006-8993(77)90205-0. [DOI] [PubMed] [Google Scholar]
- 61.Akindele MO, Evans JI, Oswald I. Mono-amine oxidase inhibitors, sleep and mood. Electroencephalogr Clin Neurophysiol. 1970;29:47–56. doi: 10.1016/0013-4694(70)90078-7. [DOI] [PubMed] [Google Scholar]
- 62.Lairy GC, Goldsteinas L, Guennoc A. Disturbances of sleep in patients with confusional and demential syndromes. Electroencephalogr Clin Neurophysiol. 1967;23:286. [PubMed] [Google Scholar]
- 63.Qiu M-H, Chen M, Lu J. Cortical neuronal activity does not regulate sleep homeostasis. Neuroscience. 2015;297:211–8. doi: 10.1016/j.neuroscience.2015.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mahowald MW, Schenck CH. Dissociated states of wakefulness and sleep. Neurology. 1992;42:44–51. discussion 2. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.