Abstract
This study investigated whether pretreatment neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and prognostic nutritional index (PNI) are prognostic factors in patients with cervical cancer who undergo concurrent chemoradiotherapy (CCRT) and radiotherapy (RT). A total of 131 patients who underwent CCRT and RT for cervical cancer were retrospectively investigated and the correlations of NLR, PLR and PNI with clinical parameters and prognosis were assessed in CCRT and RT. The CCRT and RT groups had a median progression-free survival (PFS) of 41.82 and 24.72 months, respectively, and an overall survival of 49.70 and 29.56 months, respectively. At a cut-off value of NLR≥2.85, the PFS and OS in patients with higher NLR undergoing RT were significantly shorter compared with those in patients with lower NLR (P=0.029 and P=0.017, respectively). At a cut-off value for PNI of ≤48.55 in patients undergoing CCRT and ≤45.80 in patients undergoing RT, the PFS and OS in patients with lower PNI were significantly shorter compared with those in patients with higher PNI (PFS and OS with CCRT, P<0.001 and P<0.001, respectively; PFS and OS with RT, P=0.002 and P=0.008, respectively). Multivariate analyses also identified low PNI as an independent prognostic factor for PFS and OS in patients receiving CCRT. Therefore, low PNI was shown to predict poor prognosis in patients with cervical cancer.
Keywords: cervical cancer, prognostic nutritional index, concurrent chemoradiotherapy, predictor of poor prognosis
Introduction
Cervical cancer is the fourth most common malignancy among women worldwide (1), with a 5-year recurrence rate of 28% according to the International Federation of Gynecology and Obstetrics (FIGO) (2). Poor prognostic factors for cervical cancer include stage, tumor size, histology and lymph node (LN) metastasis (3,4). However, these parameters are not sufficient to accurately predict prognosis.
Systemic inflammatory response (SIR) is an important prognostic factor for survival in various types of cancer (5,6). Neutrophils, platelets, lymphocytes and albumin play a prominent role in cancer-related inflammation. Recent evidence has indicated that relative differences in neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and prognostic nutritional index (PNI) (PNI=10 × albumin concentration +0.005 × total lymphocyte count) affect SIR and, consequently, cancer survival (7–10). Hypoalbuminemia is often observed in patients with advanced cancer and is usually considered to be a marker of malnutrition and cachexia. It has also been reported that albumin is involved in SIR and survival in various types of cancer (11,12). Although pretreatment NLR and PLR have been shown to predict outcome in gynecological cancers, such as endometrial (13,14), cervical (15,16) and ovarian cancer (17,18), PNI has not been shown to be a predictive factor in patients with cervical cancer. The aim of this study was to investigate the correlation between pretreatment NLR, PLR, PNI and prognosis in patients who had been treated with concurrent chemoradiotherapy (CCRT) or radiotherapy (RT) for cervical cancer.
Patients and methods
Patients
The study population consisted of 131 patients with primary cervical cancer who underwent CCRT or RT at the Department of Obstetrics and Gynecology of Okayama University Hospital (Okayama, Japan) between April, 2007 and March, 2013. The study protocol was approved by the Institutional Review Board of Okayama University Hospital. The patients' clinical data, including medical history, physical examination and clinical staging, were reviewed. Computed tomography (CT) and positron emission tomography-CT are widely accepted modalities for assessing the extent of LN metastasis; according to traditional criteria, LNs with short-axis length of >10.0 mm are defined as metastatic (19). The baseline pretreatment characteristics (stage, histology, LN metastasis, parametrial involvement, vaginal invasion and maximum tumor size) of the patients are listed in Table I.
Table I.
Patient and tumor characteristics.
| Baseline characteristics | All patients, no. (%) | |
|---|---|---|
| Age at diagnosis, years [mean (range)] | ||
| Stage | 61.5 (25–88) | |
| Ib1 | 7 | 5.8 |
| Ib2 | 10 | 8.3 |
| IIa1 | 7 | 5.8 |
| IIa2 | 4 | 3.3 |
| IIb | 51 | 42.1 |
| IIIa | 3 | 2.5 |
| IIIb | 33 | 27.3 |
| IVa | 6 | 4.9 |
| Histology | ||
| SCC | 104 | 85.9 |
| AD | 14 | 11.6 |
| ADSQ | 3 | 2.5 |
| Lymph node metastasis | ||
| Negative | 83 | 68.6 |
| Positive | 38 | 31.4 |
| Parametrial invasion | ||
| Negative | 30 | 24.8 |
| Positive | 91 | 75.2 |
| Vaginal invasion | ||
| Negative | 63 | 52.1 |
| Positive | 58 | 47.9 |
| Maximum tumor size, cm | ||
| ≤4.0 | 41 | 33.9 |
| >4.0 | 80 | 66.1 |
| Treatment | ||
| CCRT | 95 | 78.5 |
| RT | 36 | 21.5 |
| Chemotherapy regimen (N=95) | ||
| Weekly CDDP | 52 | 54.7 |
| Weekly NED | 28 | 29.4 |
| Ifosfamide + NED | 15 | 15.9 |
CDDP, cisplatin; NED, nedaplatin; CCRT, concurrent chemoradiotherapy; RT, radiotherapy; SCC, squamous cell carcinoma; AD, adenocarcinoma; ADSQ, adenosquamous carcinoma.
Laboratory analysis
All the patients had their white blood cell (WBC) count and albumin levels recorded within 1 week prior to treatment. Differential WBC counts and albumin levels were measured prior to treatment with RT or CCRT; WBC, neutrophil, lymphocyte and platelet counts were measured using automated blood cell counters (Bayer HealthCare, Diagnostics Division, Tarrytown, NY, USA). The levels of serum albumin were measured by latex nephelometry (LT Auto Wako, Osaka, Japan). NLR was defined as the absolute neutrophil count (µl) divided by the absolute lymphocyte count (µl), and PLR was defined as the absolute platelet count (µl) divided by the lymphocyte count (µl). The PNI was calculated as previously described (20). Briefly, PNI was defined as 10 × albumin concentration (g/dl)+0.005 × total lymphocyte count (µl). During RT or CCRT, the WBC, neutrophil, lymphocyte and platelet counts, albumin levels and weight were measured weekly. Acute toxicities were evaluated and graded using the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 (21). Seven toxicities, including leukopenia, neutropenia, lymphocytopenia, thrombocytopenia, weight loss, diarrhea and hyponatremia, were recorded based on CTCAE v.4.0.
Treatment
The patients were treated with a combination of external irradiation and intracavitary brachytherapy (ICBT) with curative intent. RT was delivered at 2.0 Gy per fraction once daily, 5 days per week, over 5 weeks. The median dose to the whole pelvis was 50.0 Gy and ICBT as the high dose rate was 24 Gy/4 times. For CCRT, the patients were treated with either cisplatin (CDDP; 40 mg/m2 infusion weekly for six cycles), nedaplatin (NED; 30 mg/m2 infusion weekly for eight cycles), or ifosfamide plus NED (IN) [ifosfamide (1 g/m2) infusion on days 1–5 and NED (80 mg/m2) infusion on day 1 of a 3-week cycle, for three cycles], as previously described (22,23). A total of 52 patients treated with CDDP, 28 treated with NED and 15 treated with IN chemotherapy were evaluated. The remaining 36 patients did not receive concurrent chemotherapy due to the presence of comorbidities or advanced age (≥75 years). CCRT was interrupted for up to 1 week in patients with WBC counts <2,000/µl, neutrophil counts <1,000/µl, or platelet counts <75,000/µl. If these side effects persisted for >1 week, no additional chemotherapy was administered. RT was suspended indefinitely in patients who exhibited a WBC count <1,000/µl, neutrophil count <500/µl, or platelet count <25,000/µl. Since the prognosis of patients with cervical cancer is associated with their hemoglobin (Hb) level during RT or CCRT (24), our treatment policy is to administer red blood cell transfusions prior to and during CCRT if the Hb level is <10.0 g/dl, until it exceeds 10 g/dl. Patients underwent follow-up examinations approximately every 1–2 months for the first 6 months, every 3 months for the next 2 years, and every 6 months thereafter.
Statistical analysis
Statistical analyses were performed using the χ2 test and the Mann-Whitney U test for comparisons with the controls and the one-factor analysis of variance, followed by Fisher's protected least-significant difference test for all pairwise comparisons. The survival curves were calculated by the Kaplan-Meier method; differences in the recurrence or survival curves were examined using the log-rank test. The analyses were performed using the SPSS software, version 20.0 (IBM SPSS, Armonk, NY, USA). P<0.05 was considered to indicate statistically significant differences.
Results
Patient characteristics
The clinicopathological characteristics, including patient age, tumor stage, histology, LN metastasis, parametrial involvement, vaginal invasion, maximum tumor size and therapy, are listed in Table I. The mean values in the CCRT group (n=95) were as follows: NLR=3.17 (range, 0.89–9.52); PLR=188.29 (range, 40.61–466.49); and PNI=49.83 (range, 39.15–62.80). The mean values in the RT group (n=36) were as follows: NLR=3.05 (range, 1.15–8.05); PLR=183.40 (range, 74.88–533.22); and PNI=47.41 (range, 30.34–56.10).
The distribution of these three values according to the patients' clinical characteristics is shown in Table II. In the CCRT group, PLR was found to be significantly associated with LN metastasis (P<0.001) and vaginal invasion (P=0.005), whereas PNI was significantly associated with stage (P=0.004), LN metastasis (P=0.031), parametrial involvement (P=0.011) and vaginal invasion (P=0.027). In the RT group, NLR was significantly associated with stage (P=0.041), histology (P=0.012), maximum tumor size (P=0.013), parametrial involvement (P<0.001) and vaginal invasion (P=0.044); PLR was associated with FIGO stage (P=0.030), histology (P=0.008), maximum tumor size (P=0.005) and parametrial involvement (P=0.016); and PNI was associated with histology (P=0.018), LN metastasis (P=0.010), maximum tumor size (P=0.001) and parametrial involvement (P<0.001; Mann-Whitney U test, P<0.05).
Table II.
Associations of NLR, PLR and PNI with clinical factors in cervical cancer.
| A, CCRT | |||||||
|---|---|---|---|---|---|---|---|
| Variables | N | NLR | P-value | PLR | P-value | PNI | P-value |
| Stage | 0.216 | 0.158 | 0.004a | ||||
| I–II | 67 | 3.05±1.46 | 179.29±70.98 | 50.65±4.50 | |||
| III–IV | 28 | 3.49±1.81 | 209.81±102.60 | 47.86±3.73 | |||
| Histology | 0.089 | 0.208 | 0.471 | ||||
| SCC | 86 | 3.23±1.63 | 191.73±83.88 | 49.67±4.40 | |||
| Non-SCC | 9 | 2.67±0.77 | 155.37±57.34 | 48.54±5.02 | |||
| LNM | 0.056 | <0.001a | 0.031a | ||||
| Negative | 57 | 2.92±1.43 | 162.82±62.51 | 50.63±4.25 | |||
| Positive | 38 | 3.55±1.73 | 226.48±93.58 | 48.63±4.54 | |||
| MTS, cm | 0.154 | 0.233 | 0.089 | ||||
| ≤4.0 | 24 | 2.78±1.42 | 170.95±72.65 | 51.17±4.48 | |||
| >4.0 | 71 | 3.31±1.61 | 194.15±84.86 | 49.38±4.39 | |||
| PI | 0.506 | 0.155 | 0.011a | ||||
| Negative | 20 | 3.00±1.19 | 169.06±60.55 | 52.04±3.69 | |||
| Positive | 75 | 3.22±1.67 | 193.41±86.69 | 49.24±4.48 | |||
| VI | 0.229 | 0.005a | 0.027a | ||||
| Negative | 48 | 2.98±1.34 | 165.19±68.46 | 50.82±4.28 | |||
| Positive | 47 | 3.37±1.77 | 211.88±88.84 | 48.81±4.44 | |||
| B, RT | |||||||
| Variables | N | NLR | P-value | PLR | P-value | PNI | P-value |
| Stage | 0.041a | 0.030a | 0.018a | ||||
| I–II | 21 | 2.58±1.55 | 151.11±53.27 | 49.38±5.61 | |||
| III–IV | 15 | 3.70±1.59 | 228.61±119.62 | 44.65±5.76 | |||
| Histology | 0.012a | 0.008a | 0.109 | ||||
| SCC | 28 | 3.31±1.74 | 198.00±100.48 | 46.77±6.55 | |||
| Non-SCC | 8 | 2.16±0.79 | 132.29±38.12 | 49.65±3.42 | |||
| LNM | 0.114 | 0.078 | 0.010a | ||||
| Negative | 26 | 2.78±1.66 | 158.49±54.16 | 48.97±5.36 | |||
| Positive | 10 | 3.75±1.46 | 248.16±140.72 | 43.37±6.20 | |||
| MTS, cm | 0.013a | 0.005a | 0.001a | ||||
| ≤4.0 | 17 | 2.35±0.84 | 139.37±36.57 | 50.62±4.67 | |||
| >4.0 | 19 | 3.68±1.94 | 222.79±112.00 | 44.54±5.81 | |||
| PI | <0.001a | 0.016a | <0.001a | ||||
| Negative | 10 | 1.96±0.72 | 140.77±36.30 | 51.83±3.26 | |||
| Positive | 26 | 3.47±1.71 | 199.79±104.42 | 45.71±6.08 | |||
| VI | 0.044a | 0.076 | 0.249 | ||||
| Negative | 15 | 2.40±1.70 | 153.01±62.18 | 48.81±6.18 | |||
| Positive | 21 | 3.51±1.48 | 205.11±107.64 | 46.42±5.94 | |||
P<0.0. CCRT, concurrent chemoradiotherapy; RT, radiotherapy; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutritional index; LNM, lymph node metastasis; MTS, maximum tumor size; PI, parametrial involvement; VI, vaginal invasion; SCC, squamous cell carcinoma.
Overall, the CCRT group had a median progression-free survival (PFS) of 41.82 months and an overall survival (OS) of 49.70 months; at the last follow-up, 59 patients in the CCRT group remained alive with no evidence of disease, 28 had succumbed to the disease and 8 were alive with disease. In the RT group, the median PFS was 24.72 months and the OS was 29.56 months; at the last follow-up, 22 patients remained alive with no evidence of disease, 13 had succumbed to the disease and 1 was alive with disease.
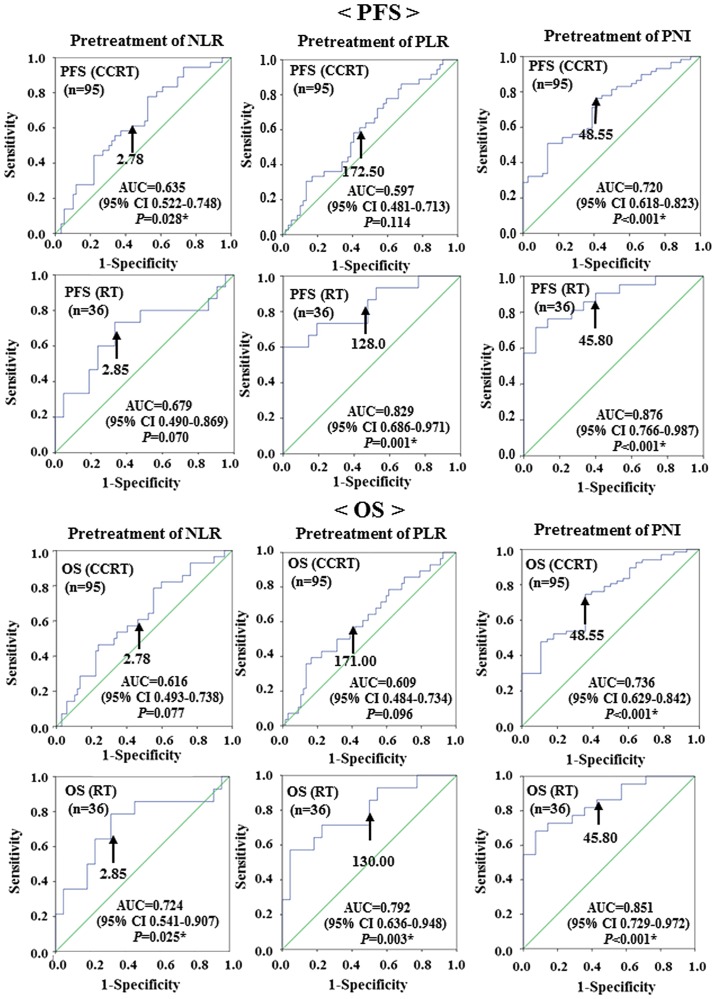
Receiver operating characteristic curve analyses were used to determine the optimal cut-off values of NLR, PLR and PNI to predict recurrence (PFS) and survival (OS). The analyses identified NLR≥2.78 [area under the curve (AUC)=0.635, sensitivity: 63.9%, specificity: 49.2%], PLR≥172.50 (AUC=0.597, sensitivity: 58.3%, specificity: 55.9%) and PNI≤48.55 (AUC=0.720, sensitivity: 72.9%, specificity: 58.3%) as the most accurate cut-off values for predicting recurrence (PFS) in the CCRT group. The analyses identified NLR≥2.85 (AUC=0.679, sensitivity: 66.7%, specificity: 66.7%), PLR≥128.00 (AUC=0.829, sensitivity: 80.0%, specificity: 52.4%) and PNI≤45.80 (AUC=0. 876, sensitivity: 85.7%, specificity: 60.0%) as the most accurate cut-off values for predicting recurrence (PFS) in the RT group.
The analyses identified NLR≥2.78 (AUC=0.616, sensitivity: 60.7%, specificity: 47.8%), PLR≥171.00 (AUC=0.609, sensitivity: 60.7%, specificity: 50.7%), and PNI≤48.55 (AUC=0.736, sensitivity: 73.1%, specificity: 64.3%) as the most accurate cut-off values for predicting survival (OS) in the CCRT group. The analyses identified NLR≥2.85 (AUC=0.724, sensitivity: 71.4%, specificity: 68.2%), PLR≥130.00 (AUC=0.792, sensitivity: 78.6%, specificity: 50.0%) and PNI≤45.80 (AUC=0.851, sensitivity: 86.4%, specificity: 57.1%), as the most accurate cut-off values for predicting survival (OS) in the RT group (Fig. 1).
Figure 1.
Receiver operating characteristic curve analysis determining the optimal cut-off values for NLR, PLR and PNI to predict recurrence (PFS) and survival (OS) in patients with cervical cancer treated with CCRT (n=95) or with RT (n=36). The optimal cut-off values for predicting recurrence (PFS) in the CCRT group were as follows: NLR=2.78 (AUC=0.635; 95% CI: 0.522–0.748; P=0.028); PLR=172.50 (AUC=0.597; 95% CI: 0.481–0.713; P=0.114); and PNI=48.55 (AUC=0.720; 95% CI: 0.618–0.823; P<0.001). The optimal cut-off values for predicting recurrence (PFS) in the RT group were as follows: NLR=2.85 (AUC=0.679; 95% CI: 0.490–0.869; P=0.070); PLR=128.00 (AUC=0.829; 95% CI: 0.686–0.971; P=0.001); and PNI=45.80 (AUC=0.876; 95% CI: 0.766–0987; P<0.001). The optimal cut-off values for predicting survival (OS) in the CCRT group were as follows: NLR=2.78 (AUC=0.616; 95% CI: 0.493–0.738; P=0.077); PLR=171.00 (AUC=0.609; 95% CI: 0.484–0.734; P=0.096); and PNI=48.55 (AUC=0.736; 95% CI: 0.629–0.842; P<0.001). The optimal cut-off values for predicting survival (OS) in the RT group were as follows: NLR=2.85 (AUC=0.724; 95% CI: 0.541–0.907; P=0.025); PLR=130.00 (AUC=0.792; 95% CI: 0.636–0.948; P=0.003); and PNI=45.80 (AUC=0.851; 95% CI: 0.729–0.972; P<0.001). PFS, progression-free survival; OS, overall survival; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutritional index; CCRT, concurrent chemoradiotherapy; RT, radiotherapy; AUC, area under the curve; CI, confidence interval.
The correlations between clinical factors and recurrence (PFS) or survival (OS) were assessed in univariate and multivariate analyses (Tables III and IV). In the univariate analysis, LN metastasis (P=0.032), histology (P=0.006), maximum tumor size (P=0.013), PNI (P=0.002) and extended radiation duration (>6 weeks; P=0.036) were significantly associated with recurrence (PFS) in the CCRT group. Moreover, histology (P=0.016), maximum tumor size (P=0.045) and PNI (P=0.012) were independent predictors of recurrence (PFS) in the CCRT group on multivariate analysis. Univariate analysis suggested that LN metastasis (P=0.013), histology (P=0.012), PNI (P=0.001) and extended radiation duration (P=0.016) were significantly associated with OS in the CCRT group. Moreover, histology (P=0.010) and PNI (P=0.003) were independent predictors of OS in the CCRT group.
Table III.
Prognostic factors for progression-free and overall survival in cervical cancer patients who underwent CCRT (n=95) by Cox's multivariate analysis.
| Progression-free survival | Overall survival | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||||
| Factors | HR | (95% CI) | P-value | HR | (95% CI) | P-value | HR | (95% CI) | P-value | HR | (95% CI) | P-value |
| Stage | 1.397 | (0.707–2.758) | 0.336 | 1.335 | (0.616–2.895) | 0.464 | ||||||
| Lymph node metastasis | 2.049 | (1.064–3.947) | 0.032a | 1.485 | (0.764–2.888) | 0.244 | 2.612 | (1.222–5.585) | 0.013a | 2.019 | (0.936–4.354) | 0.073 |
| Histology | 0.313 | (0.137–0.717) | 0.006a | 0.356 | (0.153–0.824) | 0.016a | 0.315 | (0.127–0.779) | 0.012a | 0.300 | (0.120–0.751) | 0.010a |
| Maximum tumor size | 4.510 | (1.382–14.715) | 0.013a | 3.401 | (1.027–11.267) | 0.045a | 3.270 | (0.987–10.835) | 0.053 | |||
| Parametrial involvement | 1.803 | (0.701–4.638) | 0.221 | 1.757 | (0.609–5.065) | 0.297 | ||||||
| Vaginal invasion | 0.803 | (0.416–1.550) | 0.513 | 1.275 | (0.606–2.680) | 0.522 | ||||||
| NLR | 1.423 | (0.728–2.783) | 0.303 | 1.536 | (0.717–3.289) | 0.269 | ||||||
| PLR | 1.522 | (0.784–2.954) | 0.215 | 1.634 | (0.753–3.543) | 0.214 | ||||||
| PNI | 2.796 | (1.438–5.437) | 0.002a | 2.380 | (1.212–4.676) | 0.012a | 3.754 | (1.726–8.161) | 0.001a | 3.273 | (1.481–7.237) | 0.003a |
| Leukopenia (grade 4) | 0.858 | (0.303–2.427) | 0.772 | 1.126 | (0.390–3.248) | 0.827 | ||||||
| Neutropenia (grade 4) | 0.047 | (0.000–78.691) | 0.419 | 0.047 | (0.000–219.951) | 0.478 | ||||||
| Lymphocytopenia (grade 4) | 0.874 | (0.430–1.776) | 0.709 | 1.022 | (0.462–2.258) | 0.958 | ||||||
| Thrombocytopenia (grade 4) | 1.853 | (0.445–7.720) | 0.397 | 2.102 | (0.498–8.861) | 0.312 | ||||||
| Weight loss (grade ≥2) | 1.072 | (0.446–2.577) | 0.876 | 1.211 | (0.460–3.187) | 0.699 | ||||||
| Diarrhea (grade ≥3) | 0.742 | (0.288–1.909) | 0.535 | 0.940 | (0.356–2.485) | 0.901 | ||||||
| Hyponatremia(grade ≥3) | 1.090 | (0.545–2.179) | 0.808 | 1.621 | (0.757–3.470) | 0.214 | ||||||
| ERD (>6 weeks) | 2.322 | (1.056–5.105) | 0.036a | 1.799 | (0.814–3.976) | 0.147 | 2.893 | (1.221–6.852) | 0.016a | 2.096 | (0.887–4.952) | 0.092 |
P<0.05. CCRT, concurrent chemoradiotherapy; NLR, neutrophiltolymphocyte ratio; PLR, platelettolymphocyte ratio; PNI, prognostic nutritional index; ERD, extension of radiation duration; HR, hazard ratio; CI, confidence interval.
Table IV.
Clinical factors affecting progression-free and overall survival in cervical cancer patients who underwent RT (n=36) by Cox's multivariate analysis.
| Progression-free survival | Overall survival | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||||
| Factors | HR | (95% CI) | P-value | HR | (95% CI) | P-value | HR | (95% CI) | P-value | HR | (95% CI) | P-value |
| Stage | 3.846 | (1.308–11.310) | 0.014a | 0.603 | (0.114–3.197) | 0.552 | 4.263 | (1.331–13.652) | 0.015a | 0.757 | (0.129–4.444) | 0.758 |
| Lymph node metastasis | 4.091 | (1.472–11.370) | 0.007a | 1.919 | (0.383–9.610) | 0.428 | 4.101 | (1.413–11.904) | 0.009a | 1.459 | (0.270–7.885) | 0.661 |
| Histology | 1.134 | (0.320–4.021) | 0.846 | 1.040 | (0.289–3.742) | 0.952 | ||||||
| Maximum tumor size (>4.0 cm) | 18.773 | (2.457–143.465) | 0.005a | 11.976 | (1.263–1,013.546) | 0.030a | 15.656 | (2.044–119.908) | 0.008a | 8.072 | (0.873–74.642) | 0.066 |
| Parametrial involvement | 3.075 | (0.691–13.674) | 0.140 | 2.850 | (0.635–12.788) | 0.172 | ||||||
| Vaginal invasion | 1.639 | (0.559–4.803) | 0.368 | 1.922 | (0.601–6.140) | 0.271 | ||||||
| NLR | 3.587 | (1.139–11.292) | 0.029a | 0.724 | (0.158–3.321) | 0.678 | 4.766 | (1.324–17.154) | 0.017a | 1.682 | (0.342–8.261) | 0.522 |
| PLR | 3.059 | (0.862–10.858) | 0.084 | 2.660 | (0.741–9.547) | 0.133 | ||||||
| PNI | 5.315 | (1.8741–5.074) | 0.002a | 3.127 | (0.944–10.356) | 0.062 | 4.219 | (1.456–12.228) | 0.008a | 1.783 | (0.551–5.764) | 0.334 |
| Lymphocytopenia (grade 4) | 0.047 | (0.000–16,748.526) | 0.639 | 0.047 | (0.000–25,878.809) | 0.651 | ||||||
| Weight loss (grade ≥2) | 2.770 | (0.770–9.965) | 0.119 | 2.775 | (0.762–10.106) | 0.122 | ||||||
| Diarrhea (grade ≥3) | 1.828 | (0.238–14.027) | 0.562 | 1.940 | (0.251–14.981) | 0.525 | ||||||
| Hyponatremia (grade ≥3) | 2.467 | (0.875–6.959) | 0.088 | 2.635 | (0.913–7.605) | 0.073 | ||||||
| ERD (>6 weeks) | 10.637 | (3.071–36.843) | <0.001a | 5.888 | (1.332–26.026) | 0.019a | 13.439 | (3.515–51.378) | <0.001a | 4.853 | (1.044–22.558) | 0.044a |
P<0.05. RT, radiotherapy; NLR, neutrophiltolymphocyte ratio; PLR, platelettolymphocyte ratio; PNI, prognostic nutritional index; ERD, extension of radiation duration; HR, hazard ratio; CI, confidence interval.
In the RT group, univariate analysis suggested that stage (P=0.014), LN metastasis (P=0.007), maximum tumor size (P=0.005), NLR (P=0.029), PNI (P=0.002) and extended radiation duration (P<0.001) were significantly associated with recurrence (PFS). Moreover, maximum tumor size (P=0.030) and extended radiation duration (P=0.019) were independent predictors of recurrence (PFS) in the RT group. Univariate analysis suggested that stage (P=0.015), LN metastasis (P=0.009), maximum tumor size (P=0.008), NLR (P=0.017), PNI (P=0.008) and extended radiation duration (P<0.001) were significantly associated with OS in the RT group. Moreover, extended radiation duration (P<0.001) were independent predictors of OS in the RT group.
Discussion
In cervical cancer, stage, tumor size, histological type, presence of lymphovascular invasion and metastasis to the regional LNs at the time of diagnosis are significant prognostic factors (3,4). However, the prognostic value of SIR in cervical cancer remains unknown. To the best of our knowledge, this is the first study to evaluate whether NLR, PLR and PNI are predictors of poor prognosis for patients with cervical cancer treated with CCRT or RT.
Neutrophils release inflammatory cytokines, leukocyte chemotactic factors and other phagocytic mediators that may damage cellular DNA, inhibit apoptosis and promote angiogenesis (25–28). Platelets release potent mitogens or adhesive glycoproteins, such as platelet-derived growth factor, transforming growth factor-β and vascular endothelial growth factor (29–31). The albumin levels decrease with increased levels of pro-inflammatory cytokines, such as interleukin (IL)-1, IL-6 and tumor necrosis factor, which modulate albumin production (32). Lymphocytes, such as CD3+ T cells and natural killer cells may affect tumor growth and metastasis (33). Recent evidence has shown that relative differences in neutrophil, platelet and lymphocyte counts, albumin levels, NLR, PLR, and PNI, are systemic indicators of prognosis. PNI is based on albumin and absolute lymphocyte count, which are measured routinely in clinical practice, and it is designed to assess nutritional and immunological status, which may predict prognosis (34). Mizunuma et al reported that NLR was a significant prognostic factor for PFS and OS in patients with cervical cancer treated with CCRT or RT (15).
We investigated whether pretreatment clinicopathological parameters were correlated with NLR, PLR and PNI, which reflect SIR. In the CCRT group, PLR was significantly associated with LN metastasis and vaginal invasion; and PNI was significantly associated with stage, LN metastasis, parametrial involvement and vaginal invasion. In the RT group, NLR was significantly associated with stage, histology, maximum tumor size, parametrial involvement and vaginal invasion; PLR was associated with stage, histology, maximum tumor size and parametrial involvement; and PNI was associated with stage, LN metastasis, maximum tumor size and parametrial involvement.
The present study mainly aimed to evaluate the correlation of certain parameters, such as NLR, PLR and PNI, with recurrence and survival in cervical cancer patients who underwent CCRT or RT. In the CCRT group, the PFS and OS of patients with lower PNI were significantly worse compared with those of patients with higher PNI. Multivariate analysis identified PNI as an independent prognostic factor for both PFS and OS. In the RT group, the PFS and OS of patients with higher NLR were significantly shorter compared with those of patients with lower NLR; and the PFS and OS of patients with lower PNI were significantly shorter compared with those of patients with higher PNI. Furthermore, PNI was found to be superior to NLR and PLR as a predictor of survival in the CCRT group.
There were certain limitations to our study, including the limited number of patients and the relatively short duration of follow-up. Further prospective studies with more patients and longer follow-up periods would provide more definitive data to elucidate the significance of our findings.
In conclusion, our results demonstrated that the determination of PNI may serve as a useful indicator of prognosis in cervical cancer patients who undergo CCRT.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Benedet JL, Odicino F, Maisonneuve P, Beller U, Creasman WT, Heintz AP, Ngan HY, Sideri M, Pecorelli S. Carcinoma of the cervix uteri. J Epidemiol Biostat. 2001;6:7–43. [PubMed] [Google Scholar]
- 3.Burghardt E, Pickel H, Haas J, Lahousen M. Prognostic factors and operative treatment of stages IB to IIB cervical cancer. Am J Obstet Gynecol. 1987;156:988–996. doi: 10.1016/0002-9378(87)90374-7. [DOI] [PubMed] [Google Scholar]
- 4.Van Bommel PF, Van Lindert AC, Kock HC, Leers WH, Neijt JP. A review of prognostic factors in early-stage carcinoma of the cervix (FIGO I B and II A) and implications for treatment strategy. Eur J Obstet Gynecol Reprod Biol. 1987;26:69–84. doi: 10.1016/0028-2243(87)90010-4. [DOI] [PubMed] [Google Scholar]
- 5.Acmaz G, Aksoy H, Unal D, Ozyurt S, Cingillioglu B, Aksoy U, Muderris I. Are neutrophil/lymphocyte and platelet/lymphocyte ratios associated with endometrial precancerous and cancerous lesions in patients with abnormal uterine bleeding? Asian Pac J Cancer Prev. 2014;15:1689–1692. doi: 10.7314/APJCP.2014.15.4.1689. [DOI] [PubMed] [Google Scholar]
- 6.Babu SN, Chetal G, Kumar S. Macrophage migration inhibitory factor: A potential marker for cancer diagnosis and therapy. Asian Pac J Cancer Prev. 2012;13:1737–1744. doi: 10.7314/APJCP.2012.13.5.1737. [DOI] [PubMed] [Google Scholar]
- 7.Absenger G, Szkandera J, Stotz M, Postlmayr U, Pichler M, Ress AL, Schaberl-Moser R, Loibner H, Samonigg H, Gerger A. Preoperative neutrophil-to-lymphocyte ratio predicts clinical outcome in patients with stage II and III colon cancer. Anticancer Res. 2013;33:4591–4594. [PubMed] [Google Scholar]
- 8.Migita K, Takayama T, Saeki K, Matsumoto S, Wakatsuki K, Enomoto K, Tanaka T, Ito M, Kurumatani N, Nakajima Y. The prognostic nutritional index predicts long-term outcomes of gastric cancer patients independent of tumor stage. Ann Surg Oncol. 2013;20:2647–2654. doi: 10.1245/s10434-013-2926-5. [DOI] [PubMed] [Google Scholar]
- 9.Kanda M, Fujii T, Kodera Y, Nagai S, Takeda S, Nakao A. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg. 2011;98:268–274. doi: 10.1002/bjs.7305. [DOI] [PubMed] [Google Scholar]
- 10.Zhou X, Du Y, Huang Z, Xu J, Qiu T, Wang J, Wang T, Zhu W, Liu P. Prognostic value of PLR in various cancers: A meta-analysis. PLoS One. 2014;9:e101119. doi: 10.1371/journal.pone.0101119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fathalla MF. Factors in the causation and incidence of ovarian cancer. Obstet Gynecol Surv. 1972;27:751–768. doi: 10.1097/00006254-197211000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi T, Teruya M, Kishiki T, Endo D, Takenaka Y, Tanaka H, Miki K, Kobayashi K, Morita K. Inflammation-based prognostic score, prior to neoadjuvant chemoradiotherapy, predicts postoperative outcome in patients with esophageal squamous cell carcinoma. Surgery. 2008;144:729–735. doi: 10.1016/j.surg.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 13.Haruma T, Nakamura K, Nishida T, Ogawa C, Kusumoto T, Seki N, Hiramatsu Y. Pre-treatment neutrophil to lymphocyte ratio is a predictor of prognosis in endometrial cancer. Anticancer Res. 2015;35:337–343. [PubMed] [Google Scholar]
- 14.Cummings M, Merone L, Keeble C, Burland L, Grzelinski M, Sutton K, Begum N, Thacoor A, Green B, Sarveswaran J, et al. Preoperative neutrophil: Lymphocyte and platelet: Lymphocyte ratios predict endometrial cancer survival. Br J Cancer. 2015;113:311–320. doi: 10.1038/bjc.2015.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mizunuma M, Yokoyama Y, Futagami M, Aoki M, Takai Y, Mizunuma H. The pretreatment neutrophil-to-lymphocyte ratio predicts therapeutic response to radiation therapy and concurrent chemoradiation therapy in uterine cervical cancer. Int J Clin Oncol. 2015;20:989–996. doi: 10.1007/s10147-015-0807-6. [DOI] [PubMed] [Google Scholar]
- 16.Kose M, Celik F, Kose SK, Arioz DT, Yilmazer M. Could the platelet-to-lymphocyte ratio be a novel marker for predicting invasiveness of cervical pathologies? Asian Pac J Cancer Prev. 2015;16:923–926. doi: 10.7314/APJCP.2015.16.3.923. [DOI] [PubMed] [Google Scholar]
- 17.Cho H, Hur HW, Kim SW, Kim SH, Kim JH, Kim YT, Lee K. Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol Immunother. 2009;58:15–23. doi: 10.1007/s00262-008-0516-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asher V, Lee J, Innamaa A, Bali A. Preoperative platelet lymphocyte ratio as an independent prognostic marker in ovarian cancer. Clin Transl Oncol. 2011;13:499–503. doi: 10.1007/s12094-011-0687-9. [DOI] [PubMed] [Google Scholar]
- 19.Kim SH, Kim SC, Choi BI, Han MC. Uterine cervical carcinoma: Evaluation of pelvic lymph node metastasis with MR imaging. Radiology. 1994;190:807–811. doi: 10.1148/radiology.190.3.8115631. [DOI] [PubMed] [Google Scholar]
- 20.Nozoe T, Ninomiya M, Maeda T, Matsukuma A, Nakashima H, Ezaki T. Prognostic nutritional index: A tool to predict the biological aggressiveness of gastric carcinoma. Surg Today. 2010;40:440–443. doi: 10.1007/s00595-009-4065-y. [DOI] [PubMed] [Google Scholar]
- 21.U.S. Department of Health and Human Services; National Institutes of Health. National Cancer Institute, corp. Common Terminology Criteria for Adverse Events (CTCAE) v4.0. 2010 [Google Scholar]
- 22.Kodama J, Takemoto M, Seki N, Nakamura K, Hongo A, Moriya S, Kanazawa S, Hiramatsu Y. Phase I study of chemoradiation with nedaplatin and ifosfamide in patients with advanced squamous cell carcinoma of uterine cervix. Int J Gynecol Cancer. 2008;18:1300–1304. doi: 10.1111/j.1525-1438.2008.01199.x. [DOI] [PubMed] [Google Scholar]
- 23.Kodama J, Takemoto M, Seki N, Nakamura K, Hongo A, Kanazawa S, Hiramatsu Y. Phase I study of weekly nedaplatin and concurrent pelvic radiotherapy as adjuvant therapy after radical surgery for cervical cancer. Int J Gynecol Cancer. 2008;18:1037–1041. doi: 10.1111/j.1525-1438.2007.01141.x. [DOI] [PubMed] [Google Scholar]
- 24.Münstedt K, Johnson P, Bohlmann MK, Zygmunt M, von Georgi R, Vahrson H. Adjuvant radiotherapy in carcinomas of the uterine cervix: The prognostic value of hemoglobin levels. Int J Gynecol Cancer. 2005;15:285–291. doi: 10.1111/j.1525-1438.2005.15217.x. [DOI] [PubMed] [Google Scholar]
- 25.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: Integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 26.Balkwill F, Mantovani A. Inflammation and cancer: Back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 27.Jackson JR, Seed MP, Kircher CH, Willoughby DA, Winkler JD. The codependence of angiogenesis and chronic inflammation. FASEB J. 1997;11:457–465. [PubMed] [Google Scholar]
- 28.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Assoian RK, Sporn MB. Type beta transforming growth factor in human platelets: Release during platelet degranulation and action on vascular smooth muscle cells. J Cell Biol. 1986;102:1217–1223. doi: 10.1083/jcb.102.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubernard V, Arbeille BB, Lemesle MB, Legrand C. Evidence for an alpha-granular pool of cytoskeletal protein alpha-actinin in human platelets that redistributes with the adhesive glycoprotein thrombospondin-1 during the exocytotic process. Arterioscler Tromb Vasc Biol. 1997;17:2293–2305. doi: 10.1161/01.ATV.17.10.2293. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan KL, Broekman MJ, Chernoff A, Lesznik GR, Drillings M. Platelet alpha-granule proteins: Studies on release and subcellular localization. Blood. 1979;53:604–618. [PubMed] [Google Scholar]
- 32.Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: A systematic review of the epidemiological literature. Nutr J. 2010;9:69. doi: 10.1186/1475-2891-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin X, Li W, Lai J, Okazaki M, Sugimoto S, Yamamoto S, Wang X, Gelman AE, Kreisel D, Krupnick AS. Five-year update on the mouse model of orthotopic lug transplantation: Scientific uses, tricks of the trade, and tips for success. J Thorac Dis. 2012;4:247–258. doi: 10.3978/j.issn.2072-1439.2012.06.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yao ZH, Tian GY, Wan YY, Kang YM, Guo HS, Liu QH, Lin DJ. Prognostic nutritional index predicts outcomes of malignant pleural mesothelioma. J Cancer Res Clin Oncol. 2013;139:2117–2123. doi: 10.1007/s00432-013-1523-0. [DOI] [PubMed] [Google Scholar]