Abstract
The combined effects of matrine (Mat) and cisplatin on the survival and apoptosis of rhabdomyosarcoma (RMS) RD cells, as well as the possible mechanism of the synergistic effect of Mat and cisplatin were investigated in the present study. RMS RD cells were divided and treated as follows: control group, 5 mg/l cisplatin group, Mat groups (0.5, 1.0 and 1.5 g/l), and Mat (0.5, 1.0 and 1.5 g/l) combined with 5 mg/l cisplatin groups. An MTT assay and flow cytometry were applied to detect the survival and apoptotic rates, respectively, while RT-PCR was applied to detect the expression levels of X-linked inhibitor of apoptosis protein (XIAP) mRNA in the RD cells of each group. The survival rates of RD cells in each experimental group were lower than in the control group, and the apoptotic rates were higher than those in the control group (P<0.05). An increase in drug concentrations led to the cell proliferation inhibitory and apoptotic rates of the single Mat groups increasing as a function of dose (pairwise comparison among the groups, P<0.05), while the proliferation inhibitory and apoptotic rates of Mat combined with the cisplatin groups under different concentration were significantly higher than those of the single Mat and single cisplatin groups under the same concentration (P<0.01). The expression levels of XIAP mRNA in the RD cells of each experimental group were lower than those in the control group (P<0.05). Additionally, the expression levels of XIAP mRNA in the group treated with Mat and cisplatin were significantly lower than those of the single cisplatin and single Mat groups (P<0.01). In conclusion, Mat and cisplatin are capable of inhibiting the proliferation of RD cells and inducing apoptosis by suppressing the XIAP mRNA expression levels.
Keywords: human rhabdomyosarcoma RD cells, matrine, cisplatin, X-linked apoptosis inhibition gene, chemotherapy, children
Introduction
Matrine (Mat) is one of the main active ingredients of Chinese herbal medicine (1), such as Sophora alopecuroides, Sophora tonkinensis, and Radix Sophorae Flavescentis. Its pharmacological effects are quite extensive. Previous studies have focused on Mat in depth and its antitumor effect. It was previously identified that Mat is capable of inhibiting tumor cell proliferation and inducing apoptosis (1). It can also inhibit tumor angiogenesis, invasion, and metastasis as well as reverse tumor cell resistance to pharmacological agents. In addition, combined medication can enhance its efficacy (2). A large body of studies worldwide has reported that Mat treatment results in different degrees of inhibition on melanoma, lung, gastric, breast, ovarian and bladder cancers as well as others (3–9). However, studies on the effects of Mat on rhabdomyosarcoma (RMS), as well as those on the combined effects of Mat with other chemotherapeutic drugs on RMS are limited. The present study examined cisplatin, a chemotherapeutic drug commonly used to treat clinical cases of RMS, and whether its effectiveness can be improved when used in combination with Mat, to produce an antitumor effect.
X-linked inhibitor of apoptosis protein (XIAP) is a new member of the IAP family and one of the most powerful inhibitors of apoptosis (10,11). At present, XIAP has been shown to be a protein of universal expression. It is expressed in various human tissues (12,13), but at low levels in normal cells and is increased in cancer cells (14–16). XIAP can inhibit tumor cell apoptosis and reduce tumor cell sensitivity, such as in ovarian cancer, glioma, prostate cancer, rectal cancer and childhood acute leukemia cells (17–20). However, to the best of our knowledge, there are few relevant studies reporting its role in children's RMS.
In the present study, XIAP was used as a reporter to examine the effect of Mat combined with cisplatin on human RMS cells, in order to identify a new option for the clinical treatment of RMS.
Materials and methods
Reagents
The RMS RD cell line was purchased from the Chinese Academy of Sciences Cell Bank (Shanghai, China). Mat (purity ≥98%) was purchased from Shaanxi Zhongxin Biotechnology Co., Ltd. (Shaanxi, China); the methyl hiazolyl tetrazolium (MTT) assay was purchased from Sigma (St. Louis, MO, USA); the flow cytometry kit (Annexin V-FITC kit) was purchased from Beijing Biosea Biotechnology Co., Ltd. (Beijing, China); and the total RNA extraction kit, and reverse transcription (RT)-PCR two-step kit were purchased from Tiangen Biotech (Beijing) Co., Ltd. (Beijing, China). The PCR primers were designed and produced by Sangon Biotech Co., Ltd. (Shanghai, China).
Cell culture
RD cells were grown in RPMI-1640 supplemented with 100 g/l fetal bovine serum and 10 g/l penicillin/streptomycin and maintained in a constant temperature incubator at 37°C, and 50 ml/l CO2. The medium was changed once every 3–4 days, and cells were passaged once every 5–7 days. The cells were collected and used during the logarithmic phase of growth for all the experiments.
Experimental grouping and treatments
Grouping and treatments used were: i) Control group, only RMS RD cells, without any other processing; ii) 5 mg/l cisplatin group, 5 mg/l cisplatin was added to RD cells and processed for 16 h; iii) 0.5 g/l Mat group, 0.5 g/l Mat was added to RD cells and processed for 24 h; iv) 1.0 g/l Mat group, 1.0 g/l Mat was added to RD cells and processed for 24 h; v) 1.5 g/l Mat group, 1.5 g/l Mat was added to RD cells and processed for 24 h; vi) 0.5 g/l Mat combined with cisplatin group, 0.5 g/l Mat was added to RD cells and processed for 24 h, followed by the addition of 5 mg/l cisplatin and processed for 16 h; vii) 1.0 g/l Mat combined with cisplatin group, 1.0 g/l Mat was added to RD cells and processed for 24 h, followed by the addition of 5 mg/l cisplatin and processed for 16 h; and viii) 1.5 g/l Mat combined with cisplatin group, 1.5 g/l Mat was added to RD cells and processed for 24 h, followed by the addition of 5 mg/l cisplatin and processed for 16 h.
MTT detection of RD cell proliferation inhibitory rate
RD cells were collected in the logarithmic phase of growth, and adjusted to a concentration of 1×105/l. The cell suspension (100 µl) was then inoculated into appropriate wells of a 96-well plate. Five repeats of each condition were prepared. After the cells were allowed to adhere to the plate for 24 h, they were treated in accordance with the above groupings. After 24 h, the assay was performed as previously described (21). Absorbance of each group was taken at 570 nm using a microplate reader (Bio-Rad, Hercules, CA, USA), and the cell proliferation inhibition rate of each group was calculated. The experiment was repeated three times.
Flow cytometry mediated detection of RD cell apoptotic rate
RD cells were collected in the logarithmic phase and adjusted to a concentration of 1×105/l. The cells were subsequently inoculated into 6-well plates, with each well containing 2 ml RPMI-1640 medium. After the cells were allowed to adhere to the plate, they were treated in accordance with the above groupings. After 24 h, the apoptotic rate of each group was assessed using an Annexin V-FITC kit according to the manufacturer's instructions. The experiment was repeated three times.
RT-PCR detection of XIAP mRNA
Total RNA extraction was then carried out. Briefly, harvested cells in suspension were adjusted to a concentration of 1×106/l, and inoculated into cell culture flasks. The cells were then treated in accordance with the above groupings. After 24 h, the cells were collected again and total RNA was extracted. Subsequently, 5 µl of RNA was added to 2% agarose gel for electrophoresis for detection of its quality, and the RNA concentration and purity were detected using ultraviolet spectrophotometry using GeneQuant Pro (Amersham Biosciences, Little Chalfont, UK). RT was then performed. For PCR amplification, XIAP primer sequences and internal reference sequences (GAPDH) were identified (Table I). Amplification conditions used were: Pre-degenerated under 94°C for 2 min, degenerated under 94°C for 30 sec, annealed under 55°C for 45 sec, extended under 72°C for 30 sec, for a total 32 cycles, and then extended under 72°C for 10 min. This was followed by agarose gel electrophoresis on the amplification products. The ImageJ software (National Institutes of Health, Bethesda, Maryland, USA) was used to analyze the gray level of the bands after electrophoresis, and the gene expression levels were calculated by the ratio of target gene to the level of the internal amplification product. The experiment was repeated three times.
Table I.
Primer sequence and annealing temperature of the XIAP and reference genes.
| Gene | Primer (5′→3′) | PCR amplification products size (bp) | Annealing temperature (°C) | |
|---|---|---|---|---|
| XIAP | Sense: | ATGGGATTTGATTTCAAGGA | 256 | 55 |
| Antisense: | GTCCACAAGGAATAAAAACA | |||
| GAPDH | Sense: | ACCACAGTCCATGCCATCAC | 452 | 55 |
| Antisense: | TCCACCACCCTGTTGCTGTA |
XIAP, X-linked inhibitor of apoptosis protein.
Statistical analysis
SPSS 16.0 statistical software (Chicago, IL, USA) was used for statistical analysis. Data were presented as mean ± standard deviation (SD). A normality and homogeneity test of variance was performed on the experimental data and variance analysis of two-factor factorial design was applied to make comparisons among groups. The Student-Newman-Keuls q-test was used in performing pairwise comparison. P<0.05 was considered to be statistically significant.
Results
Mat and cisplatin act synergistically to inhibit the proliferation of RD cells
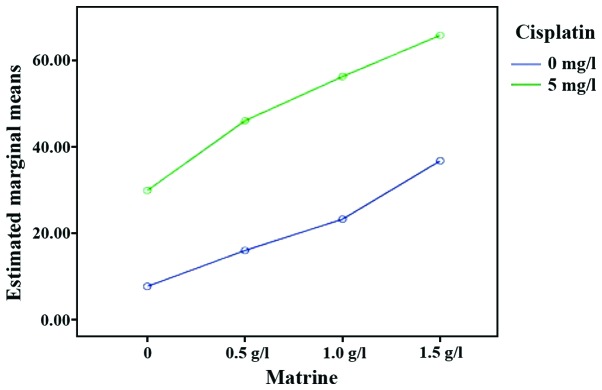
Using an MTT assay, it was observed that the proliferation of RD cells in each experimental group was inhibited to different degrees, compared with the control group. As the drug concentration increased, the proliferation inhibition effect was gradually increased (P<0.05). The proliferation inhibition rate of Mat combined with the cisplatin groups was significantly higher than those of the single Mat and single cisplatin groups under the same concentration (P<0.01). These data suggested that Mat and cisplatin act synergistically to inhibit the proliferation of RD cells (Table II and Fig. 1).
Table II.
Proliferation inhibition rate, apoptosis rate and XIAP mRNA expression level (n=3, mean ± SD) of each group.
| Group | Proliferation inhibition rate (%) | Apoptosis rate (%) | XIAP expression (FI) |
|---|---|---|---|
| Control | 7.60±6.22 | 7.80±0.85 | 0.90±0.57 |
| Cisplatin | 29.83±1.60a | 22.33±0.97a | 0.36±0.04a |
| Mat | |||
| 0.5 g/l | 15.93±2.50a | 12.63±0.85a | 0.67±0.06a |
| 1.0 g/l | 23.20±2.33a, b | 16.93±0.81a, b | 0.50±0.03a, b |
| 1.5 g/l | 36.73±2.48a, b | 27.63±0.70a, b | 0.28±0.04a, b |
| Mat + cisplatin | |||
| 0.5 g/l | 46.00±1.45a, c | 36.47±3.00a, c | 0.21±0.02a, c |
| 1.0 g/l | 56.27±2.24a,c,d | 44.03±2.06a,c,d | 0.14±0.02a,c,d |
| 1.5 g/l | 68.83±1.35a,c,d | 69.00±5.48a,c,d | 0.07±0.03a,c,d |
| F-value | 141.581 | 203.905 | 162.600 |
| P-value | 0.01 | <0.0001 | <0.0001 |
Comparison with the control group;
comparison with the 0.5 g/l Mat group;
comparison with 0.5 g/l Mat combined with the cisplatin group; P<0.05.
Comparison between the Mat group and Mat combined with cisplatin at different concentrations were statically significant (P<0.05). Mat, matrine; XIAP, X-linked inhibitor of apoptosis protein.
Figure 1.
Interaction effect sketch map (proliferation inhibition rate of RD cells). The green line is the inhibitory rate of cell proliferation in the Mat group combined with cisplatin at different concentrations. The blue line is the inhibitory rate of cell proliferation in the Mat monotherapy group at different concentrations. Mat, matrine.
Mat and cisplatin act cooperatively to induce apoptosis of RD cells
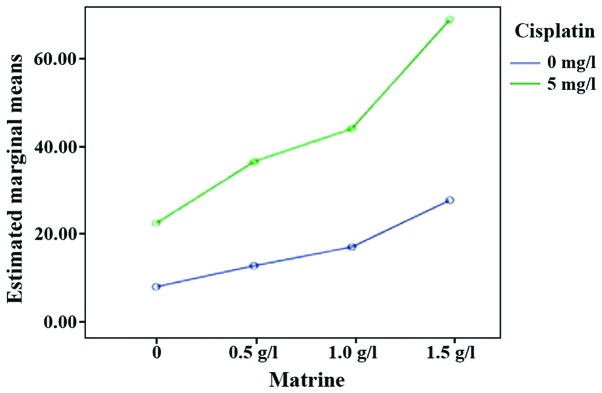
Flow cytometry results showed that, compared with the control group, the apoptotic rate of RD cells in each experimental group was increased to varying degrees (P<0.05). An increase in drug concentration, led to the apoptotic rate of RD cells in single Mat groups being gradually increased (P<0.05). The apoptotic rate of Mat combined with the cisplatin groups was significantly higher than those of the single cisplatin and single Mat groups under the same concentration (P<0.05). Consistent with the observation on the inhibition of cell proliferation, Mat and cisplatin acted cooperatively to induce the apoptosis of RD cells (Table II; Figs. 2 and 3).
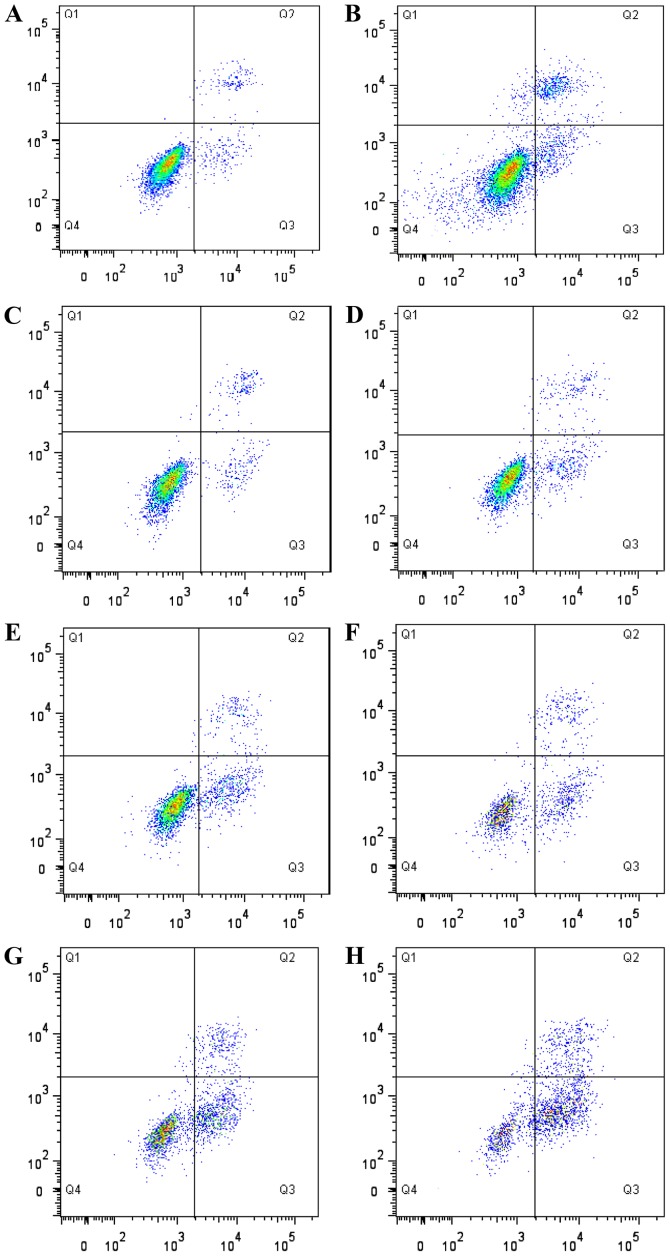
Figure 2.
Flow cytometry mediated detection of apoptosis of RD cells. (A) Positive control group, (B) 5 mg/l cisplatin group, (C) 0.5 g/l Mat group, (D) 1.0 g/l Mat group, (E) 1.5 g/l Mat group, (F) 0.5 g/l matrine combined with cisplatin group, (G) 1.0 g/l Mat combined with cisplatin group, (H) 1.5 g/l Mat combined with 5 mg/l cisplatin group. The upper left quadrant (Q1) shows cells under mechanical injury (Annexin V−/PI+); upper right quadrant (Q2) shows cells under advanced apoptosis and secondary necrosis (Annexin V+/PI+); lower left quadrant (Q4) shows surviving cells (Annexin V−/PI−); lower right quadrant shows cells undergoing early apoptosis (Annexin V+/PI−). Mat, matrine.
Figure 3.
Interaction effect sketch map (apoptosis rate of RD cells). The green line is the cell apoptosis rate in the Mat group combined with cisplatin at different concentrations. The blue line is the cell apoptotic rate in the Mat monotherapy group at different concentrations. Mat, matrine.
Mat and cisplatin act synergistically to reduce the XIAP mRNA expression levels
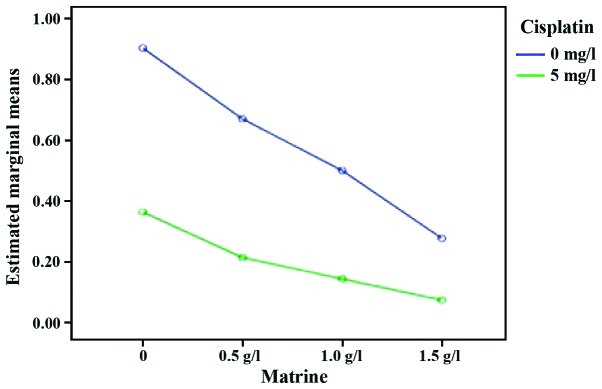
XIAP mRNA was expressed in cells of the control and each experimental group. The expression levels of XIAP mRNA in RD cells of each experimental group were lower than those in the control group (P<0.05). Compared with the single cisplatin group, the expression levels of XIAP mRNA in Mat combined with the cisplatin group were significantly reduced (P<0.05). Compared with the single Mat groups under the same concentration, the expression levels of XIAP mRNA in the Mat combined with cisplatin group were significantly reduced (P<0.01). These data showed that Mat and cisplatin act synergistically to reduce the expression levels of XIAP mRNA (Table II; Figs. 4 and 5).
Figure 4.
Interaction effect sketch map (the expression level of XIAP in RD cells). The green line is the expression level of XIAP in the Mat group combined with cisplatin at different concentrations. The blue line is the expression level of XIAP in the Mat monotherapy group at different concentrations. Mat, matrine; XIAP, X-linked inhibitor of apoptosis protein.
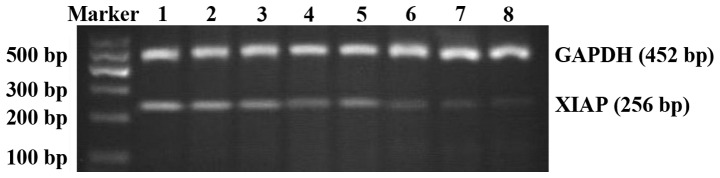
Figure 5.
XIAP mRNA expression in each group of RD cells. The upper band is the internal reference (GAPDH). The lower band is the target band (XIAP). Lane 1, control group; lane 2, 0.5 g/l Mat group; lane 3, 1.0 g/l Mat group; lane 4, 1.5 g/l Mat group; lane 5, mg/l cisplatin group; lane 6, 0.5 g/l Mat combined with cisplatin group; lane 7, 1.0 g/l Mat combined with cisplatin group; and lane 8, 1.5 g/l Mat combined with cisplatin group. XIAP, X-linked inhibitor of apoptosis protein; Mat, matrine.
Discussion
RMS is the most common soft tissue sarcoma in children, accounting for 4–8% of all malignant pediatric solid tumors (22), and 53% of soft tissue sarcomas (23). RMS can appear in any part of the body. It is most commonly seen in the head and neck of children and is characterized by high malignancy and rapid progression (24). It is challenging to diagnose the disease in its early stages. When patients arrive under hospital care, the disease is at an advanced stage and the tumor cannot be removed by surgery. At this point, comprehensive therapy by combining surgery with chemo- and radiotherapy should be utilized. Drug resistance to chemotherapy often results in treatment failure. Therefore, the need to improve the curative effect of chemotherapy drugs, and reverse the drug resistance of tumor cells has become a research hotspot.
In recent years, the traditional Chinese medicine, Mat, has increasingly aroused the interest of scholars due to its physicochemical properties of low toxicity and high efficacy. Previous findings have shown that Mat had a significant inhibitory effect on a variety of tumor cells, such as neuroblastoma, medulloblastoma, colorectal cancer, nasopharyngeal carcinoma, leukemia cells and RMS (25–31). In addition, the antitumor mechanism of Mat mainly included obstructing tumor cell cycle progression, inhibiting tumor cell proliferation, inducing tumor cell apoptosis and reversing chemotherapy drug resistance (32).
Guo et al (33) and Li et al (34) investigated the 24 and 48 h effects of different concentrations of Mat on RMS. The results have confirmed that Mat inhibited the proliferation of RMS, inducing cell apoptosis and maintaining the cell cycle in the G0/G1 phase. The results of the present study have shown that the proliferation inhibition rate and apoptosis rate of RD cells treated at different concentrations of Mat (0.5, 1.0 and 1.5 g/l) were higher than those in the control group. Additionally, as the concentration of Mat increased, the apoptosis rate of RD cells also increased, confirming that Mat suppressed the proliferation and induced the apoptosis of RMS cells. The effect was dose-dependent to a certain degree. Our experimental results were consistent with those of Guo et al and Li et al (33,34).
Thanks to its broad anticancer properties, cisplatin has occupied an extremely significant and unique position in the field of cancer treatment since 1970 (35). It is commonly regarded as an anticancer drug with epoch-making significance, particularly effective for urogenital tumors and malignant carcinomas of the neck (35). However, as the use of cisplatin became increasingly widespread, it became apparent that it had toxic side effects while killing cancer cells and that most of these side effects were related to the dose of cisplatin. The toxic side effects of cisplatin mainly manifested as a result of its application in the clinic. However, approaches regarding how to reduce the toxicity and side effects of cisplatin have become an area of interest in clinical research. Further studies on cisplatin revealed that it had synergistic effects with multiple other drugs. Drug combinations can enhance their antitumor effects, reduce the required dose of cisplatin, and reduce the toxic side effect of cisplatin. Sárosi and Lénárt showed that the combination of gemcitabine and cisplatin was effective in the treatment of advanced non-small cell lung cancer, and that the side effects were relatively minor (36). Solár et al have shown that geldanamycin enhanced the cytotoxic effect of cisplatin on tumor cells (37).
In recent years, as research on the antitumor effects of Chinese medicine progressed, investigators examined how to enhance the anticancer effect of antitumor drugs while lowering their toxicity and side effects. Several studies have shown that rhubarb, bufalin, Radix Astragali, Shengmai, Mat and other traditional Chinese medicines may enhance the anticancer effects of cisplatin to varying degrees (38–41). However, to the best of our knowledge, few studies focused on the effects of Mat combined with cisplatin on RMS. In the present study, we used Mat at different concentration (final concentrations were 0.5, 1.0 and 1.5 g/l) in combination with cisplatin (final concentration was 5 mg/l) to treat RMS RD cells cultured in vitro. We then used the MTT assay, flow cytometry and RT-PCR to measure the effects on proliferation inhibition and apoptosis of RD cells. The results of the present study show that the proliferation inhibition rate and apoptosis rate of RD cells in Mat (different concentrations) combined with cisplatin groups was significantly higher than that in the single Mat or cisplatin groups. Our study also confirmed that Mat and cisplatin produced a synergistic effect, and that the combination therapy can significantly kill RMS RD cells.
In recent years, with the extensive clinical application of cisplatin, the phenomenon of cisplatin resistance has become increasingly widespread, which has generated interest among scholars to explore the mechanisms of drug resistance. Amantana et al have shown that DU145 prostate cancer cells were resistant to cisplatin and that high expression levels of XIAP were a cause of DU145 resistance to cisplatin. The study also confirmed that antisense nucleic acid therapy of XIAP can enhance the antitumor effect of cisplatin and increase the sensitivity of the DU145 cell line to cisplatin (42). XIAP is a new member of the IAPs family and also one of the most powerful inhibitors of apoptosis (23,24). At present, XIAP has been shown to be a protein of universal expression. It is expressed in various human tissues (25,26), but at low levels in normal tissue cells and is increased in cancer cells (27–29). We have studied the effects of different concentrations of Mat in combination with cisplatin on the expression of XIAP in human RMS RD cells using RT-PCR analysis. The results showed that XIAP was expressed in RMS RD cells. Following treatment with Mat and/or cisplatin, the expression levels of XIAP in the cells were decreased. In the dose-response experiments, XIAP mRNA decreased as a function of increasing Mat concentration. The expression of XIAP mRNA was significantly reduced under the combined effect of Mat and cisplatin versus Mat or cisplatin alone.
In conclusion, our experimental results show that Mat combined with cisplatin inhibited the expression of XIAP in RMS RD cells and induced apoptosis in these tumor cells. The results also indicate that Mat may lower the tolerance of RMS RD cells to cisplatin by inhibiting the expression of XIAP, thus improving the curative effect of cisplatin. We believe this study reveals a potential new target for the study of drug-resistant RMS cell lines and may also lead to new therapeutic options for the clinical treatment of RMS.
References
- 1.Song YQ, Liu SP, Liu ZF, Hu XL. Determination of matrine and oxymatrine in Radix Sophorae Flavescentis by resonance rayleigh scattering, second-order scattering and frequency doubling scattering technique. Chem Res Chin Univ. 2011;27:746–749. [Google Scholar]
- 2.Zhaowu Z, Xiaoli W, Yangde Z, Nianfeng L. Preparation of matrine ethosome, its percutaneous permeation in vitro and anti-inflammatory activity in vivo in rats. J Liposome Res. 2009;19:155–162. doi: 10.1080/08982100902722381. [DOI] [PubMed] [Google Scholar]
- 3.Niu H, Zhang Y, Wu B, Zhang Y, Jiang H, He P. Matrine induces the apoptosis of lung cancer cells through downregulation of inhibitor of apoptosis proteins and the Akt signaling pathway. Oncol Rep. 2014;32:1087–1093. doi: 10.3892/or.2014.3273. [DOI] [PubMed] [Google Scholar]
- 4.Liu YQ, Li Y, Qin J, Wang Q, She YL, Luo YL, He JX, Li JY, Xie XD. Matrine reduces proliferation of human lung cancer cells by inducing apoptosis and changing miRNA expression profiles. Asian Pac J Cancer Prev. 2014;15:2169–2177. doi: 10.7314/APJCP.2014.15.5.2169. [DOI] [PubMed] [Google Scholar]
- 5.Li H, Li X, Bai M, Suo Y, Zhang G, Cao X. Matrine inhibited proliferation and increased apoptosis in human breast cancer MCF-7 cells via upregulation of Bax and downregulation of Bcl-2. Int J Clin Exp Pathol. 2015;8:14793–14799. [PMC free article] [PubMed] [Google Scholar]
- 6.Gao H, Guo Y, Deng N, Fei P, Qiu X, Zheng P, Feng J, Dai G. Suppressive effect of matrine on tumor invasion in N-butyl-N-4-hydroxybutyl)nitrosamine-induced urinary bladder carcinogenesis. Chemotherapy. 2014;60:119–128. doi: 10.1159/000371439. [DOI] [PubMed] [Google Scholar]
- 7.Li Q, Lai Y, Wang C, Xu G, He Z, Shang X, Sun Y, Zhang F, Liu L, Huang H. Matrine inhibits the proliferation, invasion and migration of castration-resistant prostate cancer cells through regulation of the NF-κB signaling pathway. Oncol Rep. 2016;35:375–381. doi: 10.3892/or.2015.4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rong B, Zhao C, Gao W, Yang S. Matrine promotes the efficacy and safety of platinum-based doublet chemotherapy for advanced non-small cell lung cancer. Int J Clin Exp Med. 2015;8:14701–14717. [PMC free article] [PubMed] [Google Scholar]
- 9.Wang HQ, Jin JJ, Wang J. Matrine induces mitochondrial apoptosis in cisplatin-resistant non-small cell lung cancer cells via suppression of β-catenin/survivin signaling. Oncol Rep. 2015;33:2561–2566. doi: 10.3892/or.2015.3844. [DOI] [PubMed] [Google Scholar]
- 10.Flanagan L, Sebastià J, Tuffy LP, Spring A, Lichawska A, Devocelle M, Prehn JH, Rehm M. XIAP impairs Smac release from the mitochondria during apoptosis. Cell Death Dis. 2010;1:e49. doi: 10.1038/cddis.2010.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kempkensteffen C, Jäger T, Bub J, Weikert S, Hinz S, Christoph F, Krause H, Schostak M, Miller K, Schrader M. The equilibrium of XIAP and Smac/DIABLO expression is gradually deranged during the development and progression of testicular germ cell tumours. Int J Androl. 2007;30:476–483. doi: 10.1111/j.1365-2605.2006.00742.x. [DOI] [PubMed] [Google Scholar]
- 12.Ramp U, Krieg T, Caliskan E, Mahotka C, Ebert T, Willers R, Gabbert HE, Gerharz CD. XIAP expression is an independent prognostic marker in clear-cell renal carcinomas. Hum Pathol. 2004;35:1022–1028. doi: 10.1016/j.humpath.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Hanson AJ, Wallace HA, Freeman TJ, Beauchamp RD, Lee LA, Lee E. XIAP monoubiquitylates Groucho/TLE to promote canonical Wnt signaling. Mol Cell. 2012;45:619–628. doi: 10.1016/j.molcel.2011.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng ZL, Tan LZ, Yu YP, Michalopoulos G, Luo JH. Interaction of CSR1 with XIAP reverses inhibition of caspases and accelerates cell death. Am J Pathol. 2012;181:463–471. doi: 10.1016/j.ajpath.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berezovskaya O, Schimmer AD, Glinskii AB, Pinilla C, Hoffman RM, Reed JC, Glinsky GV. Increased expression of apoptosis inhibitor protein XIAP contributes to anoikis resistance of circulating human prostate cancer metastasis precursor cells. Cancer Res. 2005;65:2378–2386. doi: 10.1158/0008-5472.CAN-04-2649. [DOI] [PubMed] [Google Scholar]
- 16.Doudican NA, Byron SA, Pollock PM, Orlow SJ. XIAP downregulation accompanies mebendazole growth inhibition in melanoma xenografts. Anticancer Drugs. 2013;24:181–188. doi: 10.1097/CAD.0b013e32835a43f1. [DOI] [PubMed] [Google Scholar]
- 17.Castells M, Milhas D, Gandy C, Thibault B, Rafii A, Delord JP, Couderc B. Microenvironment mesenchymal cells protect ovarian cancer cell lines from apoptosis by inhibiting XIAP inactivation. Cell Death Dis. 2013;4:e887. doi: 10.1038/cddis.2013.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao Z, Li X, Li J, Luo W, Huang C, Chen J. X-linked inhibitor of apoptosis protein (XIAP) lacking RING domain localizes to the nuclear and promotes cancer cell anchorage-independent growth by targeting the E2F1/Cyclin E axis. Oncotarget. 2014;5:7126–7137. doi: 10.18632/oncotarget.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liew JC, Tan WS, Alitheen NB, Chan ES, Tey BT. Over-expression of the X-linked inhibitor of apoptosis protein (XIAP) delays serum deprivation-induced apoptosis in CHO-K1 cells. J Biosci Bioeng. 2010;110:338–344. doi: 10.1016/j.jbiosc.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 20.Katragadda L, Carter BZ, Borthakur G. XIAP antisense therapy with AEG 35156 in acute myeloid leukemia. Expert Opin Investig Drugs. 2013;22:663–670. doi: 10.1517/13543784.2013.789498. [DOI] [PubMed] [Google Scholar]
- 21.Yang CL, Liu SS, Ma YG, Liu YY, Xue YX, Huang B. The influence of intraoperative pleural perfusion with matrine- cisplatin or cisplatin on stromal cell-derived factor-1 in non-small cell lung cancer patients with subclinical pleural metastasis. Med Oncol. 2012;29:574–581. doi: 10.1007/s12032-011-9849-4. [DOI] [PubMed] [Google Scholar]
- 22.Rossi S, Stoppani E, Puri PL, Fanzani A. Differentiation of human rhabdomyosarcoma RD cells is regulated by reciprocal, functional interactions between myostatin, p38 and extracellular regulated kinase signalling pathways. Eur J Cancer. 2011;47:1095–1105. doi: 10.1016/j.ejca.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Paulino AC, Okcu MF. Rhabdomyosarcoma. Curr Probl Cancer. 2008;32:7–34. doi: 10.1016/j.currproblcancer.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Panta P, Felix D. Rhabdomyosarcoma: a rapidly growing malignancy. Pan Afr Med J. 2015;22:121. doi: 10.11604/pamj.2015.22.121.7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H, Xie S, Liu X, Wu H, Lin X, Gu J, Wang H, Duan Y. Matrine alters microRNA expression profiles in SGC-7901 human gastric cancer cells. Oncol Rep. 2014;32:2118–2126. doi: 10.3892/or.2014.3447. [DOI] [PubMed] [Google Scholar]
- 26.Meng F, Zhang ZX, Xie J, Huang CB, Liu Y, Liao YG. Effects of matrine on the apoptosis and the expression of PEG10 in human hepatocarcinoma cell Line HepG2. J Pract Med. 2014;10:1523–1526. (In Chinese) [Google Scholar]
- 27.Gao XH, Xu W, Li WJ, Ma HW, Bai YL. Anti-proliferative effects of matrine on human neuroblastoma SK-N-SH cells Chin. J Exp Trad Med Formulae. 2013;23:196–199. (In Chinese) [Google Scholar]
- 28.Yu Q, Chen B, Zhang X, Qian W, Ye B, Zhou Y. Arsenic trioxide-enhanced, matrine-induced apoptosis in multiple myeloma cell lines. Planta Med. 2013;79:775–781. doi: 10.1055/s-0032-1328554. [DOI] [PubMed] [Google Scholar]
- 29.Yan F, Liu Y, Wang W. Matrine inhibited the growth of rat osteosarcoma UMR-108 cells by inducing apoptosis in a mitochondrial-caspase-dependent pathway. Tumour Biol. 2013;34:2135–2140. doi: 10.1007/s13277-013-0744-9. [DOI] [PubMed] [Google Scholar]
- 30.Guo L, Xue TY, Xu W, Gao JZ. Matrine promotes G0/G1 arrest and down-regulates cyclin D1 expression in human rhabdomyosarcoma cells. Panminerva Med. 2013;55:291–296. [PubMed] [Google Scholar]
- 31.Tan C, Qian X, Jia R, Wu M, Liang Z. Matrine induction of reactive oxygen species activates p38 leading to caspase-dependent cell apoptosis in non-small cell lung cancer cells. Oncol Rep. 2013;30:2529–2535. doi: 10.3892/or.2013.2727. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Xu Y, Ji W, Li X, Sun B, Gao Q, Su C. Anti-tumor activities of matrine and oxymatrine: literature review. Tumour Biol. 2014;35:5111–5119. doi: 10.1007/s13277-014-1680-z. [DOI] [PubMed] [Google Scholar]
- 33.Guo L, Xue TY, Xu W, Gao JZ. Effects of matrine on the proliferation and apoptosis of human rhabdomyosarcoma RD cells. Zhongguo Dang Dai Er Ke Za Zhi. 2012;14:780–784. (In Chinese) [PubMed] [Google Scholar]
- 34.Li YX. Effects and mechanisms of matrine on proliferation and apoptosis of human rhabdomyosarcoma cell RD. Seek Med Ask Med: 2012;10:295–296. [Google Scholar]
- 35.Miao L, Guo S, Zhang J, Kim WY, Huang L. Nanoparticles with precise ratiometric co-loading and co-delivery of gemcitabine monophosphate and cisplatin for treatment of bladder cancer. Adv Funct Mater. 2014;24:6601–6611. doi: 10.1002/adfm.201401076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sárosi V, Lénárt T. Gemcitabine-cisplatin combination in the first line treatment of non-small cell lung cancer. Our experience and analysis of safety. Magy Onkol. 2003;47:189–193. (In Hungarian) [PubMed] [Google Scholar]
- 37.Solár P, Horváth V, Kleban J, Koval' J, Solárová Z, Kozubík A, Fedorocko P. Hsp90 inhibitor geldanamycin increases the sensitivity of resistant ovarian adenocarcinoma cell line A2780cis to cisplatin. Neoplasma. 2007;54:127–130. [PubMed] [Google Scholar]
- 38.Huang Q, Lu G, Shen HM, Chung MC, Ong CN. Anti-cancer properties of anthraquinones from rhubarb. Med Res Rev. 2007;27:609–630. doi: 10.1002/med.20094. [DOI] [PubMed] [Google Scholar]
- 39.Yin PH, Liu X, Qiu YY, Cai JF, Qin JM, Zhu HR, Li Q. Anti-tumor activity and apoptosis-regulation mechanisms of bufalin in various cancers: new hope for cancer patients. Asian Pac J Cancer Prev. 2012;13:5339–5343. doi: 10.7314/APJCP.2012.13.11.5339. [DOI] [PubMed] [Google Scholar]
- 40.Tang MX. Reduction of metastasis of lung cancer by ‘Fufang Shengmai-compound pulse inducer’ - a preliminary report on experimental, clinical and pathologic study with reference to the ultrastructure. Zhonghua Zhong Liu Za Zhi. 1983;5:176–179. (In Chinese) [PubMed] [Google Scholar]
- 41.Xiao WL, Motley TJ, Unachukwu UJ, Lau CB, Jiang B, Hong F, Leung PC, Wang QF, Livingston PO, Cassileth BR, et al. Chemical and genetic assessment of variability in commercial Radix Astragali (Astragalus spp.) by ion trap LC-MS and nuclear ribosomal DNA barcoding sequence analyses. J Agric Food Chem. 2011;59:1548–1556. doi: 10.1021/jf1028174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amantana A, London CA, Iversen PL, Devi GR. X-linked inhibitor of apoptosis protein inhibition induces apoptosis and enhances chemotherapy sensitivity in human prostate cancer cells. Mol Cancer Ther. 2004;3:699–707. [PubMed] [Google Scholar]