Abstract
Introduction:
To determine if smoking after a cancer diagnosis makes a difference in mortality among newly diagnosed head and neck cancer patients.
Methods:
Longitudinal data were collected from newly diagnosed head and neck cancer patients with a median follow-up time of 1627 days (N = 590). Mortality was censored at 8 years or September 1, 2011, whichever came first. Based on smoking status, all patients were categorized into four groups: continuing smokers, quitters, former smokers, or never-smokers. A broad range of covariates were included in the analyses. Kaplan–Meier curves, bivariate and multivariate Cox proportional hazards models were constructed.
Results:
Eight-year overall mortality and cancer-specific mortality were 40.5% (239/590) and 25.4% (150/590), respectively. Smoking status after a cancer diagnosis predicted overall mortality and cancer-specific mortality. Compared to never-smokers, continuing smokers had the highest hazard ratio (HR) of dying from all causes (HR = 2.71, 95% confidence interval [CI] = 1.48–4.98). Those who smoked at diagnosis, but quit and did not relapse—quitters—had an improved hazard ratio of dying (HR = 2.38, 95% CI = 1.29–4.36) and former smokers at diagnosis with no relapse after diagnosis—former smokers—had the lowest hazard ratio of dying from all causes (HR = 1.68, 95% CI = 1.12–2.56). Similarly, quitters had a slightly higher hazard ratio of dying from cancer-specific reasons (HR = 2.38, 95% CI = 1.13–5.01) than never-smokers, which was similar to current smokers (HR = 2.07, 95% CI = 0.96–4.47), followed by former smokers (HR = 1.70, 95% CI = 1.00–2.89).
Conclusions:
Compared to never-smokers, continuing smokers have the highest HR of overall mortality followed by quitters and former smokers, which indicates that smoking cessation, even after a cancer diagnosis, may improve overall mortality among newly diagnosed head and neck cancer patients. Health care providers should consider incorporating smoking cessation interventions into standard cancer treatment to improve survival among this population.
Implications:
Using prospective observational longitudinal data from 590 head and neck cancer patients, this study showed that continuing smokers have the highest overall mortality relative to never-smokers, which indicates that smoking cessation, even after a cancer diagnosis, may have beneficial effects on long-term overall mortality. Health care providers should consider incorporating smoking cessation interventions into standard cancer treatment to improve survival among this population.
Introduction
Smoking is the leading cause of morbidity and mortality in the United States and is responsible for 30% of all cancer deaths among cancer patients.1 While cancer diagnosis and treatment can provide a “teachable moment” for smoking cessation,2 more than half of cancer patients who smoked prior to diagnosis fail to stop smoking or relapse after their diagnosis.3–5 Continued smoking following diagnosis is associated with decreased response to radiotherapy and chemotherapy, impaired wound healing, increased infections and circulatory problems, and late complications (eg, severe fibrosis, dysphagia).5–11 Conversely, smoking cessation after diagnosis shows several medical benefits, such as decreased fatigue and shortness of breath, increased activity level and quality of life, and improved treatment toxicity.12,13
The 2014 Surgeon General’s report “The Health Consequences of Smoking–50 Years of Progress” (SGR) acknowledged causal relationships between smoking and adverse health outcomes among cancer patients, such as increases in overall mortality, cancer-specific mortality, and second primary cancers.14 Many studies have examined the relationship between smoking and mortality among cancer patients. A history of ever smoking compared with never smoking was associated with an increase in overall mortality15–23 and cancer-specific mortality.15,16,24 However, other studies fail to show a significant difference in mortality based on smoking status after controlling for covariates.25–28
In the head and neck cancer literature, smoking significantly increases overall mortality and cancer-specific mortality,10,21,23,29–34 and pack-years of smoking has a dose–response positive relationship with mortality,23,30,35 yet some studies reported nonsignificant differences in overall mortality36–39 and cancer-specific mortality37,40 between smokers and nonsmokers. However, previous studies are limited by a one-time smoking assessment at or after diagnosis (mostly at diagnosis), their retrospective nature, inability to control for covariates, and short follow-up periods. Thus, examining smoking cessation in relation to mortality among head and neck cancer patients is crucial for making the case one way or the other for providing intensive smoking cessation interventions for head and neck cancer patients. Therefore, we used data from a large-scale longitudinal study to examine the predictive effects of smoking cessation after a cancer diagnosis on overall mortality and cancer-specific mortality among newly diagnosed head and neck cancer patients.
Methods
Study Design
This was a prospective observational cohort study of patients enrolled in the University of Michigan Head and Neck Cancer Specialized Program of Research Excellence (SPORE). The two main dependent variables were overall mortality and cancer-specific mortality censored at 8 years after a cancer diagnosis. The independent variable was smoking status, assessed every 3 months over the first 2 years following diagnosis. Covariates included demographic (age, sex, ethnicity/race, marital status, education level, and household income), health behavioral (number of cigarettes smoked at diagnosis, problem drinking, and body mass index [BMI]), and disease-related factors (cancer site, stage, comorbidities, depression, and treatment [surgery and radiation/chemotherapy]). Human subjects approval was received from the Medical School Institutional Review Board (IRBMED) at the University of Michigan, the VA Ann Arbor Healthcare System, and Henry Ford Hospital. Recruitment was conducted from January 2003 to November 2008.
Study Population
Newly diagnosed patients with squamous cell carcinoma of the head and neck were recruited. To help ensure a diverse patient sample, patients were recruited from three hospitals (Ann Arbor Health System, Ann Arbor VA Healthcare System, and Henry Ford Hospital). Exclusion criteria were those: (1) less than 18 years of age, (2) pregnant, (3) non-English speaking, (4) mentally unstable, (5) non-upper aerodigestive tract cancers (such as thyroid or skin cancer), or (6) historical diagnosis and treatment for head and neck cancer.
Out of 1185 patients approached, 934 consented to participate, yielding a response rate of 78.8%. Of those consented, 808 (86.5%) met the eligibility requirements. Analyses included only subjects with no missing survey data, leaving a sample size of 590. Mortality was censored at 8 years or on September 1, 2011, whichever came first.
Procedure
Research assistants recruited and obtained informed consent from patients in the waiting rooms of otolaryngology clinics. Patients completed written surveys regarding demographics and health behaviors. Structured assessments after diagnosis collected data on smoking status, problem drinking, and depressive symptoms every 3 months for 2 years, and yearly thereafter. A medical record review including reasons of death and treatment history was completed for each participant annually.
Measures
Independent Variables
Smoking status was determined at diagnosis and every 3 months up to 2 years after diagnosis by self-report based on a 30-day prolonged abstinence measure: if they responded “yes” to either “I currently smoke cigarettes” or “I have smoked in the past, but quit within the last 1 month” then they were considered current smokers; if they responded “yes” to either “I have smoked in the past, but quit within the last 6 months” or “I have smoked in the past, but quit within the last year” or “I have smoked in the past, but quit over a year ago” then they were considered quitters; if they responded “yes” to “I have never smoked” then they were considered never-smokers. Based on smoking status during the first 2 years after diagnosis, participants were divided into four groups: (1) continuing smokers, defined as those who smoked at any time after a cancer diagnosis; (2) quitters, defined as those who quit within the first 3 months of diagnosis and remained quit throughout the first 2 years following diagnosis; (3) former smokers, defined as those who quit longer than 1 month before a cancer diagnosis and remained quit throughout the first 2 years following diagnosis; and (4) never-smokers, defined as those who have never smoked.
Covariates
Covariates were determined based on the current literature and clinical judgement, which were then controlled by constructing multivariate Cox proportional hazard models. Covariates included demographic, health behavior, and disease-related factors. Demographics were collected from patient surveys, which included age, sex, race/ethnicity, marital status, education level, and household income. Race/ethnicity was measured using two separate questions about Hispanic/Latino origin and race. Median household income for the census tract of each subject was found using American FactFinder data for the 2000 US Census, found on the www.census.gov Web site.
Number of cigarettes smoked was assessed at diagnosis for smokers and was imputed as “0” for both former smokers and never-smokers. The previously validated 10-item instrument, Alcohol Use Disorders Identification Test (AUDIT), was used to measure alcohol misuse; the score ranges from 0 to 40 with a score of 8 or more indicating problem drinking.41 BMI (weight in kilograms divided by the square of height in meters) was calculated on the basis of self-reported height (without shoes) and weight.
Cancer site was classified into five groups: (1) oral cavity, (2) oropharynx, (3) hypo/nasopharynx, (4) larynx, and (5) other. Tumor stage (0–IV) was measured using the American Joint Committee on Cancer (AJCC) staging classification system.42 Comorbidities were assessed by the Adult Comorbidity Evaluation-27 (ACE-27) and classified as none, mild, moderate, or severe. Depressive symptoms were measured using the 5-item Geriatric Depression Scale (Short Form) (GDS-SF); a score of 4 or higher indicated probable depression. Type of curative treatment received (surgery, radiation, and/or chemotherapy) was recorded by yearly chart audit or patient self-report when treated at an outside facility.
Outcome Variables
The main outcomes were overall mortality and cancer-specific mortality. Mortality was defined as the number of days from the date of diagnosis until the date of death from either all causes (overall mortality) or any cancer-related causes (cancer-specific mortality). Patients were contacted every year to keep track of survival status (dead/alive). If patients were lost to follow-up, the Social Security Administration Death Master File was used to determine if and when they had died. Patients lost to follow-up and not found on the Death Master File were assumed alive as of September 1, 2011.
Statistical Analyses
Means and frequency distributions were examined for all variables. Associations between independent variables were conducted using chi-square tests, t tests, and analysis of variance. All variables were treated as categorical variables except age, number of cigarettes smoked per day, and BMI. Kaplan–Meier plots and the log-rank test were used to compare the independent variables and mortality. Univariate and multivariate Cox proportional hazards models were constructed to examine the relationship between smoking after diagnosis and mortality. Since hospital site was significantly correlated with income, educational level, race, and marital status, it was removed from the multivariate models to avoid multicollinearity. Variance inflation factors were evaluated to assess multicollinearity. Values for p <.05 are reported.
Results
Patient Demographics
The characteristics of the participants are described in Table 1 (N = 590). Eight-year overall mortality and cancer-specific mortality rates were 40.5% (239/590) and 25.4% (150/590), respectively. The mean age was 58.2 years. Most participants were male (78.8%), non-Hispanic White (90.9%), and married (60.5%). Just over half had some college education or more. The median household income was $47 852 ranging from $11 232 to $137 720.
Table 1.
Pretreatment Patient Characteristics of Newly Diagnosed Head and Neck Cancer Patients (N = 590)
| Variable | Mean (SD)/median | Range |
|---|---|---|
| Median follow-up time in days | 1627.2 days | 35–3099 days |
| Mean age in years | 58.2 years (10.7) | 21–92 years |
| Median household income | $47 852.2 | $11 232–$137 720 |
| N | % | |
| Smoking statusa | ||
| Continuing smokers | 146 | 24.8 |
| Quitters | 99 | 16.8 |
| Former smokers | 208 | 35.3 |
| Never-smokers | 137 | 23.2 |
| Sex | ||
| Male | 465 | 78.8 |
| Female | 125 | 21.2 |
| Race/ethnicity | ||
| Non-Hispanic White | 536 | 90.9 |
| Black | 35 | 5.9 |
| Hispanic, other (Native American) | 19 | 3.2 |
| Marital status | ||
| Married | 357 | 60.5 |
| Not married | 233 | 39.5 |
| Education level | ||
| High school or less | 270 | 45.8 |
| Some college or more | 320 | 54.2 |
| Problem drinkingb | 148 | 25.1 |
| BMI | ||
| Underweight | 25 | 4.2 |
| Normal weight | 202 | 34.2 |
| Overweight | 226 | 38.3 |
| Obese | 137 | 23.2 |
| Cancer site | ||
| Oral cavity | 134 | 22.7 |
| Oropharynx | 234 | 39.7 |
| Hypo/nasopharynx | 35 | 5.9 |
| Larynx | 134 | 22.7 |
| Other | 53 | 9.0 |
| Stage | ||
| 0 | 12 | 2.0 |
| I | 63 | 10.7 |
| II | 53 | 9.0 |
| III | 90 | 15.3 |
| IV | 372 | 63.1 |
| ACE-27 comorbidity score | ||
| None | 170 | 28.8 |
| Mild | 234 | 39.7 |
| Moderate | 130 | 22.0 |
| Severe | 56 | 9.5 |
| Depressive symptomsc | 300 | 50.9 |
| Treatment | ||
| Radiation | 513 | 87.0 |
| Chemotherapy | 402 | 68.1 |
| Head and neck surgery | 311 | 52.7 |
ACE-27 = Adult Comorbidity Evaluation-27; BMI = body mass index.
aIncludes cigarettes, cigars, and pipe tobacco.
bAlcohol Use Disorders Identification Test (AUDIT) ≥8.
cGeriatric Depression Scale (Short Form) ≥4.
About a quarter of patients continued to smoke after diagnosis (24.8%, n = 146/590), and half of the patients quit either before (35.3%, n = 208/590) or after (16.8%, n = 99/590) diagnosis and remained quit. Continuing smokers were more likely to be younger, unmarried, and have a high school education or less and low income while being classified as problem drinkers with lower BMIs and cancer of the oral cavity. One-quarter of the patients met the criteria for problem drinking. Over half of the patients were either overweight or obese.
Over one-third was diagnosed with cancer of the oropharynx, followed by cancer of the larynx (22.7%) and oral cavity (22.7%). Sixty-three percent of the patients were classified as stage IV at diagnosis. The majority of patients were scored either none (28.8%) or mild (39.7%) for ACE-27 comorbidity. About half had significant depressive symptoms on the GDS-SF. Most of the participants received at least one treatment: radiation (87.0%), chemotherapy (68.1%), or surgery (52.7%).
Univariate and Multivariate Mortality Analyses
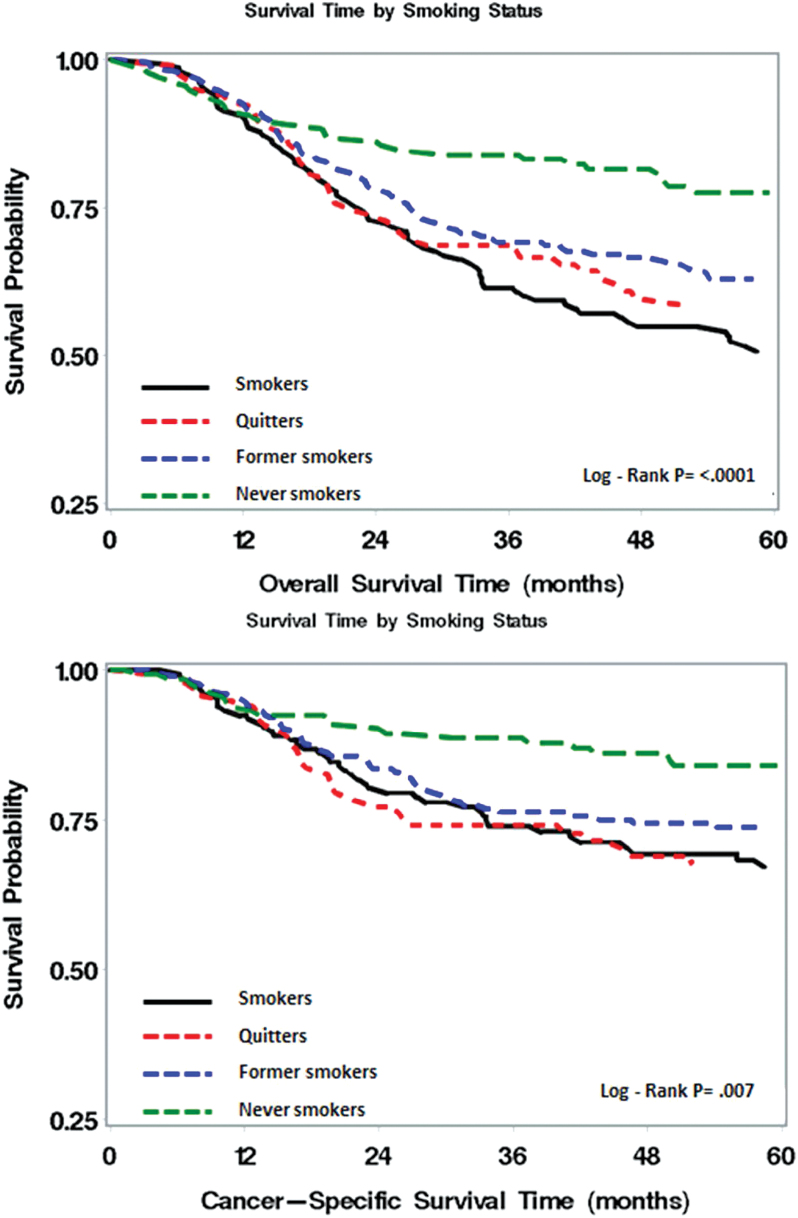
Figure 1 showed Kaplan–Meier plot for overall survival and cancer-specific survival by smoking status after a cancer diagnosis. Never-smokers had the best overall survival and cancer-specific survival, whereas current smokers had the worst survival. For overall survival, quitters and former smokers were in between. For cancer-specific survival, quitters had slightly worse survival than current smokers for the first 3 years and then followed a similar survival pattern as current smokers.
Figure 1.
Kaplan–Meier plot for overall and cancer-specific survival by smoking status.
Table 2 showed the univariate and multivariate analysis for overall mortality. Univariate analysis revealed that smoking status, age, sex, marital status, education, income, number of cigarettes smoked at diagnosis, problem drinking, BMI, cancer site, cancer stage, medical comorbidity, radiotherapy, and chemotherapy significantly predicted overall mortality among head and neck cancer patients. Compared to never-smokers, continuing smokers had the highest hazard ratio (HR) of dying from all causes (HR = 2.66, 95% confidence interval [CI] = 1.76–4.01), followed by quitters (HR = 2.03, 95% CI = 1.29–3.21) and former smokers (HR = 1.91, 95% CI = 1.27–2.88). Advanced age, less education, low income, higher cigarette consumption, problem drinking, advanced cancer stage, severe comorbidity, and having radiotherapy and chemotherapy were significantly associated with higher overall mortality. Female sex, being married, and a higher BMI were related to lower mortality.
Table 2.
Bivariate and Multivariate Cox Proportional Hazards Model for Overall Mortality, N = 590 (239 Events, 351 Censored)
| Univariate models | Multivariate models | |||||
|---|---|---|---|---|---|---|
| Variable | Hazard ratio | 95% CI | p value | Hazard ratio | 95% CI | p value |
| Smoking status (vs. never)a | ||||||
| Continuing smokers | 2.66 | 1.76–4.01 | <.0001* | 2.71 | 1.48–4.98 | .001* |
| Quitters | 2.03 | 1.29–3.21 | .002* | 2.38 | 1.29–4.36 | .005* |
| Former smokers | 1.91 | 1.27–2.88 | .002* | 1.68 | 1.12–2.56 | .015* |
| Age (in decades) | 1.42 | 1.26–1.60 | <.0001* | 1.58 | 1.36–1.83 | <.0001* |
| Female sex | 0.67 | 0.48–0.95 | .023* | 0.67 | 0.46–0.97 | .035* |
| Race/ethnicity (vs. White) | ||||||
| Black | 1.30 | 0.79–2.13 | .300 | 1.07 | 0.62–1.85 | .817 |
| Hispanic, other (Native American) | 0.85 | 0.40–1.80 | .671 | 0.71 | 0.32–1.56 | .389 |
| Married | 0.77 | 0.60–1.00 | .050* | 0.99 | 0.75–1.30 | .927 |
| High school education or less | 1.43 | 1.11–1.84 | .006* | 1.20 | 0.91–1.58 | .187 |
| Low incomeb | 1.40 | 1.06–1.85 | .018* | 1.12 | 0.83–1.52 | .448 |
| Number of cigarettes per day | 1.01 | 1.00–1.02 | .003* | 0.99 | 0.98–1.01 | .245 |
| Problem drinkingc | 1.42 | 1.08–1.87 | .013* | 1.15 | 0.84–1.59 | .388 |
| Body mass index | 0.95 | 0.93–0.97 | <.0001* | 0.97 | 0.94–0.99 | .010* |
| Cancer site (vs. oral cavity) | ||||||
| Oropharynx | 0.66 | 0.48–0.92 | .014* | 0.44 | 0.29–0.66 | <.0001* |
| Hypo/nasopharynx | 1.48 | 0.91–2.41 | .114 | 0.56 | 0.32–0.97 | .040* |
| Larynx | 0.74 | 0.51–1.07 | .108 | 0.50 | 0.33–0.76 | .001* |
| Other | 0.68 | 0.40–1.15 | .154 | 0.64 | 0.36–1.12 | .116 |
| Stage | 1.22 | 1.08–1.39 | .002* | 1.21 | 1.03–1.41 | .019* |
| ACE-27 comorbidity score | 1.43 | 1.26–1.64 | <.0001* | 1.31 | 1.14–1.51 | <.001* |
| Depressive symptomsd | 1.06 | 0.82–1.37 | .654 | 1.04 | 0.80–1.36 | .782 |
| Surgery | 0.89 | 0.69–1.14 | .357 | 0.94 | 0.69–1.27 | .670 |
| Radiation treatment | 1.61 | 1.04–2.50 | .033* | 1.12 | 0.64–1.95 | .704 |
| Chemotherapy treatment | 1.69 | 1.25–2.28 | .001* | 2.16 | 1.45–3.24 | <.001* |
ACE-27 = Adult Comorbidity Evaluation-27; CI = confidence interval.
aIncludes cigarettes, cigars, and pipe tobacco.
bLowest quartile of income ≤$35 169.
cAlcohol Use Disorders Identification Test (AUDIT) ≥8.
dGeriatric Depression Scale (Short Form) ≥4.
*Predictors with p < .05.
In the multivariate analysis controlling for covariates, smoking status after a cancer diagnosis still showed a significant dose–response relationship with overall mortality: continuing smokers (HR = 2.71, 95% CI = 1.48–4.98) had the highest hazard ratio of dying from all causes; quitters (HR = 2.38, 95% CI = 1.29–4.36) had the second-highest hazard ratio; and former smokers (HR = 1.68, 95% CI = 1.12–2.56) had the third-highest hazard ratio. A 10-year increase in age predicted a 58% increase in hazard ratio of dying (p < .0001), and females had 33% decreased hazard ratio of dying compared to males (p = .035). One-unit increase in BMI decreased the hazard ratio of dying by 3% (p = .010). As expected, more advanced cancer stage (HR = 1.21, 95% CI = 1.03–1.41), severe comorbidities (HR = 1.31, 95% CI = 1.14–1.51), and having chemotherapy (HR = 2.16, 95% CI = 1.45–3.24) predicted worse mortality. Marital status, education, income, cigarette consumption, and problem drinking were no longer significant in multivariate analysis.
Similarly, on bivariate analyses smoking status after diagnosis was significantly associated with cancer-specific mortality (Table 3). Continued smoking had the highest hazard ratio of dying from cancer-specific reasons (HR = 2.38, 95% CI = 1.41–4.04) followed by quitters (HR = 2.34, 95% CI = 1.33–4.10). Former smokers had the lowest hazard ratio of dying (HR = 1.91, 95% CI = 1.14–3.18). Older age (HR = 1.24, 95% CI = 1.06–1.44), higher cigarette consumption (HR = 1.01, 95% CI = 1.00–1.02), problem drinking (HR = 1.48, 95% CI = 1.05–2.09), low BMI (HR = 0.95, 95% CI = 0.92–0.98), advanced cancer stage (HR = 1.41, 95% CI = 1.18–1.70), receiving radiotherapy (HR = 2.85, 95% CI = 1.40–5.80) and chemotherapy (HR = 2.39, 95% CI = 1.57–3.66) were also related to higher cancer-specific mortality.
Table 3.
Bivariate and Multivariate Cox Proportional Hazards Model for Cancer-Specific Mortality, N = 590 (150 Events, 440 Censored)
| Univariate models | Multivariate models | |||||
|---|---|---|---|---|---|---|
| Variable | Hazard ratio | 95% CI | p value | Hazard ratio | 95% CI | p value |
| Smoking status (vs. never)a | ||||||
| Continuing smokers | 2.38 | 1.41–4.04 | .001* | 2.07 | 0.96–4.47 | .063 |
| Quitters | 2.34 | 1.33–4.10 | .003* | 2.38 | 1.13–5.01 | .022* |
| Former smokers | 1.91 | 1.14–3.18 | .014* | 1.70 | 1.00–2.89 | .049* |
| Age (in decades) | 1.24 | 1.06–1.44 | .006* | 1.44 | 1.19–1.74 | <.001* |
| Female sex | 0.74 | 0.49–1.13 | .165 | 0.76 | 0.48–1.21 | .246 |
| Race/ethnicity (vs. White) | ||||||
| Black | 1.22 | 0.64–2.33 | .540 | 1.02 | 0.50–2.07 | .955 |
| Hispanic, other (Native American) | 0.99 | 0.41–2.42 | .983 | 0.88 | 0.34–2.27 | .796 |
| Married | 0.76 | 0.55–1.04 | .087 | 0.91 | 0.65–1.29 | .604 |
| High school education or less | 1.35 | 0.98–1.86 | .065 | 1.20 | 0.85–1.70 | .296 |
| Low incomeb | 1.37 | 0.96–1.95 | .080 | 1.17 | 0.80–1.71 | .417 |
| Number of cigarettes per day | 1.01 | 1.00–1.02 | .023* | 1.00 | 0.98–1.01 | .595 |
| Problem drinkingc | 1.48 | 1.05–2.09 | .027* | 1.16 | 0.77–1.73 | .480 |
| Body mass index | 0.95 | 0.92–0.98 | .002* | 0.96 | 0.93–1.00 | .026* |
| Cancer site (vs. oral cavity) | ||||||
| Oropharynx | 0.67 | 0.45–1.00 | .050* | 0.39 | 0.24–0.64 | <.001* |
| Hypo/nasopharynx | 0.87 | 0.43–1.80 | .714 | 0.33 | 0.15–0.72 | .006* |
| Larynx | 0.63 | 0.39–1.01 | .054 | 0.44 | 0.25–0.75 | .003* |
| Other | 0.73 | 0.39–1.34 | .318 | 0.60 | 0.30–1.18 | .136 |
| Stage | 1.41 | 1.18–1.70 | <.001* | 1.27 | 1.03–1.57 | .027* |
| ACE-27 comorbidity score | 1.17 | 0.99–1.39 | .072 | 1.11 | 0.92–1.33 | .283 |
| Depressive symptomsd | 1.04 | 0.76–1.43 | .809 | 1.02 | 0.73–1.42 | .913 |
| Surgery | 0.94 | 0.68–1.29 | .698 | 0.94 | 0.64–1.38 | .762 |
| Radiation treatment | 2.85 | 1.40–5.80 | .004* | 1.58 | 0.68–3.68 | .289 |
| Chemotherapy treatment | 2.39 | 1.57–3.66 | <.0001* | 2.73 | 1.61–4.63 | <.001* |
ACE-27 = Adult Comorbidity Evaluation-27; CI = confidence interval.
aIncludes cigarettes, cigars, and pipe tobacco.
bLowest quartile of income ≤$35 169.
cAlcohol Use Disorders Identification Test ≥8.
dGeriatric Depression Scale (Short Form) ≥4.
*Predictors with p < .05.
In the multivariate model, smoking status remained significant: compared to never-smokers, quitters had a slightly higher hazard ratio (HR = 2.38, 95% CI = 1.13–5.01), which was similar to current smokers (HR = 2.07, 95% CI = 0.96–4.47) followed by former smokers (HR = 1.70, 95% CI = 1.00–2.89). Older age (HR = 1.44, 95% CI = 1.19–1.74), low BMI (HR = 0.96, 95% CI = 0.93–1.00), advanced cancer stage (HR = 1.27, 95% CI = 1.03–1.57), and receiving chemotherapy (HR = 2.73, 95% CI = 1.61–4.63) were independently associated with increased hazard ratio of dying from cancer-specific reasons.
Discussion
Using prospective longitudinal data from newly diagnosed head and neck cancer patients, this study showed beneficial effects of smoking cessation after a cancer diagnosis on overall mortality. Compared to never-smokers, current smokers after diagnosis had 2.7 times higher hazard ratio of dying from all causes, which is similar to Gillison et al.’s30 report where smoking during radiotherapy increased the hazard of dying by 2.2 among oropharyngeal cancer patients. These findings are also consistent with other previous studies with head and neck cancer patients10,21,23,29–34 and other cancer patients.15,24,43,44 Considering the limitations of previous studies (eg, one-time assessment of smoking, mostly at diagnosis; use of retrospective data; failure to control for covariates; short follow-up period), this study contributes to the head and neck cancer literature by identifying a more accurate measure of the magnitude of the causal relationship between smoking after a cancer diagnosis and mortality, after controlling for a broad range of covariates. However, continued smoking did not significantly predict cancer-specific mortality. This may be because, given the social stigma associated with smoking among head and neck cancer patients,45 self-reported smoking may be less accurate than biochemically verified smoking status and might introduce misrepresentation of continuing smokers.8,45–47 However, the misrepresentation of current smokers can be minimized by repeated assessment of self-report smoking status as was done in this and another study conducted with head and neck cancer patients.45
Several hypotheses have been proposed to explain the relationship between smoking after diagnosis and higher mortality. Carcinogens in tobacco products and nicotine-induced angiogenesis may promote growth and proliferation of tumor cells.15 Chronic hypoxia, due to nicotine-induced vasoconstriction and carboxyhemoglobin, leads to molecular changes21,23,48 such as increased p53 mutations and epidermal growth factor receptor expression, both of which are associated with high mortality.16,23,38,49 Chronic hypoxic tumor cells are also related to decreased efficacy of cancer treatment, especially radiation therapy.10,23
Continued smoking among cancer patients also stimulates proliferation and tumorigenesis and reduces apoptosis in cancer cells.50 Exposures of cancer cells to smoking also increase multidrug resistance, which then increase the risks for cancer recurrence and decrease response to treatment.50 Moreover, continuing smokers tend to develop more late-stage complications, such as impaired wound healing, vascular injury, or a direct irritant,10,29 which may contribute to poor mortality. Smokers tend to simultaneously engage in other risky health behaviors, such as substance abuse, problem drinking, physical inactivity, not adhering to medication regimens, and missing treatment days,10,51 which may lead to worse mortality outcomes, although this study controlled for some of these risky behaviors, including problem drinking and nutritional status (BMI).
Despite these detrimental effects of continued smoking after a cancer diagnosis, the rate of continued smoking 2 years after diagnosis was 23.4% in this sample. Even though 70% of cancer patients who smoke are motivated to quit smoking, only 56% of physicians recommend that their cancer patients who smoke stop smoking52 and most oncology providers do not provide smoking interventions beyond advice to quit.53 Our work54 and the work of others55–59 have shown that the major barriers to providers implementing smoking cessation services included lack of expertise and time. Education programs for health care providers as well as dedicated smoking cessation programs would improve tobacco treatment for cancer patients.11,60
In 2014, for the first time Surgeon General’s report “The Health Consequences of Smoking—50 Years of Progress”14,61 stated that it is imperative to address smoking among cancer patients. Recent similar reports have been endorsed by the AACR,62 American Society of Clinical Oncology (ASCO),63 and NCCN guidelines. A diagnosis of cancer can be a “teachable moment” where cancer patients are highly motivated to quit smoking as the benefits of quitting are evident.64 While several cessation interventions from brief advice/referrals to intense programs64–66 as well as pharmacotherapies66,67 have been successfully implemented to head and neck cancer patients, only few institution-wide cessation supports have been incorporated into cancer care.60
Among covariates, age, sex, BMI levels, cancer stage, medical comorbidities, and treatment predicted both/either mortality outcomes. As expected, older age, being male, cancer of the oral cavity, more advanced cancer stage, and severe medical comorbidities were associated with worse overall and/or cancer-specific mortality, while higher levels of BMI were a significant predictor of lower overall and/or cancer-specific mortality. This finding supports the evidence that excess weight among head and neck cancer patients plays a protective role for normal upper aerodigestive tissue, meaning those with higher BMIs have better survival outcomes.68 Higher BMIs may also reflect better nutritional status, which could influence treatment options. Chemotherapy was associated with higher mortality as patients with more advanced stage generally receive chemotherapy. Data were not available to analyze whether chemotherapy was used as a curative treatment or recurrence treatment, which may provide more insight in the relationship between chemotherapy and worse mortality.
An additional limitation of the study was that the analyses did not control for secondhand smoke, which plays an important role in pathogenesis of head and neck cancer29 and possibly prognosis. Using census tract data as a proxy for income is also a limitation, yet studies have shown that census data is a valid proxy for income when other sources are not available.69,70
It is also possible that selection bias affecting either the patients referred or recruited to this study, but this would be minimal because the patients were recruited from three different institutes where sociodemographic characteristics varied and overall recruitment rates were high (79%). Unfortunately, the analyses were unable to control for human papillomavirus status, which has been shown to effect mortality among oropharyngeal cancer patients,10,29,37 albeit a recent study found that smoking status remained predictive of mortality and disease progression even after controlling for human papillomavirus status.30
Conclusions
Compared to never-smokers, continuing smokers have the highest ratio of overall mortality followed by quitters and former smokers. These findings, which controlled for various covariates and tracked smoking status repeatedly for 2 years of diagnosis, support the adverse effect of continuing smoking after diagnosis and suggest that the smoking cessation after a cancer diagnosis may have beneficial effects on overall mortality for head and neck cancer patients. Health care providers should focus on incorporating smoking cessation interventions into standard cancer treatment and continuously encourage smoking cessation.
Funding
This study was funded by the US National Institutes of Health through the University of Michigan’s Head and Neck Cancer Specialized Program of Research Excellence (grant number P50 CA97248).
Declaration of Interests
None declared.
Acknowledgments
The authors wish to thank Suzan McCormick, Chelsea Hughes, Allison Dubois, Elizabeth Knight, and the staffs of the University of Michigan Hospital, Veterans Affairs Ann Arbor Healthcare System, and Henry Ford Hospital Otolaryngology clinics for their assistance with recruitment and data collection.
References
- 1. American Cancer Society. Cancer Facts and Figures 2010. Atlanta, GA: American Cancer Society; 2010. [Google Scholar]
- 2. McBride CM, Ostroff JS. Teachable moments for promoting smoking cessation: the context of cancer care and survivorship. Cancer Control. 2003;10(4):325–333. [DOI] [PubMed] [Google Scholar]
- 3. Duffy SA, Khan MJ, Ronis DL, et al. Health behaviors of head and neck cancer patients the first year after diagnosis. Head Neck. 2008;30(1):93–102. [DOI] [PubMed] [Google Scholar]
- 4. Sardari Nia P, Weyler J, Colpaert C, Vermeulen P, Van Marck E, Van Schil P. Prognostic value of smoking status in operated non-small cell lung cancer. Lung Cancer. 2005;47(3):351–359. [DOI] [PubMed] [Google Scholar]
- 5. Cataldo JK, Dubey S, Prochaska JJ. Smoking cessation: an integral part of lung cancer treatment. Oncology. 2010;78(5–6):289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gritz ER, Dresler C, Sarna L. Smoking, the missing drug interaction in clinical trials: ignoring the obvious. Cancer Epidemiol Biomarkers Prev. 2005;14(10):2287–2293. [DOI] [PubMed] [Google Scholar]
- 7. Siana JE, Rex S, Gottrup F. The effect of cigarette smoking on wound healing. Scand J Plast Reconstr Surg Hand Surg. 1989;23(3):207–209. [DOI] [PubMed] [Google Scholar]
- 8. Marin VP, Pytynia KB, Langstein HN, Dahlstrom KR, Wei Q, Sturgis EM. Serum cotinine concentration and wound complications in head and neck reconstruction. Plast Reconstr Surg. 2008;121(2):451–457. [DOI] [PubMed] [Google Scholar]
- 9. Cooley ME, Sipples RL, Murphy M, Sarna L. Smoking cessation and lung cancer: oncology nurses can make a difference. Semin Oncol Nurs. 2008;24(1):16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen AM, Chen LM, Vaughan A, et al. Tobacco smoking during radiation therapy for head-and-neck cancer is associated with unfavorable outcome. Int J Radiat Oncol Biol Phys. 2011;79(2):414–419. [DOI] [PubMed] [Google Scholar]
- 11. Gritz ER, Toll BA, Warren GW. Tobacco use in the oncology setting: advancing clinical practice and research. Cancer Epidemiol Biomarkers Prev. 2014;23(1):3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garces YI, Yang P, Parkinson J, et al. The relationship between cigarette smoking and quality of life after lung cancer diagnosis. Chest. 2004;126(6):1733–1741. [DOI] [PubMed] [Google Scholar]
- 13. Alsadius D, Hedelin M, Johansson KA, et al. Tobacco smoking and long-lasting symptoms from the bowel and the anal-sphincter region after radiotherapy for prostate cancer. Radiother Oncol. 2011;101(3):495–501. [DOI] [PubMed] [Google Scholar]
- 14. US Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 15. Kenfield SA, Stampfer MJ, Chan JM, Giovannucci E. Smoking and prostate cancer survival and recurrence. JAMA. 2011;305(24):2548–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kroeger N, Klatte T, Birkhäuser FD, et al. Smoking negatively impacts renal cell carcinoma overall and cancer-specific survival. Cancer. 2012;118(7):1795–1802. [DOI] [PubMed] [Google Scholar]
- 17. Kawaguchi T, Takada M, Kubo A, et al. Performance status and smoking status are independent favorable prognostic factors for survival in non-small cell lung cancer: a comprehensive analysis of 26,957 patients with NSCLC. J Thorac Oncol. 2010;5(5):620–630. [DOI] [PubMed] [Google Scholar]
- 18. Gajdos C, Hawn MT, Campagna EJ, Henderson WG, Singh JA, Houston T. Adverse effects of smoking on postoperative outcomes in cancer patients. Ann Surg Oncol. 2012;19(5):1430–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wells JC, Samuel TA, Morton K, Looney SW. Tobacco use and race as copredictors of overall survival in patients with breast cancer. J Clin Oncol. 2013;31(26):20. [Google Scholar]
- 20. Videtic GM, Stitt LW, Dar AR, et al. Continued cigarette smoking by patients receiving concurrent chemoradiotherapy for limited-stage small-cell lung cancer is associated with decreased survival. J Clin Oncol. 2003;21(8):1544–1549. [DOI] [PubMed] [Google Scholar]
- 21. Kumar B, Cordell KG, Lee JS, et al. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26(19):3128–3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Varadarajan R, Licht AS, Hyland AJ, et al. Smoking adversely affects survival in acute myeloid leukemia patients. Int J Cancer. 2012;130(6):1451–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoff CM, Grau C, Overgaard J. Effect of smoking on oxygen delivery and outcome in patients treated with radiotherapy for head and neck squamous cell carcinoma–a prospective study. Radiother Oncol. 2012;103(1):38–44. [DOI] [PubMed] [Google Scholar]
- 24. Warren GW, Kasza KA, Reid ME, Cummings KM, Marshall JR. Smoking at diagnosis and survival in cancer patients. Int J Cancer. 2013;132(2):401–410. [DOI] [PubMed] [Google Scholar]
- 25. Parker A, Lohse C, Cheville J, Leibovich B, Igel T, Blute M. Evaluation of the association of current cigarette smoking and outcome for patients with clear cell renal cell carcinoma. Int J Urol. 2008;15(4):304–308. [DOI] [PubMed] [Google Scholar]
- 26. Rink M, Zabor EC, Furberg H, et al. Impact of smoking and smoking cessation on outcomes in bladder cancer patients treated with radical cystectomy. Eur Urol. 2013;64(3):456–464. [DOI] [PubMed] [Google Scholar]
- 27. Meguid RA. Long-term survival outcomes by smoking status in surgical and nonsurgical patients with non-small cell lung cancer: comparing never smokers and current smokers. Chest. 2010;138(3):500–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Holmes MD, Murin S, Chen WY, Kroenke CH, Spiegelman D, Colditz GA. Smoking and survival after breast cancer diagnosis. Int J Cancer. 2007;120(12):2672–2677. [DOI] [PubMed] [Google Scholar]
- 29. Chen AM, Chen LM, Vaughan A, et al. Head and neck cancer among lifelong never-smokers and ever-smokers matched-pair analysis of outcomes after radiation therapy. Am J Clin Oncol. 2011;34(3):270–275. [DOI] [PubMed] [Google Scholar]
- 30. Gillison ML, Zhang Q, Jordan R, et al. Tobacco smoking and increased risk of death and progression for patients with p16-positive and p16-negative oropharyngeal cancer. J Clin Oncol. 2012;30(17):2102–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jerjes W, Upile T, Radhi H, et al. The effect of tobacco and alcohol and their reduction/cessation on mortality in oral cancer patients: short communication. Head Neck Oncol. 2012;4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Koole R, Calkoen EV, Kranenborg H, et al. Survival analysis of head and neck squamous cell carcinoma: influence of smoking and drinking. Head Neck. 2011;33(6):817–823. [DOI] [PubMed] [Google Scholar]
- 33. Dahlstrom KR, Calzada G, Hanby JD, et al. An evolution in demographics, treatment, and outcomes of oropharyngeal cancer at a major cancer center. Cancer. 2013;119(1):81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fortin A, Wang CS, Vigneault E. Influence of smoking and alcohol drinking behaviors on treatment outcomes of patients with squamous cell carcinomas of the head and neck. Int J Radiat Oncol Biol Phys. 2009;74(4):1062–1069. [DOI] [PubMed] [Google Scholar]
- 35. Ang KK, Harris J, Wheeler R, et al. Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. N Engl J Med. 2010;363(1):24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Friedlander PL, Schantz SP, Shaha AR, Yu G, Shah JP. Squamous cell carcinoma of the tongue in young patients: a matched-pair analysis. Head Neck. 1998;20(5):363–368. [DOI] [PubMed] [Google Scholar]
- 37. Pytynia KB, Grant JR, Etzel CJ, Roberts DB, Wei Q, Sturgis EM. Matched-pair analysis of survival of never smokers and ever smokers with squamous cell carcinoma of the head and neck. J Clin Oncol. 2004;22(19):3981–3988. [DOI] [PubMed] [Google Scholar]
- 38. Koch WM, Lango M, Sewell D, Zahurak M, Sidransky D. Head and neck cancer in nonsmokers: a distinct clinical and molecular entity. Laryngoscope. 1999;109(10):1544–1551. [DOI] [PubMed] [Google Scholar]
- 39. Mayne ST, Cartmel B, Kirsh V, W. Jarrard Goodwin J. Alcohol and tobacco use prediagnosis and postdiagnosis, and survival in a cohort of patients with early stage cancers of the oral cavity, pharynx, and larynx. Cancer Epidemiol Biomarkers Prev. 2009;18(12):3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Haughey BH, Sinha P. Prognostic factors and survival unique to surgically treated p16+ oropharyngeal cancer. Laryngoscope. 2012;122(S2):S13–S33. [DOI] [PubMed] [Google Scholar]
- 41. Saunders J, Aasland O, Babor T, de la Fuente J, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on early detection of persons with harmful alcohol consumption-II. Addiction. 1993;88(suppl 6):791–804. [DOI] [PubMed] [Google Scholar]
- 42. American Joint Committee on Cancer. AJCC 7th Ed Cancer Staging Manual. New York: Springer-Verlag; 2009. [Google Scholar]
- 43. Dobson Amato KA, Hyland A, Reed R, et al. Tobacco cessation may improve lung cancer patient survival. J Thorac Oncol. 2015;10(7):1014–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tao L, Wang R, Gao YT, Yuan JM. Impact of postdiagnosis smoking on long-term survival of cancer patients: the Shanghai cohort study. Cancer Epidemiol Biomarkers Prev. 2013;22(12):2404–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Warren GW, Arnold SM, Valentino JP, et al. Accuracy of self-reported tobacco assessments in a head and neck cancer treatment population. Radiother Oncol. 2012;103(1):45–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Khariwala SS, Carmella SG, Stepanov I, et al. Self-reported tobacco use does not correlate with carcinogen exposure in smokers with head and neck cancer. Laryngoscope. 2015;125(8):1844–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Morales NA, Romano MA, Michael Cummings K, et al. Accuracy of self-reported tobacco use in newly diagnosed cancer patients. Cancer Causes Control. 2013;24(6):1223–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kalyankrishna S, Grandis JR. Epidermal growth factor receptor biology in head and neck cancer. J Clin Oncol. 2006;24(17):2666–2672. [DOI] [PubMed] [Google Scholar]
- 49. Pukkila MJ, Kumpulainen EJ, Virtaniemi JA, et al. Nuclear and cytoplasmic p53 expression in pharyngeal squamous cell carcinoma: prognostic implications. Head Neck. 2002;24(8):784–791. [DOI] [PubMed] [Google Scholar]
- 50. Warren GW, Sobus S, Gritz ER. The biological and clinical effects of smoking by patients with cancer and strategies to implement evidence-based tobacco cessation support. Lancet Oncol. 2014;15(12):e568–e580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Spring B, Moller AC, Coons MJ. Multiple health behaviours: overview and implications. J Public Health. 2012;34(suppl 1):I3–I10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schnoll RA, Zhang B, Rue M, et al. Brief physician-initiated quit-smoking strategies for clinical oncology settings: a trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2003;21(2):355–365. [DOI] [PubMed] [Google Scholar]
- 53. Weaver KE, Danhauer SC, Tooze JA, et al. Smoking cessation counseling beliefs and behaviors of outpatient oncology providers. Oncologist. 2012;17(3):455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Duffy SA, Reeves P, Hermann C, Karvonen C, Smith P. In-hospital smoking cessation programs: what do VA patients and staff want and need? Appl Nurs Res. 2008;21(4):199–206. [DOI] [PubMed] [Google Scholar]
- 55. Borrelli B, Hecht JP, Papandonatos GD, Emmons KM, Tatewosian LR, Abrams DB. Smoking-cessation counseling in the home. Attitudes, beliefs, and behaviors of home healthcare nurses. Am J Prev Med. 2001;21(4):272–277. [DOI] [PubMed] [Google Scholar]
- 56. Willaing I, Ladelund S. Smoking behavior among hospital staff still influences attitudes and counseling on smoking. Nicotine Tob Res. 2004;6(2):369–375. [DOI] [PubMed] [Google Scholar]
- 57. Braun BL, Fowles JB, Solberg LI, Kind EA, Lando H, Pine D. Smoking-related attitudes and clinical practices of medical personnel in Minnesota. Am J Prev Med. 2004;27(4):316–322. [DOI] [PubMed] [Google Scholar]
- 58. Sarna L, Bialous SA, Wells M, Kotlerman J, Wewers ME, Froelicher ES. Frequency of nurses’ smoking cessation interventions: report from a national survey. J Clin Nurs. 2009;18(14):2066–2077. [DOI] [PubMed] [Google Scholar]
- 59. Warren GW, Marshall JR, Cummings KM, et al. Addressing tobacco use in patients with cancer: a survey of American Society of Clinical Oncology members. J Oncol Pract. 2013;9(5):258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Warren GW, Marshall JR, Cummings KM, et al. Automated tobacco assessment and cessation support for cancer patients. Cancer. 2014;120(4):562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Warren GW, Alberg AJ, Kraft AS, Cummings KM. The 2014 Surgeon General’s report: “The health consequences of smoking–50 years of progress”: a paradigm shift in cancer care. Cancer. 2014;120(13):1914–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Toll BA, Brandon TH, Gritz ER, Warren GW, Herbst RS. Assessing tobacco use by cancer patients and facilitating cessation: an American Association for Cancer Research policy statement. Clin Cancer Res. 2013;19(8):1941–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hanna N, Mulshine J, Wollins DS, Tyne C, Dresler C. Tobacco cessation and control a decade later: American Society of Clinical Oncology policy statement update. J Clin Oncol. 2013;31(25):3147–3157. [DOI] [PubMed] [Google Scholar]
- 64. Tang MW, Oakley R, Dale C, Purushotham A, Møller H, Gallagher JE. A surgeon led smoking cessation intervention in a head and neck cancer centre. BMC Health Serv Res. 2014;14:636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sharp L, Johansson H, Fagerström K, Rutqvist LE. Smoking cessation among patients with head and neck cancer: cancer as a ‘teachable moment’. Eur J Cancer Care. 2008;17(2):114–119. [DOI] [PubMed] [Google Scholar]
- 66. Duffy SA, Ronis DL, Valenstein M, et al. A tailored smoking, alcohol, and depression intervention for head and neck cancer patients. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2203–2208. [DOI] [PubMed] [Google Scholar]
- 67. National Cancer Institute. Smoking in Cancer Care—for Health Professionals www.cancer.gov/about-cancer/causes-prevention/risk/tobacco/smoking-cessation-hp-pdq#link/stoc_h2_6 Accessed February 26, 2016.
- 68. Pai PC, Chuang CC, Tseng CK, et al. Impact of pretreatment body mass index on patients with head-and-neck cancer treated with radiation. Int J Radiat Oncol Biol Phys. 2012;83(1):e93–e100. [DOI] [PubMed] [Google Scholar]
- 69. Adjemian M, Williams J. Using census aggregates to proxy for household characteristics: an application to vehicle ownership. Transportation. 2009;36(2):223–241. [Google Scholar]
- 70. Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82(5):703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]