Abstract
Study Objective:
Adverse early-life events induce behavioral psychopathologies and sleep changes in adulthood. In order to understand the molecular level mechanisms by which the maltreatment modifies sleep, valid animal models are needed. Changing pups between mothers at early age (cross-fostering) may satisfyingly model adverse events in human childhood.
Methods:
Cross-fostering (CF) was used to model mild early-life stress in male and female Wistar rats. Behavior and BDNF gene expression in the basal forebrain (BF), cortex, and hypothalamus were assessed during adolescence and adulthood. Spontaneous sleep, sleep homeostasis, and BF extracellular adenosine levels were assessed in adulthood.
Results:
CF rats demonstrated increased number of REM sleep onsets in light and dark periods of the day. Total REM and NREM sleep duration was also increased during the light period. While sleep homeostasis was not severely affected, basal level of adenosine in the BF of both male and female CF rats was lower than in controls. CF did not lead to considerable changes in behavior.
Conclusions:
Even when the consequences of adverse early-life events are not observed in tests for anxiety and depression, they leave a molecular mark in the brain, which can act as a vulnerability factor for psychopathologies in later life. Sleep is a sensitive indicator for even mild early-life stress.
Citation:
Santangeli O, Lehtikuja H, Palomäki E, Wigren HK, Paunio T, Porkka-Heiskanen T. Sleep and behavior in cross-fostering rats: developmental and sex aspects. SLEEP 2016;39(12):2211–2221.
Keywords: sleep homeostasis, depression, cross-fostering, adenosine, BDNF
Significance.
Maternal separation is an established, early-life stress-inducing animal model of depression. This is the first study to show that also other modifications of mother-infant interconnection, like cross-fostering, may predispose offspring to permanent changes at molecular level in brain, as indicated by decreased basal levels of adenosine. Sleep appeared to be a sensitive and reliable indicator of adverse early-life experience. Although cross-fostering does not lead to measurable changes in behavior, it is possible that when challenged with a new stress, the resilience of the brain is compromised, predisposing to different psychopathologies in adulthood. Thus, a more delicate treatment, as used in the present work, could be a better model reflecting poor child-parent relationships.
INTRODUCTION
Sleep problems are associated with psychological disorders, which are often rooted in adverse experiences during early-life critical periods of childhood and adolescence.1 Sexual, physical, or emotional abuse, neglect, or loss of a parent and poor child-parent relationships are important predisposing factors for development of psychological disorders in adulthood, as evidenced by both epidemiological studies and experimental studies using animal models.2,3 Sleep disturbances associated with these abuses can persist for years after the stressful events.4–6 However, the mechanisms that underlie the long-lasting vulnerability of sleep are still poorly understood. Adenosine and brain-derived neurotrophic factor (BDNF) have been proposed to participate in both regulation of sleep and depression.7–11
The gender differences in the prevalence of mental disorders are large: major depression and anxiety disorder are approximately twice as common in women as in men.12,13 While there is only modest evidence of sex differences in sleep parameters verified with EEG,14 the epidemiological studies frequently demonstrate a large difference in sleep complains between the sexes, showing that females in all age groups report more sleep complaints than males.15,16 While the reason for this discrepancy is not known, one explanation could be that women are more vulnerable to disturbed sleep.16 It is not known whether there are changes between males and females in the levels of molecules that regulate both sleep and early life stress-related behavioral outcomes, such as depression. This question could be addressed using a relevant animal model.
Several methods to induce early-life stress have been used in animal models. One of the techniques is affecting the mother-infant interaction during the first weeks of postnatal life, which corresponds to the third trimester of fetal development and early postnatal development in humans.17 Maternal separation, which inflicts a profound stress for both the pups and their mothers, is the most widespread method,18,19 and can be used to model serious childhood incidences in human subjects. The behavioral and biological consequences in adulthood resemble those of human depression. However, other modifications of the dam-pup relationships have been developed, which may better simulate the adverse childhood events that are common in human real life situations and predispose children to depression in adulthood.
One of the approaches to model early-life adverse events, based on affecting the mother-infant relationship, is cross-fostering (CF).20 In this method, the pup litters are changed between mothers closer to the end of critical postnatal period (at the age of P5–12), which is proven be the age that is sensitive to environmental stimulation. Pups that are raised by foster mothers, which have adopted them during late stages of the infancy period (from P5 on), have higher stress reactivity and impairment of cognitive functioning21,22 than pups raised by their own mothers,20 or pups which were adopted during earlier stages of their development (P1–2). Whether this procedure induces changes also in sleep and sleep-related regulatory factors in adulthood has not previously been studied.
In the present study, we asked the following questions:
what behavioral changes are induced by CF in adolescence and do these changes predict the development of depressive/anxiety-like behavior in adulthood?
are behavioral changes associated with brain BDNF gene expression levels in adolescence and/or adulthood?
does the CF procedure induce changes in sleep and/or adenosine levels in adulthood?
are there sex differences in any of these parameters?
To answer these questions, we assessed behavior and brain BDNF gene expression levels in male and female CF rats and their controls in adolescence and adulthood and sleep with its main sleep regulator, adenosine, in adulthood.
METHODS
Animals
Forty-three male and 41 female Hannover Wistar (received from formerly Harlan Laboratories B.V., now Envigo, Venray, Netherlands) rats were housed under a 12-h light/dark cycle (light on at 09:00) at constant humidity (50% to 60%) and temperature (23 ± 1°C). Food and water were available ad libitum. All animal procedures were performed according to the guidelines of the National Institute of Health Guide for the Care and Use of Laboratory Animals, and were approved by the experimental Animal Ethical Committee of Southern Finland (license ESAVI-5028-04.10.07-2014).
Cross-Fostering (CF) Model
Litters (average size 10 pups) were changed between dams20,23 on day 12 after delivery. The dams were carefully removed from their home cage and temporarily placed in empty cages equipped with nesting paper and some food pellets. The litters were placed in artificial nests while the bedding in home cages was changed. The litters were placed in the cage of their foster mothers, and the dams were returned to their home cages. The procedure was not longer than 5 min. After some reorganization of the pups and the nest, the dams calmed down and began to lactate. Another group of dams and litters served as control (CTRL) and was left undisturbed. Eight CTRL and 9 CF nests were included in this study. In adulthood, one male and one female sibling per litter was used for each measurement.
Behavioral Tests
Sucrose Preference Test
Anhedonia, one of the core symptom of human depression, can be assessed in rodents by sucrose preference test (SPT). Individually housed rats (18 CTRL and 13 CF males, 8 CTRL and 18 CF females) were subjected to sucrose preference test once during adulthood (P105). The test consisted of 2 sessions. During the 48-h training session, animals were provided simultaneously with 2 bottles in the home cage, one containing a 1% sucrose solution, the other containing tap water. The position of the bottles was changed twice a day to avoid place preference. The volume of tap and sucrose water intake was measured every 24 h. After 48 h, animals were deprived of food and water for 18 h with following test session for 2 h. The amount of water remaining in each bottle was measured at the end of the testing period. The sucrose preference score was expressed as percent of total fluid intake (sucrose preference = sucrose water consumed/total liquid consumed × 100).24
Open Field (OF) Test
Anxiety is a symptom with high prevalence in depression and can be assessed by OF test in rodents. The test provides an opportunity to assess novel environment exploration, general locomotor activity, and initial screen for anxiety-related behavior25; the activity in OF is also affected by depression.26 Rats were subjected to OF test during 2 time points, adolescence (P45) and adulthood (P100). The number of male rats in CTRL group was 22 (in adolescence) and 18 (in adulthood); in CF group—21 (in adolescence) and 14 (in adulthood). The number of female rats in CTRL group was 14 (in adolescence) and 9 (in adulthood); in CF group—24 (in adolescence) and 19 (in adulthood). Half of the adolescent animals were sacrificed a few days after the test for brain tissue collection and another half were kept alive until they reached adulthood.
The OF test apparatus, placed in a quiet room, was a white circular arena with diameter 80 cm, enclosed by white walls and divided into 19 equal sectors by lines drawn on the bottom of the arena. Rats were first weighed and then placed in the center of the OF arena. The test sessions (5 min) were video recorded for later analysis. Horizontal (number of squares crossed in the inner and outer areas, latency to leave center) and vertical (rearings [standing on hind legs]) activity, grooming (latency to start self-grooming, duration and number of self-grooming episodes), and excretory activity (defecations and urinations number) were scored. The apparatus was cleaned between tests by repeated washing with tap water followed by ethanol wiping and then sponging until dry.
Brain Tissue Collection
Rats were sacrificed by decapitation and the brains were quickly removed. Two mm coronal slices of the target areas were prepared. The basal forebrain, frontal cortex, and hypothalamus were dissected from the slices with a scalpel. The tissues were placed into RNA-stabilizing reagent RNA later (Sigma-Aldrich, Helsinki, Finland), frozen on dry ice, and stored at −80°C.
BDNF Messenger RNA Analysis
After the homogenization RNA was immediately extracted using RNA Later Lipid Mini Tissue Kit according to the protocol supplied (Qiagen). DNase treatment of the RNA fraction was carried out using the RNAse-Free DNase Set (Qiagen) as a precaution against genomic DNA contamination. The measurement of RNA fraction for purity and concentration was carried out with Nanodrop ND-1000 spectrophotometer (NanoDrop Technologies, Rockland, DE, USA). TaqMan RT-qPCR was performed using LightCycler 480 Real-Time PCR System (Roche, Espoo, Finland) and TaqMan gene expression assays (Applied Biosystems, Foster City, CA, USA). 1 μg of total RNA was reverse transcribed using a Maxima first standard cDNA synthesis kit (Thermo Scientific, Helsinki, Finland). cDNA samples were amplified using a TaqMan Universal PCR MasterMix and the commercial gene expression assays with probes for BDNF (Rn02531967_s1) and ACTB (Rn00667869_m1) from Applied Biosystems.
The PCR program was set on 50°C 2 min - 95°C 1 min and 50 cycles of repeating 95°C for 15 seconds and 60°C for 1 min. All samples were run in triplicates. Relative quantification of template was performed using the ΔΔCt method with experimental cDNA data being normalized to a housekeeping gene ACTB level.
Surgery
Fourteen male and 14 female rats were implanted with electrodes for the recording of the EEG/EMG and with a unilateral guide cannula for the microdialysis probe (CMA 11 Guide, CMA/Microdialysis, Stockholm, Sweden) aimed at the basal forebrain (BF) cholinergic area (horizontal diagonal band of Broca (HDB), the substantia innominata (SI) and the magno-cellular preoptic area (MCPO); the coordinates respective to bregma: anterior = −0.3 mm; lateral = 2.0 mm; vertical = −5.5 mm). Surgical anesthesia was achieved with isoflurane (IsoFlo Vet 100%, Abbott Laboratories Ltd, England) (5% induction; 2% maintenance). In the beginning of the surgery, rats were injected with buprenorphine (Temgesic, Indivior UK Limited, Slough, UK, 0.05 mg/kg, s.c.).
Two bipolar screw EEG electrodes were placed frontoparietally into the skull: 2 mm rostral, 2 mm lateral from bregma, and 4 mm rostral, 1 mm lateral from lambda. Two silver EMG electrodes were inserted into the neck muscles. After surgery, rats were single-housed in Plexiglas boxes; the day after the surgery they were injected with antibiotic/analgesic (1.6 g/kg A-Pen, Orion Pharma Oy, Espoo, Finland + 6 mg/kg lidocaine, Orion Pharma Oy, Espoo, Finland, i.p.), connected to recording cables through swivels, and allowed to recover up to 1 week until their behavior, EEG, and sleep-wake cycle were normal.
In Vivo Microdialysis
Microdialysis probe (CMA 11, CMA/Microdialysis, 6000 Daltons, membrane length 2 mm, diameter 0.24 mm, ∼10% mean recovery rate) was inserted through the guide cannula at least 24 h before start of the experiment, and microdialysis tubing was attached to recording cables at the same time. Half an hour prior to the sample collection, at 09:30, microdialysis tubing was connected to the pump (model R99-EMZ, Razel Scientific Instruments, Vermont, USA) and the fraction collector (Eicom EFC-82 Fraction Collector, San Diego, CA, USA). Continuous perfusion of the microdialysis probe with artificial cerebrospinal fluid (aCSF) (147 mM NaCl, 3 mM KCl, 1.2 mM CaCl2, 1 mM MgCl2) was performed at a rate of 1 μL/ min throughout the light period of baseline day. Samples were collected at 30-min intervals for 12 h starting at 09:30. The samples were stored at −80 °C until assayed.
After the experiments, the animals received a lethal dose of pentobarbital (100 mg/kg Mebunat, i.p., Orion Pharma Oy, Espoo, Finland). To verify the probe location, ink was injected through a modified microdialysis probe inserted into the guide cannula. The brains were then removed, frozen on dry ice, and stored at −80°C. Brain sections (20 μm) were cut on a freezing microtome, stained with Toluidine Blue (Sigma-Aldrich), dehydrated with ethanol, and dried. The probe locations were visually inspected under the light microscope. Only animals with probe tips located in the area of interest, BF (HDB, MCPO, and SI) were included in the analysis (n = 13 for males and n = 10 for females).
EEG/EMG Recording and Analysis
EEG/EMG activities were recorded during the microdialysis experiments. EEG/EMG signals were amplified (gain 5000), filtered (high pass: 0.3 Hz; low pass 100 Hz), and sampled at 271 Hz using the Spike2 program (version 5.19; Cambridge Electronic Devices Ltd., Cambridge, UK). EEG/EMG recordings were semi-automatically scored at 4-s epochs for non-rapid eye movement (NREM), REM sleep, and wakefulness using Autoscore-1.7 script27 and then manually checked using Sleepscore v1.01 script (Cambridge Electronic Design) according to the common criteria.28
EEG power (in square microvolts) spectra of the scored EEG data were generated in Spike2 (CED) separately for consecutive 4-s NREM epochs (fast Fourier transform routine (FFT) 512; Hanning window, 0.54 Hz resolution) within a frequency range of 1.08–29.84 Hz and averaged over the time period that was investigated. The NREM sleep power spectra in delta range (1.08–3.79 Hz) for recovery sleep during one hour of post-sleep deprivation period and respective period of baseline day were normalized to total NREM power of sleep deprivation and baseline days, respectively.
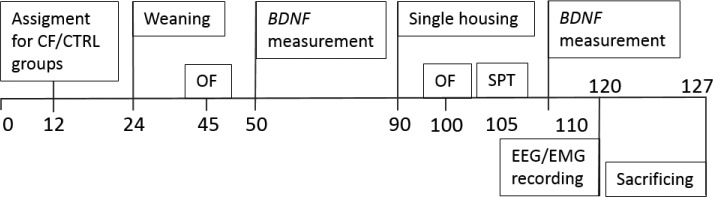
The scheme of the timeline of the experiments is represented on Figure 1.
Figure 1.
Schematic representation of the timeline of the experiments. OF, open field test; SPT, sucrose preference test; CTRL, control rats; CF, cross-fostering rats.
Experimental Days
Baseline (BL) Day
The baseline day served as a reference to which subsequent EEG recordings obtained during the sleep deprivation day were compared to. During the baseline day, the animals were allowed to sleep and wake undisturbed. The rats were connected to microdialysis tubings during 30 min after lights-on, perfusion was started at 09:30, perfusion was stopped 30 min before lights-off (i.e., 20:30).
Sleep Deprivation (SD) Day
SD day was performed on the day after the baseline day. 3 h of SD was performed by gentle handling (Franken et al., 1991) beginning 4 h after lights-on (at 13:00). The animals were kept awake by introducing novel objects into the home cage. After SD they were left undisturbed. The post-SD recovery sleep during remaining light period (16:00 to 21:00) was averaged and compared to the corresponding hours of baseline sleep.
Microdialysis Adenosine Analysis
All samples collected during the microdialysis experiments were used for measurements of adenosine using a high-performance liquid chromatography (HPLC). The analysis was done as described in Savelyev.11 The basal adenosine level was determined as the average adenosine concentration from the samples collected during the baseline day (09:30–20:30).
Statistical Analysis
All statistical analyses were performed with SPSS v.22 (IBM Corp., Armonk, NY, USA). Main effect of treatment, age and sex factors, as well as interaction between them was analyzed with factorial ANOVA. If these analyses revealed a significant effect for any of the factors or their interaction, the analysis was continued by separate ANOVAs. For comparisons of dependent variables, repeated measures ANOVA was used. A P value < 0.05 was considered statistically significant. The values reported in the figures represent mean ± SEM.
RESULTS
Sleep
Analysis of vigilance state-related parameters (wake, NREM, REM sleep duration, and number of REM sleep onsets) was performed for spontaneous sleep (separately for the light and the dark periods) and for post-SD recovery sleep. Sleep was measured in adulthood.
Spontaneous Sleep
Light period. Factorial ANOVA (factors: sex, treatment) revealed a significant effect of sex for REM sleep duration and REM sleep onsets and significant effect of treatment for all parameters.
Effect of sex. Male rats demonstrated increased REM sleep duration (F2,273 = 35.28, P < 0.001) compared to females. The number of REM sleep onsets was lower (F2,273 = 16.67, P < 0.001) in males than females. NREM and wake amount were not different between the sexes.
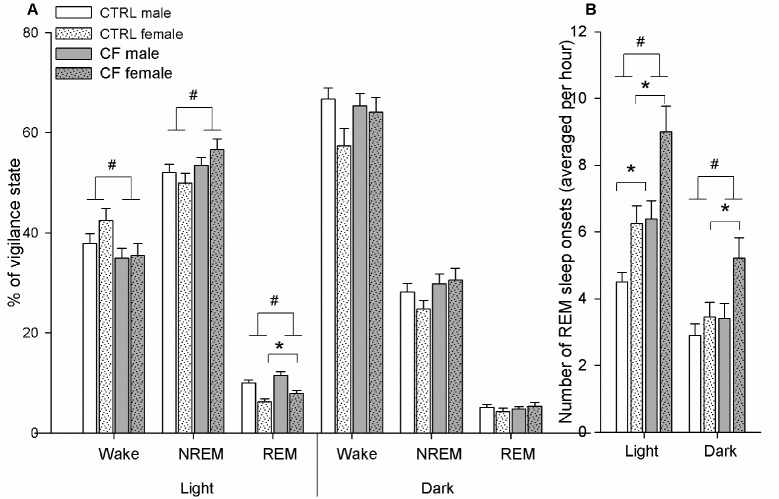
Effect of treatment. CF rats spent less time in waking (F2,273 = 4.77, P = 0.03) and more time in NREM sleep (F2,273 = 4.14, P = 0.04) and REM sleep (F2,273 = 7.17, P = 0.008) than CTRLs (Figure 2A). CF rats had higher number of REM sleep onsets than CTRL rats (F2,273 = 18.5, P < 0.001)
Figure 2.
(A) Vigilance state distribution through light-dark period and (B) number of REM sleep onsets in CTRL and CF male and female rats during BL day. Males (n = 7/control group, n = 6/CF group), females (n = 5/CTRL group, n = 5/CF group). # Difference is significant between CTRL and CF rats (factorial ANOVA (factors: sex and treatment), P < 0.05). *Difference is significant between CTRL and CF rats within same-sex group (one-way ANOVA, P < 0.05). CTRL, control rats; CF, cross-fostering rats; BL, baseline day.
One-way ANOVA within the same-sex group revealed that REM sleep duration in CF male rats tended to be higher than in CTRLs (F1,153 = 3.35, P = 0.07), while in CF females its amount was significantly higher than in CTRL females (F1,118 = 4.1, P = 0.04). Both sexes demonstrated significant increase in REM sleep onsets: males (F1,154 = 10.17, P = 0.002) and females (F1,118 = 8.64, P = 0.004) compared to respective CTRLs (Figure 2B).
No significant interaction between sex and treatment was found in any of the vigilance states (wake, NREM, REM sleep) during the light period.
Dark period. Factorial ANOVA (factors: sex, treatment) revealed a significant effect of sex on waking duration and number of REM sleep onsets, and significant effect of treatment on NREM sleep duration and number of REM sleep onsets.
Effect of sex. Male rats demonstrated longer duration of wakefulness than females (F2,273 = 4.24, P = 0.04). Females had higher number of REM sleep onsets than males (F2,273 = 6.35, P = 0.012).
Effect of treatment. CF rats tended to have longer NREM sleep duration (F2,273 = 3.46, P = 0.06) (Figure 2A) and significantly increased number of REM sleep onsets (F2,273 = 5.32, P = 0.032) compared to CTRL rats (Figure 2B).
Simple analysis of main effect of CF within the same-sex group (one-way ANOVA) revealed that the number of REM sleep onsets was significantly higher in CF females compared to CTRL females (F1,118 = 5.56, P = 0.02), but was similar be -tween CF and CTRL males (F1,154 = 0.8, P = 0.37) (Figure 2B).
No interaction between sex and treatment was found in any of the vigilance states (wake, NREM, REM sleep) during the dark period.
Recovery Sleep
Recovery sleep was analyzed during the remaining light period after ending of SD (16:00 to 21:00) separately for NREM and REM sleep.
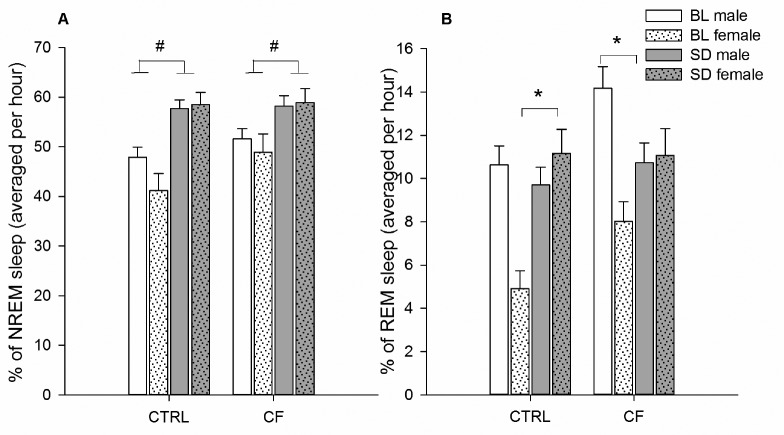
NREM sleep. Factorial repeated measures ANOVA (between-subject factors: sex and treatment, within-subject factor: sleep deprivation) revealed that normal homeostatic response to prolonged wakefulness was observed across all individuals. The effect of SD was significant: NREM sleep duration was increased after SD compared to respective baseline period (F1,20 = 35.50, P < 0.001) irrespective of sex or treatment (CTRL vs. CF) (Figure 3A).
Figure 3.
(A) NREM and (B) REM sleep duration in CTRL and CF male and female rats during recovery hours (16:00–21:00) after SD. Males (n = 7/control group, n = 6/CF group), females (n = 5/CTRL group, n = 5/CF group). # Difference is significant between BL and SD days (factorial repeated measures ANOVA (between subject factors: sex and treatment, within-subject factor: SD), P < 0.05). *Difference is significant between BL and SD days within same-sex, same-treatment group (one-way ANOVA, P < 0.05). CTRL: control rats. CF, cross-fostering rats; BL, baseline day; SD, sleep deprivation.
REM sleep. Factorial ANOVA (as above for NREM) revealed a significant effect of sex and treatment, with significant SD × sex, SD × treatment interactions. The effect of SD was suggestively significant.
Effect of SD. SD led to the tendency of increasing REM sleep duration (F1,20 = 3.93, P = 0.06).
Effect of sex. Male rats had longer REM sleep duration than females during BL day, but shorter during recovery period (F1,20 = 10.96, P = 0.003), the interaction was significant (F1,20 = 28.88, P < 0.001).
Effect of treatment. CF rats had longer REM sleep duration than CTRL rats during BL day (F1,20 = 6.72, P = 0.02) but its duration was equal during the recovery period, the interaction was significant (F1,20 = 4.96, P = 0.04).
Simple one-way ANOVA within same-sex and same-treatment group revealed that REM sleep duration was decreased in CF males after SD (F1,5 = 20.17, P = 0.006) compared to respective hours of BL day. In CTRL males no changes of REM sleep amount were observed. CTRL females demonstrated higher REM sleep amount after SD (F1,4 = 11.10, P = 0.03), but not CF females (Figure 3B).
NREM Sleep Delta Power during Recovery
NREM sleep delta power (1.08–3.79 Hz) was analyzed during one hour of post-SD period (16:00 to 17:00).
Factorial repeated measures ANOVA (between-subject factors: sex and treatment, within-subject factor: sleep deprivation) revealed a statistical significant effect of SD, sex, and interaction SD × sex. No statistically significant effect of treatment was found (F1,20 = 1.07, P = 0.313).
Effect of SD. All rats demonstrated significant increase in delta power during the recovery sleep (F1,20 = 88.392, P < 0.001) (Figure 4).
Figure 4.
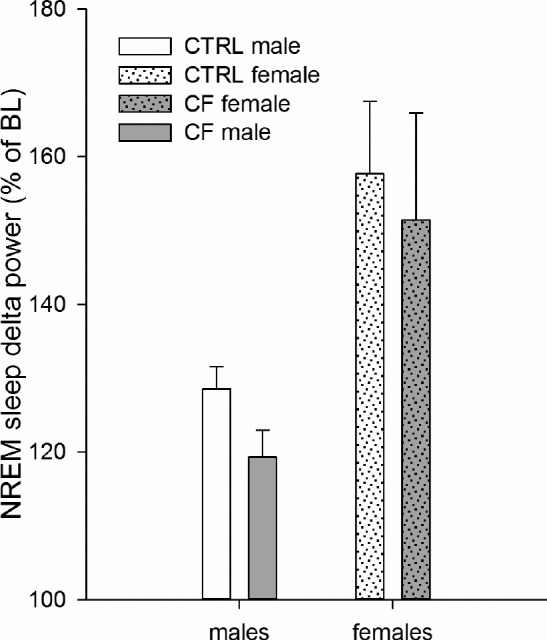
NREM sleep delta (1.08–3.79 Hz) power in CTRL and CF male and female rats during one-hour recovery sleep (16:00–17:00) after SD. Males (n = 7/control group, n = 6/CF group), females (n = 5/ CTRL group, n = 5/CF group). While there is a significant increase in NREM sleep delta power after SD (factorial repeated measures ANOVA (between subject factors: sex and treatment, within-subject factor: SD), P < 0.05) for both females and males (about 50% and 20%, respectively), there is no difference between CTRL and CF rats. CTRL, control rats. CF, cross-fostering rats; BL, baseline day; SD, sleep deprivation.
Effect of sex. Females showed higher increase in delta power than males (F1,20 = 7.129, P = 0.015). The interaction SD × sex was significant (F1,20 = 13.718, P = 0.001).
Within same-sex analysis revealed that both males (F1,11 = 75.558, P < 0.001) and females (F1,8 = 35.382, P < 0.001) demonstrated significant increase in NREM sleep delta power during first hour of recovery after SD.
Adenosine Level
Analysis of adenosine level in BF was performed for spontaneous sleep during the light period. Factorial ANOVA (factors: sex, treatment) revealed a significant effect of sex and treatment on adenosine level in BF.
Effect of sex. Basal adenosine level differed significantly between males and females (F2,195 = 37.26, P < 0.001).
Effect of treatment. Adenosine level in CF rats was lower compared to CTRLs (F2,195 = 12.14, P < 0.001).
Simple one-way ANOVA within same-sex group showed that the concentration of adenosine in the BF dialysate in CF male rats was 0.18 ± 0.01 nM, which was significantly lower (65.8 ± 4.4%) than in the CTRL males (F1,128 = 6.47, P = 0.012). In CF females the concentration of adenosine was 0.35 ± 0.03 nM, which was significantly lower (76.3 ± 5.8%) than in the CTRL females (F1,66 = 7.44, P = 0.008) (Figure 5).
Figure 5.
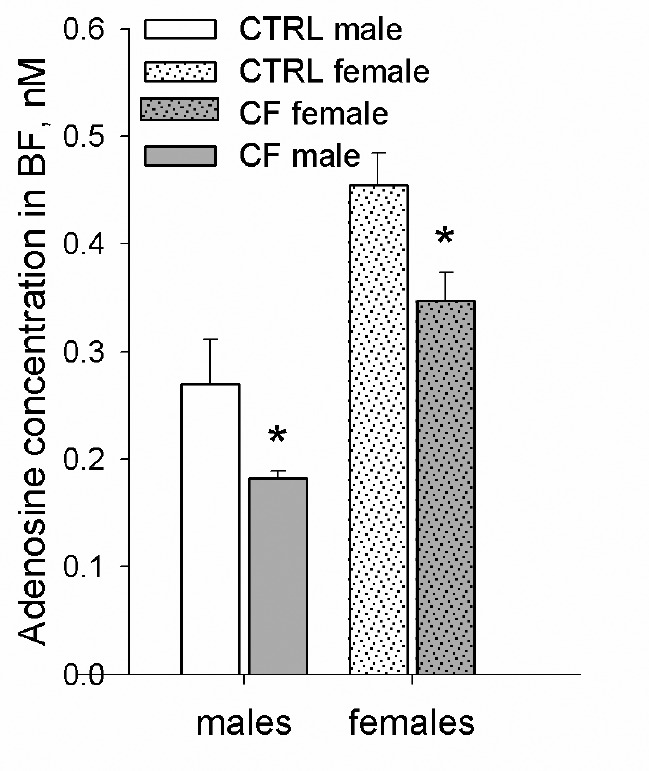
Baseline adenosine concentration (nM) in CTRL and CF male and female rats in the basal forebrain. Males: n = 7/CTRL group, n = 6/CF group, females: n = 5/CTRL group, n = 5/CF group. *Difference is significant between CTRL and CF rats within same-sex group (oneway ANOVA, P < 0.05). CTRL, control rats; CF, cross-fostering rats; BF, basal forebrain.
No interaction between treatment and sex was found (F3,194 = 0.09, P = 0.79).
BDNF Gene Expression Changes
The BDNF expression levels in males and females were measured at two time points (adolescence [P50] and adulthood [P110]) in 3 brain regions: basal forebrain, frontal cortex, and hypothalamus.
Factorial ANOVA (factors: sex, age and treatment) revealed differences in the BF for age and treatment factors, but not for sex factor. No differences were found in other brain areas.
Effect of age. Rats had higher BDNF gene expression during adolescence compared to adulthood (F3,41 = 7.75, P = 0.009).
Effect of treatment. CF rats tended to have lower BDNF gene expression compared to CTRLs (F3,41 = 3.17, P = 0.08) (Figure 6).
Figure 6.
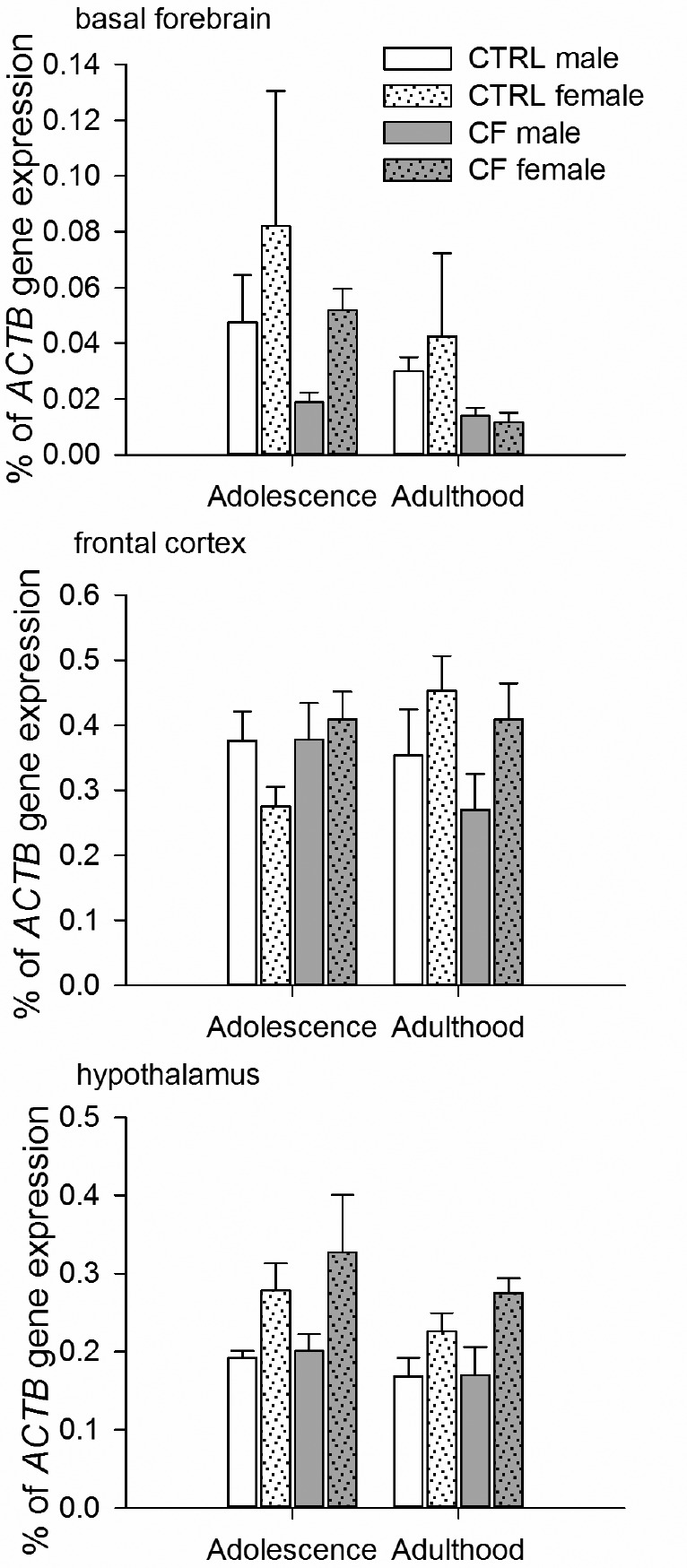
BDNF gene expression in the basal forebrain, frontal cortex and hypothalamus in male and female rats during adolescence and adulthood. Males: n = 5/CTRL group, n = 6/CF group, females: n = 6/CTRL group, n = 6/CF group. In the basal forebrain, BDNF gene expression level was higher in adolescence than in adulthood (factorial ANOVA (factors: sex, age and treatment), P < 0.05) and tended to be lower in CF rats than in CTRLs (factorial ANOVA (factors: sex, age and treatment), P = 0.08). Such differences were not observed in other brain areas. CTRL, control rats; CF, cross-fostering rats.
Behavioral Changes
Sucrose Preference Test
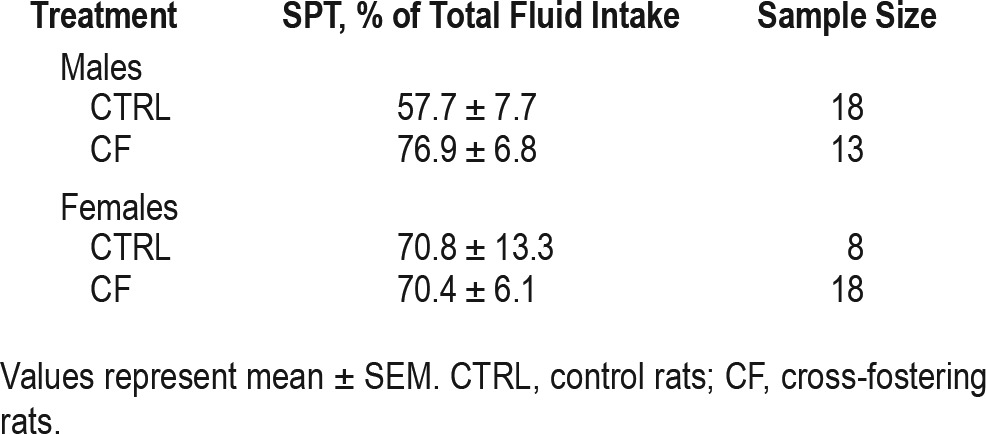
SPT was performed in adulthood. Factorial ANOVA (factors: sex, treatment) revealed no statistically significant effect of sex (F2,54 = 0.05, P = 0.83), CF treatment (F2,54 = 1.74, P = 0.19) or interaction (F2,53 = 1.39, P = 0.24) between them (Table 1).
Table 1.
Effect of CF on sucrose preference in adulthood in males and females.
Open Field Test
Factorial ANOVA (factors: sex, age, and treatment) revealed a statistically significant effect of sex on 3 parameters in the OF test: number of squares crossed in outer area, total number of squares crossed in OF, and number of defecations. The effect of age was significant for number of squares crossed in outer area, total number of squares crossed by rats, grooming number and duration, defecation number. No significant effect of CF treatment was found in any studied parameter of OF test.
Effect of sex. The number of squares crossed in outer area (F3,137 = 10.51, P = 0.001) and total number of squares crossed (F3,137 = 9.02, P = 0.002) was higher in females compared with males. The number of defecations was higher in males (F3,137 = 24.93, P < 0.001) (Table 2, Table 3).
Table 2.
Effect of CF on behavior in the OF test in adolescent and adult males.
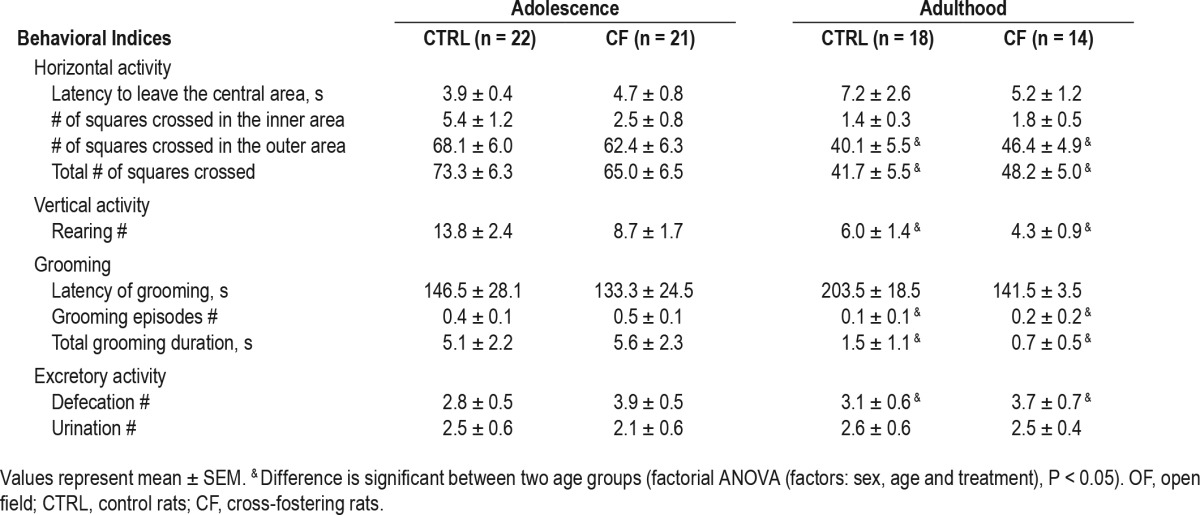
Table 3.
Effect of CF on behavior in the OF test in adolescent and adult females.
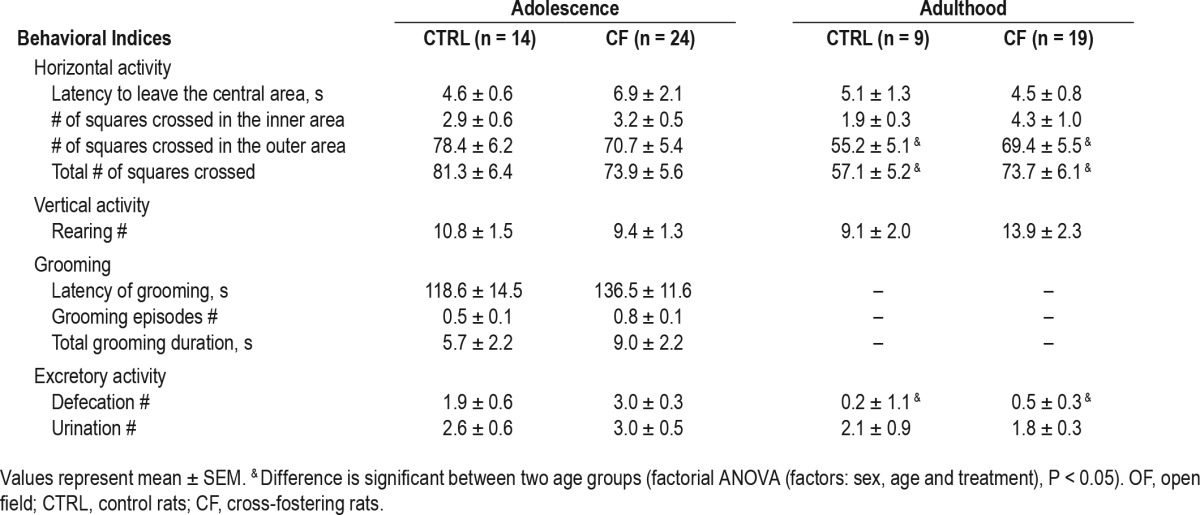
Effect of age. The number of squares crossed in outer area (F3,137 = 14.07, P < 0.001), the total number of squares crossed (F3,137 = 14.34, P < 0.001), grooming number (F3,137 = 24.17, P < 0.001) and duration (F3,137 = 17.46, P < 0.001), and defecation number (F3,137 = 6.3, P = 0.013) decreased with age (Table 2, Table 3).
The interaction between sex and age was observed for defecation number (F6,134 = 9.32, P = 0.003).
Body Weight Changes
Factorial ANOVA (factors: sex, age, and treatment) revealed statistically significant effect of all the factors: sex, age, and treatment on weight of the animals.
Effect of sex. The body weight was significantly higher in males than in females (F3,110 = 99.99, P < 0.001) (Table 4).
Table 4.
Effect of CF on body weight in adolescent and adult male and female rats.
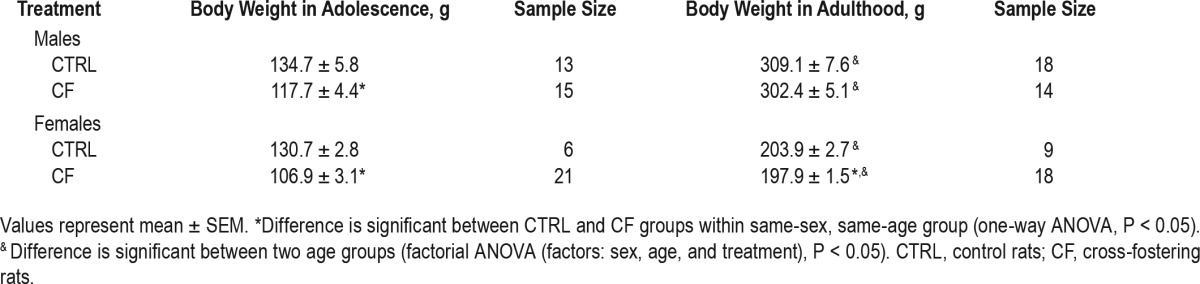
Effect of age. The body weight was significantly higher in adulthood than in adolescence (F3,110 = 562.21, P < 0.001).
The interaction between sex and age was significant (F3,107 = 172.98, P < 0.001).
Effect of treatment. The effect of CF treatment was significant (F3,110 = 4.11, P = 0.04). Simple one-way ANOVA within same-sex and same-age group, analysis revealed that adolescent CF males had lower weight than CTRLs (F1,26 = 5.63, P = 0.03), but in adulthood this difference disappeared (F1,30 = 0.46, P = 0.5). CF females had lower body mass in both adolescence (F1,25 = 15.79, P = 0.001) and adulthood (F1,25 = 4.61, P = 0.04).
DISCUSSION
The main finding of the study was that even a relatively mild adverse early-life event, namely the cross-fostering procedure, on the twelfth day of life induced considerable changes in sleep architecture and brain adenosine levels in adulthood. These changes were found in both female and male animals. Interestingly, no changes were found in the open field and sucrose preference tests, leaving the sleep modifications as predominant consequences of this mild early-life event and indicating that sleep is a very sensitive indicator of early-life stress events in adulthood.
Many previous studies have demonstrated the effects of maternal separation on parameters of spontaneous and stress-induced sleep.29–34 The core of the reported, slightly variable, separation protocols is a daily separation of the pups from their mothers for 180 min during a period of several days at the age of 2–14 (16) days. This procedure inflicts considerable stress, as evidenced by hyper responsivity of the hypothalamic-pituitary-adrenal axis and increased corticosterone levels in adulthood.35,36 The effects of maternal separation on sleep in adulthood have reported to be relatively uniform: the majority of the changes manifest as increases in different parameters of REM sleep; one study reported decrease of REM sleep.31 Some studies also reported changes in NREM sleep.29,31,32
In the present study, although the cross-fostering model deviates from the maternal separation, the results were analogous: REM sleep onsets during spontaneous sleep were increased in both male and female CF treated rats in light and dark periods. Moreover, the total amount of time spent in REM and NREM sleep during the light period was elevated in CF males and females, which was reflected as a decrease in waking. Total amount of NREM sleep was also slightly increased in CF rats during the dark period.
Effects of maternal separation on sleep homeostasis (sleep after a prolonged period of wakefulness) have not previously been addressed. In a pharmacologically induced depression model in males (clomipramine administration in early life) sleep homeostasis was impaired and characterized by a reduced increase in NREM sleep duration and delta power during recovery sleep.11 The present study demonstrated a normal homeostatic NREM sleep response with increased delta power in CF treated male and female rats. Sleep deprivation for three hours is considered a minimum deprivation time, which causes physiological sleep homeostasis reflected in an increased NREM sleep duration and intensity, as well as in elevated basal forebrain adenosine level.11,37 Longer sleep deprivation (6–24 h) could potentially induce different homeo-static responses in CF and control rats.
Early-life stress affects sleep also in humans4–6: high stress load during childhood decreases sleep efficiency and increases nocturnal activity.4,38 Most human studies on the effects of adverse childhood experiences on sleep have been obtained from self-reported and/or actigraphy data, and do not contain polysomnographic studies.
Adenosine is a sleep regulatory factor,8,39 but it can also be involved in development of psychopathologies.40,41 Previous studies have shown that its basal level is decreased in the clomipramine model of depression.11
In the present study, the baseline adenosine level in the basal forebrain of adult male and female CF rats was lower than in CTRLs. The basal forebrain is a specific site for adenosine-mediated regulation of sleep homeostasis,8 and the low adenosine level there could contribute to the sleep changes observed in the adult CF rats. However, sleep homeostasis was not severely affected, as both control and CF rats demonstrated increase in slow wave activity during recovery period after SD.
The BDNF gene expression level in the basal forebrain of CF rats tended to be lower compared to CTRL rats during both age points: adolescence and adulthood. Interestingly, BDNF gene expression levels in other brain areas did not change either with age or by the treatment. Such trend in BDNF expression level, particularly in the basal forebrain, may reflect dysfunction of the cholinergic neurotransmission that may account for the development of cognitive impairment often associated with psychopathologies42 and usually observed in cognitive tests. As the basal forebrain is also a specific site for regulation of sleep homeostasis,8 we can speculate that this structure is one of the common regulatory sites for both sleep and psychopathologies. Sex-specific, long-term effect of maternal separation on BDNF expression has been previously reported.43
The quality of maternal care has long-lasting effects on the pups. The effects of late adoption (during days 5–12 of life) are similar to those of early maternal separation, and are characterized by prolonged stress-induced corticosterone secretion in adult offspring.20 Low level of maternal care increases anxiety-and depression-like behavior44,45 by epigenetic changes in glucocorticoid receptor in the hippocampus of adult offspring and cause the impairment of negative feedback in hypothalamic-pituitary adrenal axis.46,47 Such changes can lead to decline in BDNF expression not only in hippocampus,48 but probably also in other brain regions, potentially contributing to low BDNF expression levels observed in the present study.
Open field test is used to assess general locomotor activity as well as emotional, and exploratory behavior in rodents. Horizontal and vertical activities are measures of exploration level, while activity in the central or peripheral sectors reflects the proportion between exploratory behavior and harm avoidance, respectively.49,50 Excretory activity (defecations and urinations) is used as indicator of sympathetic nervous activity.49 These parameters give an estimate of the emotionality and anxiety level of the animal. The interpretation of the relationship between emotionality level (fear) and exploration varies from the assumption that emotionality (or fear) and exploration are inversely related (i.e., high emotionality inhibits exploration and low emotionality facilitates it), to the opinion that high fear facilitates exploration.49
The overall motor activity of CF rats did not significantly differ from CTRL rats either in adolescence or adulthood. Motor activity of CTRL rats decreased from adolescence to adulthood, as evidenced by decreased horizontal activity. This result is in line with previous studies.51,52 No significant differences were found in open field test between CTRL and CF animals.
Sucrose preference test, which in animal studies is used as a measure of sensitivity to reward53 and anhedonia,54 did not show significant changes in CF rats compared to CTRLs, indicating that CF rats did not develop depressive-like behavior.
In summary, behavioral tests did not reveal differences between CTRL and CF treated groups under normal conditions. However, considering the molecular level changes after CF, it can be suggested that under stressful conditions, brain resilience can be compromised, predisposing to different psycho-pathologies in adulthood.
Weight disorder is a common sign of psychopathology in both humans55 and animals.11,56,57 In the present study, both male and female CF rats weighed in adolescence less than CTRLs. This difference remained to adulthood in females, while male CF rats in adulthood had equal weight to CTRLs. A meta-analysis of human studies showed higher association of body weight disorder with psychopathology in adolescent girls than in boys.58
Strengths and Limitations
Both female and male rats were used in the study, and some of the parameters were measured in both adolescence and adulthood. High variability was observed in some parameters, particularly in females (e.g., BDNF expression in the basal forebrain). This variability may at least partially be due to different BDNF levels in the course of estrous cycle.59 This may have been reflected also to the variability in sleep stages.60 The female rats in the study were not cycled because of the potential effects of this procedure on the result outcome. In future, the studies should be repeated with cycled females.
Sleep-wake disorders, which are related to psychiatric disorders like anxiety and depression, are often associated with elevated levels of cortisol (in humans) or corticosterone (in animals). Measurement of corticosterone levels in the present study could have clarified the stress level induced by the CF treatment, and potentially added information to the observed sex differences.
CONCLUSIONS
Even when the consequences of adverse early-life events cannot in adulthood be detected from behavioral signs used to monitor depression and/or anxiety, they at a molecular level leave permanent changes in brain, as in the present study evidenced by decreased adenosine level. This molecular “scar” may act as a predisposing factor in development of psychopathologies later in life. Sleep, particularly REM sleep, appears to be the most sensitive behavioral indicator of the early-life events in adulthood.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was supported by the Centre for International Mobility Fellowships programme (CIMO, 2013), Finska Läkaresällskapet (2014) and the Academy of Finland foundation (#137575). Dr. Porkka-Heiskanen consults for the Orion Company. The other authors have indicated no financial conflicts of interest. The work was performed at the Department of Physiology, Faculty of Medicine, University of Helsinki, Helsinki, Finland.
ACKNOWLEDGMENTS
The authors thankfully acknowledge Pirjo Saarelainen for the excellent technical help and Dr. Stanislav Rozov for his assistance with the HPLC measurements.
REFERENCES
- 1.Arnow BA. Relationships between childhood maltreatment, adult health and psychiatric outcomes, and medical utilization. J Clin Psychiat. 2004;65:10–5. [PubMed] [Google Scholar]
- 2.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–39. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 3.McCormick CM, Smith C, Mathews IZ. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behav Brain Res. 2008;187:228–38. doi: 10.1016/j.bbr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Chapman DP, Wheaton AG, Anda RF, et al. Adverse childhood experiences and sleep disturbances in adults. Sleep Med. 2011;12:773–9. doi: 10.1016/j.sleep.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 5.Greenfield EA, Lee C, Friedman EL, Springer KW. Childhood abuse as a risk factor for sleep problems in adulthood: evidence from a U.S. national study. Ann Behav Med. 2011;42:245–56. doi: 10.1007/s12160-011-9285-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koskenvuo K, Hublin C, Partinen M, Paunio T, Koskenvuo M. Childhood adversities and quality of sleep in adulthood: a population-based study of 26,000 Finns. Sleep Med. 2010;11:17–22. doi: 10.1016/j.sleep.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Lopez J, Hoffmann R, Armitage R. Reduced sleep spindle activity in early-onset and elevated risk for depression. J Am Acad Child Adolesc Psychiatry. 2010;49:934–43. doi: 10.1016/j.jaac.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1265–8. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dwivedi Y. Brain-derived neurotrophic factor: role in depression and suicide. Neuropsychiatr Dis Treat. 2009;5:433–49. doi: 10.2147/ndt.s5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castren E, Rantamaki T. The role of BDNF and its receptors in depression and antidepressant drug action: reactivation of developmental plasticity. Dev Neurobiol. 2010;70:289–97. doi: 10.1002/dneu.20758. [DOI] [PubMed] [Google Scholar]
- 11.Savelyev SA, Rantamaki T, Rytkonen KM, Castren E, Porkka-Heiskanen T. Sleep homeostasis and depression: studies with the rat clomipramine model of depression. Neuroscience. 2012;212:149–58. doi: 10.1016/j.neuroscience.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 12.Piccinelli M, Wilkinson G. Gender differences in depression. Critical review. Br J Psychiatry. 2000;177:486–92. doi: 10.1192/bjp.177.6.486. [DOI] [PubMed] [Google Scholar]
- 13.Vesga-Lopez O, Schneier FR, Wang S, et al. Gender differences in generalized anxiety disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) J Clin Psychiatry. 2008;69:1606–16. [PMC free article] [PubMed] [Google Scholar]
- 14.Goel N, Kim H, Lao RP. Gender differences in polysomnographic sleep in young healthy sleepers. Chronobiol Int. 2005;22:905–15. doi: 10.1080/07420520500263235. [DOI] [PubMed] [Google Scholar]
- 15.Zhang B, Wing YK. Sex differences in insomnia: a meta-analysis. Sleep. 2006;29:85–93. doi: 10.1093/sleep/29.1.85. [DOI] [PubMed] [Google Scholar]
- 16.Krishnan V, Collop NA. Gender differences in sleep disorders. Curr Opin Pulm Med. 2006;12:383–9. doi: 10.1097/01.mcp.0000245705.69440.6a. [DOI] [PubMed] [Google Scholar]
- 17.Bath KG, Schilit A, Lee FS. Stress effects on BDNF expression: effects of age, sex, and form of stress. Neuroscience. 2013;239:149–56. doi: 10.1016/j.neuroscience.2013.01.074. [DOI] [PubMed] [Google Scholar]
- 18.Wilber AA, Southwood CJ, Sokoloff G, Steinmetz JE, Wellman CL. Neonatal maternal separation alters adult eyeblink conditioning and glucocorticoid receptor expression in the interpositus nucleus of the cerebellum. Dev Neurobiol. 2007;67:1751–64. doi: 10.1002/dneu.20549. [DOI] [PubMed] [Google Scholar]
- 19.Wilber AA, Wellman CL. Neonatal maternal separation alters the development of glucocorticoid receptor expression in the interpositus nucleus of the cerebellum. Int J Dev Neurosci. 2009;27:649–54. doi: 10.1016/j.ijdevneu.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Barbazanges A, Vallee M, Mayo W, et al. Early and later adoptions have different long-term effects on male rat offspring. J Neurosci. 1996;16:7783–90. doi: 10.1523/JNEUROSCI.16-23-07783.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maccari S, Piazza PV, Kabbaj M, Barbazanges A, Simon H, Le Moal M. Adoption reverses the long-term impairment in glucocorticoid feedback induced by prenatal stress. J Neurosci. 1995;15:110–6. doi: 10.1523/JNEUROSCI.15-01-00110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barros VG, Berger MA, Martijena ID, et al. Early adoption modifies the effects of prenatal stress on dopamine and glutamate receptors in adult rat brain. J Neurosci Res. 2004;76:488–96. doi: 10.1002/jnr.20119. [DOI] [PubMed] [Google Scholar]
- 23.Darnaudery M, Koehl M, Barbazanges A, Cabib S, Le Moal M, Maccari S. Early and later adoptions differently modify mother-pup interactions. Behav Neurosci. 2004;118:590–6. doi: 10.1037/0735-7044.118.3.590. [DOI] [PubMed] [Google Scholar]
- 24.Bhagya V, Srikumar BN, Raju TR, Shankaranarayana Rao BS. Neonatal clomipramine induced endogenous depression in rats is associated with learning impairment in adulthood. Behav Brain Res. 2008;187:190–4. doi: 10.1016/j.bbr.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 25.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- 26.Kalueff AV, Tuohimaa P. Experimental modeling of anxiety and depression. Acta Neurobiol Exp. 2004;64:439–48. doi: 10.55782/ane-2004-1526. [DOI] [PubMed] [Google Scholar]
- 27.Rytkonen KM, Zitting J, Porkka-Heiskanen T. Automated sleep scoring in rats and mice using the naive Bayes classifier. J Neurosci Methods. 2011;202:60–4. doi: 10.1016/j.jneumeth.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 28.Zant JC, Rozov S, Wigren HK, Panula P, Porkka-Heiskanen T. Histamine release in the basal forebrain mediates cortical activation through cholinergic neurons. J Neurosci. 2012;32:13244–54. doi: 10.1523/JNEUROSCI.5933-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sampath D, Sabitha KR, Hegde P, et al. A study on fear memory retrieval and REM sleep in maternal separation and isolation stressed rats. Behav Brain Res. 2014;273:144–54. doi: 10.1016/j.bbr.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 30.Tiba PA, Tufik S, Suchecki D. Effects of maternal separation on baseline sleep and cold stress-induced sleep rebound in adult Wistar rats. Sleep. 2004;27:1146–53. doi: 10.1093/sleep/27.6.1146. [DOI] [PubMed] [Google Scholar]
- 31.Reyes Prieto NM, Romano Lopez A, Perez Morales M, et al. Oleamide restores sleep in adult rats that were subjected to maternal separation. Pharmacol Biochem Behav. 2012;103:308–12. doi: 10.1016/j.pbb.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 32.Mrdalj J, Pallesen S, Milde AM, et al. Early and later life stress alter brain activity and sleep in rats. PLoS One. 2013;8:e69923. doi: 10.1371/journal.pone.0069923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tiba PA, Tufik S, Suchecki D. Long lasting alteration in REM sleep of female rats submitted to long maternal separation. Physiol Behav. 2008;93:444–52. doi: 10.1016/j.physbeh.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Tiba PA, Palma BD, Tufik S, Suchecki D. Effects of early handling on basal and stress-induced sleep parameters in rats. Brain Res. 2003;975:158–66. doi: 10.1016/s0006-8993(03)02630-1. [DOI] [PubMed] [Google Scholar]
- 35.Liu D, Diorio J, Tannenbaum B, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–62. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 36.Roque S, Mesquita AR, Palha JA, Sousa N, Correia-Neves M. The behavioral and immunological impact of maternal separation: a matter of timing. Front Behav Neurosci. 2014;8:192. doi: 10.3389/fnbeh.2014.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalinchuk AV, Lu Y, Stenberg D, Rosenberg PA, Porkka-Heiskanen T. Nitric oxide production in the basal forebrain is required for recovery sleep. J Neurochem. 2006;99:483–98. doi: 10.1111/j.1471-4159.2006.04077.x. [DOI] [PubMed] [Google Scholar]
- 38.Schafer V, Bader K. Relationship between early-life stress load and sleep in psychiatric outpatients: a sleep diary and actigraphy study. Stress Health. 2013;29:177–89. doi: 10.1002/smi.2438. [DOI] [PubMed] [Google Scholar]
- 39.Urade Y. The role of adenosine in sleep-wake regulation. J Pharmacol Sci. 2011;115:40. [Google Scholar]
- 40.Kessler RC, Aguilar-Gaxiola S, Alonso J, et al. The global burden of mental disorders: an update from the WHO World Mental Health (WMH) Surveys. Epidemiol Psichiat S. 2009;18:23–33. doi: 10.1017/s1121189x00001421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gass N, Ollila HM, Utge S, et al. Contribution of adenosine related genes to the risk of depression with disturbed sleep. J Affect Disord. 2010;126:134–9. doi: 10.1016/j.jad.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 42.Dagyte G, Den Boer JA, Trentani A. The cholinergic system and depression. Behav Brain Res. 2011;221:574–82. doi: 10.1016/j.bbr.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 43.Hill RA, Klug M, Kiss Von Soly S, Binder MD, Hannan AJ, van den Buuse M. Sex-specific disruptions in spatial memory and anhedonia in a “two hit” rat model correspond with alterations in hippocampal brain-derived neurotrophic factor expression and signaling. Hippocampus. 2014;24:1197–211. doi: 10.1002/hipo.22302. [DOI] [PubMed] [Google Scholar]
- 44.Caldji C, Diorio J, Meaney MJ. Variations in maternal care in infancy regulate the development of stress reactivity. Biol Psychiatry. 2000;48:1164–74. doi: 10.1016/s0006-3223(00)01084-2. [DOI] [PubMed] [Google Scholar]
- 45.Newport DJ, Stowe ZN, Nemeroff CB. Parental depression: animal models of an adverse life event. Am J Psychiatry. 2002;159:1265–83. doi: 10.1176/appi.ajp.159.8.1265. [DOI] [PubMed] [Google Scholar]
- 46.Weaver IC, Cervoni N, Champagne FA, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–54. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 47.Anacker C, O'Donnell KJ, Meaney MJ. Early life adversity and the epigenetic programming of hypothalamic-pituitary-adrenal function. Dialogues Clin Neurosci. 2014;16:321–33. doi: 10.31887/DCNS.2014.16.3/canacker. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schaaf MJ, De Kloet ER, Vreugdenhil E. Corticosterone effects on BDNF expression in the hippocampus. Implications for memory formation. Stress. 2000;3:201–8. doi: 10.3109/10253890009001124. [DOI] [PubMed] [Google Scholar]
- 49.Archer J. Tests for emotionality in rats and mice: a review. Animal Behav. 1973;21:205–35. doi: 10.1016/s0003-3472(73)80065-x. [DOI] [PubMed] [Google Scholar]
- 50.Redina OE, Smolenskaya SE, Maslova LN, Sakharov DG, Markel AL. The characteristics of motor activity in ISIAH rats in an open field test are controlled by genes on chromosomes 2 and 16. Neurosci Behav Physiol. 2009;39:57–64. doi: 10.1007/s11055-008-9100-8. [DOI] [PubMed] [Google Scholar]
- 51.Masur J, Schutz MT, Boerngen R. Gender differences in open-field behavior as a function of age. Dev Psychobiol. 1980;13:107–10. doi: 10.1002/dev.420130202. [DOI] [PubMed] [Google Scholar]
- 52.Hilakivi LA, Sinclair JD, Hilakivi IT. Effects of neonatal treatment with clomipramine on adult ethanol related behavior in the rat. Brain Res. 1984;317:129–32. doi: 10.1016/0165-3806(84)90148-2. [DOI] [PubMed] [Google Scholar]
- 53.Harris RBS, Zhou J, Youngblood BD, Smagin GN, Ryan DH. Failure to change exploration or saccharin preference in rats exposed to chronic mild stress. Physiol Behav. 1997;63:91–100. doi: 10.1016/s0031-9384(97)00425-3. [DOI] [PubMed] [Google Scholar]
- 54.Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology. 1987;93:358–64. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- 55.de Wit LM, van Straten A, Lamers F, Cuijpers P, Penninx BW. Depressive and anxiety disorders: associated with losing or gaining weight over 2 years? Psychiatr Res. 2015;227:230–7. doi: 10.1016/j.psychres.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 56.Amani M, Samadi H, Doosti MH, et al. Neonatal NMDA receptor blockade alters anxiety- and depression-related behaviors in a sex-dependent manner in mice. Neuropharmacology. 2013;73:87–97. doi: 10.1016/j.neuropharm.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 57.Doosti MH, Bakhtiari A, Zare P, et al. Impacts of early intervention with fluoxetine following early neonatal immune activation on depression-like behaviors and body weight in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2013;43:55–65. doi: 10.1016/j.pnpbp.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 58.Burke NL, Storch EA. A meta-analysis of weight status and anxiety in children and adolescents. J Dev Behav Pediatr. 2015;36:133–45. doi: 10.1097/DBP.0000000000000143. [DOI] [PubMed] [Google Scholar]
- 59.Luine V, Frankfurt M. Interactions between Estradiol, Bdnf and Dendritic Spines in Promoting Memory. Neuroscience. 2013;239:34–45. doi: 10.1016/j.neuroscience.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fang JD, Fishbein W. Sex differences in paradoxical sleep: influences of estrus cycle and ovariectomy. Brain Res. 1996;734:275–85. [PubMed] [Google Scholar]