Abstract
Small ubiquitin-like modifier (SUMO)/sentrin-specific protease 1 (SENP1), a member of the SENP family, is highly expressed in several neoplastic tissues. However, the effect of SENP1 in acute promyelocytic leukemia (APL) has not been elucidated. In the present study, it was observed that SENP1 deficiency had no effect on the spontaneous apoptosis or differentiation of NB4 cells. Arsenic trioxide (As2O3) could induce the upregulation of endoplasmic reticulum (ER) stress, resulting in the apoptosis of NB4 cells. Additionally, knockdown of SENP1 significantly increased As2O3-induced apoptosis in NB4 cells transfected with small interfering RNA targeting SENP1. SENP1 deficiency also increased the accumulation of SUMOylated X-box binding protein 1 (XBP1), which was accompanied by the downregulation of the messenger RNA expression and transcriptional activity of the XBP1 target genes endoplasmic reticulum-localized DnaJ 4 and Sec61a, which were involved in ER stress and closely linked to the apoptosis of NB4 cells. Taken together, these results revealed that the specific de-SUMOylation activity of SENP1 for XBP1 was involved in the ER stress-mediated apoptosis caused by As2O3 treatment in NB4 cells, thus providing insight into potential therapeutic targets for APL treatment via manipulating XBP1 signaling during ER stress by targeting SENP1.
Keywords: SENP1, acute promyelocytic leukemia, As2O3, SUMOylation, XBP1, apoptosis
Introduction
Acute myeloid leukemia (AML) is composed of a group of diseases with marked morphological and cytogenetic heterogeneity that account for ~20% of childhood and adolescent acute leukemias (1,2). Acute promyelocytic leukemia (APL) is considered a distinct subtype of AML, with a particular cytological morphology (M and M3 variant in the French-American-British classification) (3). APL is characterized by a specific chromosome translocation, t(15;17), which results in the rearrangement of the promyelocytic leukemia (PML) gene and the retinoic acid receptor α (RARα) gene, followed by the expression of the PML-RARα chimeric protein (4). Despite the fact that majority of APL cases can achieve complete remission when treated with conventional chemotherapy by all-trans retinoic acid (ATRA) and/or arsenic trioxide (As2O3) (5–8), certain clinical cases exhibit acquired resistance and APL relapse (5,6,8), and the detailed molecular mechanisms have not been completely elucidated.
The small ubiquitin-like modifier (SUMO)/sentrin-specific protease (SENP) family includes six SENPs named SENP1, SENP2, SENP3, SENP5, SENP6 and SENP7, which have different substrate specificities and subcellular localizations in mammalian cells (9). It has been reported that SENP1 is important in placental development and erythropoiesis (10,11). In addition, SENP1 could control adipocyte differentiation via de-SUMOylation modification (12), and is important in protecting against myocardial ischemia/reperfusion injury via a hypoxia-inducible factor 1α-dependent pathway (13). Numerous studies have demonstrated that SENP1 is overexpressed and contributes significantly to the development and progression of tumors, including prostatic intraepithelial neoplasia, colon cancer and pancreatic cancer (14–16). Previous studies have revealed that deficiency or downregulation of SENP1 could induce radiosensitization in lung cancer cells (17), promote endoplasmic reticulum (ER) stress-induced apoptosis in MEF and HEK 293T cells (18), and increase apoptosis in Burkitt lymphoma cells (19). Furthermore, SENP1 is also involved in the formation of PML protein nuclear bodies (20,21), which were originally characterized as part of a fusion protein with PML-RARα in patients with APL (22). However, whether SENP1 is a potential drug target for APL treatment still remains unclear.
ER stress has been implicated in diverse diseases, including cancer, diabetes, cerebral ischemia, and neurodegenerative and cardiovascular diseases (23,24). In addition to the intrinsic and extrinsic apoptosis pathways, ER stress-induced apoptosis has recently been reported as another major pathway mediating cell apoptosis (25), and is involved in arsenic-induced apoptosis of osteoblasts, myoblasts, pancreatic cells, myeloma cells, and drug-sensitive and drug-resistant leukemia cells (26–30). It has been suggested that As2O3 can induce human lens cell apoptosis by the ER stress-initiated process (31).
In the present study, the NB4 cell line (an APL cell line from a relapsed APL patient) was utilized as an in vitro model (32). It was demonstrated that As2O3 could induce ER stress-initiated apoptosis in NB4 cells, which was significantly upregulated by SENP1 knockdown. In addition, it was observed that SENP1 specifically de-SUMOylated X-box binding protein 1 (XBP1) and played a critical role during As2O3-induced ER stress. Taken together, our results revealed the roles of SENP1 in APL and the potential effects of clinical APL treatment by targeting SENP1.
Materials and methods
Antibodies and reagents
RPMI-1640 medium, Dulbecco's modified Eagle medium, trypsin and TRIzol were obtained from Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Puromycin and fetal bovine serum (FBS) were purchased from Gibco (Thermo Fisher Scientific, Inc.). As2O3 was provided by Beijing Shuanglu Pharmaceutical Co., Ltd. (Beijing, China). Radioimmunoprecipitation assay lysates were purchased from Beyotime Institute of Biotechnology (Haimen, China). ATRA, phenylmethylsulfonyl fluoride, aprotinin, leupeptin and pepstatin were acquired from Sigma-Aldrich (Merck Millipore, Darmstadt, Germany). Protein A/G PLUS-Agarose was obtained from Roche Diagnostics (Indianapolis, IN, USA). Anti-XBP1 antibody (cat. no. H00007494-D01) and anti-SUMO-1 antibody (cat. no. AJ1746a) were purchased from Abnova (Taipei City, Taiwan) and Abgent Biotech Co., Ltd. (Suzhou, China), respectively. The FITC Annexin V Apoptosis Detection kit and the anti-cluster of differentiation (CD) 11b antibody (cat. no. C09-550019) were commercially available from BD Pharmingen (San Diego, CA, USA). The PrimeScript RT reagent kit and SYBR Green PCR Master Mix were commercially available from Takara Bio, Inc. (Otsu, Japan).
Cell culture
Human APL NB4 cells (American Type Culture Collection, Manassas, VA, USA) were suspended at 5×105 cells/ml in RPMI-1640 medium supplemented with 10% FBS. Retrovirus containing SENP1 small interfering RNA (siRNA) or nonspecific control (NC) siRNA, as described previously (10), was transfected into NB4 cells to generate si-SENP1-transfected NB4 cells (si-SENP1) or NC siRNA-transfected cells (si-NC) upon puromycin (0.75 µg/ml) selection. All cells were cultured in RPMI-1640 medium with 10% FBS at 37°C in 5% CO2.
As2O3 and ATRA treatment
NB4 cells were seeded into a 6-well plate and then incubated with As2O3 (1 µM) or ATRA (1 µM) for different time periods, as indicated. A total of 106 cells were harvested at different time points subsequent to As2O3 or ATRA treatment.
Flow cytometry
For apoptosis assay, cells treated with As2O3 at each indicated time point were washed twice with ice-cold phosphate-buffered saline, and the apoptotic cells were detected with a flow cytometer (Merck Millipore) using the FITC Annexin V Apoptosis Detection kit according to the manufacturer's protocol. For CD11b assay, cells were incubated with fluorescein isothiocyanate (FITC)-conjugated anti-CD11b antibody (1:100) after treatment with ATRA for 24 h. Appropriate isotype control IgG2b antibodies (cat. no. NB810-82278; Novus Biologicals LLC, Littleton, CO, USA) were used. The percentage of apoptotic cells and the differentiation marker of cell surface expression were analyzed using Guava 1.0 software (Guava Technologies, Inc., Hayward, CA, USA).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated with TRIzol, and complementary DNA was synthesized using TaKaRa RNA PCR kit (Takara Bio, Inc.) according to the manufacturer's protocol. The sequences of the PCR primers used in the amplification of the target genes are shown in Table I. RT-qPCR was performed with the double-stranded DNA dye SYBR Green PCR core reagents using the ABI ViiA 7 system (PerkinElmer, Inc., Waltham, MA, USA). Thermal cycler conditions were 95°C for 30 sec, and 40 cycles of 95°C for 5 sec and 60°C for 30 sec. Relative gene expression was determined by the delta delta Cq method (Applied Biosystems; Thermo Fisher Scientific, Inc.), with β-actin as the endogenous control using the Applied Biosystems ViiA™ 7 Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.) (16). Three independent experiments were performed, and the standard deviations (SDs) representing experimental errors were calculated. All data were analyzed using GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA).
Table I.
Sequences of the primers used in the amplification of the target genes.
| Locus | Primers (5′-3′) |
|---|---|
| β-actin | (F) CTTTTCCAGCCTTCCTTCTTGG |
| (R) CAGCACTGTGTTGGCATAGAGG | |
| SENP1 | (F) ATCAGGCAGTGAAACGTTGGAC |
| (R) ATCAGGCAGTGAAACGTTGGAC | |
| GADD153 | (F) GAAACGGAAACAGAGTGGTCATTCCCC |
| (R) GTGGGATTGAGGGTCACATCATTGGCA | |
| ATF6 | (F) ATGAAGTTGTGTCAGAGAAC |
| (R) GGGTGCTATTGTAATGACTCA | |
| GRP78 | (F) AGTTGATATTGGAGGTGGGC |
| (R) CATTGAAGTAAGCTGGTACAGTAAC | |
| Erdj4 | (F) CCCCAGTGTCAAACTGTACCAG |
| (R) AGCGTTTCCAATTTTCCATAAATT | |
| Sec61a | (F) CTATTTCCAGGGCTTCCGAGT |
| (R) AGGTGTTGTACTGGCCTCGGT | |
| Edem | (F) AAGCCCTCTGGAACTTGCG |
| (R) AACCCAATGGCCTGTCTGG |
F, forward; R, reverse; SENP1, Small ubiquitin-like modifier/sentrin-specific protease 1; GADD153, growth arrest and DNA damage inducible protein 153; ATF6, activating transcription factor 6; GRP78, 78 kDa glucose-regulated protein; ErdJ4, ER-localized DnaJ 4; Edem, ER degradation enhancer, mannosidase; ER, endoplasmic reticulum.
Immunoprecipitation
Treated cells were lysed with lysis buffer, and immunoprecipitation using anti-XBP1 and anti-SUMO-1 antibodies was performed as previously reported (33,34). Briefly, cell lysates were centrifuged at 10,800 × g for 15 min at 4ºC, and the supernatant was collected. Protein concentrations were determined by means of Lowry protein assay. Equal amounts of protein samples (1 mg) were incubated with 2 µg antibody for 3 h at 4ºC, followed by addition of pre-equilibrated Protein A/G PLUS-Agarose beads (20 µl) and incubation overnight. The immunoprecipitates were washed four times with lysis buffer, and the bound proteins were boiled in 15 µl 2X sodium dodecyl sulfate (SDS) sample buffer, eluted and resolved on 10% SDS-polyacrylamide gel electrophoresis. The proteins were transferred onto a polyvinylidene fluoride membrane and probed with horseradish peroxidase (HRP)-phytohemagglutinin-L (Thermo Fisher Scientific, Inc.) and HRP-concanavalin A lectin (Abnova), and then detected using an enhanced chemiluminescence kit (Pierce Biotechnology, Inc., Rockford, IL, USA).
Statistical analyses
The data were presented as the mean ± SD. Statistical analysis was performed using GraphPad Prism 5 software. The paired t-test was used to compare the difference between two different groups. P<0.05 was considered to indicate a statistically significant difference.
Results
SENP1 deficiency has no effect on the spontaneous apoptosis or differentiation of NB4 cells
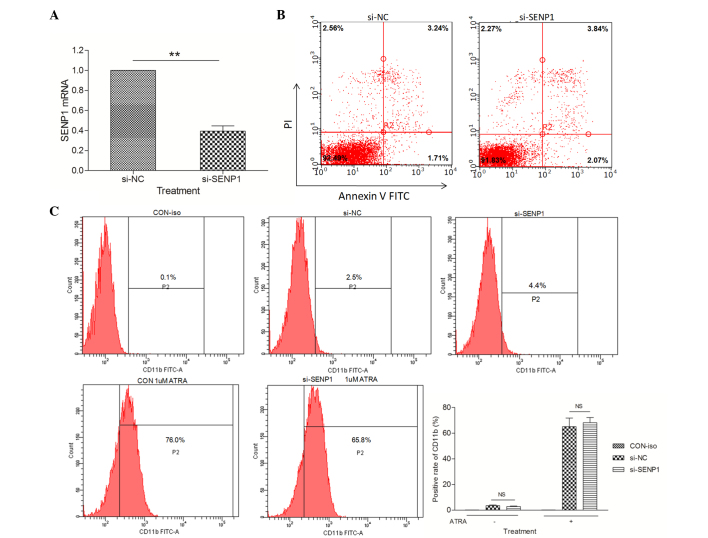
The fact that SENP1 was overexpressed in several neoplastic tissues (14–16) raised the question whether SENP1 plays any role in the pathogenesis of APL. Thus, a SENP1-knockdown NB4 cell line (si-SENP1) was generated by stably transfecting SENP1 siRNA into NB4 cells. Compared with the NB4 cells transfected with NC scrambled siRNA (si-NC), the messenger RNA (mRNA) expression of SENP1 was significantly downregulated to ~45% in si-SENP1 NB4 cells (Fig. 1A). A series of flow cytometry assays were performed to explore whether SENP1 deficiency would promote spontaneous apoptosis and differentiation in NB4 cells. Firstly, the percentage of apoptotic si-NC and si-SENP1 NB4 cells was examined by flow cytometry following annexin V-FITC and propidium iodide (PI) staining. As shown in Fig. 1B, knockdown of SENP1 had no effect on the spontaneous apoptosis of NB4 cells compared with si-NC cells. Next, the effects of SENP1 deficiency on NB4 cell differentiation were examined by detecting the expression levels of a differentiation marker, CD11b. The results from cytometry revealed no difference in the expression of CD11b between si-NC and si-SENP1 NB4 cells, even after treatment with 1 µΜ ATRA (a differentiation inducing agent) for 24 h (Fig. 1C). These results suggested that the downregulation of SENP1 has no effect on the spontaneous apoptosis or differentiation of NB4 cells.
Figure 1.
SENP1 deficiency has no effect on the spontaneous apoptosis or differentiation of NB4 cells. (A) mRNA expression level of SENP1 in cells transfected with si-SENP1 and cells transfected with si-NC. (B) Spontaneous apoptosis of NB4 cells transfected with si-SENP1 and si-NC. (C) Positive expression rate of CD11b in NB4 cells induced by ATRA for 72 h. **P<0.01, t-test. SENP1, small ubiquitin-like modifier/sentrin-specific protease 1; mRNA, messenger RNA; NC, nonspecific control; si, small interfering; PI, propidium iodide; FITC, fluorescein isothiocyanate; CD, cluster of differentiation; ATRA, all-trans retinoic acid; CON, control; iso, isotype; NS, not significant.
SENP1 deficiency promotes As2O3-induced NB4 cell apoptosis
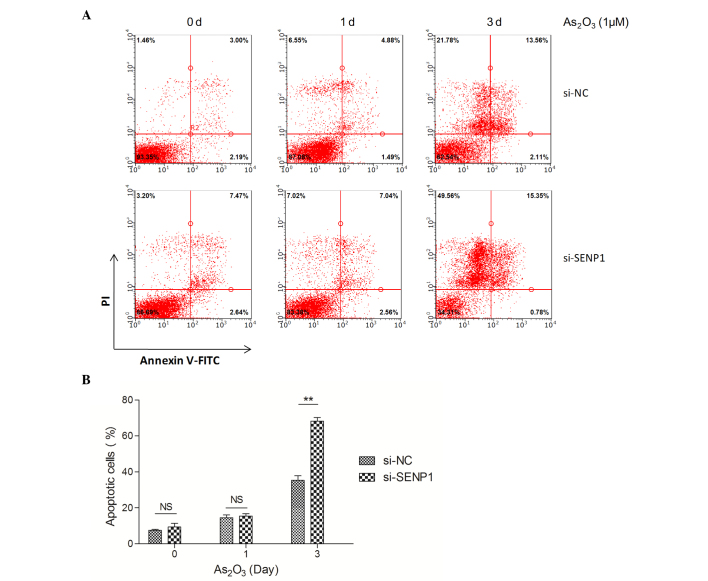
As2O3 has been successfully used in the treatment of APL (7,35,36), and therapeutic doses of As2O3 could effectively induce complete molecular remission in vivo and trigger the apoptotic death of APL cells (37). Thus, we sought to investigate if the knockdown of SENP1 affects As2O3-induced apoptosis in NB4 cells. Therefore, normal and SENP1 knocked down NB4 cells treated with As2O3 were collected and subjected to flow cytometry assay upon annexin V-FITC and PI staining. As shown in Fig. 2A, after treatment with As2O3 for 3 days, the apoptotic cells in the si-NC NB4 group only increased by 2.90-fold (from 12.92±1.00 to 37.00±2.00%), while in the si-SENP1 NB4 group, the apoptotic cells increased by ≤4.00-fold (from 16.62±1.00 to 66.00±3.00%). The total number of apoptotic cells was ~30% in si-SENP1 cells compared with that in si-NC cells (Fig. 2B), suggesting that SENP1 is essential for NB4 cell survival during treatment with As2O3.
Figure 2.
SENP1 deficiency promotes As2O3-induced NB4 cell apoptosis. (A) Apoptosis of the si-NC and si-SENP1 NB4 cells was analyzed by flow cytometry. (B) The number of apoptotic si-SENP1 cells was significantly higher than that of the si-NC cells, following treatment with As2O3 for 3 d.**P<0.01, t-test. SENP1, small ubiquitin-like modifier/sentrin-specific protease 1; NC, nonspecific control; si, small interfering; PI, propidium iodide; FITC, fluorescein isothiocyanate; NS, not significant; As2O3, arsenic trioxide; d, day.
SENP1 regulates the expression of XBP1 target genes in the ER stress-mediated apoptotic pathway
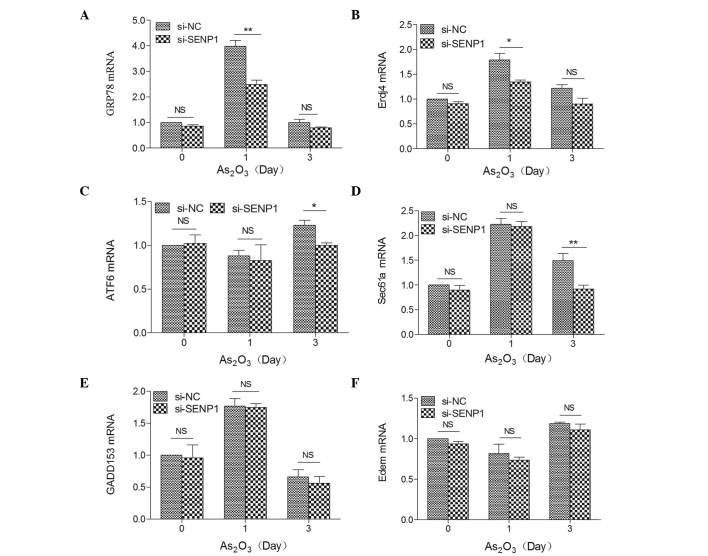
Numerous studies have revealed that As2O3 induces cell apoptosis through the ER stress pathway (28,29,31). Thus, we assumed that As2O3-induced apoptosis may also accompany ER stress. Several key players, including CCAAT-enhancer-binding protein homologous protein [also known as growth arrest and DNA damage inducible protein 153 (GADD153)], activating transcription factor 6 (ATF6) and 78 kDa glucose-regulated protein (GRP78), as well as XBP1 target genes, including ER-localized DnaJ 4 (Erdj4), Sec61a and ER degradation enhancer, mannosidase (Edem), are common markers that reflect the unfolded protein response during ER stress (38,39). Therefore, to investigate the effects of SENP1 on the ER stress-mediated apoptotic pathway, the mRNA expression levels of the above proteins were detected by RT-qPCR in si-NC and si-SENP1 NB4 cells subsequent to treatment with As2O3 for different time periods. As shown in Fig. 3, the mRNA expression levels of these markers were highly increased by ~4.00-fold (GRP78), 1.77-fold (GADD153), 1.80-fold (Erdj4) and 1.80-fold (Sec61a) in si-NC cells after As2O3 treatment for 1 day, but no effect on ATF6 or Edem (Fig. 3C and F) were observed, and upregulation was also observed in si-SENP1 NB4 cells, which revealed that As2O3 induced NB4 cell apoptosis through the ER stress-mediated apoptotic pathway. Additionally, among the XBP1 target genes, the mRNA expression levels of Erdj4 and Sec61a were significantly reduced by knockdown of SENP1 following As2O3 treatment in si-SENP1 NB4 cells (Fig. 3B and D).
Figure 3.
Expression levels of ER stress-related key markers (A) GRP78, (B) Erdj4, (C) ATF6, (D) Sec61a, (E) GADD153 and (F) Edem in SENP1-deficient cells treated with As2O3. **P<0.01; *P<0.05, t-test. SENP1, small ubiquitin-like modifier/sentrin-specific protease 1; GADD153, growth arrest and DNA damage inducible protein 153; ATF6, activating transcription factor 6; GRP78, 78 kDa glucose-regulated protein; ErdJ4, ER-localized DnaJ 4; Edem, ER degradation enhancer, mannosidase; NC, nonspecific control; si, small interfering; NS, not significant; As2O3, arsenic trioxide; mRNA, messenger RNA; ER, endoplasmic reticulum.
SENP1 deficiency upregulates XBP1 SUMOylation in si-SENP1 NB4 cells
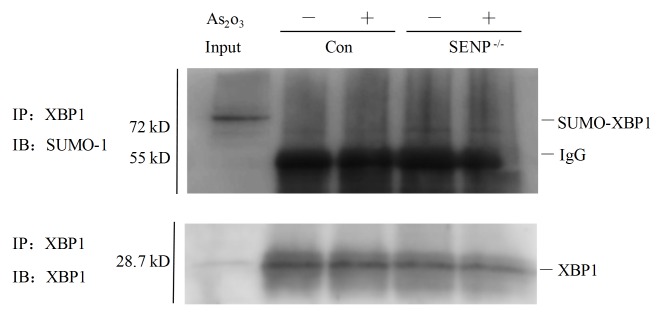
Since the mRNA expression levels of XBP1 target genes, including Erdj4 and Sec61a, were significantly reduced in si-SENP1 NB4 cells upon As2O3 treatment, the present study next sought to explore whether XBP1 could be SUMOylated in si-SENP1 NB4 cells. As shown in Fig. 4, in contrast to si-NC cells (Fig. 4, lanes 2 and 3), XBP1 was partly SUMOylated following immunoprecipitation with an anti-SUMO-1 antibody in si-SENP1 NB4 cells (Fig. 4, lanes 4 and 5). The input sample for immunoprecipitation was directly loaded to reveal the appropriate location of the protein bands of interest (Fig. 4, lane 1). These findings suggested that SENP1 deficiency may downregulate XBP1 transactivation by increasing XBP1 SUMOylation.
Figure 4.
SENP1 deficiency upregulates XBP1 SUMOylation in NB4 si-NC (lanes 2 and 3) and si-SENP1 (lanse 4 and 5) cells treated with (lanes 3 and 5) or without (lanes 2 and 4) As2O3. SENP1, SUMO/sentrin-specific protease 1; SUMO, small ubiquitin-like modifier; XBP1, X-box binding protein 1; Con, control; IgG, immunoglobulin; IP, immunoprecipitation; IB, immunoblotting; As2O3, arsenic trioxide; si, small interfering.
Discussion
APL is characterized by a specific t(15;17) chromosomal translocation that yields the PML/RARA fusion gene (3). It has been reported that >97% of APL patients have the t(15;17) translocation, and several rare variant translocations observed in the remaining APL patients always involve RARα (3,40). As2O3 is an effective treatment for this disease, since it could induce SUMO-dependent ubiquitin-mediated proteasomal degradation of PML-RARα (41). However, knockdown of SENP1 had no effect on the SUMOylation of PML-RARα in NB4 cells, even after NB4 cells were treated with 1 µΜ As2O3 (data not shown), suggesting the possibility that other members of the SENP family may be involved in regulating the level of SUMOylated PML-RARα, which remains to be further investigated. It has been reported that knockdown of SENP1 could promote drug-induced cell apoptosis in MEF and HEK 293T cells, as well as lung cancer and Burkitt lymphoma cells (17–19). However, our results revealed that downregulation of SENP1 had no effect on the spontaneous apoptosis or differentiation of NB4 cells (Fig. 1). Furthermore, it was observed that downregulation of SENP1 had no impact on drug-induced apoptosis or differentiation in NB4 cells (Fig. 1).
The metabolism of As2O3 in APL cells is complex (42). Production of intracellular methylated metabolites and epigenetic changes of DNA methylation during As2O3 metabolism may contribute to the therapeutic efficacy of As2O3 in APL (43). The signaling cascades and transcription factors related to As2O3-induced apoptosis were caused by reactive oxygen species formation (44,45), resulting in the stimulation of apoptosis in leukemia cells. In contrast to the observation that inhibition of phosphoinositide 3-kinase (PI3K)/Akt could increase As2O3-induced apoptosis of APL cells (46,47), in the present study, it was observed that downregulation of SENP1 could promote As2O3-induced NB4 cell apoptosis, suggesting that there may be pathways other than PI3K/Akt contributing to the effect of SENP1 on As2O3-treated APL cells. Numerous studies have demonstrated that As2O3 could induce cell apoptosis through the ER stress pathway (28,29,31,48). Consistently, in our study, the upregulation of key markers of the ER stress-mediated apoptotic pathway in si-SENP1 NB4 cells indicated that As2O3 could induce NB4 cell apoptosis through the ER stress-mediated apoptotic pathway. Notably, the high ER stress induced by As2O3 treatment at day 1 in the present study led to significant cell apoptosis at day 3, which may be explained by the sustained effect of As2O3-induced apoptosis. SUMOylation is a dynamic process that is reversed by proteins of the SENP family, which can be catalyzed by SUMO-specific activating (E1), conjugating (E2) and ligating (E3) enzymes (49). The modification of proteins with SUMO plays pivotal roles in modulating the activation, function and subcellular localization of these proteins (50). The transcription factor XBP1 is a key component of the ER stress response and a critical transcription factor that drives the transcription of various genes (51,52). Additionally, XBP1 can be activated in MEF and HEK 293T cells via the de-SUMOylation function of SENP1 (18). Therefore, it would be reasonable to assume the existence of an intrinsic mechanism by which the downregulation of SENP1 affects cell apoptosis in the treatment of APL with As2O3. Our data suggested that SENP1 regulated XBP1 transactivation by regulating the expression of XBP1 target genes, and that SENP1 may play a specific role in regulating XBP1 activity during As2O3-induced apoptosis. Of note, SENP1 downregulation also reduced the expression of the transcription factor GRP78, which was involved in the ER stress response after As2O3 treatment for 1 day (Fig. 3C), suggesting that SENP1 and its downstream signaling pathway may affect other transcription factors involved in ER stress.
Taken together, our study provided the first evidence for As2O3-induced NB4 cell apoptosis through the ER stress-mediated apoptotic pathway, and demonstrated that SENP1 downregulation promoted As2O3-induced NB4 cell apoptosis. The knockdown of SENP1 also increased the levels of accumulated SUMOylated XBP1, which was accompanied by downregulation of the mRNA expression and transcriptional activity of XBP1 target genes, which were involved in ER stress response and closely linked to the apoptosis of NB4 cells. Our data also provided insight into potential therapeutic targets for APL treatment via manipulating XBP1 signaling during ER stress by targeting SENP1.
Acknowledgements
The present study was supported by Shanghai Health Bureau of Scientific Research Projects (Shanghai, China; grant no. 20124Y110), the Scientific Research Foundation of XinHua Hospital Group (Shanghai, China; grant no. 12XJ22005) and the National Natural Science Foundation of China (Beijing, China; grant nos. 81401572 and 81201450, awarded to Q.C.).
References
- 1.Kömür M, Erbey F, Bayram I, Tanyeli A. Incidence and prognostic importance of molecular genetic defects in children with acute myeloblastic leukemia. Asian Pac J Cancer Prev. 2010;11:1393–1395. [PubMed] [Google Scholar]
- 2.Gilliland DG. Molecular genetics of human leukemia. Leukemia. 1998;12(Suppl 1):S7–S12. [PubMed] [Google Scholar]
- 3.Rowley JD, Golomb HM, Dougherty C. 15–17 translocation, a consistent chromosome change in acute promyelocytic leukemia. Lancet. 1977;1:549–50. doi: 10.1016/S0140-6736(77)91415-5. [DOI] [PubMed] [Google Scholar]
- 4.Melnick A, Licht JD. Deconstructing a disease: RARalpha, its fusion partners and their roles in the pathogenesis of acute promyelocytic leukemia. Blood. 1999;93:3167–3215. [PubMed] [Google Scholar]
- 5.Burnett AK, Grimwade D, Solomon E, Wheatley K, Goldstone AH. Presenting white blood cell count and kinetics of molecular remission predict prognosis in acute promyelocytic leukemia treated with all-trans retinoic acid: Result of the Randomized MRC Trial. Blood. 1999;93:4131–4143. [PubMed] [Google Scholar]
- 6.Fenaux P, Chastang C, Chevret S, Sanz M, Dombret H, Archimbaud E, Fey M, Rayon C, Huguet F, Sotto JJ, et al. A randomized comparison of all transretinoic acid (ATRA) followed by chemotherapy and ATRA plus chemotherapy and the role of maintenance therapy in newly diagnosed acute promyelocytic leukemia. The European APL Group. Blood. 1999;94:1192–1200. [PubMed] [Google Scholar]
- 7.Chen GQ, Zhu J, Shi XG, Ni JH, Zhong HJ, Si GY, Jin XL, Tang W, Li XS, Xong SM, et al. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RAR alpha/PML proteins. Blood. 1996;88:1052–1061. [PubMed] [Google Scholar]
- 8.Lam MS, Ignoffo RJ. Arsenic trioxide for the treatment of acute promyelocytic leukemia. Cancer Pract. 2001;9:155–157. doi: 10.1046/j.1523-5394.2001.009003155.x. [DOI] [PubMed] [Google Scholar]
- 9.Bawa-Khalfe T, Yeh ET. SUMO losing balance: SUMO proteases disrupt SUMO homeostasis to facilitate cancer development and progression. Genes Cancer. 2010;1:748–752. doi: 10.1177/1947601910382555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng J, Kang X, Zhang S, Yeh ET. SUMO-specific protease 1 is essential for stabilization of HIF1alpha during hypoxia. Cell. 2007;131:584–595. doi: 10.1016/j.cell.2007.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu L, Ji W, Zhang H, Renda MJ, He Y, Lin S, Cheng EC, Chen H, Krause DS, Min W. SENP1-mediated GATA1 deSUMOylation is critical for definitive erythropoiesis. J Exp Med. 2010;207:1183–1195. doi: 10.1084/jem.20092215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu B, Wang T, Mei W, Li D, Cai R, Zuo Y, Cheng JK. Small ubiquitin-like modifier (SUMO) protein-specific protease 1 de-SUMOylates Sharp-1 protein and controls adipocyte differentiation. J Biol Chem. 2014;289:22358–22364. doi: 10.1074/jbc.M114.571950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu J, Fan Y, Liu X, Zhou L, Cheng J, Cai R, Xue S. SENP1 protects against myocardial ischaemia/reperfusion injury via a HIF1α-dependent pathway. Cardiovasc Res. 2014;104:83–92. doi: 10.1093/cvr/cvu177. [DOI] [PubMed] [Google Scholar]
- 14.Bawa-Khalfe T, Cheng J, Lin SH, Ittmann MM, Yeh ET. SENP1 induces prostatic intraepithelial neoplasia through multiple mechanisms. J Biol Chem. 2010;285:25859–25866. doi: 10.1074/jbc.M110.134874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Y, Li J, Zuo Y, Deng J, Wang LS, Cheng GQ. SUMO-specific protease 1 regulates the in vitro and in vivo growth of colon cancer cells with the upregulated expression of CDK inhibitors. Cancer Lett. 2011;309:78–84. doi: 10.1016/j.canlet.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 16.Ma C, Wu B, Huang X, Yuan Z, Nong K, Dong B, Bai Y, Zhu H, Wang W, Ai K. SUMO-specific protease 1 regulates pancreatic cancer cell proliferation and invasion by targeting MMP-9. Tumour Biol. 2014;35:12729–12735. doi: 10.1007/s13277-014-2598-1. [DOI] [PubMed] [Google Scholar]
- 17.Wang RT, Zhi XY, Zhang Y, Zhang J. Inhibition of SENP1 induces radiosensitization in lung cancer cells. Exp Ther Med. 2013;6:1054–1058. doi: 10.3892/etm.2013.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang Z, Fan Q, Zhang Z, Zou Y, Cai R, Wang Q, Zuo Y, Cheng J. SENP1 deficiency promotes ER stress-induced apoptosis by increasing XBP1 SUMOylation. Cell Cycle. 2012;11:1118–1122. doi: 10.4161/cc.11.6.19529. [DOI] [PubMed] [Google Scholar]
- 19.Huang BB, Gao QM, Liang W, Xiu B, Zhang WJ, Liang AB. Downregulation of SENP1 expression increases apoptosis of Burkitt lymphoma cells. Asian Pac J Cancer Prev. 2012;13:2045–2049. doi: 10.7314/APJCP.2012.13.5.2045. [DOI] [PubMed] [Google Scholar]
- 20.Sharma P, Murillas R, Zhang H, Kuehn MR. N4BP1 is a newly identified nucleolar protein that undergoes SUMO-regulated polyubiquitylation and proteasomal turnover at promyelocytic leukemia nuclear bodies. J Cell Sci. 2010;123:1227–1234. doi: 10.1242/jcs.060160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohbayashi N, Kawakami S, Muromoto R, Togi S, Ikeda O, Kamitani S, Sekine Y, Honjoh T, Matsuda T. The IL-6 family of cytokines modulates STAT3 activation by desumoylation of PML through SENP1 induction. Biochem Biophys Res Commun. 2008;371:823–828. doi: 10.1016/j.bbrc.2008.04.179. [DOI] [PubMed] [Google Scholar]
- 22.Kastner P, Perez A, Lutz Y, Rochette-Egly C, Gaub MP, Durand B, Lanotte M, Berger R, Chambon P. Structure, localization and transcriptional properties of two classes of retinoic acid receptor alpha fusion proteins in acute promyelocytic leukemia (APL): Structural similarities with a new family of oncoproteins. EMBO J. 1992;11:629–642. doi: 10.1002/j.1460-2075.1992.tb05095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mollereau B, Manié S, Napoletano F. Getting the better of ER stress. J Cell Commun Signal. 2014;8:311–321. doi: 10.1007/s12079-014-0251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gorman AM, Healy SJ, Jäger R, Samali A. Stress management at the ER: Regulators of ER stress-induced apoptosis. Pharmacol Ther. 2012;134:306–316. doi: 10.1016/j.pharmthera.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Mathur A, Abd Elmageed ZY, Liu X, Kostochka ML, Zhang H, Abdel-Mageed AB, Mondal D. Subverting ER-stress towards apoptosis by nelfinavir and curcumin coexposure augments docetaxel efficacy in castration resistant prostate cancer cells. PLoS One. 2014;9:e103109. doi: 10.1371/journal.pone.0103109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kondo S, Hino SI, Saito A, Kanemoto S, Kawasaki N, Asada R, Izumi S, Iwamoto H, Oki M, Miyagi H, et al. Activation of OASIS family, ER stress transducers, is dependent on its stabilization. Cell Death Differ. 2012;19:1939–1949. doi: 10.1038/cdd.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin L, Wang Z, Tao L, Wang Y. ER stress negatively regulates AKT/TSC/mTOR pathway to enhance autophagy. Autophagy. 2010;6:239–247. doi: 10.4161/auto.6.2.11062. [DOI] [PubMed] [Google Scholar]
- 28.Chiu HW, Tseng YC, Hsu YH, Lin YF, Foo NP, Guo HR, Wang YJ. Arsenic trioxide induces programmed cell death through stimulation of ER stress and inhibition of the ubiquitin-proteasome system in human sarcoma cells. Cancer Lett. 2015;356:762–772. doi: 10.1016/j.canlet.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 29.Li K, Zhang L, Xiang X, Gong S, Ma L, Xu L, Wang G, Liu Y, Ji X, Liu S, et al. Arsenic trioxide alleviates airway hyperresponsiveness and promotes apoptosis of CD4+ T lymphocytes: Evidence for involvement of the ER stress-CHOP pathway. Ir J Med Sci. 2013;182:573–583. doi: 10.1007/s11845-013-0928-8. [DOI] [PubMed] [Google Scholar]
- 30.Doudican NA, Wen SY, Mazumder A, Orlow SJ. Sulforaphane synergistically enhances the cytotoxicity of arsenic trioxide in multiple myeloma cells via stress-mediated pathways. Oncol Rep. 2012;28:1851–1858. doi: 10.3892/or.2012.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang H, Duncan G, Wang L, Liu P, Cui H, Reddan JR, Yang BF, Wormstone IM. Arsenic trioxide initiates ER stress responses, perturbs calcium signalling and promotes apoptosis in human lens epithelial cells. Exp Eye Res. 2007;85:825–835. doi: 10.1016/j.exer.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 32.Fan YH, Chen M, Meng J, Yu L, Tu Y, Wan L, Fang K, Zhu W. Arsenic trioxide and resveratrol show synergistic anti-leukemia activity and neutralized cardiotoxicity. PLoS One. 2014;9:e105890. doi: 10.1371/journal.pone.0105890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu M, Yang Y, Wang C, Sun L, Mei C, Yao W, Liu Y, Shi Y, Qiu S, Fan J, et al. The effect of epidermal growth factor receptor variant III on glioma cell migration by stimulating ERK phosphorylation through the focal adhesion kinase signaling pathway. Arch Biochem Biophys. 2010;502:89–95. doi: 10.1016/j.abb.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 34.Wang C, Yang Y, Yang Z, Liu M, Li Z, Sun L, Mei C, Chen H, Chen L, Wang L, Zha X. EGF-mediated migration signaling activated by N-acetylglucosaminyltransferase-V via receptor protein tyrosine phosphatase kappa. Arch Biochem Biophys. 2009;486:64–72. doi: 10.1016/j.abb.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 35.Ghaffari SH, Bashash D, Dizaji MZ, Ghavamzadeh A, Alimoghaddam K. Alteration in miRNA gene expression pattern in acute promyelocytic leukemia cell induced by arsenic trioxide: A possible mechanism to explain arsenic multi-target action. Tumour Biol. 2012;33:157–172. doi: 10.1007/s13277-011-0259-1. [DOI] [PubMed] [Google Scholar]
- 36.Ghaffari SH, Momeny M, Bashash D, Mirzaei R, Ghavamzadeh A, Alimoghaddam K. Cytotoxic effect of arsenic trioxide on acute promyelocytic leukemia cells through suppression of NFkβ-dependent induction of hTERT due to downregulation of Pin1 transcription. Hematology. 2012;17:198–206. doi: 10.1179/1607845412Y.0000000008. [DOI] [PubMed] [Google Scholar]
- 37.Zhao XY, Yang S, Chen YR, Li PC, Dou MM, Zhang J. Resveratrol and arsenic trioxide act synergistically to kill tumor cells in vitro and in vivo. PLoS One. 2014;9:e98925. doi: 10.1371/journal.pone.0098925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.So AY, de la Fuente E, Walter P, Shuman M, Bernales S. The unfolded protein response during prostate cancer development. Cancer Metastasis Rev. 2009;28:219–223. doi: 10.1007/s10555-008-9180-5. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto K, Tashiro E, Imoto M. Quinotrierixin inhibited ER stress-induced XBP1 mRNA splicing through inhibition of protein synthesis. Biosci Biotechnol Biochem. 2011;75:284–288. doi: 10.1271/bbb.100622. [DOI] [PubMed] [Google Scholar]
- 40.Nasr R, Lallemand-Breitenbach V, Zhu J, Guillemin MC, de Thé H. Therapy-induced PML/RARA proteolysis and acute promyelocytic leukemia cure. Clin Cancer Res. 2009;15:6321–6326. doi: 10.1158/1078-0432.CCR-09-0209. [DOI] [PubMed] [Google Scholar]
- 41.Geoffroy MC, Jaffray EG, Walker KJ, Hay RT. Arsenic-induced SUMO-dependent recruitment of RNF4 into PML nuclear bodies. Mol Biol Cell. 2010;21:4227–4239. doi: 10.1091/mbc.E10-05-0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen GQ, Shi XG, Tang W, Xiong SM, Zhu J, Cai X, Han ZG, Ni JH, Shi GY, Jia PM, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): I. As2O3 exerts dose-dependent dual effects on APL cells. Blood. 1997;89:3345–3353. [PubMed] [Google Scholar]
- 43.Khaleghian A, Ghaffari SH, Ahmadian S, Alimoghaddam K, Ghavamzadeh A. Metabolism of arsenic trioxide in acute promyelocytic leukemia cells. J Cell Biochem. 2014;115:1729–1739. doi: 10.1002/jcb.24838. [DOI] [PubMed] [Google Scholar]
- 44.Sumi D, Shinkai Y, Kumagai Y. Signal transduction pathways and transcription factors triggered by arsenic trioxide in leukemia cells. Toxicol Appl Pharmacol. 2010;244:385–392. doi: 10.1016/j.taap.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 45.Wang J, Li L, Cang H, Shi G, Yi J. NADPH oxidase-derived reactive oxygen species are responsible for the high susceptibility to arsenic cytotoxicity in acute promyelocytic leukemia cells. Leuk Res. 2008;32:429–436. doi: 10.1016/j.leukres.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 46.Tabellini G, Cappellini A, Tazzari PL, Falà F, Billi AM, Manzoli L, Cocco L, Martelli AM. Phosphoinositide 3-kinase/Akt involvement in arsenic trioxide resistance of human leukemia cells. J Cell Physiol. 2005;202:623–634. doi: 10.1002/jcp.20153. [DOI] [PubMed] [Google Scholar]
- 47.Tabellini G, Tazzari PL, Bortul R, Evangelisti C, Billi AM, Grafone T, Martinelli G, Baccarani M, Martelli AM. Phosphoinositide 3-kinase/Akt inhibition increases arsenic trioxide-induced apoptosis of acute promyelocytic and T-cell leukaemias. Br J Haematol. 2005;130:716–725. doi: 10.1111/j.1365-2141.2005.05679.x. [DOI] [PubMed] [Google Scholar]
- 48.Binet F, Chiasson S, Girard D. Evidence that endoplasmic reticulum (ER) stress and caspase-4 activation occur in human neutrophils. Biochem Biophys Res Commun. 2010;391:18–23. doi: 10.1016/j.bbrc.2009.10.141. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, Dasso M. SUMOylation and deSUMOylation at a glance. J Cell Sci. 2009;122:4249–4252. doi: 10.1242/jcs.050542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Verger A, Perdomo J, Crossley M. Modification with SUMO. A role in transcriptional regulation. EMBO Rep. 2003;4:137–142. doi: 10.1038/sj.embor.embor738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quentin T, Steinmetz M, Poppe A, Thoms S. Metformin differentially activates ER stress signaling pathways without inducing apoptosis. Dis Model Mech. 2012;5:259–269. doi: 10.1242/dmm.008110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ibuki T, Yamasaki Y, Mizuguchi H, Sokabe M. Protective effects of XBP1 against oxygen and glucose deprivation/reoxygenation injury in rat primary hippocampal neurons. Neurosci Lett. 2012;518:45–48. doi: 10.1016/j.neulet.2012.04.053. [DOI] [PubMed] [Google Scholar]