Abstract
The incidence of colorectal cancer is on the increase owing to changes in daily diet. In the present study, the methylation status of caudal type homeobox transcription factor 2 (CDX2) gene in lesion tissue of colorectal cancer (CRC) was investigated. Additionally, the correlation between the promoter methylation of CDX2 gene, CRC and gene expression in patients with CRC and normal population was examined. Between April 2014 and May 2015 78 cases with CRC were enrolled in the study. Using methylation-specific polymerase chain reaction (PCR), the promoter methylation of CDX2 in normal tissues and colorectal tissues was examinned. Through the fluorescence quantitative PCR technique, the expression levels of CDX2 gene were determined in a normal population and lesion tissue of patients with CRC. At the same time, we evaluated the levels of the CDX2 gene product in the normal population and lesion tissue of patients with CRC. The results showed that the methylation rate of the promoter region of CDX2 gene in normal colorectal tissue was 43.5%, whereas that in the lesion tissue of CRC was 78.5%. The result was statistically significant (P<0.05). The quantity of mRNA and protein expression of CDX2 gene in colorectal and normal tissue was significantly different (P<0.05). In conclusion, the methylation of the CDX2 gene promoter region was associated with risk of CRC, i.e., methylation of the promoter region of CDX2 gene favors the occurrence of CRC.
Keywords: colorectal, CDX2 gene, promoter, methylation, gene expression
Introduction
High fat diet and low fiber has become increasingly widespread in the daily diet, which to some extent, accelerates the incidence of colorectal cancer (1). Colorectal cancer (CRC) is one of the most common malignant tumors worldwide. Its incidence rate in China ranks fourth and its mortality rate ranks third (2). Therefore, the prevention and treatment of CRC have important practical significance for improving quality of life of patients. In its early stage, CRC mainly infiltrates locally while at its advanced stage it is characterized by lymphatic spread, with surgical resection being the only viable treatment option (3). However, the postoperative recurrence rate of patients with CRC following surgical resection is approximately 45.3–73.5%, which challenges the survival rate within 5 years of surgery (4). Therefore, investigation into the pathogenesis of CRC is crucial. Caudal type homeobox transcription factor 2 (CDX2) in the human body is a type of transcription factor for regulating gene transcription and expression in gastrointestinal embryo pathways (5–7). CDX2 gene plays an important role in some relevant digestive system cancers, including gastric and colon cancer (8). The downregulation of CDX2 gene expression may lead to the loss of relevant differentiation function and the increase of value-added ability in the process of the occurrence of CRC, and it may also co-operate with other genes to participate in regulating the occurrence of relevant tumors (9). It is believed that the methylation of DNA promoter to a certain extent can regulate the expression of relevant genes (10). However, the transcriptional regulation of many genes in the human system specifically expressed in differentiation is de-methylated (11), but it is methylated in specific cells, not requiring the expression of genes.
Previous studies on CDX2 gene were primarily focused on gastric and colon cancer. At present, few studies are available on the function of CDX2 gene in CRC and on the methylation of the promoter region of CDX2 gene in CRC. To the best of our knowledge, the present study, for the first time, examined the methylation of promoter region of CDX2 gene in CRC to provide preliminary data on the correlation between the methylation of the promoter regions of CDX2 and CRC. We also aimed to determine strategies in the prevention and treatment of CRC in advanced stage.
Patients and methods
Patients
In total 78 cases with CRC (tested by relevant pathology) were selected at the Luoyang Central Hospital (Henan, China) from May 2014 to April 2015. The patietns comprised 43 men and 35 women, with an average age of 42±7.9 years. Additionally, a total of 52 (31 men and 21women, with an average age of 45±9.3 years) participants who were diagnosed with colorectal polyps served as controls (normal population). The study subjects were not undergoing treatment in the form of surgery in vitro, chemotherapy and radiotherapy.
Reagents
The following reagents were used: Mouse anti-human CDX2 monoclonal antibody (Roche Diagnostics, Indianapolis, IN, USA), goat anti-mouse HRP secondary antibody (Roche Diagnostics); RT-polymerase chain reaction (PCR) kit (Takara Bio, Inc., Otsu, Japan); mouse anti-human immunohistochemistry kit (Roche Diagnostics); DBA staining kit (Thermo Fisher Scientific, Waltham, MA, USA); animal cell genomic extraction kit (Axygen Scientific Inc., Union City, CA, USA); CpG methylation enzyme (New England Biolabs, Ipswich, MA, USA); MethylDetector bisulfite modification kit (Active Motif, Carlsbad, CA, USA).
Detection of methylation
DNA extracted from the sample tissue was in accordance with the instructions of the animal cell genomic extraction kit, purchased from Axygen Scientific Inc. The modification of DNA bisulfite was performed in accordance with the instructions of the MethylDetector bisulfite modification kit (Active Motif).
The methylation primer was designed according to the supplier's protocol (12). The primers are listed in Table I. The reaction system of methylation-specific PCR was 25 µl, i.e., ddH2O 8.5 µl, DNA 2 µl, Premix Ex Taq DNA polymerase mixture 12.5 µl, methylation- or nonmethylation-specific primer 2 µl. The PCR reaction conditions were: denaturation at 95°C for 3 min, annealing at 95°C for 30 sec, 58°C for 30 sec, and extension 72°C for 30 sec, for a total of 30 cycles. A final extension was conducted at 72°C for 10 min, and final heat preservation was at 4°C. The PCR products was recovered and sequenced using gel electrophoresis (Invitrogen Life Technologies, Carlsbad, CA, USA).
Table I.
Primer of methylation-specific polymerase chain reaction.
| Primers | Length of products (bp) |
|---|---|
| Methylation forward primer | |
| 5′-CGAAAATAAATCACTACGACG-3′ | 200 |
| Non-methylation forward primer | |
| 5′-ATTCAAAATAAAAATCACTACAACA-3′ | 204 |
| Common reverse primer | |
| 5′-AAAGGATATTGGAGAGTATTTTAG-3′ |
Fluorescence quantitative PCR (qPCR)
RNA extraction was performed as described previously (13). Fluorescence qPCR was performed according to the manufacturer's instructions (Takara fluorescent qPCR; Takara Bio, Inc.).
Enzyme-linked immune reaction
The procedure was performed according to the instructions of ELISA kit. An assay buffer of PBST (Biosharp, Hefei, China) was used to prepare dilutions of 1:25, and a standard curve was then designed. Firstly, the sample to be measured was diluted at a 1:100 ratio, and 100 µl of sample to be measured was mixed with 50 µl of detection solution in each well. The samples were kept for 2 h at 20°C, followed by the addition of TMB to measure light absorption at 495 nm using a microplate reader ((Model 3550; Bio-Rad Labs, Hercules, CA, USA). The concentration of CDX2 in every sample was measured according to the standard curves.
Western blotting
The animal cell protein extraction kit (Roche Diagnostics) was used to extract the total proteins. Western blot analysis was performed as described previously (13). After protein quantitation using the Lowery protein assay, equal amounts of proteins were separated by SDS-PAGE and blotted onto nitrocellulose membranes by the semi-dry blotting method using a three-buffer system. The membranes were incubated with primary antibody at a dilution of 1:500 (anti-CDX2, RayBiotech, Inc., Norcross, GA, USA; cat no.: 119-14329) overnight at 4°C. The membrane was washed with TBST and incubated with a peroxidase-conjugated secondary polyclonal goat-anti-mouse antibody (1:1000) (Santa Cruz Biotechnology, Inc. CA, USA; cat no.: sc-395763) for 1 h. Western blot film was scanned and the membrane was stripped and reprobed with antibody against β-actin (1:1500) (Santa Cruz Biotechnology, Inc.; cat no.: sc-8432) to confirm equal sample loading
Immunohistochemical staining
The procedure was performed according to the mouse anti-human immunohistochemistry kit (Roche Diagnostics). After staining, the nucleus appeared yellow, which was considered positive. The staining criteria were classified as: (−) negative, unstained cells or stained very lightly and there was no noticeable difference between the cell and the background; (+) extensively weakly colored after staining, the color of cell was slightly higher than that of the background; (++) extensively moderately colored after staining, the color of cell was markedly higher than that of the background; and (+++) extensively strong colored after staining, where the cells were dark brown. In the study, all the (+) (++) (+++) were considered positive. The final score was evaluated by two experts independently, and the average score was considered as final.
Statistics analysis
The SPSS 20.0 software (IBM SPSS, Armonk, NY, USA) was used to process the obtained data. Measurement data were presented as mean ± standard deviation and the measurement data was tested using the χ2 test.
Results
Methylation status of CDX2 gene in tissue of CRC and normal population
As shown in Fig. 1, the controls had a lower degree of CDX2 promoter methylation, but there was a high degree of methylation for CDX2 gene in the lesion tissue of patients with CRC. Table II shows the rate of CDX2 promoter methylation in the colorectal tissue of the normal population, which was ~43.5%, but the rate in the lesion tissue of CRC was ~78.5%. The results were statistically significant.
Figure 1.
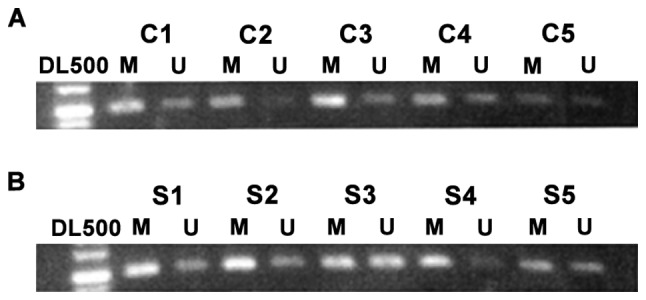
Methylation of promoter region of CDX2 gene in (A) normal colorectal and (B) colorectal cancer tissue. C1-C5 are samples of colorectal tissue of different normal population; S1-S5 are samples of colorectal tissue of patients with colorectal cancer. M indicates methylation of promoter; U indicates Un-methylation of promoter; appearance of both M and U simultaneously indicates the methylated promoter.
Table II.
Relative expression quantity of mRNA of CDX2 in colorectal tissue in normal population and patients with colorectal cancer (use GAPDH as internal control).a
| CDX2 gene | Promoter methylation | ||||
|---|---|---|---|---|---|
| Group | Cases | Methylation | Non-methylation | χ2 | P-value |
| Normal population | 63 | 27 | 36 | 5.8855 | 0.023b |
| Patients with colorectal cancer | 78 | 61 | 17 |
State of promoter methylation of CDX2 in tissue of normal colorectal and colorectal cancer.
P<0.05 is of noticeable difference.
Fluorescence qPCR
By comparing the levels of CDX2 gene in controls and patients with CRC, we found that the level of CDX2 gene was higher in colorectal tissue of the normal population and lower in colorectal tissue of the patients with CRC (Fig. 2). The result indicates that there may be a correlation between CDX2 gene and the disease of CRC.
Figure 2.
Fluorescence quantitative polymerase chain reaction for the relative expression of CDX2 protein in controls and patients with colorectal cancer.
Enzyme-linked immune response and western blotting
The protein in controls and patients with CRC were determined by ELISA and western blotting (Figs. 3 and 4). In comparison to CRC patients, the expression of CDX2 was higher in the controls and a similar observation was made with the western blotting and fluorescence qPCR results.
Figure 3.
ELISA for the relative expression of CDX2 protein in controls and patients with colorectal cancer.
Figure 4.

Western blotting, the data shows the expression of CDX2 protein in controls and patients with colorectal cancer. CK is the colorectal tissue of normal population; S1-S7 are colorectal tissue of different patients with colorectal cancer.
Immunohistochemistry
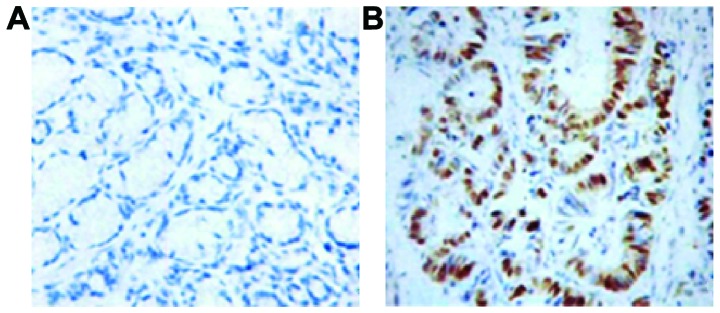
By comparing the results of colorectal tissue staining in CRC patients and controls, we found that the positive expression of CDX2 primarily exists in colonic epithelial goblet cells (Fig. 5). Staining showed brownish yellow granules. We observed that CDX2 protein mainly exists in the colorectal tissue of the normal population and has a lower expression in CRC tissue.
Figure 5.
Immunohistochemical staining for CDX2 protein in colorectal tissue of patients with colorectal cancer and normal population. (A) Tissue of colorectal cancer; (B) normal tissue.
Discussion
The occurrence and aggravation of CRC involves numerous factors and is not only influenced by physical conditions but also by diet, and mental state (14). The increase of studies of genomics with a focus on the occurrence of CRC have led to investigations at gene level. CDX2, a tail-type homologous box gene family, is a type of transcription factor of intestinal epithelial cell specificity in the human body (15). CDX2 gene in human body is involved in the regulation of the expression of intestine-specific genes, intestinal development, and the differentiation and proliferation of intestinal epithelial cells (16). CDX2 gene has also been found to be involved in the occurrence of some tumors and cancers in the human body (17). It has been suggested that CDX2 gene has a higher expression in intestinal metaplasia associated with chronic atrophic gastritis and is expressed in gastric cancer (18). CDX2 may be associated with gastric mucosal epithelial transformation and gastric mucosal carcinogenesis (19–22). Previous findings have shown that transfection of CDX2 cDNA, and human HT29 CRC cell line to express CDX2 protein, indicated the oncogenic potential of the abovementioned cells, and metastasis of related cells markedly decreased while cell sensitivity for apoptosis significantly increased (23).
In summary, to the best of our knowledge, for the first time, we examined a correlation between methylation of the promoter region of CDX2 in CRC and CRC. The results have shown that in comparison to the normal population, the degree of methylation of the promoter region of CDX2 in lesion tissue of patients with CRC was higher than that of the normal population. The protein expression in the control and lesion sections of CRC patients showed that the expression level of CDX2 in the lesion section of patients with CRC was lower. This finding suggested that there was a certain correlation between CDX2 and CRC, or the decrease in the degree of CDX2 gene promoter methylation to a certain extent, promotes the risk of CRC.
Acknowledgements
The present study was supported by the Scientific and Technologic Development Programme of Luoyang City (No.: 1401087A-3).
References
- 1.Gregoire R, Yeung KS, Stadler J, Stern HS, Kashtan H, Neil G, Bruce WR. Effect of high-fat and low-fiber meals on the cell proliferation activity of colorectal mucosa. Nutr Cancer. 1991;15:21–26. doi: 10.1080/01635589109514107. [DOI] [PubMed] [Google Scholar]
- 2.Liu S, Zheng R, Zhang M, Zhang S, Sun X, Chen W. Incidence and mortality of colorectal cancer in China, 2011. Chin J Cancer Res. 2015;27:22–28. doi: 10.3978/j.issn.1000-9604.2015.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dawson H, Galván JA, Helbling M, Muller DE, Karamitopoulou E, Koelzer VH, Economou M, Hammer C, Lugli A, Zlobec I. Possible role of Cdx2 in the serrated pathway of colorectal cancer characterized by BRAF mutation, high-level CpG Island methylator phenotype and mismatch repair-deficiency. Int J Cancer. 2014;134:2342–2351. doi: 10.1002/ijc.28564. [DOI] [PubMed] [Google Scholar]
- 4.Wang XT, Xie YB, Xiao Q. siRNA targeting of Cdx2 inhibits growth of human gastric cancer MGC-803 cells. World J Gastroenterol. 2012;18:1903–1914. doi: 10.3748/wjg.v18.i16.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baba Y, Nosho K, Shima K, Freed E, Irahara N, Philips J, Meyerhardt JA, Hornick JL, Shivdasani RA, Fuchs CS, et al. Relationship of CDX2 loss with molecular features and prognosis in colorectal cancer. Clin Cancer Res. 2009;15:4665–4673. doi: 10.1158/1078-0432.CCR-09-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saandi T, Baraille F, Derbal-Wolfrom L, Cattin AL, Benahmed F, Martin E, Cardot P, Duclos B, Ribeiro A, Freund JN, et al. Regulation of the tumor suppressor homeogene Cdx2 by HNF4α in intestinal cancer. Oncogene. 2013;32:3782–3788. doi: 10.1038/onc.2012.401. [DOI] [PubMed] [Google Scholar]
- 7.Kim SP, Park JW, Lee SH, Lim JH, Jang BC, Lee SH, Jang IH, Freund JN, Suh SI, Mun KC, et al. Homeodomain protein CDX2 regulates COX-2 expression in colorectal cancer. Biochem Biophys Res Commun. 2004;315:93–99. doi: 10.1016/j.bbrc.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 8.Kawai H, Tomii K, Toyooka S, Yano M, Murakami M, Tsukuda K, Shimizu N. Promoter methylation downregulates CDX2 expression in colorectal carcinomas. Oncol Rep. 2005;13:547–551. [PubMed] [Google Scholar]
- 9.Brabletz T, Spaderna S, Kolb J, Hlubek F, Faller G, Bruns CJ, Jung A, Nentwich J, Duluc I, Domon-Dell C, et al. Down-regulation of the homeodomain factor Cdx2 in colorectal cancer by collagen type I: An active role for the tumor environment in malignant tumor progression. Cancer Res. 2004;64:6973–6977. doi: 10.1158/0008-5472.CAN-04-1132. [DOI] [PubMed] [Google Scholar]
- 10.Li F, Wang Y, Zhang G, Zhou J, Yang L, Guan H. Expression and methylation of DNA repair genes in lens epithelium cells of age-related cataract. Mutat Res 766-767. 2014:31–36. doi: 10.1016/j.mrfmmm.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Zhang JF, Zhang JG, Kuai XL, Zhang H, Jiang W, Ding WF, Li ZL, Zhu HJ, Mao ZB. Reactivation of the homeotic tumor suppressor gene CDX2 by 5-aza-2′-deoxycytidine-induced demethylation inhibits cell proliferation and induces caspase-independent apoptosis in gastric cancer cells. Exp Ther Med. 2013;5:735–741. doi: 10.3892/etm.2013.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wojdacz TK, Hansen LL, Dobrovic A. A new approach toprimer design for the control of PCR bias in methylation studies. BMC Res Notes. 2008;1:54. doi: 10.1186/1756-0500-1-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Padda RS, Gkouvatsos K, Guido M, Mui J, Vali H, Pantopoulos K. A high-fat diet modulates iron metabolism but does not promote liver fibrosis in hemochromatotic Hjv¯/¯ mice. Am J Physiol Gastrointest Liver Physiol. 2015;308:G251–G261. doi: 10.1152/ajpgi.00137.2014. [DOI] [PubMed] [Google Scholar]
- 14.Sugimoto M, Yamaoka Y, Furuta T. Influence of interleukin polymorphisms on development of gastric cancer and peptic ulcer. World J Gastroenterol. 2010;16:1188–1200. doi: 10.3748/wjg.v16.i10.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gendron FP, Mongrain S, Laprise P, McMahon S, Dubois CM, Blais M, Asselin C, Rivard N. The CDX2 transcription factor regulates furin expression during intestinal epithelial cell differentiation. Am J Physiol Gastrointest Liver Physiol. 2006;290:G310–G318. doi: 10.1152/ajpgi.00217.2005. [DOI] [PubMed] [Google Scholar]
- 16.Mutoh H, Hayakawa H, Sakamoto H, Sugano K. Homeobox protein CDX2 reduces Cox-2 transcription by inactivating the DNA-binding capacity of nuclear factor-kappaB. J Gastroenterol. 2007;42:719–729. doi: 10.1007/s00535-007-2088-y. [DOI] [PubMed] [Google Scholar]
- 17.Qin R, Wang NN, Chu J, Wang X. Expression and significance of homeodomain protein Cdx2 in gastric carcinoma and precancerous lesions. World J Gastroenterol. 2012;18:3296–3302. doi: 10.3748/wjg.v18.i25.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bai YQ, Yamamoto H, Akiyama Y, Tanaka H, Takizawa T, Koike M, Yagi O Kenji, Saitoh K, Takeshita K, Iwai T, et al. Ectopic expression of homeodomain protein CDX2 in intestinal metaplasia and carcinomas of the stomach. Cancer Lett. 2002;176:47–55. doi: 10.1016/S0304-3835(01)00753-4. [DOI] [PubMed] [Google Scholar]
- 19.Asano N, Imatani A, Watanabe T, Fushiya J, Kondo Y, Jin X, Ara N, Uno K, Iijima K, Koike T, et al. Cdx2 expression and intestinal metaplasia induced by H. Pylori infection of gastric cells is regulated by NOD1-Mediated innate immune responses. Cancer Res. 2016;76:1135–1145. doi: 10.1158/0008-5472.CAN-15-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mutoh H, Sakurai S, Satoh K, Tamada K, Kita H, Osawa H, Tomiyama T, Sato Y, Yamamoto H, Isoda N, et al. Development of gastric carcinoma from intestinal metaplasia in Cdx2-transgenic mice. Cancer Res. 2004;64:7740–7747. doi: 10.1158/0008-5472.CAN-04-1617. [DOI] [PubMed] [Google Scholar]
- 21.Mizoshita T, Tsukamoto T, Nakanishi H, Inada K, Ogasawara N, Joh T, Itoh M, Yamamura Y, Tatematsu M. Expression of Cdx2 and the phenotype of advanced gastric cancers: Relationship with prognosis. J Cancer Res Clin Oncol. 2003;129:727–734. doi: 10.1007/s00432-003-0499-6. [DOI] [PubMed] [Google Scholar]
- 22.Zheng J, Sun X, Wang W, Lu S. Hypoxia-inducible factor-1α modulates the down-regulation of the homeodomain protein CDX2 in colorectal cancer. Oncol Rep. 2010;24:97–104. [PubMed] [Google Scholar]
- 23.Soubeyran P, Mallo GV, Moucadel V, Dagorn JC, Iovanna JL. Overexpression of Cdx1 and Cdx2 homeogenes enhances expression of the HLA-I in HT-29 cells. Mol Cell Biol Res Commun. 2000;3:271–276. doi: 10.1006/mcbr.2000.0226. [DOI] [PubMed] [Google Scholar]