Abstract
The aim of the study was to investigate the anti-tumoral activity of morelloflavone substances with different structures. We also studied the possible link between morelloflavone structure and its function. Various types of chromatographic techniques were used to isolate and screen morelloflavone substances from the extracts of gambogic tree trunk and the morelloflavone structures were identified by analytical techniques such as high resolution mass spectrometry and nuclear magnetism. Anti-tumoral activities of different compounds were investigated and the link between the antitumor activity and the structure of compound was exaimed. Our results showed that the isolated morelloflavone substances demonstrated a certain level of antitumor activity. The compound no. 1 had the strongest effect to inhibit glioma U87 and C6 cells followed by compound no. 2 while compound no. 5 was the weakest among them. We conducted a preliminary analysis on the structure-function relationship through the structure comparison and we concluded that the antitumor effects of morelloflavone substances with different structures were significantly different from each other. Thus, the glucose chain in C4 position of biflavone structure can enhance the antitumor activity of the compound in glioma cells. Additionally, the formation of intramolecular hydrogen bonds in biflavone compounds may also play a role in enhancing the antitumor activity and inhibition rate.
Keywords: biflavone, glioma, structure-function relationship
Introduction
Glioma originates from nerve epithelium-derived cells (1), accounting for 44.6% of the central nervous system tumors. It is the most common malignant tumor in the central nervous system with a high incidence and high mortality rate (2). Despite several achievements in cancer therapy in recent years, no effective treatment for glioma has been found. Currently, surgery-based comprehensive treatment is the treatment of choice for glioma, but the prognosis of comprehensive treatment remains poor. Nevertheless, this treatment can improve the recovery rate of glioma to a certain extent with 5-year survival rate of <5% (3,4). Therefore, there is a huge interest in developing a new means of treatment for brain glioma. The application of traditional Chinese medicine for treating the malignant tumors has a long history in China, but traditional Chinese medicine has failed to be recognized in the medical field due to the complexity of Chinese herbal medicinal ingredients and unclear antitumor mechanism. Thus, there are several restrictions in conducting clinical trials for traditional Chinese remedies (5,6). In recent years, several studies have been conducted on the adverse side effects associated with semi-synthetic and synthetic chemotherapy drugs used for treatment of tumors. As a result, there is interest in research on the natural antitumor compounds extracted from Chinese medicines. The Garcinia plants are known for their therapeutic effects and there is great interest in the medical effects of Garcinia. There are studies on using Garcinia for its anti-HIV, antibiosis, antioxidation, anti-inflammation, anti-malarial, insecticide and antitumor activities (7–10). Morelloflavone substances in the Garcinia plant have been shown to have broad-spectrum antitumor effects, although their action mechanism remains to be determined (11).
We extracted various plant substances from Garcinia plants and then screened the flavonoid substances using LC-MS. The specific structure of these compounds was analyzed using nuclear magnetic resonance and the effects of different compounds on glioma U87 cells and rat glioma C6 cells were investigated. We also analyzed the relationship between the structure of compounds and their anti-glioma effects. This study provides a theoretical reference for further research on anti-glioma agents.
Materials and methods
Experimental materials
The materials used for the present study were: Ethyl alcohol, methyl alcohol (chromatographic grade; Sinopharm Chemical Ltd., Shanghai, China); ultra-pure water (Milli-Q); gambogic tree trunk; deuterated methanol, dimethyl sulfoxide (DMSO); and human glioma U87 and rat glioma C6 cells (ATCC).
Experimental method
Extraction of active substances of gambogic tree trunk
Gambogic tree trunk extract (50.00 g) was broken into pieces and 70% of ethyl alcohol was added. It was transferred to a water bath (80°C) for 8 h, and the leaching liquid was collected and the supernatant after the leaching liquid was centrifuged at 2,000 × g for 15 min and filtrated. A rotary evaporator was used to remove the solvent for freeze-drying, and it was stored at −20°C.
Chromatographic separation of gambogic leaching liquid
Based on Waters preparative high performance liquid chromatography (Pre-HPLC), the XBridge C18 (5 µm, 21.2×250 mm) chromatographic column (Waters, Milford, MA, USA) was used to isolate the gambogic leaching liquid. Conditions for chromatography were as follows: Column temperature was 40°C; flow velocity was 4 ml/min; mobile A phase was methyl alcohol, mobile B phase was 0.1% formic acid aqueous solution, pH 5.0; time 0 min, A (40%), B (60%); 3 min, A (40%), B (60%); 25 min, A (40%), B (60%); 27 min, A (100%), B (0%); 37 min, A (40%), B (60%); and 45 min, A (40%), B (60%). The effluent components were collected according to the chromatographic peak and C18 solid phase extraction column was used to desalt each component. Components were freeze-dried by degreasing solvent and stored at −20°C.
Screening the biflavone substances
High resolution mass spectrometry (HRMS TOF; Waters, Milford, MA, USA) was employed to identify the collected components. Each component was dissolved in the methyl alcohol by connecting the HRMS to the ultra-performance liquid chromatography (UPLC; Waters). Waters BEH C18 (1.7 µm, 2×150 mm) chromatographic column was used for injecting samples directly and each component was identified with HRMS (MassLynx 4.1 analysis method) and by consulting literature and comparing CAS database.
Structure identification
The biflavone substances were prepared again to make the quantity of biflavone substances to reach the concentration necessary for nuclear magnetic resonance. Magnetic resonance spectroscopy (NMR; Bruker ARX 400; Karlsruhe, Germany) was used to confirm and analyze their structure. The sample solvent of nuclear magnetic resonance was CD3OD and the internal sample standard was TMS. Data acquired were analyzed by MestreNova 4.1 (San Diego, CA, USA).
Inhibition rate of U87 cells of biflavone substances by MTT detection
The biflavone substances with different structures were dissolved in DMSO, and the compound concentration was 0, 10, 20, 40 and 80 µmol/l. The sample of 0 µmol/l was taken as the blank control. The specific experimental operation methods were similar to those reported by Vandamme et al (12).
The calculation method for obtaining the inhibition rate was: IR inhibition rate (%) = (1 - OD value of experimental port/OD value of blank control port) × 100%. The mean of the inhibition rate was the end value and the inhibition rate of blank control port was 0 according to the calculation.
Preparation of glioma animal model
To prepare rat glioma cell C6 xenograft tumor model we followed the method reported by Li et al (13) with a slight modification. Approval for the animal experiments was received from The First Affiliated Hospital of Liaoning Medical University (Liaoning, China).
Statistical analysis
SPSS 18.0 statistical software (Chicago, IL, USA) was used to conduct statistical analysis. The mean ± standard deviation (SD) was used to record the independent experimental data and the comparison among various groups was analyzed using one-way ANOVA. Inter-group comparisons were tested by t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
Pre-HPLC isolation of gambogic extraction solution
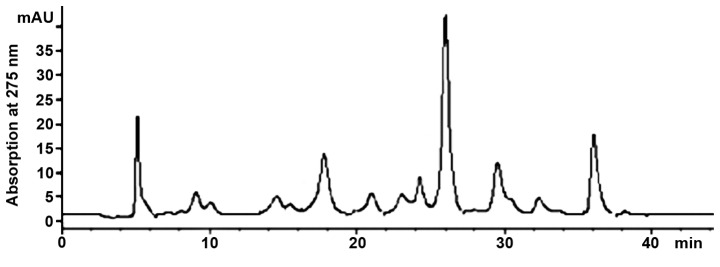
The ethanol water solution was used to extract the active ingredients from the gambogic tree trunk. Due to the fact that flavonoids contain benzene and pyranoid rings in their structure we used 275 nm wavelength to detect the ultraviolet absorption band. To ensure rapid isolation of flavonoids, the gradient elution method was used. The gradient of methanol started from 40% and gradually increased to 100% in order to elute all components. Results for final chromatographic separation are presented in Fig. 1. The chromatogram showed that the separation degree between the chromatographic peaks was improved. Components were eluted from chromatographic column and collected according to the chromatographic peaks (18 components in total). Due to the presence of formic acid in the mobile phase, collected components were desalinated and purified, and C18 solid phase extraction column was used to absorb active components. Ultra-pure water was then used to elute salts and impurities and finally the methanol was used to elute active substances from the solid phase extraction column. Active substances were freeze-dried after the solvent was removed and then stored at −20°C.
Figure 1.
Chromatographic separation of gambogic extraction solution.
Screening of biflavone substances
We used HRMS to analyze the structure of biflavones. Due to the fact that all 18 components mentioned in the present study were isolated and purified by liquid chromatography, the direct injection technique was used for HRMS. Components structures were determined according to the accurate molecular mass and ion fragments information, and finally the biflavone substances were determined by comparing our data with those available in the literature (14,15) and CAS database. Five biflavone substances were finally selected (Table I).
Table I.
HRMS information of biflavone substances.
| Substancesa | Retention time | Measured molecular mass | Actual molecular mass | Molecular formula | Error m/z (ppm) | Molecular fragment |
|---|---|---|---|---|---|---|
| 1 | 17.8 | 751.1505 | 751.1510 | C36H31O18 | −0.7 | 151.0031, 285.0392, 435.0708, 437.0866, 445.0556 |
| 2 | 18.6 | 735.1564 | 735.1561 | C36H31O17 | +0.4 | 125.0237, 269.0448, 403.0818, 429.0609, 447.0717 |
| 3 | 24.6 | 589.0972 | 589.0982 | C30H22O13 | −1.7 | 125.0237, 151.0028, 285.0393, 435.0713, 463.0660 |
| 4 | 26.6 | 573.1043 | 573.1033 | C30H22O12 | +1.7 | 125.0237, 296.0313, 419.0761, 447.0711 |
| 5 | 32.7 | 557.1084 | 557.1083 | C30H22O11 | +0.1 | 125.2038, 269.0443, 296.0317, 403.0813, 431.0764 |
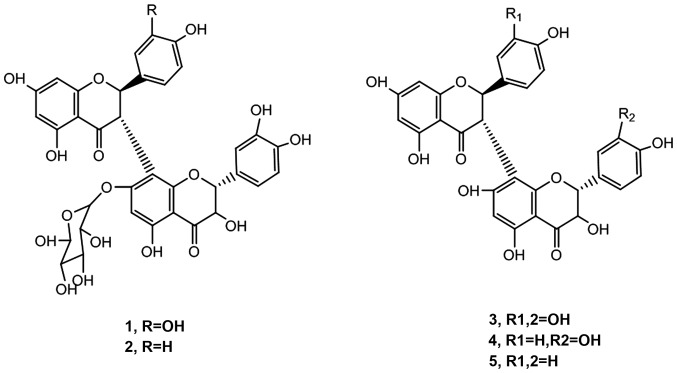
Corresponding to the structure of number of substances as shown in Fig. 2. HRMS, high resolution mass spectrometry.
Although HRMS is a fast and effective method to determine the molecular structure of different compounds, structure of biflavones cannot be completely determined just by mass spectrometry. In order to determine the specific structure and position of each functional group they should be analyzed by nuclear magnetic resonance. Therefore, we used a relatively HRMS and prepared large quantities of samples to for nuclear magnetic resonance.
Structure determination of biflavone substances by nuclear magnetic resonance
Five biflavone substances were dissolved in CD3OD and 1H and 13C spectrum was then analyzed by nuclear magnetic resonance and the specific structures of these compounds were determined by comparing them with literature (16–18) and spectrum database (Fig. 2). After determination of the biflavone molecular structures, we investigated the inhibition effects of these substances on glioma and then analyzed the possible relationship between the structure and the function.
Figure 2.
Structure information of biflavone substances.
Inhibition effect on U87 cells
Different concentrations of each biflavone substance were added to cells and after 24 h the inhibition rate on glioma U87 cells was determined (Table II). Our results showed that the five biflavone substances demonstrated some inhibitory activities on U87 glioma cells. The inhibition rate of compound no. 1 was the highest followed by compound no. 2. The inhibition rate of compound no. 3 had no significant difference from that of compound no. 4, while the inhibition rate of compound no. 5 was the lowest.
Table II.
Proliferation inhibition effect of biflavone substances on glioma U87 cell (%, mean ± SD).
| Concentration (µmol/l) | Compound 1 | Compound 2 | Compound 3 | Compound 4 | Compound 5 |
|---|---|---|---|---|---|
| 0 | 0.13±0.11 | 0.14±0.09 | 0.08±0.12 | 0.11±0.10 | 0.09±0.15 |
| 10 | 12.24±0.24 | 14.10±0.55 | 6.75±0.41 | 6.12±0.33 | 2.23±0.54 |
| 20 | 19.78±0.28 | 17.64±0.86 | 8.17±0.96 | 8.43±0.35 | 1.85±0.21 |
| 40 | 25.66±0.68 | 32.73±0.94 | 12.31±0.68 | 11.92±0.68 | 4.31±0.35 |
| 80 | 55.64±0.65a | 49.82±0.91a,b | 21.17±0.84a–c | 22.78±0.75a–c | 9.87±0.66a–d |
As for the comparison on single factor inter-class variance, the same letters (P>0.05), indicate that there is no significant difference; the different letters (P<0.05), indicate that there is a significant difference.
Inhibition of five biflavone substances on cell C6
To verify the inhibition activity of biflavone substances on xenograft tumor cells, the axillary xenograft tumor model of rat glioma C6 cells was used. Rats were injected with 1 mg/kg of cisplatin and 800 µg/kg of biflavone on the seventh day after the inoculation of glioma C6 cells. The results are presented in Table III.
Table III.
Inhibition effect of biflavone substances on the growth of rat glioma cell C6 xenograft tumors.
| Groups | Dosage (µg/kg) | Weight (g) | Tumor change (g) | Inhibition rate (%) |
|---|---|---|---|---|
| Control | 0 | 7.13 | 2.33±1.11 | 0 |
| Compound 1 | 800 | 5.62 | 0.95±0.55 | 66.73±0.12a |
| Compound 2 | 800 | 6.77 | 1.23±0.62 | 65.21±0.35a |
| Compound 3 | 800 | 4.89 | 2.28±0.47 | 52.12±0.51a,b |
| Compound 4 | 800 | 5.12 | 2.59±0.41 | 53.31±0.28a,b |
| Compound 5 | 800 | 6.08 | 4.41±0.53 | 31.84±0.33a–c |
Compared with the control, P<0.05.
Compared with compound 1, P<0.05.
Compared with compound 3, P<0.05.
Our results showed that the inhibition activity of biflavone substances on glioma C6 cells was similar to the inhibition effect in the case of U87 cells. Compound no. 1 was the most potent compound among the five. Compound no. 2 was the second and compound no. 3 had no significant difference compared to compound no. 4. The inhibition rate of compound no. 5 was the lowest.
Discussion
Glioma is one of the most common malignant tumors occurring in the central nervous system, and the incidence of glioma accounts for 50–60% of brain tumors (19,20). Although surgery remains the method of choice for treating malignant glioma, the efficacy of surgery has not greatly improved in recent years. Thus, the trial of drug therapy or assisted surgery therapy has a certain practical significance and broad applications. There are studies on adverse effects associated with synthetic and semi-synthetic drugs used in chemotherapy, therefore people are gradually paying more attention to the research on natural antitumor compounds extracted from traditional Chinese medicine. As type of traditional Chinese medicine, the Garcinia has various types of medical effects and the biflavone substances that the carcinia contains have broad-spectrum antitumor effects, but the action mechanism of the these biflavone substances are still unclear.
Our results showed that the inhibition effects of biflavone substances on glioma U87 and C6 cells were alike. The inhibition effect of compound no. 1 was the strongest followed by compound no. 2 whose inhibition effect was significantly greater than that of compounds nos. 3–5. From studying the structure of all five compounds we detected the presence of a glucose chain in the structure of compound nos. 1 and 2. The preliminary structure-function relationship showed that the circumscribed glucose chain in the biflavone compounds could enhance the inhibition effect. It is possible that the glucose chain was responsible for these adverse effect on the cell DNA (21).
Compound no. 5, had R1=R2=H, while compounds nos. 1–4 R1=R2=hydroxyl group. It was hypothesized that the reduction of hydroxyl group in biflavone substances may reduce their inhibition effects. Additionally, the inhibition activity of compound no. 2 was significantly lower than that of compound no. 1. The existence of hydroxyl group can stimulate the formation of external as well as internal hydrogen bonds within and between molecules and indirectly enhance the interaction between molecules and improved the inhibition rate on glioma.
In future, more biflavone substances will be isolated and screened from different parts of Garcinia plants and their anti-tumoral activity will be verified. This may lay a more solid foundation for the further verification and exploration of the possible link between biflavone structure and its function.
References
- 1.Johnson BE, Mazor T, Hong C, Barnes M, Aihara K, McLean CY, Fouse SD, Yamamoto S, Ueda H, Tatsuno K, et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2014;343:189–193. doi: 10.1126/science.1239947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee Titsworth W, Murad GJ, Hoh BL, Rahman M. Fighting fire with fire: the revival of thermotherapy for gliomas. Anticancer Res. 2014;34:565–574. [PubMed] [Google Scholar]
- 3.Mirimanoff RO. High-grade gliomas: reality and hopes. Chin J Cancer. 2014;33:1–3. doi: 10.5732/cjc.013.10215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giovagnoli AR, Meneses RF, Silvani A, Milanesi I, Fariselli L, Salmaggi A, Boiardi A. Quality of life and brain tumors: what beyond the clinical burden? J Neurol. 2014;261:894–904. doi: 10.1007/s00415-014-7273-3. [DOI] [PubMed] [Google Scholar]
- 5.Ji B, Shi J, Cheng X, Zhou J, Zhou Q, Cao C, Pang J. Association analysis of two candidate polymorphisms in the tumour necrosis factor-α gene with osteoarthritis in a Chinese population. Int Orthop. 2013;37:2061–2063. doi: 10.1007/s00264-013-1931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin SZ. Era of stem cell therapy for regenerative medicine and cancers: an introduction for the special issue of Pan Pacific Symposium on Stem Cells and Cancer Research. Cell Transplant. 2015;24:311–312. doi: 10.3727/096368915X686814. [DOI] [PubMed] [Google Scholar]
- 7.Boakye PA, Brierley SM, Pasilis SP, Balemba OB. Garcinia buchananii bark extract is an effective anti-diarrheal remedy for lactose-induced diarrhea. J Ethnopharmacol. 2012;142:539–547. doi: 10.1016/j.jep.2012.05.034. [DOI] [PubMed] [Google Scholar]
- 8.Castro J, Balemba OB, Blackshaw LA, Brierley SM. Garcinia buchananii bark extract inhibits nociceptors, with greater efficacy during inflammation. Gastroenterology. 2011;140(Suppl 1):S866. [Google Scholar]
- 9.Kisangau DP, Lyaruu HV, Hosea KM, Joseph CC. Use of traditional medicines in the management of HIV/AIDS opportunistic infections in Tanzania: a case in the Bukoba rural district. J Ethnobiol Ethnomed. 2007;3:29–37. doi: 10.1186/1746-4269-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye ZN, Yu MY, Kong LM, Wang WH, Yang YF, Liu JQ, Qiu MH, Li Y. Biflavone Ginkgetin, a novel Wnt inhibitor, suppresses the growth of medulloblastoma. Nat Prod Bioprospect. 2015;5:91–97. doi: 10.1007/s13659-015-0056-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pang X, Yi T, Yi Z, Cho SG, Qu W, Pinkaew D, Fujise K, Liu M. Morelloflavone, a biflavonoid, inhibits tumor angiogenesis by targeting rho GTPases and extracellular signal-regulated kinase signaling pathways. Cancer Res. 2009;69:518–525. doi: 10.1158/0008-5472.CAN-08-2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vandamme M, Robert E, Pesnel S, Barbosa E, Dozias S, Sobilo J, Lerondel S, Le Pape A, Pouvesle JM. Antitumor effect of plasma treatment on U87 glioma xenografts: preliminary results. Plasma Process Polym. 2010;7:264–273. doi: 10.1002/ppap.200900080. [DOI] [Google Scholar]
- 13.Li XQ, Ouyang ZG, Zhang SH, Liu H, Shang Y, Li Y, Zhen YS. Synergistic inhibition of angiogenesis and glioma cell-induced angiogenesis by the combination of temozolomide and enediyne antibiotic lidamycin. Cancer Biol Ther. 2014;15:398–408. doi: 10.4161/cbt.27626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stark TD, Losch S, Frank O, Balemba OB, Hofmann T. Purification procedure for (2R,3S,2′R,3′R)-manniflavanone and its minor (2R,3S,2′S,3′S)-isomer from Garcinia buchananii stem bark extract. Eur Food Res Technol. 2015;240:1075–1080. doi: 10.1007/s00217-014-2411-9. [DOI] [Google Scholar]
- 15.Xu G, Kan WL, Zhou Y, Song JZ, Han QB, Qiao CF, Cho CH, Rudd JA, Lin G, Xu HX. Cytotoxic acylphloroglucinol derivatives from the twigs of Garcinia cowa. J Nat Prod. 2010;73:104–108. doi: 10.1021/np9004147. [DOI] [PubMed] [Google Scholar]
- 16.Bing L, Gao-Sheng H, Ling-Ling H, He-Yu L, Jing-Ming J. Extraction and determination of biflavone components in ginkgo leaves. Chinese Herbal Med. 2014;17:2014. [Google Scholar]
- 17.Xueping J, Keli C. Research on biflavone compounds in Selaginella moellendorfii Hieron. Chin J Pharmaceuticals. 2009;1:2009. [Google Scholar]
- 18.Lianzhu Z. Treatment and possible effect of total flavonoids of Selaginella tamariscina and amentoflavone in cognitive disorder model (unpublished PhD dissertation) Jilin University. 2013 [Google Scholar]
- 19.Lipeng L. Experimental research on the treatment of C6brain glioma by nimustine combined with cisplatin (unpublished Master dissertation) Tianjin Medical University. 2008 [Google Scholar]
- 20.Flavahan WA, Drier Y, Liau BB, Gillespie SM, Venteicher AS, Stemmer-Rachamimov AO, Suvà ML, Bernstein BE. Insulator dysfunction and oncogene activation in IDH mutant gliomas. Nature. 2016;529:110–114. doi: 10.1038/nature16490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]