Abstract
Estrogens affect dopamine transmission in the striatum, increasing dopamine availability, maintaining D2 receptor density, and reducing the availability of the dopamine transporter. Some of these effects of estrogens are rapid, suggesting that they are mediated by membrane associated receptors. Recently our group demonstrated that there is extra-nuclear labeling for ERα, ERβ, and GPER1 in the striatum, but that ERα and GPER1 are not localized to dopaminergic neurons in this region. GABAergic neurons are the most common type of neuron in the striatum, and changes in GABA transmission affect dopamine transmission. Thus, to determine whether ERα or GPER1 are localized to GABAergic neurons, we double labeled the striatum with antibodies for ERα or GPER1 and GABA and examined them using electron microscopy. Ultrastructural analysis revealed that ERα and GPER1 are localized exclusively to extranuclear sites in the striatum, and ~35% of the dendrites and axon terminals labeled for these receptors contain GABA immunoreactivity. Binding at membrane-associated ERα and GPER1 could account for rapid estrogen-induced decreases in GABA transmission in the striatum, which, in turn, could affect dopamine transmission in this region.
Keywords: GPR30, membrane estrogen receptor alpha, γ-Aminobutyric acid, electron microscopy
1. Introduction
Estrogens can increase dopamine transmission in the dorsal striatum (STR). Increases in estrogens across the estrous cycle, and estradiol (E2) replacement in ovariectomized (OVX) rats, attenuate dopamine reuptake in the STR [1–3], possibly by reducing the availability of the dopamine transporter [4]. Furthermore, chronic E2 treatment results in significant increases in dopamine D2 receptor binding in the STR [5], and systemic injections of E2 are associated with higher levels of amphetamine-induced dopamine release in the STR [1,6]. These E2-induced increases in dopamine release occur rapidly, which suggests that estrogens act through non-genomic mechanisms in this region [1,3].
Our recent electron microscopic study [7] demonstrates that membrane-associated ERα and GPER1 are prevalent in the STR, and membrane associated ERβ is also present at lower levels. ERα, ERβ, and GPER1 were observed exclusively at extranuclear sites, and were localized predominantly to presynaptic profiles, either axons or axon terminals, suggesting that estrogens alter striatal transmission via presynaptic mechanisms [7]. These membrane-associated estrogen receptors provide a potential mechanism for the rapid effects of E2 in the STR. A dual labeling study observed no colocalization of immunoreactivity for ERα or GPER1 and TH, suggesting that these estrogen receptors (ERs) are not localized to dopamine neurons in the STR [7]. A second experiment was conducted examining cholinergic interneurons, identified using an immunolabel for VAChT, which demonstrated that ~10% of ERα and GPER1 -labelled profiles in the STR are cholinergic [7]. Therefore a low proportion of ERs are localized to cholinergic interneurons, but a large proportion of ERs in the STR is localized to an unknown neuron type.
Previous research suggests that the remaining ERs in the STR may be localised to GABA neurons, as this has been observed in other brain regions. Membrane-associated ERα has been observed in the hypothalamus using electron microscopy [8], and light microscopy has demonstrated that ERα is localized to GABAergic neurons in preoptic and periventricular nuclei of the hypothalamus (for review see 14). Membrane-associated ERα and GPER1 are observed throughout the hippocampus, primarily in interneurons of the CA1 and dentate gyrus [10,11]. The majority of neurons in the STR are GABAergic interneurons and projection neurons [12], so it seems likely that the remaining membrane-associated ERs are in these GABA neurons. Systemic injections of E2 rapidly reduce GABA concentration in the STR [12,13], and antagonizing GABAergic neurons in the STR increases DA availability [14]. These results indicate that E2 may alter GABAergic transmission in the STR, supporting the hypothesis that the remaining estrogen receptor labelled profiles are associated with GABA neurons. This experiment used electron microscopy with dual labeling for ERα or GPER1 and GABA in tissue from the STR to determine if ERs are localized to GABAergic neurons. Similar to our previous study, ERβ was not examined in this dual labelling experiment because sufficiently low levels of ERβ were observed in the previous single labeling study [7], to make it unlikely that colocalization between with GABA would be observed (see 4.1 Methodological Considerations).
2. Method
2.1 Animals
Three adult female Sprague Dawley rats from Charles River Laboratories (Wilmington, MA), approximately 225–250g on arrival, were pair-housed with ad libitum access to food and water. Previous experiments have demonstrated that a sample size of 3 rats is sufficient for electron microscopy studies like the one presented here [11, 24–26]. In the animal colony, there was a 12:12 light/dark cycle, with lights on at 6:00am. Tissue for this experiment was taken from rats in the diestrus phase of the estrous cycle. Estrous phase was verified by measuring uterine weights and plasma estradiol levels from blood samples [15]. All procedures were in accordance with the National Institutes of Health guidelines and approved by the Weill Cornell Medicine Institutional Animal Care and Use Committee. The rats used in these experiments are the same as those used in a previous experiment [15].
2.2 Antisera
ERα
A rabbit polyclonal antiserum (AS409) produced against almost the full peptide for the native rat ERα (aa 61 through the carboxyl terminus) was supplied by S. Hayashi. This antibody was previously tested for specificity, and shown to recognize both ligand bound and unbound receptors [18,19]. On immunoblots of uterine lysates, this antiserum recognizes one major band migrating at ~67kD (the molecular weight of ERα; [10]). Preadsorption of the antibody with purified ERα resulted in no detectable bands in any of these locations [10].
GPER1
These experiments used a rabbit polyclonal antiserum generated against a synthetic peptide, CAVIPDSTEQSDVRFSSAV (Multiple Peptide Systems, San Diego, CA), derived from the C-terminus of the human GPER-1 receptor [18]. In Western blots, this affinity purified antibody specifically recognizes a 38-kD band that corresponds to the mature 351-amino acid GPER-1 polypeptide, and does not recognize either estrogen receptor α or β [18]. In brains fixed with 4% paraformaldehyde, immunoreactivity was greatly reduced when the antibody was preadsorbed with 10mg/ml of purified C-terminal peptide [19].
GABA
A third antibody, provided by A. Towle, formerly affiliated with Dept. of Neurology at Cornell University Medical College, was used for identification of GABA. A rat polyclonal antiserum was produced against GABA-glutaraldehyde-hemocyanin conjugates, and was specificity tested using preadsorption with GABA-BSA, which eliminated immunoreactivity [20]. Additionally immunoreactivity of this antiserum has been reported to be consistent with the specificity of other GABA-antisera [20].
2.3 Tissue preparation
Rats were deeply anesthetised with sodium pentobarbital (150 mg/kg, i.p.) and were perfused through the ascending aorta sequentially with: 10ml heparin (1000 U/ml) in saline; 50 ml of 3.75% acrolein (Polysciences, Washington, PA) in 2% paraformaldehyde and 0.1 M phosphate buffer (PB; pH 7.4), and 200 ml of 2% paraformaldehyde in PB. Brains were removed, cut into four 5mm blocks, and postfixed in 2% paraformaldehyde in PB for 30 minutes. The brains were sectioned coronally at 40 μm thickness on a vibrating microtome (Vibratome; Leica) and stored in 30% sucrose and 30% ethylene glycol in PB [21] at −80°C.
Tissue sections containing the STR (Fig 1) were rinsed in PB and coded with hole punches so that they could be pooled in single containers. Additionally, a tissue section containing the ventromedial and arcuate nuclei of the hypothalamus was included in the immunohistochemical procedure as a positive control. Abundant ERα immunoreactivity (IR) is present in this region [22], so the success of immunolabelling could be confirmed prior to processing the STR for EM. Sections were incubated in 1% sodium borohydride in PB for 30 minutes to remove any active aldehydes. Tissue then was rinsed in PB, followed by 0.1M Tris-buffered saline (TBS; pH 7.6), and was incubated for 30 minutes in 1% bovine serum albumin (BSA) in TBS to reduce non-specific labeling.
Figure 1.
The area of the dorsal striatum examined in this experiment.
2.4 Immunohistochemical labeling and tissue fixation and embedding
Free floating tissue sections containing the STR from three rats were processed for immunohistochemical localization of ERα or GPER1 [23]. Briefly, sections were incubated in anti-rabbit ERα (1:10,000 dilution) or GPER1 (1:1000) in 0.1% BSA in TBS for 24 hours at room temperature, and 4 days at 4°C. One day prior to processing GABA antisera (1:2000 dilution) was added to the diluent. For immunoperoxidase labeling, sections were incubated in 1) biotinylated donkey anti-rabbit immunoglobulin (IgG; diluted 1:400; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) in 0.5% BSA in TBS, 30 minutes; 2) avidin-biotin complex (Vector, Burlingame, CA), 30 minutes; and 3) 3,3-diaminobenzidine (DAB, Aldrich, Milwaukee, WI) and H2O2 in TBS, 6–7 minutes. For immunogold labeling sections were incubated in a 1:50 dilution donkey anti-rat conjugated to 1-nm colloidal gold particles (Electron Microscopy Sciences [EMS], Fort Washington, PA) and a 0.001% gelatin and 0.08% BSA in 0.01M phosphate buffered saline (PBS) for 2hrs. Sections were rinsed in PBS and incubated in 1.25% glutaraldehyde in PBS for 10 minutes. Sections were then rinsed in PBS, washed in 0.2M sodium citrate (pH 7.4), and incubated in a silver solution for ~7min (IntenSE; GE Healthcare) to enhance the conjugated gold particles. Following immunolabelling sections were fixed for 60min in 2% osmium tetroxide in PB, dehydrated through a graded series of ethanols and propylene oxide, and embedded in EMbed 812 (EMS) between two sheets of Aclar [21]. Ultrathin sections (~70nm thick) were taken through the dorsal region of the STR (Fig 1) using a Leica UCT ultratome. The tissue was collected on copper grids (EMS) and then was counterstained using Reynolds’ lead citrate and uranyl acetate.
2.5 Analyses
Sections from the STR were examined under a Philips CM10 electron microscope with an AMT digital camera. The subcellular distribution of each ER was examined in two sections per rat; a 54μm2 area of each section was counted in each section and categorized as: dendrites, dendritic spines, axons, axon terminals, or glia. The total number of labelled profiles were summed for the two sections, and averaged across the three rats. Tissue selected for analysis was taken from a depth of 0.2–1.5μm from the plastic-tissue interface, and only samples thin sectioned evenly across the plastic tissue interface were included in analyses. Soma were not included in the quantification analyses, as they frequently occupy more than half of the area analyzed, reducing the overall number of estrogen receptor immunoreactive profiles observed. Final photomicrographs were generated from digital images, where brightness and contrast were adjusted using GIMP 2.8. Figures were assembled in Microsoft PowerPoint 2013.
The type of neuronal profile was determined using the description of ultrastructural morphology from Peters et al. [24]. Dendrites were large profiles (usually between 1.0 and 2.0 μm) that contained regular microtubule arrays and were sometimes contacted by terminals. Dendritic spines were small (usually between 0.3 and 0.4 μm) and sometimes contained a spine apparatus or budded from dendritic shafts and formed synaptic contacts with axon terminals. Axon profiles were less than 0.2μm in diameter, contained a few small vesicles, and did not form synapses within the plane of section. Axon terminals had a cross-sectional diameter greater than 0.3μm and contained numerous synaptic vesicles, and sometimes formed synapses with other neuronal profiles. Glial profiles were recognized by their conformation to the boundaries of other profiles, and their lack of microtubules. Finally, soma were identified by their extremely large size, a lack of microtubules and high numbers of cellular organelles. All sections were assessed for nuclear labeling, however, soma were not included in the quantitative analyses, as they frequently occupy more than half of the area counted for analysis, reducing the overall number of estrogen receptor immunoreactive profiles.
3. Results
The proportions of both ERα and GPER1 immunoreactive profiles observed in the STR were comparable to those observed in the previous study [7]. The majority of ERα- and GPER1- IRs were observed at presynaptic sites, associated with axons (<0.15μm) or axon terminals (0.4–1.5μm; see Table 1). Semi-quantitative analysis demonstrated that 36% of ERα-IR was observed in axons and 32% was localized to axon terminals, while 40% of GPER1-IR was localized to axons and 32% was localized to axon terminals. ERα- and GPER1- IRs were often observed at the membrane in axons and axon terminals, and in close proximity to small synaptic vesicles (SSVs) in axon terminals. Immunolabelling for ERα and GPER1 was also observed at postsynaptic sites, in dendrites and dendritic spines. Dendritic shafts accounted for 10% of ERα-labeled profiles and 13% of GPER1 labeled profiles, while dendritic spines accounted for 7% and 3 % of ERα and GPER1 immunoreactive profiles, respectively. In dendritic shafts, peroxidase reaction product was often affiliated with the mitochondrial and plasma membranes, and with microtubules. In dendritic spines, immunolabelling for these ERs accumulated in the spine head, and was observed on the plasma membrane particularly near the post-synaptic density. Additionally, 13% of profiles labeled for ERα and 11% of the profiles labeled for GPER1 were glia. Both ERα- and GPER1- IRs were observed at mitochondrial membranes, again paralleling findings from the previous study in the STR [7]. Also consistent with previous findings, GABA-IR was commonly observed in terminals, dendrites, and neuronal perikarya in the STR; axons and dendritic spines with GABA-IR were infrequently observed [25,26].
Table 1.
Distribution of ERα or GPER1 and GABA in the dorsal striatum
| Receptor | ERα | GPER1 | ERα + GABA | GPER1 + GABA | ||
|---|---|---|---|---|---|---|
| Dendrites | % | 10.8 | 13.0 | 35.3 | 44.1 | |
|
|
||||||
| # SEM | 28.3 ±0.3 | 34.0 ±1.7 | 10.0 ± 0.1 | 15.0 ±1.0 | ||
|
| ||||||
| Spines | % | 7.01 | 3.44 | 7.27 | 7.41 | |
|
|
||||||
| # SEM | 18.33 ±1.2 | 9.0 ±0.4 | 1.3 ±0.3 | 0.7 ±0.7 | ||
|
| ||||||
| Axons | % | 36.1 | 40.0 | 7.1 | 3.8 | |
|
|
||||||
| # SEM | 94.3 ±2.9 | 104.7 ±3.7 | 6.7 ±1.2 | 4.0 ±0.6 | ||
|
| ||||||
| Terminals | % | 32.5 | 32.1 | 27.1 | 30.2 | |
|
|
||||||
| # SEM | 85.0 ±0.6 | 84.0 ±9.9 | 23.0 ±3.1 | 25.3 ±3.3 | ||
|
| ||||||
| Glia | % | 13.2 | 11.3 | 4.8 | 14.6 | |
|
|
||||||
| # SEM | 34.7 ±5.5 | 29.7 ±5.7 | 1.7 ±0.7 | 4.3 ±1.9 | ||
|
| ||||||
| Total | % | 100 | 100 | 16.9 | 20.2 | |
|
|
||||||
| # SEM | 261.3 ±7.8 | 262.0 ±9.8 | 44.3 ±2.0 | 53.0 ±5.6 | ||
Percentage of total IR profiles and number of IR profiles, and the corresponding standard error, observed in ~6000nm area of the dorsal STR, averaged across rats.
3.1 ERα and GABA most frequently colocalize in dendrites and terminals
There are moderate levels of colocalization between ERα and GABA in the STR. Colocalization of ERα- and GABA- IRs was most commonly observed in dendritic shafts (Fig. 2 A, B), with 35.3% of ERα immunoreactive dendrites containing GABA-IR (see Table 1). Colocalization of GABA- and ERα- IRs was also frequently (27.1%) observed in axon terminals (Fig 2B). Comparatively, lower levels of colocalization (~7%) between ERα- and GABA- IRs were observed in axons and dendritic spines. Glial profiles containing ERα- and GABA- IRs were infrequently (4.8%). Additionally, ERα-IR was occasionally observed in GABA immunoreactive soma, associated with the membrane or with mitochondria, although these profiles were not included in quantitative analyses.
Figure 2.
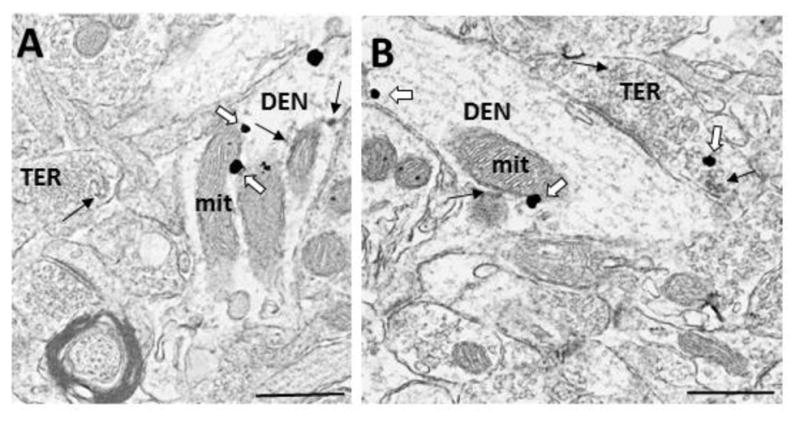
Electron micrographs demonstrating colocalization of ERα and GABA in the dorsal striatum. A) A dendrite (DEN) containing immunogold labeling for GABA and immunoperoxidase labeling for ERα, which is associated with the membrane and a mitochondrion, forms a synapse with a GABA- and ERα immunoreactive terminal (TER). B) ERα- and GABA -IRs in a dendrite (DEN) that forms a synapse with an unlabeled terminal, and a terminal (TER) containing ERα-IR. Black arrow = immunoperoxidase for ERα, White arrow = immunogold for GABA, bar = 500nm.
3.2 GPER1 and GABA most frequently colocalize in dendrites and terminals
There were moderate levels of colocalization between GPER1- and GABA- IRs in the STR. GPER1-IR was most frequently (40.1%) colocalized with GABA-IR in dendritic shafts (Fig 3A; Table 1). GPER1- and GABA- IRs also colocalized in axon terminals (30.2%; Fig 3B). Similar to the findings with ERα, low levels of colocalization were observed between GABA- and GPER1- IRs in dendritic spines and axons. Specifically, 7.4% of GPER1-labeled dendritic spines contained GABA-IR, and 3.8% of GPER1-labeled axons contained GABA-IR. Glia also colocalized GPER1- and GABA- IRs (14.6%). Finally, GPER1-IR was observed in GABAergic soma (Fig 3C), associated with the cell membrane an cellular organelles, including mitochondria and Golgi bodies, but soma were not included in quantitative analyses.
Figure 3.
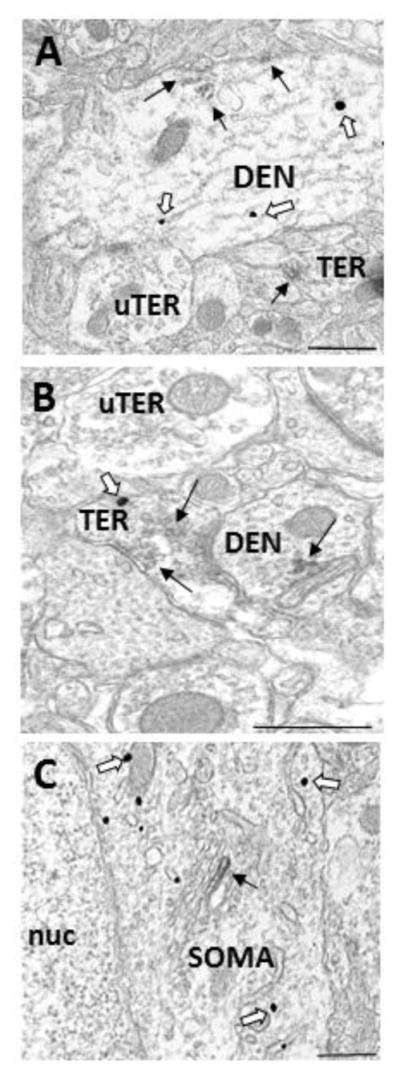
Electron micrographs demonstrating colocalization of GPER1 and GABA in the dorsal striatum. A) A dendrite (DEN) containing GABA- and GPER1- IRs associated with microtubules and the plasma membrane. B) A terminal (TER) containing GABA- and GPER1- IRs associated with small synaptic vesicles, which forms a synapse with a GPER1 immunoreactive dendrite (DEN). C) GABA-IR in a soma (SOM) containing GPER1-IR that is associated with a Golgi body. Black arrow = immunoperoxidase for GPER1, White arrow = immunogold for GABA, bar = 500nm.
4. Discussion
A moderate proportion of both ERα and GPER1 were localized to GABAergic neuronal profiles in the STR, suggesting a possible mechanism for previous research demonstrating that estrogens rapidly decreased GABA availability in the STR [14,27]. The results of this study are similar to the results of our previous experiments examining ERs in the STR [7], increasing confidence in these findings. The greatest proportion of ERα and GPER1 colocalization with GABA was observed in dendritic shafts, and a substantial proportion of ERα and GPER1 immunoreactive axon terminals were GABAergic. Additionally, GPER1 was localized to glial cells that also contained GABA-IR. There were low levels of colocalization between these estrogen receptors and GABA in axons and dendritic spines, likely due to the low levels of GABA-IR observed in these profiles. The findings of this experiment clearly demonstrate that a proportion of ERα and GPER1 in the STR are localized to GABAergic neurons, where they are observed exclusively at extranuclear sites.
4.1 Methodological Considerations
Methodological considerations are discussed in detail in our previous publication [7]. Briefly, the immunolabelling methods used here lead to excellent preservation of cellular morphology allowing for discrete localization of antigens [28]. All tissue sections were identical in size and were taken near the plastic tissue interface to ensure that differences in antigen penetration did not affect the results of these experiments. Immunoreactivity for ERα and GPER1 is discrete and does not fill the entire profile. Thus, lack of immunoreactivity for ERs in a single 70 nm thick section does not demonstrate that the profile lacks ERs. Additionally, for the dual-labeling analyses, the probability of detecting both immunomarkers in the same plane of section is decreased, particularly for small profiles. Consequently, the quantification analyses presented here are conservative, underestimating the number of ERs, and the frequency with which these receptors are localized to GABAergic profiles.
4.2 ERα and GPER1 are localized to GABAergic neurons in the STR
Approximately one third of axon terminals with ERα- and GPER1- IRs were GABAergic. These ERs are positioned to directly affect transmitter release from terminals, which corresponds to previous research that demonstrates that systemic injections of E2 rapidly (<30min) attenuate K+ -evoked GABA release in the STR [12,27]. There is evidence that dopamine release in the STR is inhibited by GABA [29,30], thus E2-induced decreases in GABA could increase dopamine availability in the STR. More recently it was shown that E2-induced decreases in GABA affect rotational behaviour, which is dependent on dopamine transmission in the striatum, suggesting that E2 –induced changes in GABA alter dopamine transmission in this region [27]. The localization of ERα and GPER1 to GABAergic terminals provides a potential mechanism for estrogens’ effects on GABA transmission, and a means by which estrogens could indirectly affect dopamine transmission in the STR.
Additionally, approximately one third of the ERα and GPER1 immunoreactive dendrites were GABAergic. This suggests that estrogens can also affect postsynaptic transmission in GABAergic neurons of the STR. The localization of ERs to dendrites of GABAergic neurons is of particular interest because previous research has demonstrated that the majority of dopaminergic synapses in the STR are onto GABAergic medium spiny neurons [38,39]. Additionally, 70% of the dopamine terminals in the STR synapse on dendritic shafts, potentially dendrites of GABA neurons [38]. Thus, ERs localized to GABAergic dendrites could be in close proximity to dopamine-GABA synapses, ideally positioned to modify dopamine-GABA interactions in the STR. This suggests a second potential mechanism through which binding at ERs could affect GABA transmission in the STR.
In the STR, GABAergic spiny projection neurons and interneurons are the majority (~90%) while the cholinergic interneurons are the remainder [31]. Terminals within the STR can arise from these interneurons as well as dopaminergic or glutamatergic projection neurons from the substantia nigra and cortex, respectively [31,32]. In a previous experiment ERα and GPER1 were not observed in dopaminergic neurons in the STR, and a low proportion of these receptors were observed in cholinergic interneurons (~10%) [7]. In this study ~30% of ER-immunoreactive terminals and ~40% of ER-immunoreactive dendrites were GABAergic, but it is very likely that these are conservative estimates due to the nature of the technique. These electron microscopy studies observe the highest proportion of ERs in GABA neurons, which are the most common neuron type in this brain region, suggesting that estrogens may elicit the majority of their effects in the STR by altering GABA transmission. Membrane-associated ERs commonly are associated with GABAergic neurons in the hippocampus [33,34], hypothalamus [8,9], and now in the STR, and E2 affects GABA transmission in all three of these brain regions [12,27,34–37]. As such, this experiment provides further support for the hypothesis that GABAergic neurons are major targets for estrogens actions in the central nervous system [35].
Finally, it is interesting that his experiment observed GPER1, and to a lesser extent ERα, in glia containing GABA-IR. Previous research has demonstrated that there is non-synaptic GABA release from astrocytes, which is important for the maintenance of tonic GABA tone in the cerebellum, and is also implicated in tonic GABA levels in the thalamus, and hippocampus [40]. Since GABA is important in neuron-glial interactions, and GPER1 are localized to GABA-containing glia, it is possible that estrogens could affect glial GABA release, although research is needed to confirm this speculation.
4.3 Conclusion
This experiment demonstrated that ERα and GPER1 are localized to GABA neurons in the STR. ERs were observed in GABAergic terminals and dendrites, where they are positioned to modulate transmission at GABA synapses. These ERs on GABAergic profiles are a possible mechanism for the rapid E2-induced decreases in GABA in the STR, and suggest that estrogens may indirectly affect dopamine transmission in this region by decreasing GABA availability.
Highlights.
Estrogen receptors have been shown to be membrane associated in the dorsal striatum
Here we confirm that and show that they are localized to GABA neurons
Acknowledgments
GRANT SUPPORT: Supported by a discovery grant from the Natural Sciences and Engineering Research Council (NSERC) of Canada (WGB) and NIH grants DA08259, HL096571, HL098351, AG016765 (TAM). The CSBN is a “groupe de recherche” funded by the Fonds de Recherche du Québec – Santé.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Becker JB, Rudick CN. Rapid effects of estrogen or progesterone on the amphetamine-induced increase in striatal dopamine are enhanced by estrogen priming: a microdialysis study. Pharmacol Biochem Behav. 1999;64:53–57. doi: 10.1016/s0091-3057(99)00091-x. http://www.ncbi.nlm.nih.gov/pubmed/10494997. [DOI] [PubMed] [Google Scholar]
- 2.Thompson TL. Attenuation of dopamine uptake in vivo following priming with estradiol benzoate. Brain Res. 1999;834:164–167. doi: 10.1016/s0006-8993(99)01508-5. [DOI] [PubMed] [Google Scholar]
- 3.Becker JB. Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotational behavior during microdialysis. [accessed January 27, 2016];Neurosci Lett. 1990 118:169–71. doi: 10.1016/0304-3940(90)90618-j. http://www.ncbi.nlm.nih.gov/pubmed/2125712. [DOI] [PubMed] [Google Scholar]
- 4.Watson CS, Alyea RA, Hawkins BE, Thomas ML, Cunningham KA, Jakubas AA. Estradiol effects on the dopamine transporter – protein levels, subcellular location, and function. J Mol Signal. 2006;1:5. doi: 10.1186/1750-2187-1-5. http://www.ncbi.nlm.nih.gov/pubmed/17224081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landry M, Lévesque D, Di Paolo T. Estrogenic properties of raloxifene, but not tamoxifen, on D2 and D3 dopamine receptors in the rat forebrain. Neuroendocrinology. 2002;76:214–222. doi: 10.1159/000065951. http://www.karger.com/doi/10.1159/000065951. [DOI] [PubMed] [Google Scholar]
- 6.Becker JB. Direct effect of 17 beta-estradiol on striatum: sex differences in dopamine release. Synap New York Ny. 1990;5:157–164. doi: 10.1002/syn.890050211. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=2309159. [DOI] [PubMed] [Google Scholar]
- 7.Almey A, Filardo EJ, Milner TA, Brake WG. Estrogen Receptors Are Found in Glia and at Extranuclear Neuronal Sites in the Dorsal Striatum of Female Rats: Evidence for Cholinergic But Not Dopaminergic Colocalization. Endocrinology. 2012;153:5373–5383. doi: 10.1210/en.2012-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blaustein JD, Lehman MN, Turcotte JC, Greene G. Estrogen receptors in dendrites and axon terminals in the guinea pig hypothalamus. Endocrinology. 1992;131:281–90. doi: 10.1210/endo.131.1.1612006. [DOI] [PubMed] [Google Scholar]
- 9.Herbison AE. Estrogen regulation of GABA transmission in rat preoptic area. [accessed January 27, 2016];Brain Res Bull. 1997 44:321–6. doi: 10.1016/s0361-9230(97)00210-4. http://www.ncbi.nlm.nih.gov/pubmed/9370195. [DOI] [PubMed] [Google Scholar]
- 10.Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429:355–371. http://www.ncbi.nlm.nih.gov/pubmed/11116225. [PubMed] [Google Scholar]
- 11.Waters EM, Thompson LI, Patel P, Gonzales AD, Ye HZ, Filardo EJ, et al. G-protein-coupled estrogen receptor 1 is anatomically positioned to modulate synaptic plasticity in the mouse hippocampus. J Neurosci. 2015;35:2384–97. doi: 10.1523/JNEUROSCI.1298-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu M, Watson CJ, Kennedy RT, Becker JB. Estradiol attenuates the K+-induced increase in extracellular GABA in rat striatum. Synapse. 2006;59:122–4. doi: 10.1002/syn.20221. [DOI] [PubMed] [Google Scholar]
- 13.Schultz KN, von Esenwein SA, Hu M, Bennett AL, Kennedy RT, Musatov S, et al. Viral vector-mediated overexpression of estrogen receptor-alpha in striatum enhances the estradiol-induced motor activity in female rats and estradiol-modulated GABA release. J Neurosci. 2009;29:1897–903. doi: 10.1523/JNEUROSCI.4647-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adermark L. Subregion-specific modulation of excitatory input and dopaminergic output in the striatum by tonically activated glycine and GABAA receptors. Front Syst Neurosci. 2011;5:85. doi: 10.3389/fnsys.2011.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams TJ, Torres-Reveron A, Chapleau JD, Milner TA. Hormonal regulation of delta opioid receptor immunoreactivity in interneurons and pyramidal cells in the rat hippocampus. Neurobiol Learn Mem. 2011;95:206–20. doi: 10.1016/j.nlm.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okamura H, Yamamoto K, Hayashi S, Kuroiwa A, Muramatsu M. A polyclonal antibody to the rat oestrogen receptor expressed in Escherichia coli: characterization and application to immunohistochemistry. J Endocrinol. 1992;135:333–341. doi: 10.1677/joe.0.1350333. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=1474341. [DOI] [PubMed] [Google Scholar]
- 17.Alves SE, Weiland NG, Hayashi S, McEwen BS. Immunocytochemical localization of nuclear estrogen receptors and progestin receptors within the rat dorsal raphe nucleus. [accessed January 27, 2016];J Comp Neurol. 1998 391:322–34. http://www.ncbi.nlm.nih.gov/pubmed/9492203. [PubMed] [Google Scholar]
- 18.Filardo EJ, Quinn JA, Bland KI, Frackelton AR. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol Balt Md. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. http://www.ncbi.nlm.nih.gov/pubmed/11043579. [DOI] [PubMed] [Google Scholar]
- 19.Hammond R, Gibbs RB. GPR30 is positioned to mediate estrogen effects on basal forebrain cholinergic neurons and cognitive performance. Brain Res. 2011;1379:53–60. doi: 10.1016/j.brainres.2010.11.098. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3046317&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lauder JM, Han VK, Henderson P, Verdoorn T, Towle AC. Prenatal ontogeny of the GABAergic system in the rat brain: an immunocytochemical study. [accessed January 27, 2016];Neuroscience. 1986 19:465–93. doi: 10.1016/0306-4522(86)90275-7. http://www.ncbi.nlm.nih.gov/pubmed/3022187. [DOI] [PubMed] [Google Scholar]
- 21.Milner TA, Waters EM, Robinson DC, Pierce JP. Degenerating processes identified by electron microscopic immunocytochemical methods. Methods Mol Biol Clift Nj. 2011;793:23–59. doi: 10.1007/978-1-61779-328-8_3. [DOI] [PubMed] [Google Scholar]
- 22.Kritzer MF. Regional, laminar, and cellular distribution of immunoreactivity for ER alpha and ER beta in the cerebral cortex of hormonally intact, adult male a... - PubMed - NCBI, Cereb. [accessed January 27, 2016];Cortex. 2002 doi: 10.1093/cercor/12.2.116. http://www.ncbi.nlm.nih.gov/pubmed/?term=Regional%2C+laminar%2C+and+cellular+distribution+of+immunoreactivity+for+ER+alpha+and+ER+beta+in+the+cerebral+cortex+of+hormonally+intact%2C+adult+male+and+female+rats. [DOI] [PubMed]
- 23.Milner TA, Waters EM, Robinson DC, Pierce JP. Degenerating processes identified by electron microscopic immunocytochemical methods. Methods Mol Biol. 2011;793:23–59. doi: 10.1007/978-1-61779-328-8_3. [DOI] [PubMed] [Google Scholar]
- 24.Peters A, Palay SL, Webster HF. The fine structure of the nervous system: neurons and their supporting cells. Oxford University Press; 1991. https://books.google.ca/books?id=eapqAAAAMAAJ. [Google Scholar]
- 25.Delle Donne KT, Sesack SR, Pickel VM. Ultrastructural immunocytochemical localization of neurotensin and the dopamine D2 receptor in the rat nucleus accumbens. J Comp Neurol. 1996;371:552–66. doi: 10.1002/(SICI)1096-9861(19960805)371:4<552::AID-CNE5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 26.Gundersen V, Ottersen OP, Storm-Mathisen J. Selective excitatory amino acid uptake in glutamatergic nerve terminals and in glia in the rat striatum: quantitative electron microscopic immunocytochemistry of exogenous (D)-aspartate and endogenous glutamate and GABA. [accessed January 27, 2016];Eur J Neurosci. 1996 8:758–65. doi: 10.1111/j.1460-9568.1996.tb01261.x. http://www.ncbi.nlm.nih.gov/pubmed/9081627. [DOI] [PubMed] [Google Scholar]
- 27.Schultz KN, von Esenwein SA, Hu M, Bennett AL, Kennedy RT, Musatov S, et al. Viral Vector-Mediated Overexpression of Estrogen Receptor- in Striatum Enhances the Estradiol-Induced Motor Activity in Female Rats and Estradiol-Modulated GABA Release. J Neurosci. 2009;29:1897–1903. doi: 10.1523/JNEUROSCI.4647-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pickel VMLC. Electron Microscopic Preembedding Double-Immunostaining Methods in Neuroanatomical Tract-Tracing Methods 2. Springer US; Boston, MA: 1989. [DOI] [Google Scholar]
- 29.Whitehead KJ, Rose S, Jenner P. Involvement of intrinsic cholinergic and GABAergic innervation in the effect of NMDA on striatal dopamine efflux and metabolism as assessed by microdialysis studies in freely moving rats. [accessed January 27, 2016];Eur J Neurosci. 2001 14:851–60. doi: 10.1046/j.0953-816x.2001.01702.x. http://www.ncbi.nlm.nih.gov/pubmed/11576189. [DOI] [PubMed] [Google Scholar]
- 30.Smolders I, De Klippel N, Sarre S, Ebinger G, Michotte Y. Tonic GABA-ergic modulation of striatal dopamine release studied by in vivo microdialysis in the freely moving rat. [accessed January 27, 2016];Eur J Pharmacol. 1995 284:83–91. doi: 10.1016/0014-2999(95)00369-v. http://www.ncbi.nlm.nih.gov/pubmed/8549640. [DOI] [PubMed] [Google Scholar]
- 31.Gerfen CR, Bolam JP. Chapter 1 - The Neuroanatomical Organization of the Basal Ganglia. Handb Behav Neurosci. 2010:3–28. http://dx.doi.org/10.1016/B978-0-12-374767-9.00001-9.
- 32.Hassler R, Haug P, Nitsch C, Kim JS, Paik K. Effect of Motor and Premotor Cortex Ablation on Concentrations of Amino Acids, Monoamines, and Acetylcholine and on the Ultrastructure in Rat Striatum. A Confirmation of Glutamate as the Specific Cortico-Striatal Transmitter. J Neurochem. 1982;38:1087–1098. doi: 10.1111/j.1471-4159.1982.tb05352.x. [DOI] [PubMed] [Google Scholar]
- 33.Hart SA, Patton JD, Woolley CS. Quantitative analysis of ER alpha and GAD colocalization in the hippocampus of the adult female rat. [accessed January 29, 2016];J Comp Neurol. 2001 440:144–55. doi: 10.1002/cne.1376. http://www.ncbi.nlm.nih.gov/pubmed/11745614. [DOI] [PubMed] [Google Scholar]
- 34.Hart SA, Snyder MA, Smejkalova T, Woolley CS. Estrogen mobilizes a subset of estrogen receptor-alpha-immunoreactive vesicles in inhibitory presynaptic boutons in hippocampal CA1. J Neurosci. 2007;27:2102–11. doi: 10.1523/JNEUROSCI.5436-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wagner EJ, Ronnekleiv OK, Bosch MA, Kelly MJ. Estrogen biphasically modifies hypothalamic GABAergic function concomitantly with negative and positive control of luteinizing hormone release. [accessed January 27, 2016];J Neurosci. 2001 21:2085–93. doi: 10.1523/JNEUROSCI.21-06-02085.2001. http://www.ncbi.nlm.nih.gov/pubmed/11245692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, et al. Rapid Signaling of Estrogen in Hypothalamic Neurons Involves a Novel G-Protein-Coupled Estrogen Receptor that Activates Protein Kinase C. [accessed January 27, 2016];J Neurosci. 2003 23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. http://www.jneurosci.org/content/23/29/9529.long. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wójtowicz T, Mozrzymas JW. Estradiol and GABAergic transmission in the hippocampus. Vitam Horm. 2010;82:279–300. doi: 10.1016/S0083-6729(10)82015-1. [DOI] [PubMed] [Google Scholar]
- 38.Pickel VM, Towle AC, Joh TH, Chan J. Gamma-aminobutyric acid in the medial rat nucleus accumbens: ultrastructural localization in neurons receiving monosynaptic input from catecholaminergic afferents. J Comp Neurol. 1988;272:1–14. doi: 10.1002/cne.902720102. [DOI] [PubMed] [Google Scholar]
- 39.Pickel VM, Chan J. Spiny neurons lacking choline acetyltransferase immunoreactivity are major targets of cholinergic and catecholaminergic terminals in rat striatum. J Neurosci Res. 1990;25:263–280. doi: 10.1002/jnr.490250302. [DOI] [PubMed] [Google Scholar]
- 40.Vélez-Fort M, Audinat E, Angulo MC. Central role of GABA in neuron-glia interactions. Neuroscientist. 2012;18:237–50. doi: 10.1177/1073858411403317. [DOI] [PubMed] [Google Scholar]


