Abstract
Introduction:
Direct-to-consumer personal genomic testing has the potential to influence health behaviors, including smoking. Critics of this testing highlight limited evidence to support positive behavioral benefits and caution that genomic results may provide false reassurance, leading to unhealthy behaviors. This study investigates interest in genetic risks of smoking-related diseases and changes in smoking behaviors among genomic testing consumers.
Methods:
From 2012 to 2013, a longitudinal series of web surveys was conducted. A total of 1464 customers of 23andMe and Pathway Genomics completed a survey prior to viewing genomic test results, of which 1002 participants provided data on smoking behaviors 6 months after receiving results.
Results:
At baseline, 64% of participants were never smokers, 29% were former smokers, and 7% were current smokers. Most baseline current smokers were very interested in genetic risk results for lung cancer (65%) and heart disease (72%). For lung cancer, this interest was significantly greater than former (50% very interested) and never smokers (37% very interested) (p < .0001). Even though participants were interested in smoking-related disease genetic risks, 96% reported the same smoking status at baseline and 6-month follow-up. Importantly, only 1% (n = 13/916) of former and never smokers became current smokers at 6 months and 22% (n = 14/64) of current smokers reported quitting.
Conclusions:
Overall, smokers show a high level of interest in genetic risks of smoking-related illnesses. The experience of receiving direct-to-consumer genomic health risks does not appear to have obvious harms related to smoking behaviors, with some potential benefits.
Implications:
In the setting of ongoing controversy surrounding direct-to-consumer genomic testing, this study provides evidence that consumers are interested in genetic risk results of smoking-related diseases. Receiving genomic testing results does not lead to smoking initiation among never smokers or reinitiation among former smokers and may be associated with a higher quit rate among current smokers at 6-month follow-up than the general population. These findings ease concerns that direct-to-consumer genomic testing could lead to false reassurance and unhealthy behaviors related to smoking.
Introduction
Over the last decade, we have witnessed an explosion in the popularity and availability of personal genomic risk information, especially in the context of direct-to-consumer testing. This rapid growth has sparked controversy regarding the regulation of direct-to-consumer testing companies,1–3 leading the Food and Drug Administration to order 23andMe to remove health-related interpretations from its services in 20134 before 23andMe adopted offerings to meet regulations in 2015.5 Studies on the behavioral implications of direct-to-consumer genomic testing are warranted to inform current policies.
The question of whether personal genomic risk information can be harnessed to positively influence health behaviors has received considerable attention.6–9 Early empirical studies on the effect of returning genomic information to motivate health behaviors, including healthy dietary intake, physical activity, alcohol consumption reduction, and smoking cessation, have overall been underwhelming,10–12 with only a few studies showing a positive behavioral impact of genetic risk information.13,14 Limited studies have examined potential negative consequences of returning personal genomic risks, and critics question whether results may lead to false reassurance and unhealthy behaviors.9,15,16
As a leading cause of preventable death in the United States and worldwide,17,18 smoking is an ideal health behavior for studying the influence of personal genomic information. This study investigates: (1) interest in genetic risk for smoking-related disease among current, former, and never smokers and (2) changes in smoking behaviors among customers of two genomic testing companies. By examining the important behavior of cigarette smoking, this analysis seeks to elucidate the potential benefits and harms of direct-to-consumer genomic testing.
Methods
Participants and Procedures
From March to July 2012, the Impact of Personal Genomics (PGen) Study recruited new customers of two personal genomic testing companies (23andMe and Pathway Genomics), which provided health results directly to consumers during the study period. Details of the PGen Study are published elsewhere.19,20 A total of 1464 participants completed a baseline survey prior to viewing health-related genomic testing results and were eligible for follow-up. Smoking variables were available on all but one participant at baseline, who was dropped in analyses. Six months following receipt of genomic results, participants received a follow-up survey. A total of 1042 participants responded to this survey (71% of baseline participants), of which 1002 provided smoking measures.
Survey Instruments
Baseline measures included demographics and interest in genetic risk information. Participants were asked “How interested are you in learning about your genetic risk” for lung cancer and heart disease (coronary artery disease), with response choices of not at all, somewhat, and very interested. We focused on these two complex diseases because smoking status is highly related to risk, smoking cessation is critical for prevention, and both companies returned genetic risk information about these diseases.
At both baseline and 6-month follow-up, smoking status was evaluated. Never smokers were individuals who had not smoked 100 cigarettes in their lifetime. Former smokers had smoked 100 cigarettes, but did not smoke anymore. Current smokers had smoked 100 cigarettes and reported smoking some days or every day. Among current smokers, smoking quantity was assessed with the question “How many cigarettes per day do you smoke?” with the responses 10 or fewer, 11–20, 21–30, or 31 or more.
Genetic Risk Estimates
Participants received their personal genomic results online directly from the companies along with information on the average population risk and environmental risk factors. Lung cancer genetic risk was based on one variant in 23andMe (rs8034191) and three variants in Pathway Genomics (rs3117582, rs1051730, and rs2736100). Heart disease genetic risk was based on multiple variants in 23andMe (rs10757278, rs12526453, rs1746048, rs1122608, rs9982601, rs17465637, rs6725887, rs2306374, rs3798220, rs11556924, rs579459, rs12413409, rs964184, rs4773144, and rs2895811) and Pathway Genomics (rs8055236, rs2259816, rs501120, rs3008621, rs2943634, rs383830, rs17411031, rs1333049, rs9818870, rs6922269, rs688034, and rs17228212).
Participants’ genetic risk results from 23andMe and Pathway Genomics were linked with PGen survey responses. We evaluated a previously described dichotomous outcome according to whether individuals received an elevated versus average or reduced genetic risk estimate for lung cancer or heart disease.20 These dichotomous genetic risk outcomes were developed after consultation with both testing companies and used a relative risk threshold, where individuals with a risk of 1.2 or more for each respective disease were considered elevated.
Statistical Analysis
Statistical analyses were performed using Statistical Analysis System (SAS v. 9.3, Cary, NC). Across the smoking status categories (never, former, current), differences in dichotomous demographic variables were evaluated using chi-square goodness of fit. For continuous demographic variables, differences were evaluated using analysis of variance. Logistic regression was used to model the effect of smoking status on interest level of lung cancer and heart disease genetic risk. These models were adjusted for significant differences in demographic factors between groups, and the proportional odds assumption was satisfied. Power calculations indicated more than 90% power with an alpha of 0.05 to detect an effect of smoking status on interest level in lung cancer or heart disease risk with an odds ratio of 1.5. Changes in smoking status occurred when participants switched categories (never, former, current) between baseline and follow-up interviews. In secondary analyses, we examined associations between dichotomous genetic risk scores and changes in smoking behaviors using Fisher’s exact test with two-sided p values. Power calculations indicated 54%–98% power to detect a threefold increase (reference proportion of participants with elevated risk ranged from 0.17 to 0.30).
Results
Participant Characteristics
The majority of PGen Study participants were female (61%), self-reported White race (90%), US residents (98%), health insured (95%), and college graduates (78%) (n = 1463, Table 1). At baseline, 64% were never smokers, 29% were former smokers, and 7% were current smokers. These smoking status groups differed by age (p < .0001), self-identified White race (p < .05) and Asian race (p < .05), college education (p < .0001), and household income (p < .005).
Table 1.
Baseline Characteristics of Never, Former, and Current Smokers Enrolled in the PGen Study
| Characteristics | Never smokers (N = 940, 64%) | Former smokers (N = 427, 29%) | Current smokers (N = 96, 7%) | Entire sample (N = 1463) |
|---|---|---|---|---|
| Sex, N (%) | ||||
| Males | 352 (37%) | 177 (41%) | 37 (39%) | 566 (39%) |
| Females | 587 (63%) | 250 (59%) | 59 (61%) | 896 (61%) |
| Age, years*** | ||||
| Mean ± SD | 45±15 | 54±15 | 43±13 | 48±16 |
| Range | 19–94 | 22–91 | 22–71 | 19–94 |
| Self-reported racea, N (%) | ||||
| White* | 830 (88%) | 395 (93%) | 90 (94%) | 1315 (90%) |
| Asian* | 55 (6%) | 13 (3%) | 2 (2%) | 70 (5%) |
| Black or African American | 43 (5%) | 11 (3%) | 2 (2%) | 56 (4%) |
| Otherb | 94 (10%) | 29 (7%) | 8 (8%) | 131 (9%) |
| Self-reported Hispanic, N (%) | 54 (6%) | 20 (5%) | 7 (7%) | 81 (6%) |
| Residence in the United States, N (%) | 921 (98%) | 417 (98%) | 94 (98%) | 1432 (98%) |
| Has health insurance, N (%) | 897 (95%) | 400 (94%) | 88 (92%) | 1385 (95%) |
| College degree or more advanced education, N (%)*** | 777 (83%) | 308 (72%) | 59 (61%) | 1144 (78%) |
| Household income categoryc,** | ||||
| Mean ± SD | 3.1±1.3 | 3.1±1.4 | 2.6±1.4 | 3.1±1.3 |
| Range | 1–6 | 1–6 | 1–6 | 1–6 |
| Genetic testing company, N (%) | ||||
| 23andMe | 607 (65%) | 286 (67%) | 54 (56%) | 947 (65%) |
| Pathway Genomics | 333 (35%) | 141 (33%) | 42 (44%) | 516 (35%) |
| Interest in lung cancer genetic risk, N (%)*** | ||||
| Not at all interested | 200 (21%) | 66 (15%) | 6 (6%) | 272 (19%) |
| Somewhat interested | 395 (42%) | 147 (35%) | 27 (28%) | 569 (39%) |
| Very interested | 344 (37%) | 214 (50%) | 63 (65%) | 621 (42%) |
| Interest in heart disease genetic risk, N (%) | ||||
| Not at all interested | 50 (5%) | 17 (4%) | 9 (9%) | 76 (5%) |
| Somewhat interested | 246 (26%) | 125 (29%) | 18 (19%) | 389 (26%) |
| Very Interested | 643 (68%) | 285 (67%) | 69 (72%) | 997 (68%) |
| Elevated lung cancer genetic risk | 152 (17%) | 71 (17%) | 15 (17%) | 238 (17%) |
| Elevated heart disease genetic risk | 181 (21%) | 99 (24%) | 27 (30%) | 307 (22%) |
Chi-squared test used to evaluate differences between smoking status and sex, self-reported race groups, Hispanic ethnicity, residence, health insurance status, college education, genomic testing company, and elevation in lung and heart disease genetic risk. Analysis of variance was used to evaluate the differences between smoking status and age or household income. Logistic regression was used to evaluate how smoking status influenced interest in lung cancer and heart disease genetic risk.
aParticipants could report multiple races so numbers do not add up to 100%.
bOther includes the responses of American Indian/Native Alaskan, Hawaiian or Pacific Islander, or other.
cTotal household income over last 12-months category 1 < $40 000, 2 = $40 000–$69 999, 3 = $70 000–$99 999, 4 = $100 000–$199 999, 5 = $200 000–$500 000, 6 > $500 000.
Characteristics that are significantly different between never, former, and current smokers are indicated by *p < .05; **p < .005; ***p < .0001.
Comparing participants with 6-month follow-up information on smoking variables (n = 1042) to those without (n = 421), there were no differences in smoking status or genetic risk for lung cancer or heart disease (chi-square p > .3). An additional 22 participants (2%) were excluded because of inconsistent responses (reported smoking 100 cigarettes at baseline but never smoking at follow-up).
Interest in Genetic Risk for Lung Cancer and Heart Disease
Current smokers expressed the most interest in their lung cancer genetic risk (65% very, 28% somewhat, and 6% not at all), compared to former smokers (50% very, 35% somewhat, and 15% not at all), and never smokers (37% very, 42% somewhat, and 21% not at all) (Table 1). This ordered effect was significant (odds ratio = 1.8, 95% CI = 1.5–2.1, p < .0001) after controlling for differences in demographic variables.
Overall, participants were more interested in their heart disease genetic risk (68% very, 26% somewhat, and 5% not at all) compared to lung cancer (42% very, 39% somewhat, 19% not at all) (Table 1). Across smoking status groups, there was no significant difference in interest for heart disease genetic risk (p = .8).
Changes in Smoking Behavior
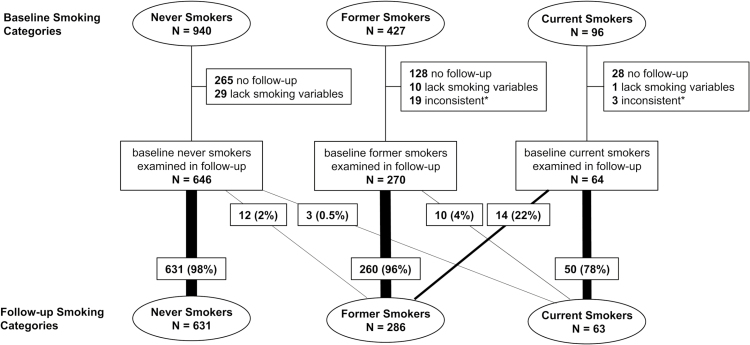
The majority of participants examined at 6-month follow-up (96%, n = 941/980) had the same smoking status as at baseline (Figure 1). Only 13 of the 916 baseline never and former smokers (1%) were current smokers at follow-up. Among the 64 baseline current smokers, 14 (22%) reported that they had quit at 6 months after receiving genetic results. An additional six baseline current smokers reduced their smoking quantity, and only two baseline current smokers increased their smoking quantity.
Figure 1.
Changes in smoking behaviors reported at baseline and 6-month follow-up after receiving genetic results. *Individuals with inconsistent responses were defined as those who reported smoking 100 cigarettes in lifetime at baseline, but not at follow-up.
Secondary analyses revealed that neither lung cancer nor heart disease genetic risk results were significantly different (p > .05) among those who initiated (or reinitiated) smoking compared to those who remained never or former smokers at follow-up. Similarly, these genetic risk results were not significantly different between those who quit smoking and those who remained current smokers. However, these secondary analyses were only powered to detect large differences.
Discussion
This study examined interest in genetic risk information for lung cancer and heart disease, as well as the impact of personal testing on smoking behaviors in more than 1000 23andMe and Pathway Genomics customers. The majority of baseline current smokers were very interested in receiving lung cancer (65%) and heart disease (72%) genetic risk results. This interest in lung cancer risk was significantly greater in current smokers than former and never smokers, possibly reflecting an understanding of the connection between cigarette consumption and lung cancer. For heart disease risk, all groups were very interested in receiving genetic results for this common disease. Our observation that smokers were interested in genetic results for smoking-related illnesses complements recent findings from a high-risk population of smokers. Hartz et al.14 found that 61% of participants were interested in genetic results of smoking-related diseases from Pathway Genomics. Given this interest among smokers, receiving direct-to-consumer genomic results may present an opportunity for multicomponent smoking cessation interventions. One of the few trials showing positive behavioral change with return of genetic results also provided nicotine patches and counseling,13 highlighting that genomic information may be more likely to motivate cessation when combined with other interventions.6
Even though PGen Study participants were interested in smoking-related disease risks, the majority (96%) had the same smoking status 6 months after receiving results as at baseline. Importantly, among baseline never and former smokers, only 1% (n = 13/916) reported being a current smoker at follow-up, suggesting that false reassurance from genomic findings did not lead consumers to initiate (or reinitiate) smoking. Furthermore, secondary analyses found that rates of elevated lung cancer and heart disease genetic risk were similar between those who started smoking and those who did not. If false reassurance led to smoking initiation, we would expect initiators and reinitiators to more frequently have received an average (vs. elevated) risk of smoking-related diseases. To our knowledge, no previous study has provided evidence that personal genomic testing does not lead to smoking initiation among never smokers or reinitiation among former smokers. These findings may help ease concerns that genomic testing contributes to false reassurance of health risks, which in turn, may lead to an uptake of smoking behaviors.
Among baseline current smokers, 22% (n = 14/64) reported quitting at 6-month follow-up, a higher rate than observed in the general US population. For example, the National Health Interview Survey examined more than 25 000 US adults and found that although 52% of smokers made a quit attempt in the last year, only 6% successfully quit.21 Though the baseline sample of current smokers in this study was small, these promising results suggest that genomic health information can motivate quitting. However, meta-analyses have shown that although genetic results may increase cessation motivation11 and behaviors within 6 months,22,23 these results are not associated with abstinence at longer follow-ups. A next step is to examine how to propagate these positive behavioral changes over the long term. Importantly, future studies should assess whether genomic risk information in conjunction with psychosocial and pharmacological treatments can support long-term quitting.
This study has limitations. First, we used an available sample recruited through consumers of two genomic testing companies. These early adopters of genomic testing are predominantly White, college-educated individuals with health insurance, and are not representative of the general population. Their interest in testing may bias the results toward more favorable outcomes and limit generalizability. Future research should focus on engaging diverse populations, and previous work highlights that personal contact, incentives, and community engagement are effective strategies for recruitment of minority populations.24 Second, this longitudinal observational study did not have a control group who did not receive direct-to-consumer testing, limiting conclusions regarding the causality of genomic information on smoking behaviors. Third, a personal or family history of heart disease or lung cancer may influence interest in these conditions. Future research may take advantage of these powerful risk factors for health behaviors that incorporate genetic and environmental influences. Fourth, these analyses are based on one 6-month follow-up and do not provide information on long-term smoking behaviors. Fifth, outcome measures are subject to the limitations of self-reported health behaviors. It is possible that our observed high smoking quit rate reflects health-conscious participants anticipating future actions or expected responses.
This study is a first step toward understanding the impact of direct-to-consumer genomic health testing on cigarette smoking. In the midst of ongoing controversy surrounding direct-to-consumer testing, these results suggest that for the behavior of smoking, genomic results are of interest and have no clear harms with some possible benefits.
Funding
This work was supported by the National Human Genome Research Institute (R01HG005092 to RCG and JSR; U01HG006500, U19HD077671, and R01HG002213 to RCG); the National Institute of Drug Abuse (R01DA036583 to LJB; and K08DA032680 to SH); the National Cancer Institute (P30CA091842 supporting LJB); the National Institute on Alcohol Abuse and Alcoholism (F30AA023685 to EO); the National Institute of General Medical Sciences (T32GM07200 supporting EO); the National Center for Advancing Translational Sciences (UL1TR000448 and TL1TR000449 supporting EO); DAC is funded by a Canadian Institutes of Health Research Postdoctoral Fellowship award and the Michael G. DeGroote Postdoctoral Fellowship from McMaster University.
Declaration of Interests
LJB is listed as an inventor on issued US Patent 8,080,371, “Markers for Addiction” covering the use of certain SNPs in determining the diagnosis, prognosis, and treatment of addiction. RCG is compensated for speaking or advisory services by AIA, Helix, Illumina, Invitae, Prudential and Roche. The other authors declare no conflict of interest.
Acknowledgments
Members of the PGen Study at the time of publication are as follows: Kurt D. Christensen, Robert C. Green, Margaret H. Helm, Joel B. Krier, Lisa S. Lehmann, Erica Schonman (Harvard Medical School, Brigham and Women’s Hospital); Peter Kraft (Harvard School of Public Health); Mick P. Couper, J. Scott Roberts, Wendy R. Uhlmann (University of Michigan); Lan Q Le, Mack T. Ruffin IV, Jenny Ostergren (University of Michigan School of Public Health); Deanna Alexis Carere (McMaster University); Amy K. Kiefer, Joanna L. Mountain (23andMe); Glenn D. Braunstein (Pathway Genomics); Scott D. Crawford (Survey Sciences Group); Clara A. Chen, L. Adrienne Cupples, Catharine Wang (Boston University School of Public Health); Stacy W. Gray (Dana-Farber Cancer Institute); Barbara A. Koenig (University of California San Francisco); Kimberly Kaphingst (University of Utah); Sarah Gollust (University of Minnesota).
References
- 1. Evans JP, Green RC. Direct to consumer genetic testing: avoiding a culture war. Genet Med. 2009;11(8):568–569. doi:10.1097/GIM.0b013e3181afbaed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kuehn BM. Risks and benefits of direct-to-consumer genetic testing remain unclear. JAMA. 2008;300(13):1503–1505. doi:10.1001/jama.300.13.1503. [DOI] [PubMed] [Google Scholar]
- 3. Green RC, Farahany NA. Regulation: the FDA is overcautious on consumer genomics. Nature. 2014;505(7483):286–287. doi:10.1038/505286a. [DOI] [PubMed] [Google Scholar]
- 4. Gutierrez A. Warning Letter. 2013. www.fda.gov/iceci/enforcementactions/warningletters/2013/ucm376296.htm Accessed September 10, 2014. [Google Scholar]
- 5. FDA News Release. FDA Permits Marketing of First Direct-to-Consumer Genetic Carrier Test for Bloom Syndrome. 2015. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/UCM435003 Accessed April 29, 2016. [Google Scholar]
- 6. Bloss CS, Madlensky L, Schork NJ, Topol EJ. Genomic information as a behavioral health intervention: can it work? Per Med. 2011;8(6):659–667. doi:10.2217/pme.11.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bryan AD, Hutchison KE. The role of genomics in health behavior change: challenges and opportunities. Public Health Genomics. 2012;15(3–4):139–145. doi:10.1159/000335226. [DOI] [PubMed] [Google Scholar]
- 8. McBride CM, Bryan AD, Bray MS, Swan GE, Green ED. Health behavior change: can genomics improve behavioral adherence? Am J Public Health. 2012;102(3):401–405. doi:10.2105/AJPH.2011.300513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Graves KD, Hay JL, O’Neill SC. The promise of using personalized genomic information to promote behavior change: is the debate over, or just beginning? Personal Med. 2014;11(2):173–185. doi:10.2217/pme.13.110. [DOI] [PubMed] [Google Scholar]
- 10. Bloss CS, Schork NJ, Topol EJ. Effect of direct-to-consumer genomewide profiling to assess disease risk. N Engl J Med. 2011;364(6):524–534. doi:10.1056/NEJMoa1011893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marteau TM, French DP, Griffin SJ, et al. Effects of communicating DNA-based disease risk estimates on risk-reducing behaviours. Cochrane Database Sys Rev. 2010(10):CD007275. doi:10.1002/14651858.CD007275.pub2. [DOI] [PubMed] [Google Scholar]
- 12. Hollands GJ, French DP, Griffin SJ, et al. The impact of communicating genetic risks of disease on risk-reducing health behaviour: systematic review with meta-analysis. BMJ. 2016;352:i1102. doi:http://dx.doi/10.1136/bmj.i1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McBride CM, Bepler G, Lipkus IM, et al. Incorporating genetic susceptibility feedback into a smoking cessation program for African-American smokers with low income. Cancer Epidemiol Biomarkers Prev. 2002;11(6):521–528. [PubMed] [Google Scholar]
- 14. Hartz SM, Olfson E, Culverhouse R, et al. Return of individual genetic results in a high-risk sample: enthusiasm and positive behavioral change. Genet Med. 2015;17(5):374–379. doi:10.1038/gim.2014.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hogarth S, Javitt G, Melzer D. The current landscape for direct-to-consumer genetic testing: legal, ethical, and policy issues. Annu Rev Genomics Hum Genet. 2008;9:161–182. doi:10.1146/annurev.genom.9.081307.164319. [DOI] [PubMed] [Google Scholar]
- 16. Frueh FW, Greely HT, Green RC, Hogarth S, Siegel S. The future of direct-to-consumer clinical genetic tests. Nat Rev Genet. 2011;12(7):511–515. doi:10.1038/nrg3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. WHO. Media Center: Tobacco. World Health Organization; 2014. www.who.int/mediacentre/factsheets/fs339/en/ Accessed October 16, 2014. [Google Scholar]
- 18. CDC. Tobacco-Related Mortality. Centers for Disease Control and Prevention; 2014. www.cdc.gov/tobacco/data_statistics/fact_sheets/health_effects/tobacco_related_mortality/ Accessed October 16, 2014. [Google Scholar]
- 19. Lehmann LS, Kaufman DJ, Sharp RR, et al. Navigating a research partnership between academia and industry to assess the impact of personalized genetic testing. Genet Med. 2012;14(2):268–273. doi:10.1038/gim.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carere DA, Couper MP, Crawford SD, et al. Design, methods, and participant characteristics of the Impact of Personal Genomics (PGen) Study, a prospective cohort study of direct-to-consumer personal genomic testing customers. Genome Med. 2014;6(12):96. doi:10.1186/s13073-014-0096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. CDC. Quitting smoking among adults—United States, 2001–2010. MMWR Morb Mortal Wkly Rep. 2011;60(44):1513–1519. [PubMed] [Google Scholar]
- 22. Smerecnik C, Grispen JE, Quaak M. Effectiveness of testing for genetic susceptibility to smoking-related diseases on smoking cessation outcomes: a systematic review and meta-analysis. Tob Control. 2012;21(3):347–354. doi:10.1136/tc.2011.042739. [DOI] [PubMed] [Google Scholar]
- 23. de Viron S, Van der Heyden J, Ambrosino E, Arbyn M, Brand A, Van Oyen H. Impact of genetic notification on smoking cessation: systematic review and pooled-analysis. PLoS One. 2012;7(7):e40230. doi:10.1371/journal.pone.0040230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yancey AK, Ortega AN, Kumanyika SK. Effective recruitment and retention of minority research participants. Annu Rev Public Health. 2006;27:1–28. doi:10.1146/annurev.publhealth.27.021405.102113. [DOI] [PubMed] [Google Scholar]