Abstract
Glycosaminoglycans (GAGs), including chondroitin sulfate (CS), dermatan sulfate, heparin, heparan sulfate and keratan sulfate (KS) are linear sulfated repeating disaccharide sequences containing hexosamine and uronic acid [or galactose (Gal) in the case of KS]. Among the GAGs, CS shows structural variations, such as sulfation patterns and fucosylation, which are responsible for their physiological functions through CS interaction with CS-binding proteins. Here, we solved the structure of KS-branched CS-E derived from a clam, Mactra chinensis. KS disaccharide [d-GlcNAc6S-(1→3)-β-d-Gal-(1→] was attached to the C-3 position of GlcA, and consecutive KS-branched disaccharide sequences were found in a CS chain. KS-branched polysaccharides clearly exhibited resistance to degradation by chondroitinase ABC or ACII (at low concentrations) compared with typical CS structures. Furthermore, KS-branched polysaccharides stimulated neurite outgrowth of hippocampal neurons. These results strongly suggest that M. chinensis is a rich source of KS-branched CS, and it has important biological activities.
Keywords: chondroitin sulfate, chondroitinase, Glycobiology, keratan sulfate, proteoglycan
Introduction
Glycosaminoglycans (GAGs), a group of structurally related polysaccharides, are primarily found as the glycan moieties of proteoglycan (PG) glycoconjugates. GAGs, which include chondroitin sulfate (CS), dermatan sulfate (DS), heparin, heparan sulfate and keratan sulfate (KS) are linear sulfated polysaccharides comprising 50–200 repeating disaccharides of hexosamine and uronic acid [or galactose (Gal) in the case of KS] [1]. CS consists of a distinctive repeating disaccharide unit [→4)-β-d-GlcA-(1→3)-β-d-GalNAc-(1→]n, where GlcA is glucuronic acid, and GalNAc is N-acetylgalactosamine, and some hydroxy groups in disaccharide units are replaced with sulfo groups (S) responsible for the diversity of disaccharide units, namely, GlcA-GalNAc (O-type unit), GlcA-GalNAc (4S) (A-type unit: CS-A), GlcA-GalNAc (6S) (C-type units: CS-C), GlcA (2S)-GalNAc (6S) (D-type unit: CS-D), GlcA-GalNAc (4S, 6S) (E-type unit: CS-E), GlcA (3S)-GalNAc (4S) (K-type unit: CS-K) [2–4]. CS biosynthesis is initiated once GalNAc is transferred by CSGalNAcT-1 or -2 to the common linkage tetrasaccharide, GlcAβ1-3Galβ1-3Galβ1-4xylose (Xyl)β1-O-Ser in PGs, and chain elongation is then catalyzed by the CHSY(1-3)/CHPF heterodimer [5,6]. After the polymerization, the majority of the GalNAc residues are 4-O-sulfated by chondroitin 4-O-sulfotransferases (C4ST-1, -2 and -3) or 6-O-sulfated by 6-O-sulfotransferases (C6ST-1 and -2). In addition, resulting A- or C-type units can be further sulfated by GalNAc 4-sulfate 6-O-sulfotransferase (GalNAc 4S-6ST) or chondroitin uronyl 2-O-sulfotransferase (UST), generating di-sulfated disaccharides, E-type unit or D-type unit, respectively. A disaccharide unit containing iduronic acid (IdoA) in place of GlcA is commonly found in DS, a stereoisoform of CS that differs in the C-5 configuration of the hexuronic acid moieties [7]. This epimerization of GlcA is catalyzed by DS epimerase (DS-epi1 and DS-epi2), and resulting IdoA-GalNAc is subsequently sulfated by D4ST-1. Resulting IdoA-GalNAc (4S) (iA-type unit) is further sulfated by UST, generating IdoA (2S)-GalNAc (4S) (B-type unit). Because CS function can be achieved through the interaction with growth factors, receptors, and other CS-binding proteins, it is assumed that consecutive and highly sulfated disaccharide units are critical for these mammalian physiologies. However, the degree of sulfation of CS by sulfotransferases is intricately regulated at multiple levels in mammals, because most disaccharide units in CS/DS from mammals are A-type/iA-type units, and di-sulfated disaccharide levels are very low despite their high affinity for growth factors [8]. Thus, the distribution and number of protein-binding sites containing IdoA and di-sulfated CS disaccharides are important for the mammalian physiologies [9]. In contrast, highly sulfated and rare structures of CS have been identified from marine organisms. For example, DS from adult and embryonic sea urchin are composed of 59% of iC-type units (IdoA-GalNAc (6S)) and 74% of iE-type units (IdoA-GalNAc (4S,6S)), and B-type units (66%) and iD-type units (IdoA-GalNAc (2S,6S)) (>90%) are predominant structures of DS from the ascidian Spathoglottis plicata and Ascidia nigra, respectively [10–12]. In addition, GlcA[(Fuc(4S)-Fuc(3S,4S)]-GalNAc (4S, 6S) oligosaccharides were found in CS from sea cucumbers [13]. Hikino et al. [14] reported that consecutive highly sulfated CS (or DS) derived from sea urchin, ascidian and sea cucumber possesses the neurite outgrowth promotion activities of E16 hippocampal neurons; however, physiological functions of CS structures in their origin are not fully understood.
It is of interest to note that CS, having a fucosylated GlcA, a sulfated 3-position of GlcA residue and IdoA residues, shows different susceptibility to chondroitinases (Chases) compared with other types of CS. It is well known that Chase ABC can depolymerize almost all types of natural CS, and it can afford the unsaturated disaccharides, including ΔDi-0S, ΔDi-4S, ΔDi-6S, ΔDi-diSE, ΔDi-diSB and ΔDi-diSD [3]. Interestingly, CS-K, (1-3)-linkage of K-type units and (1-4)-linkage of disaccharides are completely digested by Chase ABC, resulting in GalNAc (4S) residues being produced from K-type units [4]. However, (1-4)-linkage between GalNAc and 3-O-sulfated GlcA in CS-K shows resistance to depolymerization by Chase ACII [15,16]. In addition, (1-4)-linkage between GalNAc and IdoA in DS is also resistant to the action of Chase ACII [17]. Therefore, unsaturated disaccharides and resistant oligosaccharides, containing IdoA residue or K-type units, can be obtained after the treatment of Chase ACII from DS or CS-K [16,17]. In contrast, fucosylated CS, derived from sea cucumber, shows resistance to both Chase ABC and ACII, and partial acid hydrolysis is required to prepare the unsaturated disaccharide units [13].
We have purified CS (or DS) from a variety of animal tissues and characterized their structures to better understand their biological distribution and to explore new sources of polysaccharides as functional foods, nutraceuticals, cosmetics and drugs [16–22].
In this study, we have identified novel CS-E from a clam, Mactra chinensis, containing KS disaccharide unit [d-GlcNAc6S-(1→3)-β-d-Gal-(1→] at the C-3 position of GlcA. KS-branched CS exhibits resistance to degradation by both Chase ABC and ACII (at low concentrations), and KS-branched disaccharides are clustered in the CS chain. Furthermore, KS-rich polysaccharides promoted neurite outgrowth of hippocampal neurons. These results demonstrate that M. chinensis is a rich source of KS-branched CS having important biological activities.
Materials and methods
Chemicals
M. chinensis in dry powder form was obtained from Futtsu City Fishery Association in Chiba, Japan. Actinase E was purchased from Kaken Pharmaceutical Co., Ltd, Tokyo, Japan. Chondroitinase ABC (Chase ABC) from Proteus vulgaris, chondroitinase ACII (Chase ACII) from Arthrobacter aurescens, unsaturated disaccharides (ΔDi-0S, ΔDi-4S, ΔDi-6S, ΔDiUA-2S, ΔDi-diSE, ΔDi-diSB, ΔDi-diSD, ΔDi-TriS), CS-A (6.33% of ΔDi-0S, 74.2% of Δdi-4S, 19.5% of ΔDi-6S, 0.3% of ΔDi-diSE) from whale cartilage, CS-E (10.0% of ΔDi-0S, 17.0% of Δdi-4S, 8.31% of ΔDi-6S, 63.6% of ΔDi-diSE, 1.08% of ΔDi-diSD) from squid cartilage and KS from bovine cornea were purchased from Seikagaku Corp., Tokyo, Japan [3]. The other analytical reagents used were of analytical grade.
Isolation of crude glycosaminoglycans from viscera of M. chinensis
The hot water extract of the viscera of M. chinensis was used in the present study. The dried powder (30 g) was proteolyzed at 45°C with actinase E (10 mg/g dry powder) in 50 mM Tris/acetate (pH 8.0) for 18 h. After proteolysis, the β-elimination reaction, on the reducing termini of peptidoglycan chains, was performed with 0.5 M NaOH, containing 0.3 M sodium borohydride (20 ml/g of dry sample) at 4°C for 18 h. The reaction mixture was then neutralized with 1.0 M HCl. The resulting GAG chains were precipitated by the addition of 5% cetylpyridinium chloride (CPC; final concentration 0.1%) containing 30 mM NaCl at 4°C for 16 h. The GAG–CPC complex was collected by centrifugation at 2300 × g for 15 min. The GAG chains were extracted from the GAG–CPC complex by the addition of 2.5 M NaCl, and the mixture was centrifuged at 2300 × g for 15 min. The GAG chains were precipitated from the supernatant by the addition of 11 volumes of 85% ethanol at 4°C for 16 h, and they were collected by centrifugation at 2300 × g for 15 min. The GAG chains were then isolated through dialysis against distilled water at room temperature for 16 h followed by lyophilization to afford partially purified GAG.
The crude GAG sample (∼30 mg of dry powder) in 2 ml of water was applied at a flow rate of 2 ml/min on a HiPrep DEAE FF (16 mm internal diameter × 100 mm, obtained from GE Healthcare Europe GmbH) and fractionated to prepare the highly sulfated CS polysaccharides. The eluents were (A) 50 mM sodium phosphate, (B) 2.0 M NaCl in 50 mM sodium phosphate. The gradient program was 0–30 min (5% B), 30–150 min (5–100% B), and 150–180 min (100% B). Fractionated samples were collected at 30 min-intervals, followed by concentration with a rotary evaporator, dialyzed, freeze-dried and kept stored at 4°C.
High-performance liquid chromatography
Disaccharide composition analysis of GAGs was performed as follows. GAGs (5 μg) were incubated in the reaction mixture (35 μl), which contained 28.6 mM Tris/acetate (pH 8.0), 50 mU of Chase ABC and/or 50 mU of Chase ACII. After 16 h at 37°C, depolymerized samples were boiled and evaporated, resuspended in 10 μl of water. The HPLC system was constructed with a high-pressure pump (LC-10Ai, Shimadzu, Kyoto, Japan), Intelligent Fluorescence detector (FP-920S, Jasco, Tokyo, Japan), a dry reaction bath (DB-3, Shimamura Instruments Co., Japan), double plunger pumps for reagent solution (NP-FX (II)-1U, Nihon Seimitsu Kagaku Co. Ltd., Tokyo, Japan), a chromato-integrator (D-2500, Hitachi High-Technologies Corp., Tokyo, Japan) and a sample injector with a 20 μl loop (Model 7725i, Rheodyne, CA, USA). A gradient was applied at a flow rate of 1.0 ml/min on Senshu Pak Docosil (4.6 mm × 150 mm; Senshu Scientific, Tokyo, Japan) at 60°C. The eluent buffers were as follows: A, 10 mM tetra-n-butylammonium hydrogen sulfate in 12% methanol; B, 0.2 M NaCl in buffer A. The gradient program was as follows: 0–10 min (1% B), 10–11 min (1–10% B), 11–30 min (10% B), 30–35 min (10–60% B), and 35–40 min (60% B). Aqueous (0.5% (w/v)) 2-cyanoacetamide solution and 1 M NaOH were added to the eluent at the same flow rates (0.25 ml/min), using a double plunger pump. The effluent was monitored fluorometrically (excitation, 346 nm; emission, 410 nm).
Liquid chromatography–mass spectrometry
Freeze-dried oligosaccharides obtained by Chase ACII treatment were labeled with 2-aminoacridone (AMAC) by slightly modifying the method of Yang et al. [23] with minor modifications. Oligosaccharides (60 μg) were added to 15 μl of 0.1 M AMAC solution in acetic acid/dimethyl sulfoxide (DMSO) (3:17, v/v), and mixed by vortexing for 5 min. Next, 15 μl of 1 M NaBH3CN was added in the reaction mixture, and incubated at 45°C for 4 h. Finally, the AMAC-tagged oligosaccharide mixture was diluted, using 50% (v/v) aqueous DMSO. LC–MS analysis was performed on an amaZon series SL ion-trap mass spectrometer (Bruker Daltonics, Bremen, Germany). MS data were collected by the following conditions: ESI interface, negative mode; capillary voltage, 3500 V; end plate offset 500 V; gas flow, 6.0 l/min. The column used was an Acquity UPLC BEH C18 column (2.1 mm internal diameter × 150 mm, 1.7 µm) at 50°C in a column oven CO 631A (GL Science Corp., Tokyo, Japan), and the flow rate was 0.1 ml/min. The eluent buffers were as follows: eluent A, 80 mM ammonium acetate/methanol (88/12, v/v) and B, 80 mM ammonium acetate/methanol (12/88, v/v). The gradient program was as follows: 0–4 min (0% eluent B), 4–12.5 min (0–4% eluent B), 12.5–25 min (4–15% eluent B), 25–50 min (15–100% eluent B).
Methylation analysis
Partially methylated alditol acetates (PMAAs) of the monosaccharide components from oligosaccharides were prepared according to the method of Anumula and Taylor [24]. Gas chromatography–mass spectrometry (GC–MS) analysis was performed by an Agilent Technologies 7890A GC system (Agilent Technologies, CA, USA) and Time-of-flight mass spectrometer JMS-T100GCV (JEOL, Tokyo, Japan). Mass spectra were obtained by electro impact ionization (70 eV) with the following program parameters: column, ZB-5 ms column (0.25 µm film thickness, 0.25 µm i.d. × 30 m) (Phenomenex, CA, USA); carrier gas, helium at 1.2 ml/min; column oven temperature program: 3 min at 100°C, with an increase at 4°C/min to 160°C, 1 min at 160°C followed by an increase at 0.5°C/min to 180°C, and a final increase at 20°C/min to 260°C and held for 10 min at 260°C.
1H-NMR spectroscopy
1H-NMR spectra of the purified polysaccharides were recorded on a JEOL JNM-ECA600 (600 MHz) spectrometer in the FT mode. Each polysaccharide (∼6 mg) for NMR analysis was dissolved in deuterium oxide (D2O, 99.90% D), and lyophilized three times to replace exchangeable protons with deuterons. Then the lyophilized samples were dissolved in 0.6 ml of D2O and transferred to the NMR tube. The one- and two-dimensional (1D and 2D) NMR experiments were recorded with a relaxation time of 1.5 s at a probe temperature of 30°C. 1D 1H-NMR spectra were recorded with an acquisition time of 1.45 s and many 300 scans. 2D 1H/1H COSY (correlation spectroscopy) and TOCSY (total correlation spectroscopy) spectra were recorded with many 112 and 156 scans, respectively. 2D TOCSY spectra were run with a mixing time of 100 ms. The proton signals were assigned, using the 2D 1H/1H COSY and TOCSY spectra.
Effect of CS derived from M. chinensis on neurite outgrowth of hippocampal neurons
All animal experiments were approved by the Institutional Animal Care and Use Committee of Chiba University and carried out according to the guidelines for Animal Research of Chiba University. GAG precoating in an eight-well chamber slide and evaluation of CS on neurite outgrowth of mouse hippocampal neurons were performed as described previously [17]. Briefly, eight-well chamber slides were pre-coated with 50 μg/ml poly-d,l-ornithine in 0.1 M sodium borate (pH 8.0), and then 0.5 μg/well of the CS (Fr. 4 in Figure 1B and remaining polysaccharides in Figure 2B) derived from M. chinensis, CS-E and CS-A were coated at 4°C overnight. Subsequently, the cells were seeded on coverslips at a density of 16 000 cells/cm2 and cultured for 18 h. Thereafter, the cells were fixed by 4% (w/v) paraformaldehyde for 30 min, the neurites were visualized by immunochemical staining, using anti-microtubule-associated protein-2 (Lieco Technologies Inc., St. Louis, MO, USA) and anti-neurofilament (Sigma–Aldrich, St. Louis, MO, USA). Fifty cells with at least one neurite longer than the cell body were chosen at random to determine the length of the longest neurite. At least three independent experiments per condition were carried out.
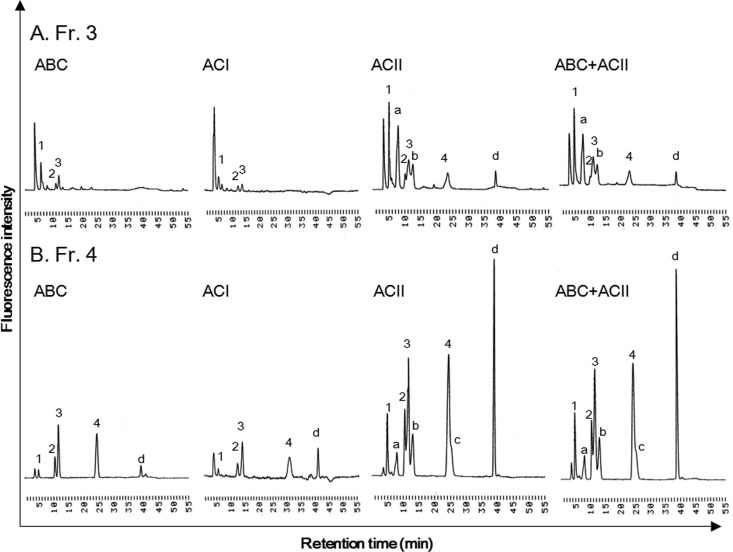
Figure 1. Disaccharide composition of CS of M. chinensis after chondroitinase ABC, ACI and ACII treatment.
Chromatograms of unsaturated disaccharides of Fr. 3 (A) and Fr. 4 (B) of CS obtained by weak anion-exchange chromatography (see Supplementary Figure S1). Unsaturated disaccharide analysis was performed as follows. CS (5 µg) were incubated in the reaction mixture (35 µl), which contained 28.6 mM Tris/acetate (pH 8.0) and 25 mU of Chase ABC, ACI or ACII. After incubation, depolymerized samples were submitted to gradient HPLC with fluorescence detection as described previously [17]. Experiments were repeated in triplicate with reproducible results. Peaks: 1, ΔDi-0S; 2, ΔDi-4S; 3, ΔDi-6S; 4, ΔDi-diSE; a–d, unknown peaks.
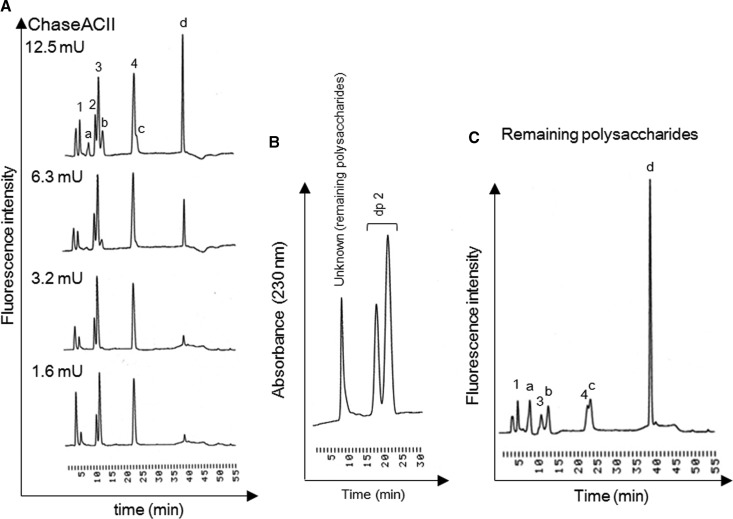
Figure 2. Different sensitivities of existing and unknown polysaccharides to chondroitinase ACII.
(A) Chromatogram of unsaturated disaccharides of clam CS (Fr. 4) obtained by Chase ACII. Clam CS (2.5 μg) in reaction mixture (17.5 μl) was treated with Chase ACII at the specified concentrations, and resulting unsaturated disaccharides were analyzed by HPLC. (B) Clam CS has consecutive repeating unknown structures. After incubation of RT mix (17.5 μl) containing 2.5 μg of clam CS and 1.6 mU of Chase ACII, remaining polysaccharides and unsaturated disaccharides were separated using HiTrap™ Desalting column. The isocratic elution condition was as follows: eluent, 10 mM ammonium bicarbonate; flow rate, 1.0 ml/min. To obtain the remaining polysaccharide, 3 mg of CS (Fr. 4) was treated with 3 units of Chase ACII. (C) Chromatogram of unknown structure treated with Chase ACII. Remaining polysaccharides (2.5 μg) were treated with 12.5 mU of Chase ACII in RT mix (17.5 μl). To collect the unknown peaks (c) and (d), 200 μg of remaining polysaccharides was degraded and fractionated (see Supplementary Figure S2). Peaks: 1, ΔDi-0S; 2, ΔDi-4S; 3, ΔDi-6S; 4, ΔDi-diSE; a–d, unknown peaks.
Results
Composition of CSs derived from M. chinensis
Crude GAGs were extracted from dry samples (30 g) from the viscera of M. chinensis by actinase E digestion, and recovered by ethanol precipitation. The dried pellet, including crude GAGs, was weighed after dialysis and freeze-drying. As a result, 353.8 mg GAG/g of dry samples was recovered. The recovery of crude GAGs from the viscera of M. chinensis was confirmed repeatedly. Crude GAGs (30 mg) were further fractionated using anion-exchange chromatography, and 18.5 mg of Fr. 3 and 3.73 mg of Fr. 4 were obtained. Disaccharide composition analysis of each fraction was performed using Chase ABC treatment, and a significant amount of ΔDi-diSE was observed in Fr. 4 (Figure 1B). The disaccharide composition of clam CS in Fr. 4 was 2.1% of ΔDi-0S, 7.4% of ΔDi-4S, 30.7% of ΔDi-6S and 59.8% of ΔDi-diSE. However, recovery of total amount of CS disaccharides in Fr. 3 and Fr.4 was very low, with clam CS contents in Fr. 3 and Fr. 4 of 2.97% (551 μg/18.5 mg dry powder) and 17.2% (640 μg/3.73 mg dry powder), respectively (Supplementary Figure S1). In addition, a small unidentified peak eluting at 38.1 min was also found in Fr. 4 (Figure 1B). Thus, disaccharide analysis of Fr. 3 and Fr. 4 was carried out, using Chase ACI or Chase ACII. When CS in Fr. 3 or Fr. 4 was treated with Chase ACII but not Chase ABC or ACI, a significant amount of four kinds of unidentified peaks were observed (Figure 1). In particular, unidentified peak (d) is a major component in clam CS, and detection of unidentified peaks (a–c) is Chase ACII treatment-dependent. Next, clam CS was treated with Chase ACII at the specified concentrations to characterize the unidentified structures. When a small amount (1.6 mU) of Chase ACII was added to the reaction mixture (17.5 μl) containing 2.5 μg of clam CS, well-known CS structures were completely degraded to unsaturated disaccharides, including ΔDi-0S, ΔDi-4S, ΔDi-6S, ΔDi-diSE (Figure 2A). In contrast, a higher concentration of Chase ACII (12.5 mU) was needed to detect the four kinds of unidentified peaks. Unidentified peaks were also detectable at 230 nm (Supplementary Figure S2), suggesting the existence of C4–C5 unsaturated uronate residue at the non-reducing end, and reducing end was GalNAc because Chase ACII can recognize the (1–4)-linkage between GalNAc and GlcA. Next, we tested whether unidentified structures represented a consecutive sequence in clam CS. After the treatment of 1.6 mU of Chase ACII, degraded samples were separated by HiTrap desalting column, and monitored at 230 nm. As a result, single peak of remaining polysaccharide (unknown peak) was observed (Figure 2B). In addition, disaccharide analysis with 12.5 mU of Chase ACII shows that unidentified peak (d) is the main component of remaining polysaccharides (Figure 2C). Thus, molecular masses of parent CS (Fr. 4 in Figure 1B) and remaining polysaccharides were determined by GPC–HPLC analysis. The chromatogram of parent CS shows a high structural diversity and high polydispersity with the number average molecular mass of ∼12 kDa (Supplementary Figure S3). Interestingly, the remaining polysaccharides show a high structural diversity and high polydispersity with the average molecular mass of ∼8 kDa, suggesting a cluster of previously unidentified structures, such as DS having IdoA-rich domains in the polysaccharide backbone [17,25]. The remaining polysaccharides with large chain length heterogeneity consisted of more than 20 saccharides, given that the retention time of remaining polysaccharides (20.76 min) is shorter than that of dp20 (molecular mass 4960) from CS (21.84 min; Supplementary Figure S3). Taken together, clam CS from M. chinensis has unknown consecutive repeating structures that exhibit different chondroitinase susceptibilities when compared with other types of CS structures.
Analysis of CS oligosaccharides having unknown structures by UPLC–MS/MS
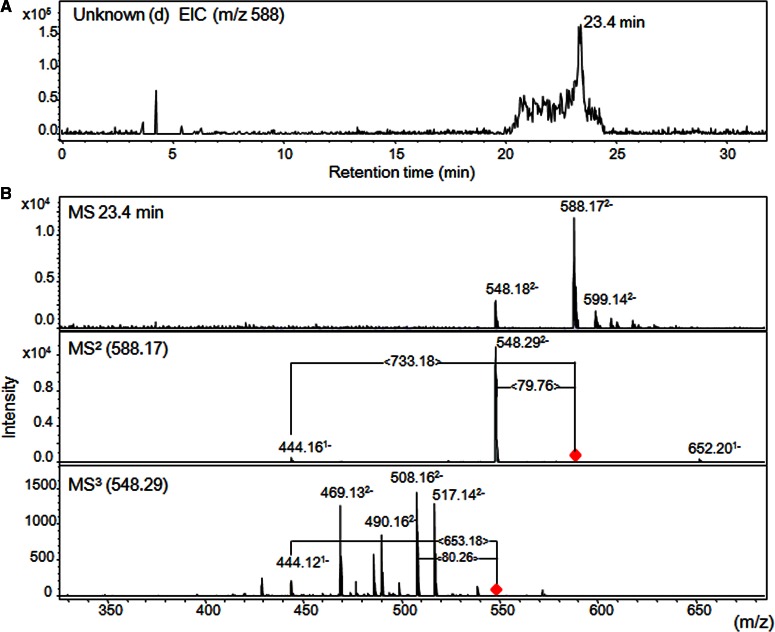
UPLC–MS/MS analysis of AMAC-labeled sample was performed to obtain the mass of unidentified peaks (c) and (d) in Figure 2C. Before UPLC–MS/MS analysis, unidentified peaks (c) and (d) in Figure 2C were fractionated (Supplementary Figure S2) and reacted with AMAC according to the methods of Yang et al. [23]. When the labeled sample containing unidentified peak (d) was analyzed, a single peak with m/z 588.1 [M-2H]2− was observed at 23.4 min (Figure 3A). The product ions, including m/z 548.29 [M-2H]2− and m/z 444.16 [M-H]−, were obtained by the fragmentation (MS2) of m/z 588.17 [M-2H]2−, whereas product ion m/z 444.12 [M-H]− was also observed by further fragmentation (MS3) of m/z 548.29 [M-2H]2− (Figure 3B). The structures, theoretical molecular masses and observed ions shown in Figure 3 are summarized in Table 1. The product ions at m/z 548.29 [M-2H]2− or 444.16 [M-H]− from m/z 588.1 [M-2H]2− were probably obtained by neutral loss of a sulfo group or AMAC-labeled di-sulfated disaccharides (ΔUA+GalNAc+2SO3+AMAC), respectively (Figure 3B). The product ion 444.12 [M-H]− was also obtained through the neutral loss of AMAC-labeled mono-sulfated disaccharides (ΔUA+GalNAc+SO3+AMAC) from m/z 548.29 [M-2H]2−. The remaining 444.16 [M-H]− peak was suggested to be a compound of Hex and HexNAc with one sulfo group. In the case of unidentified peak (c), two peaks having m/z 548.44 [M-2H]2− were unexpectedly observed at 24.6 and 25.4 min, and fragmentation (MS2) of m/z 548.44 [M-2H]2− afforded the product ions that were similar to those from unidentified peak (d) (Supplementary Figure S4). There was no difference between the two peaks at 24.6 and 25.4 min in unidentified peak (c) fraction in terms of mass spectra and fragment patterns (data not shown). On the basis of these observations, we speculated that unidentified peaks (c) and (d) were tetrasaccharides containing an unsaturated disaccharide, a neutral sugar, a HexNAc and two or three sulfo groups (Table 1).
Figure 3. LC–MS/MS analysis of AMAC-labeled unknown peak (d).
(A) Extracted ion chromatograms (EICs) of unknown peak (d) obtained by partial degradation of Chase ACII (see Figure 2C). AMAC labeling and LC–MS analysis were performed according to the method of Yang et al. [23]. A single peak (m/z 588) at 23.4 min was also observed in TIC. (B) Mass spectra of unknown peak (d). MS2 or MS3 was performed using m/z 588.172− or 548.292− as a precursor ion.
Table 1. The comparison of theoretical and calculated ions with observed ions from M. chinensis.
| Structure | Theoretical mass | Calculated ions (charge) | Observed ions (charge) | |
|---|---|---|---|---|
| Unknown peak (c)* | Unknown peak (d) | |||
| ΔUA+GalNAc | 379.1 | 378.1 (−) | ||
| ΔUA+GalNAc+AMAC | 573.1 | 572.1 (−) | ||
| ΔUA+GalNAc+SO3+AMAC | 653.1 | 652.1 (−) | 652.2 (−) | |
| ΔUA+GalNAc+2SO3+AMAC | 733.0 | 732.1 (−) | ||
| Hex+HexNAc+SO3-H2O | 445.1 | 444.1 (−) | 444.1 (−) | 444.1 (−) |
| ΔUA+GalNAc+Hex+HexNAc+2SO3+AMAC | 1098.3 | 1097.3 (−) | ||
| 548.1 (2−) | 548.4 (2−) | 548.3 (2−) | ||
| ΔUA+GalNAc+Hex+HexNAc+3SO3+AMAC | 1178.3 | 1177.3 (−) | ||
| 588.1 (2−) | 588.2 (2−) | |||
LC–MS/MS data of unknown peak (c) are shown in Supplementary Figure S4.
Methylation analysis
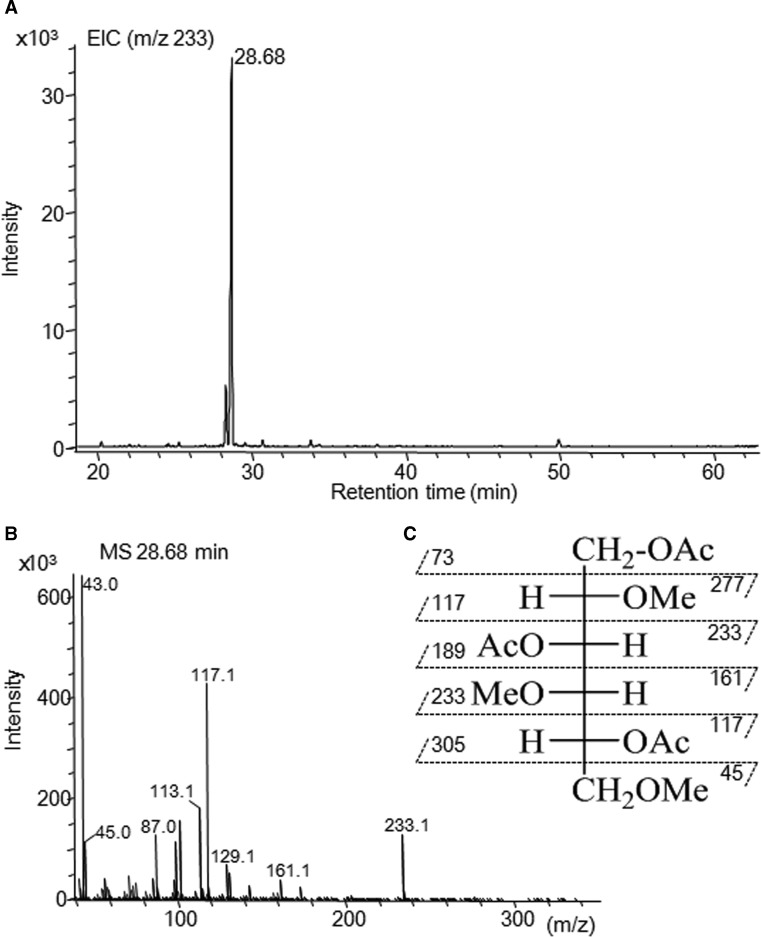
GC–MS analysis of the reaction products of PMAA derived from the polysaccharides has been shown to represent a powerful tool to investigate the glycosidic linkages. On the basis of this technique, fractionated unidentified peak (d) in Figure 2C was derivatized to PMAAs, and the resulting products were subsequently subjected to GC–MS analysis. When the fragment ion at m/z 233 from PMAAs was monitored, a single peak was observed at 28.68 min (Figure 4A). The mass spectrum of the single peak at 28.68 min is shown in Figure 4B. On the basis of the observation of certain fragment ions (m/z 45, 117, 161, 233) and earlier reported [26], it is suggested that the peak at 28.68 min is 1,3,5-tri-O-acetyl-2,4,6-tri-O-methyl-galactitol [→3) Galactose (Gal) (1→]. Because KS consists of a distinctive repeating disaccharide unit [→3)-β-d-Gal-(1→4)-β-d-GlcNAc-(1→]n [27], PMAAs of KS from bovine cornea were analyzed as a control to confirm the PMAA of [→3) Gal (1→] at 28.68 min. When the fragment ion at m/z 233 from PMAAs was monitored, four peaks eluted at 28.62, 29.64, 39.65 and 50.55 min were observed (Supplementary Figure S5A). The fragment ion pattern of eluted peak at 28.62 min resembles that of Figure 4B (Supplementary Figure S5B), confirming that unidentified peak (d) was [→3) Gal (1→]. The peak corresponding to the 1,4,5-tri-O-acetyl-2-(acetylmethylamino)-2-deoxy-3,6-di-O-methyl-d-glucitol [→4) GlcNAc (1→] was also observed at 50.55 min in PMAA analysis of cornea KS (Supplementary Figure S5A); however, there was no peak of [GlcNAc (1→] in PMAA analysis of fractionated unidentified peak (d) (Figure 4A).
Figure 4. Methylation analysis of unknown (d) peak.
PMAA from unknown (d) peak were performed as described in the Materials and methods section. To obtain PMAAs, 100 μg of unknown (d) peak was used. (A) EIC (m/z 233) of unknown (d) peak. (B) Mass spectrum of PMAA at 28.68 min. (C) Theoretical mass fragment pattern of 1,3,5-tri-O-acetyl-2,4,6-tri-O-methyl-galactitol [26].
2D NMR analysis of unknown structure-rich polysaccharide
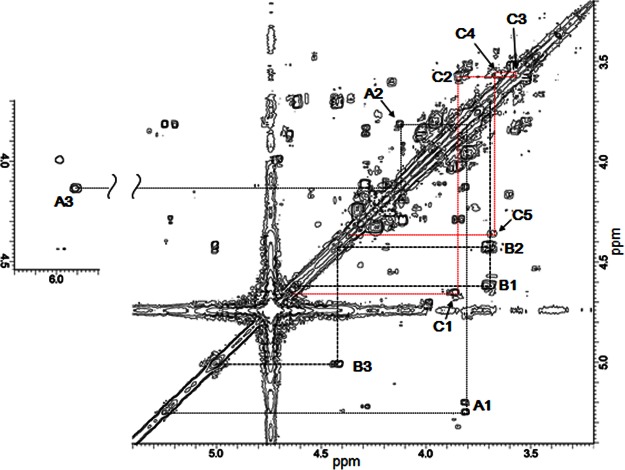
The structure of the remaining polysaccharides obtained in Figure 2B was investigated by COSY and TOCSY (Figure 5 and Supplementary Figure S6). The proton chemical shifts obtained are summarized in Table 2. Because partial degradation of Chase ACII afforded various lengths of remaining polysaccharides, we anticipated multiple proton signals of ΔUA, GlcA and GalNAc in the COSY spectrum. Among these, cross-peaks of H-1/H-2, H-2/H-3, H-3/H-4 signals, corresponding to ΔUA and GalNAc residues, were readily assigned (Figure 5). Since GC–MS analysis suggested the possibility of the presence of KS disaccharide in the remaining polysaccharides (Figure 4), assignment of 1H-chemical shifts of Gal and GlcNAc6S residues was performed. As shown in Table 2 and Figure 5, 1H-chemical shifts of GlcNAc6S observed in the remaining polysaccharides are almost the same as non-reducing end of GlcNAc6S in hexasaccharides of KS as previously reported by Hounsell et al. [28]. Unfortunately, there was no signal corresponding to the Gal residue. In contrast, the cross peak of H-2/H-3 signals corresponding to GlcA was almost absent from the COSY spectrum of remaining polysaccharides. This result is consistent with the observation that two signals at 3.39 and 3.59 ppm, attributable to H-2 and H-3, respectively, of non-substituted glucuronic acid residues are almost absent from the 1H-NMR spectrum of the fucosylated CS [29]. Thus, the 3-OH position of GlcA residues in remaining polysaccharides must be replaced with a Gal residue.
Figure 5. 2D COSY spectra of remaining polysaccharides obtained after the digestion of clam CS by chondroitinase ACII.
COSY spectra were recorded in D2O at 30°C. Proton signals of GlcNAc were assigned according to the result of Hounsell et al. [28]. The cross-peaks are assigned as: (A1) ΔUA-H1/H2; (A2) ΔUA-H2/H3; (A3) ΔUA-H3/H4; (B1) GalNAc-H1/H2; (B2) GalNAc-H2/H3; (B3) GalNAc-H3/H4; (C1) GlcNAc-H1/H2; (C2) GlcNAc-H2/H3; (C3) GlcNAc-H3/H4; (C4) GlcNAc-H4/H5; (C5) GlcNAc-H5/H6.
Table 2. 1H chemical shifts of remaining polysaccharides obtained after the digestion of clam CS by chondroitinase ACII.
| Residue | Proton assignment | Chemical shift | Chemical shift* | ||
|---|---|---|---|---|---|
| α | β | α | β | ||
| ppm | |||||
| ΔUA | H-1 | 5.248 | 5.200 | ||
| H-2 | 3.813 | ||||
| H-3 | 4.122 | ||||
| H-4 | 5.904 | ||||
| GalNAc | H-1 | – | 4.622 | ||
| H-2 | 3.694 | ||||
| H-3 | 4.424 | ||||
| H-4 | 5.008 | ||||
| H-5 | – | ||||
| H-6 | – | ||||
| GlcNAc | H-1 | 4.652 | 4.76–4.70 | ||
| H-2 | 3.849 | 3.84–3.76 | |||
| H-3 | 3.584 | 3.59 | |||
| H-4 | 3.555 | 3.56 | |||
| H-5 | 3.678 | 3.7 | |||
| H-6, H-6′ | 4.366 | 4.34–4.25 | |||
Hounsell et al. [28].
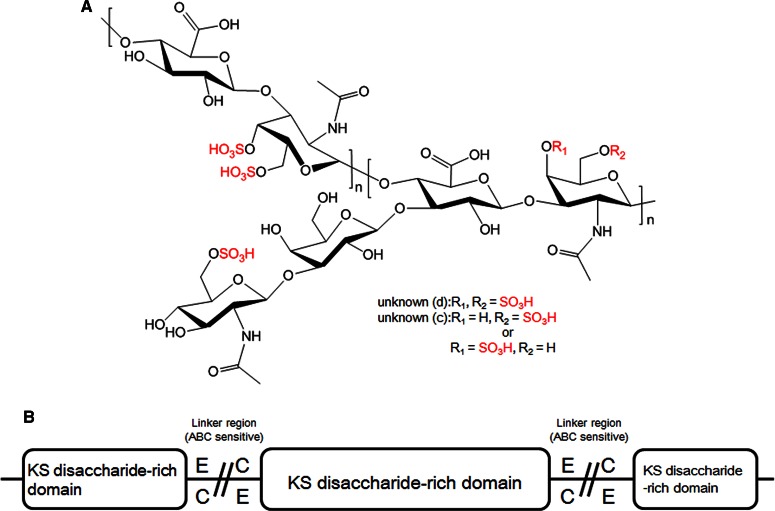
To summarize the data obtained by LC–MS, GC–MS and NMR, we can conclude that KS disaccharide [d-GlcNAc6S-(1→3)-β-d-Gal-(1→] is attached to a GlcA residue, and a consecutive KS-branched disaccharide sequence is present in the CS chain (Figure 6). Because, (1) unidentified peak (d) is the main component in remaining polysaccharides obtained by the Chase ACII treatment (Figure 2C and Supplementary Figure S3), (2) unidentified peak (d) is suggested to be ΔUA+GalNAc+3SO3+Hex+HexNAc by LC–MS (Figure 3 and Table 1), (3) Hex is suggested to be [→3) Gal (1→] by GC–MS (Figure 4) and (4) HexNAc is suggested to be [GlcNAc6S (1→] by COSY experiment (Figure 5). From these observations, we refer to the remaining polysaccharides as KS-branched polysaccharides.
Figure 6.
Proposed structure of KS disaccharide-branched CS (A) and of consecutively branched disaccharides in CS of M. chinensis (B).
Stimulation of neurite outgrowth by clam CS
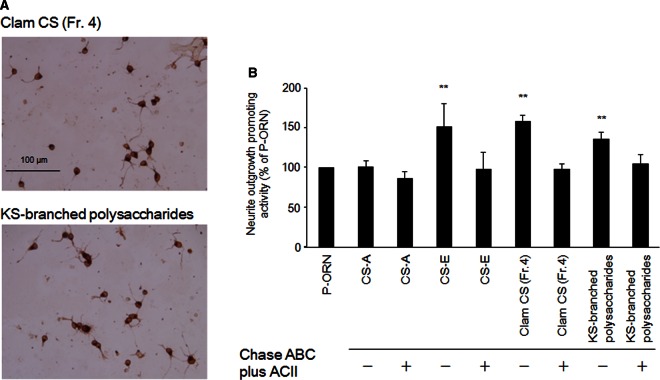
The effect of CS on neurite outgrowth-promoting (NOP) activity was examined, using E16 embryonic mouse hippocampal neurons, because M. chinensis CS contained significant amount of E-type units (Figure 1B). Thus, hippocampal neurons were cultured with clam CS (Fr. 4, see Figure 1B), and KS-branched polysaccharide (remaining polysaccharides, see Figure 2C), CS-E (a positive control) and CS-A (a negative control) that were each immobilized onto coverslips precoated with poly-d,l-ornithine. As a result, CS-E, clam CS (Fr. 4) and KS-branched polysaccharides stimulated neurite outgrowth, and their NOP activities were completely disappeared by the Chase ABC and ACII treatment (Figure 7).
Figure 7. Effect of clam CS (Fr. 4) or KS-branched polysaccharides on neurite outgrowth of hippocampal neurons.
(A) Representative morphological features of E16 hippocampal neurons cultured with clam CS (Fr.4) or KS-branched polysaccharides. E16 hippocampal neuronal cells (16 000 cells/cm2) were cultured for 18 h on various substrates coated on poly-d,l-ornithine, fixed and immunostained as described in the Materials and methods section. (B) The length of the longest neurite of the randomly selected 50–100 individual neurons was measured. The values obtained from the three separate experiments are expressed as the means ± SEM. Mann–Whitney's U-test was used to evaluate the significance of differences between means (**P < 0.01).
Discussion
It has been known that commercially available CS, including CS-A (50–80% of A-type unit), CS-C (50–70% of C-type unit), CS-D (20–40% of D-type unit) and CS-E (63.6% of E-type unit), is sensitive to digestion by Chase ABC or ACII, while DS and CS-K (13.9% of K-type unit) are resistant to Chase ACII [16,17]. In contrast, fucosylated CS exhibits resistance to Chase ABC and ACII, respectively [13]. In this study, we identified new CS from M. chinensis that lacks chondroitinase susceptibility. M. chinensis CS having KS disaccharide units [d-GlcNAc6S-(1→3)-β-d-Gal-(1→] showed the resistance to Chase ABC and lower concentrations of Chase ACII, and higher concentrations of Chase ACII were required to obtain KS-branched disaccharide units (Figures 1 and 2). In addition, KS-branched disaccharide units represent consecutive sequences, such as IdoA-rich domains like CS/DS from shark fin or DS from porcine skin [17,25], because the remaining polysaccharides were obtained by the partial degradation at a lower concentration of Chase ACII (Figure 2 Supplementary Figure S3).
Unfortunately, determination of the structure of unidentified peaks (a) to (b) failed, because their contents were very low in remaining polysaccharides (Figure 2C and Supplementary Figure S2). However, LC–MSn analysis showed that the difference between the unidentified peaks (c) and (d) is the number of sulfo groups on the unsaturated disaccharide unit. On the basis of fragmentation patterns of each ion, unidentified peak (d) contains an E-type unit, because the major component in the CS from M. chinensis was an E-type unit (Figure 1). In contrast, there were two distinguishable peaks in the (c) fraction in LC–MS experiments (Supplementary Figure S4). Because unidentified (c) peaks contain mono-sulfated CS disaccharide, these peaks are likely to result from tetrasaccharide having ΔDi-4S or ΔDi-6S. This idea can be supported by the retention time of each peak observed in HPLC, that is, (d) peak which has three sulfo groups elutes close to ΔDi-TriS (data not shown), while (c) peak which has two sulfo groups appeared beside ΔDi-diSE (Figure 1B). For this reason, we speculate that unidentified peak (b) is ΔDi-0S plus KS disaccharide unit, and (a) is ΔDi-0S plus KS disaccharide unit (‒SO3), respectively. Further analysis is required to elucidate the complete CS structure of M. chinensis.
E- and K-type units are likely to be subject to further modifications in marine organisms. For instance, almost all CS from sea cucumbers are fucosylated [30], and a d-glucose branch at C-3 position of GalNAc was also found in CS-E from squid cartilage [31]. K-type units of CS from the cartilage of king crab and octopus were also fucosylated [4,16]. Thus, lower marine organisms are rich sources of CS-E and CS-K having these unique structures; however, biological functions of unique CS in individual organisms remain unclear. Vieira et al. [13] proposed that these branches serve to prevent digestion of polysaccharide by microorganisms. Considering that KS-branched CS was abundant in the gut of the M. chinensis, the previous assumption is quite reasonable because Chase ABC and ACII produced by bacteria fail to completely degrade it.
CS plays an important role in enhancing or preventing the elongation of axons [5,6]. Recent studies have focused on the molecular mechanism by which CS PGs stimulate or prevent axonal elongation, and several receptors for CS have been identified at the neuronal cell surface. In the case of E16 embryonic mouse hippocampal neurons, there are several reports that NOP activities of highly sulfated CS are observed when highly sulfated CS is pre-coated [4,8,14,16,17]. Because the barrier effect of KS PG to neurite outgrowth was reported [32], the effect of clam CS, having KS disaccharide unit, was examined (Figure 7). However, NOP activities of KS-branched polysaccharides were moderately stimulated when compared with those of CS-E and clam CS (Fr. 4), and it was also observed that Chase ABC and ACII treatment diminished its NOP activities, suggesting the importance of consecutive E-type unit sequence but not the KS disaccharide unit in KS-branched polysaccharides. Experiments are in progress to identify the specific biological activities of M. chinensis CS.
Abbreviations
- AMAC
2-aminoacridone
- Chase
chondroitinase
- COSY
correlation spectroscopy
- CPC
cetylpyridinium chloride
- CS
chondroitin sulfate
- DMSO
dimethyl sulfoxide
- DS
dermatan sulfate
- GAGs
glycosaminoglycans
- Gal
galactose
- IdoA
iduronic acid
- KS
keratan sulfate
- NOP
neurite outgrowth-promoting
- PG
proteoglycan
- PMAAs
partially methylated alditol acetates
- TIC
total ion chromatography
- TOCSY
total correlation spectroscopy
- UST
uronyl 2-O-sulfotransferase
- ΔDi-0S
deoxy-α-l-threo-hex-4-enopyranosyluronic acid (ΔUA) (1→3) N-acetylgalactosamine (GalNAc)
- ΔDi-4S, ΔUA (1→3) GalNAc4S
where S is sulfo, ΔDi-6S is ΔUA (1→3) GalNAc6S
- ΔDi-diSB
ΔUA2S (1→3) GalNAc4S
- ΔDi-diSD
ΔUA2S (1→3) GalNAc6S
- ΔDi-diSE
ΔUA (1→3) GalNAc4S6S
- ΔDi-TriS
ΔUA2S (1→3) GalNAc4S6S
- ΔDi-UA2S
ΔUA2S (1→3) GalNAc.
Author Contribution
K.H. and T.T. designed the experiments. K.H., K.T., R.J.L. and T.T. interpreted data and wrote the manuscript. K.T. performed much of experiments. K.H., K.T., A.M., Y.O. and S.M. performed the biochemical and the analytical experiments.
Funding
A part of the present study was supported by the Grant-in-aid for Scientific Research from the Ministry of Education, Culture, Sport, Science and Technology of Japan (TT) and the Inohana Foundation, Chiba University (K.H.).
Competing Interests
The Authors declare that there are no competing interests associated with the manuscript.
References
- 1.Roden L. (1980) Structure and Metabolism of Connective Tissue Proteoglycans, Plenum Press, New York [Google Scholar]
- 2.Volpi N.E. (2006) Chondroitin Sulfate: Structure, Role and Pharmacological Activity, Elsevier, London, UK [Google Scholar]
- 3.Higashi K., Okamoto Y., Mano T., Wada T. and Toida T. (2014) A simple HPLC method for identification of the origin of chondroitin sulfate in health food. Jpn. J. Food Chem. Saf. 21, 187–194 [Google Scholar]
- 4.Fongmoon D., Shetty A.K., Basappa, Yamada S., Sugiura M., Kongtawelert P. et al. (2007) Chondroitinase-mediated degradation of rare 3-O-sulfated glucuronic acid in functional oversulfated chondroitin sulfate K and E. J. Biol. Chem. 282, 36895–36904 doi: 10.1074/jbc.M707082200 [DOI] [PubMed] [Google Scholar]
- 5.Mizumoto S., Yamada S. and Sugahara K. (2015) Molecular interactions between chondroitin-dermatan sulfate and growth factors/receptors/matrix proteins. Curr. Opin. Struct. Biol. 34, 35–42 doi: 10.1016/j.sbi.2015.06.004 [DOI] [PubMed] [Google Scholar]
- 6.Maeda N. (2015) Proteoglycans and neuronal migration in the cerebral cortex during development and disease. Front. Neurosci. 9, 98 doi: 10.3389/fnins.2015.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malmström A., Bartolini B., Thelin M.A., Pacheco B. and Maccarana M. (2012) Iduronic acid in chondroitin/dermatan sulfate: biosynthesis and biological function. J. Histochem. Cytochem. 60, 916–925 doi: 10.1369/0022155412459857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bao X., Nishimura S., Mikami T., Yamada S., Itoh N. and Sugahara K. (2004) Chondroitin sulfate/dermatan sulfate hybrid chains from embryonic pig brain, which contain a higher proportion of l-iduronic acid than those from adult pig brain, exhibit neuritogenic and growth factor binding activities. J. Biol. Chem. 279, 9765–9776 doi: 10.1074/jbc.M310877200 [DOI] [PubMed] [Google Scholar]
- 9.Coles C.H., Shen Y., Tenney A.P., Siebold C., Sutton G.C., Lu W. et al. (2011) Proteoglycan-specific molecular switch for RPTPσ clustering and neuronal extension. Science 332, 484–488 doi: 10.1126/science.1200840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pavão M.S.G., Mourão P.A.S., Mulloy B. and Tollefsen D.M. (1995) A unique dermatan sulfate-like glycosaminoglycan from ascidian: its structure and the effect of its unusual sulfation pattern on anticoagulant activity. J. Biol. Chem. 270, 31027–31036 doi: 10.1074/jbc.270.52.31027 [DOI] [PubMed] [Google Scholar]
- 11.Pavão M.S.G., Aiello K.R.M., Werneck C.C., Silva L.C.F., Valente A.-P., Mulloy B. et al. (1998) Highly sulfated dermatan sulfates from Ascidians. Structure versus anticoagulant activity of these glycosaminoglycans. J. Biol. Chem. 273, 27848–27857 doi: 10.1074/jbc.273.43.27848 [DOI] [PubMed] [Google Scholar]
- 12.Vilela-Silva A.C., Werneck C.C., Valente A.P., Vacquier V.D. and Mourão P.A.S. (2001) Embryos of the sea urchin Strongylocentrotus purpuratus synthesize a dermatan sulfate enriched in 4-O- and 6-O-disulfated galactosamine units. Glycobiology 11, 433–440 doi: 10.1093/glycob/11.6.433 [DOI] [PubMed] [Google Scholar]
- 13.Vieira R.P., Mulloy B. and Mourão P.A. (1991) Structure of a fucose-branched chondroitin sulfate from sea cucumber. Evidence for the presence of 3-O-sulfo-beta-d-glucuronosyl residues. J. Biol. Chem. 266, 13530–13536 PMID: [PubMed] [Google Scholar]
- 14.Hikino M., Mikami T., Faissner A., Vilela-Silva A.-C.E.S., Pavão M.S.G. and Sugahara K. (2003) Oversulfated dermatan sulfate exhibits neurite outgrowth-promoting activity toward embryonic mouse hippocampal neurons: implications of dermatan sulfate in neuritogenesis in the brain. J. Biol. Chem. 278, 43744–43754 doi: 10.1074/jbc.M308169200 [DOI] [PubMed] [Google Scholar]
- 15.Sugahara K., Tanaka Y., Yamada S., Seno N., Kitagawa H., Haslam S.M. et al. (1996) Novel sulfated oligosaccharides containing 3-O-sulfated glucuronic acid from king crab cartilage chondroitin sulfate K. Unexpected degradation by chondroitinase ABC. J. Biol. Chem. 271, 26745–26754 PMID: [DOI] [PubMed] [Google Scholar]
- 16.Higashi K., Okamoto Y., Mukuno A., Wakai J., Hosoyama S., Linhardt R.J. et al. (2015) Functional chondroitin sulfate from Enteroctopus dofleini containing a 3-O-sulfo glucuronic acid residue. Carbohydr. Polym. 134, 557–565 doi: 10.1016/j.carbpol.2015.07.082 [DOI] [PubMed] [Google Scholar]
- 17.Higashi K., Takeuchi Y., Mukuno A., Tomitori H., Miya M., Linhardt R.J. et al. (2015) Composition of glycosaminoglycans in elasmobranchs including several deep-sea sharks: identification of chondroitin/dermatan sulfate from the Dried Fins of Isurus oxyrinchus and Prionace glauca. PLoS One 10, e0120860 doi: 10.1371/journal.pone.0120860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ha Y.W., Jeon B.T., Moon S.H., Toyoda H., Toida T., Linhardt R.J. et al. (2005) Characterization of heparan sulfate from the unossified antler of Cervus elaphus. Carbohydr. Res. 340, 411–416 doi: 10.1016/j.carres.2004.11.011 [DOI] [PubMed] [Google Scholar]
- 19.Kim Y.S., Aln M.Y., Wu S.J., Kim D.-H., Toida T., Teesch L.M. et al. (1998) Determination of the structure of oligosaccharides prepared from acharan sulfate. Glycobiology 8, 869–877 doi: 10.1093/glycob/8.9.869 [DOI] [PubMed] [Google Scholar]
- 20.Sakai S., Kim W.S., Lee I.S., Kim Y.S., Nakamura A., Toida T. et al. (2003) Purification and characterization of dermatan sulfate from the skin of the eel, Anguilla japonica. Carbohydr. Res. 338, 263–269 doi: 10.1016/S0008-6215(02)00442-1 [DOI] [PubMed] [Google Scholar]
- 21.Warda M., Mao W., Toida T. and Linhardt R.J. (2003) Turkey intestine as a commercial source of heparin? Comparative structural studies of intestinal avian and mammalian glycosaminoglycans. Comp. Biochem. Physiol. B, Biochem. Mol. Biol. 134, 189–197 doi: 10.1016/S1096-4959(02)00250-6 [DOI] [PubMed] [Google Scholar]
- 22.Warda M., Gouda E.M., Toida T., Chi L. and Linhardt R.J. (2003) Isolation and characterization of raw heparin from dromedary intestine: evaluation of a new source of pharmaceutical heparin. Comp. Biochem. Physiol. C, Toxicol. Pharmacol. 136, 357–365 doi: 10.1016/j.cca.2003.10.009 [DOI] [PubMed] [Google Scholar]
- 23.Yang B., Chang Y., Weyers A.M., Sterner E. and Linhardt R.J. (2012) Disaccharide analysis of glycosaminoglycan mixtures by ultra-high-performance liquid chromatography–mass spectrometry. J. Chromatogr. A. 1225, 91–98 doi: 10.1016/j.chroma.2011.12.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anumula K.R. and Taylor P.B. (1992) A comprehensive procedure for preparation of partially methylated alditol acetates from glycoprotein carbohydrates. Anal. Biochem. 203, 101–108 doi: 10.1016/0003-2697(92)90048-C [DOI] [PubMed] [Google Scholar]
- 25.Zhao X., Yang B., Solakyildirim K., Solakylidirim K., Joo E.J., Toida T. et al. (2013) Sequence analysis and domain motifs in the porcine skin decorin glycosaminoglycan chain. J. Biol. Chem. 288, 9226–9237 doi: 10.1074/jbc.M112.437236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Björndal H., Hellerqvist C.G., Lindberg B. and Svensson S. (1970) Gas-liquid chromatography and mass spectrometry in methylation analysis of polysaccharides. Angew. Chem. Int. Ed. Engl. 9, 610–619 doi: 10.1002/anie.197006101 [DOI] [Google Scholar]
- 27.Pomin V.H. (2015) Keratan sulfate: an up-to-date review. Int. J. Biol. Macromol. 72, 282–289 doi: 10.1016/j.ijbiomac.2014.08.029 [DOI] [PubMed] [Google Scholar]
- 28.Hounsell E.F., Scudder P., Tang P.W., Feizi T. and Feeney J. (1986) 1H-NMR studies at 500 MHz of a neutral disaccharide and sulphated di-, tetra-, hexa- and larger oligosaccharides obtained by endo-β-galactosidase treatment of keratan sulphate. Eur. J. Biochem. 157, 375–384 doi: 10.1111/j.1432-1033.1986.tb09679.x [DOI] [PubMed] [Google Scholar]
- 29.Mourão P.A.S., Pereira M.S., Pavão M.S.G., Mulloy B., Tollefsen D.M., Mowinckel M.-C. et al. (1996) Structure and anticoagulant activity of a fucosylated chondroitin sulfate from echinoderm. Sulfated fucose branches on the polysaccharide account for its high anticoagulant action. J. Biol. Chem. 271, 23973–23984 doi: 10.1074/jbc.271.39.23973 [DOI] [PubMed] [Google Scholar]
- 30.Myron P., Siddiquee S. and Al Azad S. (2014) Fucosylated chondroitin sulfate diversity in sea cucumbers: a review. Carbohydr. Polym. 112, 173–178 doi: 10.1016/j.carbpol.2014.05.091 [DOI] [PubMed] [Google Scholar]
- 31.Habuchi O., Sugiura K. and Kawai N. (1977) Glucose branches in chondroitin sulfates from squid cartilage. J. Biol. Chem. 252, 4570–4576 PMID: [PubMed] [Google Scholar]
- 32.Cole G.J. and McCabe C.F. (1991) Identification of a developmentally regulated keratan sulfate proteoglycan that inhibits cell adhesion and neurite outgrowth. Neuron 7, 1007–1018 doi: 10.1016/0896-6273(91)90345- [DOI] [PubMed] [Google Scholar]