Abstract
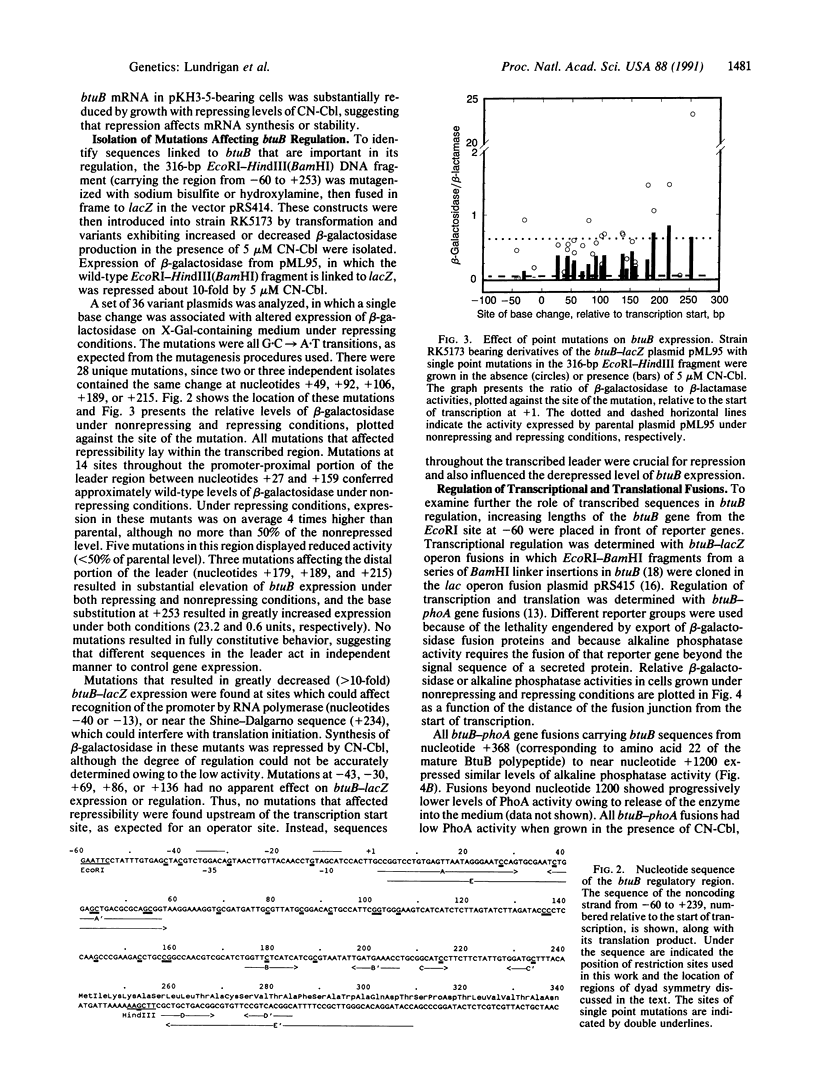
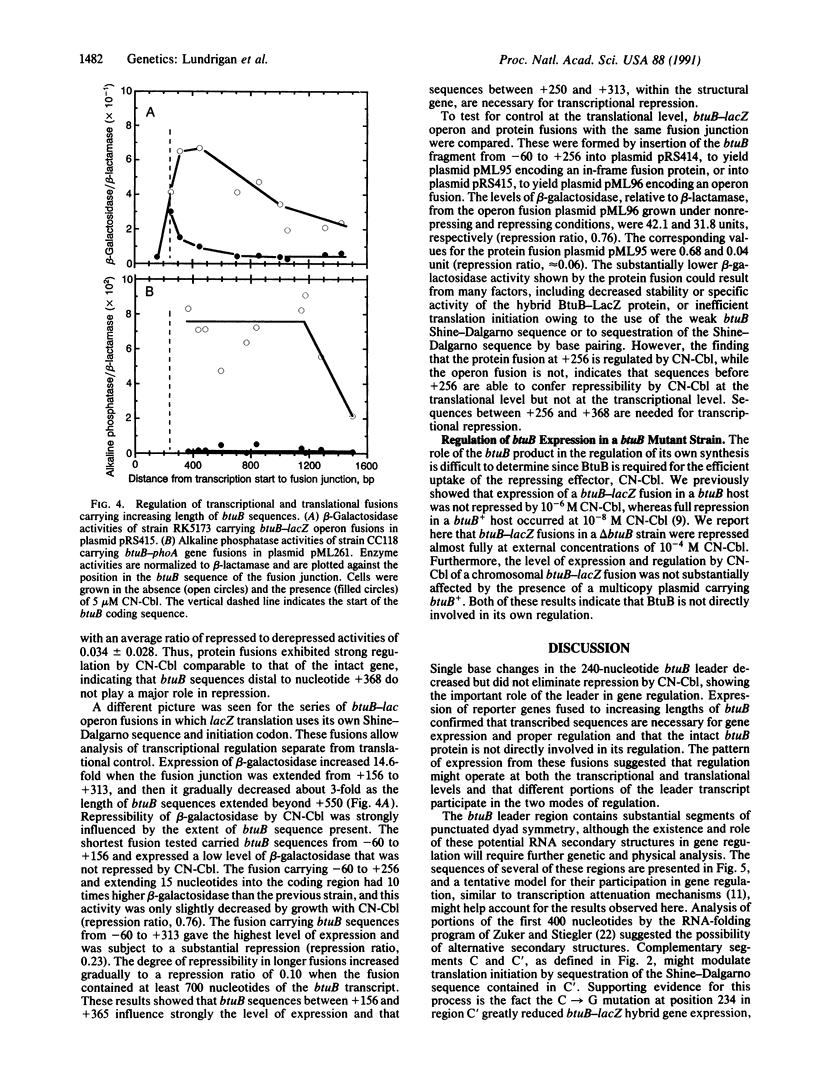
The Escherichia coli btuB gene product is an outer membrane protein required for the active transport of vitamin B12 and other cobalamins. Synthesis of BtuB is repressed when cells are grown in the presence of cobalamins. Mapping of the 5' end of the btuB transcript revealed that a 240-nucleotide transcribed leader precedes the coding sequence. Point mutations causing increased expression under repressing conditions were isolated by use of a btuB-lacZ gene fusion. Mutations at many sites within the leader region affected btuB-lacZ regulation, whereas some base changes upstream of the start of transcription affected the absolute level of expression but not its repressibility. Analysis of btuB-phoA gene fusions and btuB-lacZ operon and gene fusions of various lengths showed that sequences within the btuB coding region (between nucleotides +250 and +350) had to be present for proper expression and transcriptional regulation. Sequences within the leader region (up to +250) conferred regulation of translational fusions. These results indicate that btuB expression is controlled at both the transcriptional and translational levels and that different but possibly overlapping sequences in the transcribed region, including the coding region for the transport protein itself, mediate these two modes of regulation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Adhya S., de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem. 1981 Nov 25;256(22):11905–11910. [PubMed] [Google Scholar]
- Aufrère R., Tempête M., Bohin J. P. Regulation of expression of the gene for vitamin B12 receptor cloned on a multicopy plasmid in Escherichia coli. Mol Gen Genet. 1986 Nov;205(2):358–365. doi: 10.1007/BF00430451. [DOI] [PubMed] [Google Scholar]
- Bagg A., Neilands J. B. Molecular mechanism of regulation of siderophore-mediated iron assimilation. Microbiol Rev. 1987 Dec;51(4):509–518. doi: 10.1128/mr.51.4.509-518.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassford P. J., Jr, Bradbeer C., Kadner R. J., Schnaitman C. A. Transport of vitamin B12 in tonB mutants of Escherichia coli. J Bacteriol. 1976 Oct;128(1):242–247. doi: 10.1128/jb.128.1.242-247.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Masi D. R., White J. C., Schnaitman C. A., Bradbeer C. Transport of vitamin B12 in Escherichia coli: common receptor sites for vitamin B12 and the E colicins on the outer membrane of the cell envelope. J Bacteriol. 1973 Aug;115(2):506–513. doi: 10.1128/jb.115.2.506-513.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudmundsdottir A., Bell P. E., Lundrigan M. D., Bradbeer C., Kadner R. J. Point mutations in a conserved region (TonB box) of Escherichia coli outer membrane protein BtuB affect vitamin B12 transport. J Bacteriol. 1989 Dec;171(12):6526–6533. doi: 10.1128/jb.171.12.6526-6533.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudmundsdottir A., Bradbeer C., Kadner R. J. Altered binding and transport of vitamin B12 resulting from insertion mutations in the Escherichia coli btuB gene. J Biol Chem. 1988 Oct 5;263(28):14224–14230. [PubMed] [Google Scholar]
- Hantke K. Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol Gen Genet. 1981;182(2):288–292. doi: 10.1007/BF00269672. [DOI] [PubMed] [Google Scholar]
- Heller K., Kadner R. J. Nucleotide sequence of the gene for the vitamin B12 receptor protein in the outer membrane of Escherichia coli. J Bacteriol. 1985 Mar;161(3):904–908. doi: 10.1128/jb.161.3.904-908.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller K., Mann B. J., Kadner R. J. Cloning and expression of the gene for the vitamin B12 receptor protein in the outer membrane of Escherichia coli. J Bacteriol. 1985 Mar;161(3):896–903. doi: 10.1128/jb.161.3.896-903.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadner R. J. Repression of synthesis of the vitamin B12 receptor in Escherichia coli. J Bacteriol. 1978 Dec;136(3):1050–1057. doi: 10.1128/jb.136.3.1050-1057.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundrigan M. D., De Veaux L. C., Mann B. J., Kadner R. J. Separate regulatory systems for the repression of metE and btuB by vitamin B12 in Escherichia coli. Mol Gen Genet. 1987 Mar;206(3):401–407. doi: 10.1007/BF00428878. [DOI] [PubMed] [Google Scholar]
- Lundrigan M. D., Kadner R. J. Altered cobalamin metabolism in Escherichia coli btuR mutants affects btuB gene regulation. J Bacteriol. 1989 Jan;171(1):154–161. doi: 10.1128/jb.171.1.154-161.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. TnphoA: a transposon probe for protein export signals. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray J. M., Yanofsky C., Bauerle R. Mutational analysis of the catalytic and feedback sites of the tryptophan-sensitive 3-deoxy-D-arabino-heptulosonate-7-phosphate synthase of Escherichia coli. J Bacteriol. 1988 Dec;170(12):5500–5506. doi: 10.1128/jb.170.12.5500-5506.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds P. R., Mottur G. P., Bradbeer C. Transport of vitamin B12 in Escherichia coli. Some observations on the roles of the gene products of BtuC and TonB. J Biol Chem. 1980 May 10;255(9):4313–4319. [PubMed] [Google Scholar]
- Shortle D., DiMaio D., Nathans D. Directed mutagenesis. Annu Rev Genet. 1981;15:265–294. doi: 10.1146/annurev.ge.15.120181.001405. [DOI] [PubMed] [Google Scholar]
- Simons R. W., Houman F., Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53(1):85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]




