Abstract
The aim of the present systematic review was to analyze the potential impact of mammalian target of rapamycin (mTOR) inhibitors on the treatment of cervical squamous cell carcinoma (CSCC). A systematic literature search was conducted in PubMed, PMC, Scopus, Cochrane Library, LILACS, Web of Science, Google Scholar and ScienceDirect on January 19, 2015, without time and language restrictions. Studies that evaluated women of any age with CSCC and who received mTOR inhibitors alone or in association with other treatments were considered. Randomized and non-randomized clinical trials were included, and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist was followed. Selected studies were methodologically appraised according to the Grades of Recommendation, Assessment, Development and Evaluation method to assess the quality of evidence. Of 642 identified citations, 43 studies were fully reviewed; however, only 3 studies met the inclusion criteria and were used for qualitative analysis. Of these, two studies were phase 1 and one was a phase 2 clinical trial. The studies included were not conclusive with regard to the association between mTOR inhibitor treatment and cervical cancer. The main analysis of secondary endpoints revealed that individuals treated with other drugs in association with mTOR inhibitors achieved partial responses (15.4–33.3%) or stable disease (17.6–28%). Treatment with mTOR inhibitors in general was well tolerated in patients with metastatic disease. The predominant toxicities were grade 1 and 2. The phase 1 trials included in this review demonstrated that mTOR inhibitor treatments are feasible and safe. However, the currently available evidence is insufficient to determine the effect of mTOR inhibitors on CSCC, and further investigation in high-quality, randomized clinical trials is required.
Keywords: mammalian target of rapamycin inhibitors, cervical cancer, systematic review, evidence-based medicine
Introduction
Cervical cancer (CC) is a major public health concern, representing the fourth most commonly diagnosed cancer in women and the seventh overall, with an estimated 528,000 new cases worldwide in 2012 (1). Globally, ~266,000 mortalities from CC occurred in 2012, accounting for 7.5% of all female cancer mortalities; this number is expected to increase to 410,000 by the year 2030 (2).
Although the systemic treatment of cervical squamous cell carcinoma (CSCC) has advanced into an era of targeted drugs, such as erlotinib (3) and bevacizumab (4), the antitumor efficacies of current therapies are limited, most likely due to the high degree of cancer clonal heterogeneity, intratumoral genetic heterogeneity and cell signal complexity (5). In this context, there is an urgent necessity for more active treatment and rationally designed targeted therapies (6).
More than 95% of CSCC patients are positive for oncogenic human papillomavirus (HPV) DNA. HPV infection plays a central role in the development of this cancer, particularly infection with the high-risk subtypes, HPV 16 and 18 (7). The HPV infection has multiple intracellular effects in different signaling pathways. The phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) pathway is often dysregulated in gynecological cancers, particularly in HPV-associated tumors (6). Alterations that cause the activation and dysregulation of the PI3K/AKT/mTOR pathway may act as potentially drug-treatable targets (8).
The PI3K/AKT/mTOR signaling pathway, which functions in mammal cells, acts to coordinate important cell activities (6). Tumor cells may have a greater sensitivity to mTOR inhibitors than normal cells as a consequence of the dysregulation of mTOR and other proteins associated with this pathway in solid tumors (9). Mechanisms for pathway activation include loss of function of the tumor suppressor gene phosphatase and tensin homolog (PTEN), amplification or mutation of PI3K, amplification or mutation of AKT, activation of growth factor receptors and exposure to carcinogens (10,11).
Rapamycin was the first mTOR inhibitor to be defined; however, other analogs of rapamycin have been developed, including temsirolimus and everolimus (12). Certain natural compounds also possess mTOR inhibitor properties, such as curcumin, resveratrol and epigallocatechin gallate (13). Temsirolimus and everolimus have already been incorporated into clinical practice to treat kidney and breast cancer (14–16). Therefore the present systematic review was conducted to verify the potential effect of mTOR inhibitors on CSCC, and thereby provide support for future rationally designed strategies that may involve this pathway.
Materials and methods
Protocol and registration
This systematic review followed the the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist (17). The protocol was registered in PROSPERO (no. CRD42015016329) (18).
Eligibility criteria
Randomized and non-randomized clinical trials that evaluated women of any age with CSCC who received mTOR inhibitors alone or in association with other treatments (drugs or radiotherapy) were included.
Studies were excluded for the following reasons: i) Different target conditions, such as studies that did not use mTOR inhibitors to treat CSCC or did not verify the association between mTOR inhibitors and CSCC; ii) study assessed associations between mTOR inhibitor treatment and CSCC in vitro or in vivo in animal studies; iii) insufficient information provided regarding histological type, response or treatment.
Information sources and search strategies
Detailed individual search strategies were developed for each of the following bibliographic electronic databases: Cochrane Library (http://www.cochranelibrary.com), Google Scholar (https://scholar.google.com.br), LILACS (http://lilacs.bvsalud.org), PMC (https://www.ncbi.nlm.nih.gov/pmc/), PubMed (https://www.ncbi.nlm.nih.gov/pubmed/), ScienceDirect (http://www.sciencedirect.com), Scopus (https://www.scopus.com) and Web of Science (http://login.webofknowledge.com/). The search strategy for Pubmed included the following terms: ‘Cervical cancer’ or ‘uterine cancer’ or ‘cervix cancer’ or ‘cervical neoplasm’ or ‘cervix neoplasm’; and ‘mTOR’. The reference lists in the selected articles were also searched to identify any additional references that may have been missed in the electronic databases searches. The search was conducted through January 19th, 2015, across all databases, without date and language restrictions. The references were managed and the duplicates removed using appropriate software (EndNote; Thomson Reuters, New York, NY, USA).
Study selection
Studies were considered for inclusion in two phases. In the first phase, two reviewers (D.X.A. and S.T.E.) independently reviewed the titles and abstracts of all references. These authors selected articles that met the inclusion criteria based on their titles and abstracts. In the second phase, the two authors read the full text of all selected articles and excluded studies that did not meet the inclusion criteria. The same two authors independently reviewed all full text articles. Disagreements were resolved by consensus of the authors or by a third reviewer (E.N.S.G.).
Data collection process and data items
One reviewer (D.X.A.) collected the required information from the selected articles, including the following: Author, year, country, study design, treatment agents, number of patients with CC and CSCC included, patient population with number of prior treatments, maximum tolerated dose (MTD) of treatment, recommended dose of treatment (RD), number of partial responses (PRs), percentage of patients with stable disease (SD) lasting ≤6 months, time to treatment failure (TTF) or duration of progression-free survival (PFS), complications, main conclusions and clinical application. A second reviewer (S.T.E.) crosschecked all retrieved information. Disagreements were resolved by author consensus or by a third reviewer (E.N.S.G.).
Risk of bias in individual studies
The Grades of Recommendation, Assessment, Development and Evaluation (GRADE) approach was used to assess the quality of evidence (19). Two authors (D.X.A. and S.T.E.) completed the required criteria necessary to qualify the selected articles, which were categorized as ‘high’, ‘moderate’, ‘low’ or ‘very low’, according to the analysis of each study. The third reviewer (E.N.S.G.) was involved when required to make a final decision.
Summary measures
Any reported outcome or efficacy measurements were considered, including MTD, RD, response rate (RR), percentage of patients with SD lasting ≥6 months, PFS time, TTF and complications.
Synthesis of results
A meta-analysis was planned since the data from the included studies was considered relatively homogeneous.
Results
Study selection
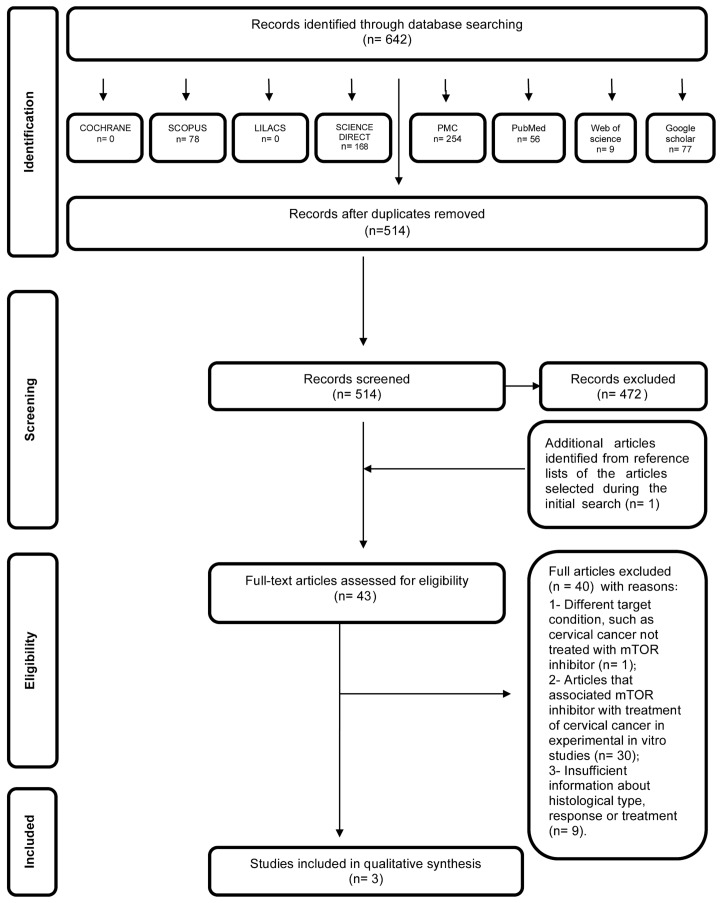
In the first phase of study selection, 642 citations were identified across the seven electronic databases and Google Scholar. Following the removal of duplicates, 514 citations remained. Comprehensive evaluation of the title and abstracts was completed and 472 articles were excluded; thus, 42 articles remained after the first phase. One additional study was included from the reference lists of the identified studies. From the 43 articles retrieved, full text reviews were conducted. This process excluded 40 studies (20–59). Finally, 3 studies were selected (60–62). A flow chart detailing the process of identification, inclusion and exclusion of studies is shown in Fig. 1.
Figure 1.
Flow diagram of literature search and selection criteria adapted from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (17).
Study characteristics
The selected studies were conducted in two countries: The USA (60,61) and Canada (62). All 3 studies were published recently, in 2011 (60), 2013 (62), 2014 (61), and all were written in English. All included articles were non-randomized clinical trials; two studies were phase 1 and one was phase 2. A summary of the descriptive characteristics of the included studies is given in Table I.
Table I.
Summary of descriptive characteristics of clinical studies in included studies.
| Author, year (ref.), country | Study design | Treatment agents | No. of patients with CC/CSCC | Patient population | MTD | RD | PR, n (%a) | SD ≥6 months | TTF or PFS | Complications | Main conclusions | Clin. app.b |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moroney et al, 2011 (60), USA | Phase 1, NR | Liposomal doxorubicin, bevacizumab and temsirolimus | 13 CC/10 CSCC | Metastatic disease; median 4 prior regimens | Level 6: Bevacizumab, 15 mg/kg, d1; liposomal doxorubicin 30 mg/m2, d1; and temsirolimus, 25 mg IV, d1, 8 and 15 | Bevacizumab, 15 mg/kg, d1; liposomal doxorubicin, 20–30 mg/ m2, d1; and temsirolimus, 25 mg IV, d1, 8 and 15 | 2 among CSCC patients (15.4%) | 17.6% (among all patients) | 112 days (among all patients)b; 172 days (among patients with PR)b | Grade 3 and 4 toxicities: Thrombocytopenia (9.5%), mucositis (6.7%), cardiac (4.1%), genitourinary (1.4%), bowel perforation (2.7%) | Combination is well tolerated with manageable side effects; 2 PRs among 13 CC patients | 2 |
| Piha-Paul et al, 2014 (61), USA | Phase 1, NR | Bevacizumab and temsirolimus | 6 CC/4 CSCC | Metastatic disease; median 4 prior regimens | Dose level 13: Temsirolimus, 25 mg IV, d1, 8 and 15; bevacizumab, 15 mg/kg IV was reached and no MTD was obtained | N/A | 2 among CSCC patients (33.3%) | 20% (among all patients) | N/A | Grade 3 and 4 toxicities: Thrombocytopenia (10%), mucositis (2%), hypertension (2%), hypercholesterolemia (2%), fatigue (7%), increased AST (2%), neutropenia (2%) | Bevacizumab and temsirolimus were well tolerated; 2 PRs among 6 CC patients | 2 |
| Tinker et al, 2013 (62), Canada | Phase 2, NR | Temsirolimus | 38 CC/22 CSCC | Metastatic disease; ≤1 prior therapy permitted | N/A | N/A | 0 | 28% (95% confidence interval, 14–43%) among all patients | 3.52 months (among all patients) | Grade 3 toxicity: fatigue (5.4%), mucositis (5.4%), rash (2.7%), lymphopenia (43.2%), anemia (16.2%), leucopenia (2.7%), hyponatremia (16.2%), hypertriglyceridemia (5.4%), hypokalemia (10.8%) | Temsirolimus was not active in this population as defined by RECIST. SD rates were notably high. | 3 |
Data not available in the original article; the authors calculated data from information available in the article (organ specific histological subtypes grouped to determine denominator).
Clinical application of mTOR inhibitors was classified as follows: 1, potential effect in cervical cancer treatment; 2, inconclusive; and 3, evidence not supportive as a drug for CSCC treatment. CC, cervical cancer; CSCC, cervical squamous cell carcinoma; MTD, maximum tolerated dose; RD, recommended dose; PR, partial response; SD ≥6 months, stable disease lasting ≥6 months; TTF, time to treatment failure; PFS, progression-free survival; NR, non-randomized; N/A, not available; d, day; IV, intravenously; AST, aspartate aminotransferase; RECIST, Response Criteria in Solid Tumors version 1.1.
Risk of bias within studies
The GRADE approach (19) was used to assess the quality of evidence of the included studies, as outlined in Table II. All studies were categorized as having a low quality level of evidence (60–62). All had serious issues with regard to the design, none had control groups, and all lacked blinding. Imprecision occurred in one study due to the extremely small number of patients included (61).
Table II.
Assessment of the quality of evidence for intervention.
| GRADE factors | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Author, year (ref.) | Study design | Limitations in study design and/or executiona | Inconsistency | Indirectness | Imprecision | Publication bias | Moderate/large effect size | Dose effect | Overall quality |
| Moroney et al, 2011 (27) | Phase 1, non-randomized | P | N | N | N | N | Not present | Not present | ++ |
| Piha-Paul et al, 2014 (28) | Phase 1, non-randomized | P | N | N | P (very few patients were included) | N | Not present | Not present | + |
| Tinker et al, 2013 (29) | Phase 2, non-randomized | P | N | N | N | N | Not present | Not present | ++ |
None of the included clinical trials had control groups, all lacked blinding and all were non-randomized. For GRADE factors (19): N, no serious limitations; P, presence of serious limitation. For overall quality (of evidence): +, very low; ++, low; +++, moderate; ++++, high.
The clinical application of the studies was also evaluated. The mTOR inhibitor application in CC was classified as 1 (potential effect in CSCC treatment), 2 (inconclusive or 3 (evidence not supportive of mTOR inhibitors a drug for CSCC treatment). A summary of descriptive characteristics of the studies is given in Table I. All studies were classified as inconclusive with regard to the effect of mTOR inhibitors in CSCC (Table I).
Synthesis of results
Study 1
The study by Moroney et al (60) evaluated 74 patients with gynecological and breast malignancies who were treated with liposomal doxorubicin, bevacizumab and temsirolimus. This included 13 CC patients, of whom 10 had CSCC. The study was a non-randomized, phase 1 clinical trial, for which the primary endpoints were to establish the MTD and characterize dose-limiting toxicities. Secondary endpoints included a preliminary assessment of antitumor efficacy. All 74 patients were heavily pretreated with a median of 4 previous lines of chemotherapy. There were two PRs in the group of patients with CSCC [treated with dose level 6: Bevacizumab, 15 mg/kg intravenously (IV), day 1; liposomal doxorubicin, 30 mg/m2 IV, day 1; and temsirolimus, 25 mg IV, days 1, 8 and 15]. The MTD for the study was reached at level 6. The RD for the corresponding phase 2 clinical trial study was as follows: Bevacizumab, 15 mg/kg, day 1; liposomal doxorubicin, 20–30 mg/m2, day 1; and temsirolimus, 25 mg IV, days 1, 8 and 15. The overall RR in this heavily pretreated population was 20.3%. Among all 74 patients included, 17.6% had SD lasting ≥6 months. The TTFs were 112 days [95% confidence interval (CI), 89–147 days] among all patients, and 172 among patients with PRs. The median overall survival (OS) time was 214 days (95% CI, 185–312 days).
All 74 patients (100%) experienced ≥1 adverse event that was at least possibly drug-related. These events were predominantly grades 1 or 2 and reversible. The treatment combination was relatively safe and well tolerated. Among the 15 responders (complete response plus PR), PI3K catalytic subunit α (PIK3CA) and PTEN statuses were known in 9 (60%) and 5 (33.3%), respectively. Of the 9 responders for whom PIK3CA mutational status was known, 4 (44.4%) were positive. Of the 5 responders for whom PTEN status was known, 3 (60%) were found to have PTEN loss. The tumor molecular analysis is listed in Table III. As the molecular alterations were not reported for each type of cancer separately, it is not possible to conclude anything regarding treatment responses and mutations in CSCC.
Table III.
Tumor molecular analysis of the included studies.
| Moroney et al (60) | Piha-Paul et al (61) | Tinker et al (62) | ||||
|---|---|---|---|---|---|---|
| Variable | n (%) | Response comments | n (%) | Response comments | n (%) | Response comments |
| Total patients included | 74 | N/A | 41 | N/A | 38 | N/A |
| KRAS mutation status | ||||||
| Number tested | 49 | N/A | 17 | N/A | 0 | N/A |
| Number with mutation | 8 (16.3%) | 8 KRAS mutation-positive patients (100%) achieved a response | 1 (5.9%) | KRAS mutation-positive patient did not achieve SD ≥6 months/PR | N/A | KRAS mutation was not tested |
| NRAS mutation status | ||||||
| Number tested | 0 | N/A | 17 | N/A | 0 | N/A |
| Number with mutation | N/A | NRAS mutation was not tested | 1 (5.9%) | NRAS mutation-positive patient did not achieve SD ≥6 months/PR | N/A | NRAS mutation was not tested |
| PIK3CA mutation status | ||||||
| Number tested | 57 | N/A | 25 | N/A | 33 | N/A |
| Number with mutation | 16 (28.1%) | 4 PIK3CA mutation-positive patients (25%) achieved a response | 1 (4.0%) | The PIK3CA mutation-positive patient achieved a PR | 8 (24.2%)a | The single patient with PR did not have PIK3CA mutation |
| PTEN status | ||||||
| Number tested | 25 | N/A | 2 | N/A | 33 | N/A |
| Number with loss | 11 (44.0%) | 5 PTEN loss-positive patients (45.5%) achieved a response | 0 (0.0%) | No PTEN loss was detected | 3 (9.1%)a | The single patient with PR did not have PTEN loss |
Data not available in the original article; the authors calculated data from information available in the article. N/A, not applicable; KRAS, Kirsten rat sarcoma viral oncogene homolog; NRAS, neuroblastoma RAS viral oncogene homolog; PIK3CA, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit α; PTEN, phosphatase and tensin homolog; PR, partial response; SD, stable disease.
Study 2
Piha-Paul et al (61) evaluated 41 patients with advanced gynecological malignancies who were treated with bevacizumab and temsirolimus. There were 6 patients with CC included, of whom 4 had CSCC. This study was a non-randomized, phase 1 clinical trial. The primary endpoints were to establish the MTD and to characterize dose-limiting toxicities. Secondary endpoints included a preliminary assessment of antitumor efficacy. All patients were heavily pretreated with a median of 4 previous lines of chemotherapy. Among all patients included, 20% had SD lasting ≥6 months. Analysis of the mutational statuses of PTEN, PIK3CA, RAS and RAF was not performed for all included patients. Of the 2 patients who achieved PRs, the mutational status was not determined in 1, while the other patient was negative for PIK3CA, RAS and RAF mutations. The 5 responders for whom PTEN status was known were found to have PTEN loss. Tumor molecular analysis is listed in Table III. As the molecular alterations were not reported for each type of cancer, conclusions regarding the association of responses and mutations in CSCC are not possible.
Grade 1 and 2 toxicities were described in 71% of the patients. The highest dose escalation was obtained (dose level 13: Bevacizumab, 15 mg/kg IV, day 1; and temsirolimus, 25 mg/kg IV, days 1, 8 and 15), and the MTD was not reached. All 41 patients experienced ≥1 adverse event that was possibly drug-related. These events were predominantly grades 1 or 2 and reversible.
Study 3
Tinker et al (62) evaluated 38 patients with CC, of whom 22 had CSCC. The study was a non-randomized, phase 2 clinical trial. The primary endpoint was the objective RR, as determined by Response Evaluation Criteria in Solid Tumors (version 1.1). Up to 1 prior line of chemotherapy for metastatic or recurrent disease was permitted. Patients were treated with temsirolimus (25 mg IV, weekly) in 4-week cycles. Only 1 PR occurred, and this patient had cervical adenocarcinoma. The median duration of SD was 6.5 months (range, 2.4–12.0 months) and the proportion of patients with SD lasting ≥6 months was 28% (95% CI, 14–43%). The median PFS time was 3.52 months (95% CI, 1.81–4.7 months). There were 11 serious adverse events among 7 patients that were possibly related to the therapy protocol. Original diagnostic material was available for molecular analysis of 33 patients. No significant association was found between any of the markers and response to temsirolimus therapy. The 5 responders for whom PTEN status was known had PTEN loss. Tumor molecular analysis is presented in Table III.
Risk of bias across studies
The selected studies used similar methods, which reduced the possibility of misinterpretation. The studies selected for this analysis were considered to be relatively homogeneous; however, they did not provide compatible data that would allow a meta-analysis.
Discussion
The present study reviewed the available evidence regarding the potential impact of mTOR inhibitors in the treatment of CSCC. Palliation with platinum-based chemotherapy remains the standard of care for inoperable patients who have advanced disease (63). Few advances in medical management have occurred in recent years in the treatment of advanced recurrent gynecological malignancies, and a poor prognosis remains (12). Rationally designed molecularly targeted therapy is an emerging and important option in this setting (12).
mTOR is a serine/threonine protein kinase of the PI3K/AKT signaling pathway, with a critical role in controlling cancer cell growth, metabolism and cell cycle progression. Aberrant PI3K-dependent signaling occurs frequently in a wide range of tumor types, including ovarian, endometrial and cervical cancers (6,12).
HPV infection of the uterine cervix is linked to the pathogenesis of CC. Preclinical in vitro and in vivo studies using HPV-containing human cervical carcinoma cell lines have demonstrated that rapamycin is able to induce growth delay of xenografts. Activation of Akt and mTOR in CSCC, and expression of phosphorylated mTOR have been reported to serve as a markers to predict response to chemotherapy and survival of CC patients (8).
Several studies have provided evidence of the association between the activated PI3K/AKT/mTOR pathway and CC (8,12,64,65); however, few studies have analyzed the effect of treatment with mTOR inhibitors in CC patients (26,33–36, 44, 46,47, 53,60–62). Research has also been conducted in the field of combined treatment with mTOR inhibitors, chemotherapy and radiotherapy for locally advanced CC (66).
The present review identified only two phase 1 (60,61) and one phase 2 (62) clinical trials that met the inclusion criteria. All studies were non-randomized and included treatment with temsirolimus as the mTOR inhibitor agent. No control groups were included. The toxicities were manageable, and the predominant grade 3 and 4 toxicities included hematological and hepatic side effects.
The analysis of responses in these three studies was compromised due to the design of the studies and the lack of control groups. The two phase 1 studies had RR as secondary endpoints, and were thus not powered to detect differences in response. These studies revealed that a number of patients treated with chemotherapy or bevacizumab in association with mTOR inhibitors achieved PRs (15.4–33.3% of cases) or SD lasting ≥6 months (17.6–28% of cases). Patients were heavily pretreated in two studies (60,61), thus it is possible that RRs could be improved with these agents if used in earlier lines of treatment.
One serious limitation in determining the activity of temsirolimus in these two phase 1 studies, aside from the lack of statistical power to analyze RR, is the lack of a control arm. In the 22 CSCC patients included in the phase 2 study, there was no PR following treatment, only SD, and this study also did not include a control arm (62). The two phase 1 studies used temsirolimus combined with bevacizumab. Therefore, it cannot be affirmed whether the benefit obtained was due to the mTOR inhibitor, the combination of agents, or bevacizumab alone. In the study by Moroney et al (60), liposomal doxorubicin was also included, making this analysis further complicated. The other important limitation is that only the phase 2 trial included >20 CSCC patients, but this study reported no responses to temsirolimus in CSCC patients (62). The phase 1 studies included ≤10 CSCC patients (60,61). Thus, it is not possible to conclude the effectiveness of temsirolimus in the treatment of CSCC based on the phase 1 studies, and the phase 2 study indicates that treatment is inactive.
There is evidence that tumor PIK3CA mutation status may predict response to PI3K/AKT/mTOR inhibitors (34). Only the study by Tinker et al (62) could be used to evaluate molecular alterations and responses, as the studies by Piha-Paul et al (61) and Moroney et al (60) did not report the results of molecular analysis in a separate manner with regard to the different types of tumor. No established association between PI3K pathway activating mutations, loss of PTEN and treatment response could be determined in the study by Tinker et al (62).
In another study, Moroney et al (67) at the MD Anderson Cancer Center (University of Texas, Houston, TX, USA) described their experience of treatment with mTOR inhibitors in a phase 1 clinical trials of solid tumors (67). Patients with PIK3CA mutations were treated, whenever possible, with agents targeting the PI3K/AKT/mTOR pathway (36). In patients with CSCC, the presence of PIK3CA mutations was associated with a significantly longer OS time (median, 9.4 months) than the absence of PIK3CA mutations (median, 4.2 months; P=0.019). Identifying patients who may, or more importantly may not, benefit from a molecularly targeted agent is highly desirable. Furthermore, evaluation of PI3K/AKT/mTOR pathway-targeted therapy is warranted, particularly in metastatic or recurrent CSCC (33).
The study of genomic and molecular characteristics of cervical tumors is underway by The Cancer Genome Atlas (TGCA; http://cancergenome.nih.gov/cancersselected). This will confirm the genomic and molecular alterations in the disease and provide rationale for specific targeted therapies. The evaluation of the PI3K/AKT/mTOR pathway by the TCGA is of great importance, as new classes of drugs targeted to this pathway are in the process of development, including PI3K inhibitors.
In summary, the current study is the first systematic review of the potential impact of mTOR inhibitors on CSCC treatment. Some serious methodological limitations of this review should be considered, such as that the studies were non-randomized, had only one arm, included a limited number of patients and lacked a control group. All studies were categorized as having a low or very low quality level of evidence. The currently available evidence is inconclusive with regard to the effects of mTOR inhibitors on CSCC. The phase 2 study in women with recurrent, unresectable, locally advanced or metastatic carcinoma of the cervix indicated that temsirolimus was inactive in this population. Investigation of PI3K/AKT/mTOR pathway-targeted therapies is warranted, and future studies should include information regarding mutations. Randomized, high-quality clinical trials are necessary to confirm the efficacy of mTOR inhibitors in the treatment of CSCC patients.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Forman DBF, Brewster DH, Mbalawa C Gombe, Kohler B, Piñeros M, Steliarova-Foucher E, Swaminathan R, Ferlay J. GLOBOCAN 2012: Estimated Cancer Incidence. http://globocan.iarc.fr Mortality and Prevalence Worldwide in 2012. 2015 Mar 6th; accessed. Available at.
- 3.Nogueira-Rodrigues A, Moralez G, Grazziotin R, Carmo CC, Small IA, Alves FV, Mamede M, Erlich F, Viegas C, Triginelli SA, Ferreira CG. Phase 2 trial of erlotinib combined with cisplatin and radiotherapy in patients with locally advanced cervical cancer. Cancer. 2014;120:1187–1193. doi: 10.1002/cncr.28471. [DOI] [PubMed] [Google Scholar]
- 4.Tewari KS, Sill MW, Long HJ, III, Penson RT, Huang H, Ramondetta LM, Landrum LM, Oaknin A, Reid TJ, Leitao MM, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370:734–743. doi: 10.1056/NEJMoa1309748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Movva S, Rodriguez L, Arias-Pulido H, Verschraegen C. Novel chemotherapy approaches for cervical cancer. Cancer. 2009;115:3166–3180. doi: 10.1002/cncr.24364. [DOI] [PubMed] [Google Scholar]
- 6.Husseinzadeh N, Husseinzadeh HD. mTOR inhibitors and their clinical application in cervical, endometrial and ovarian cancers: A critical review. Gynecol Oncol. 2014;133:375–381. doi: 10.1016/j.ygyno.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 7.Bosch FX, Lorincz A, Muñoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–265. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng W, Duan X, Liu J, Xiao J, Brown RE. Morphoproteomic evidence of constitutively activated and overexpressed mTOR pathway in cervical squamous carcinoma and high grade squamous intraepithelial lesions. Int J Clin Exp Pathol. 2009;2:249–260. [PMC free article] [PubMed] [Google Scholar]
- 9.Advani SH. Targeting mTOR pathway: A new concept in cancer therapy. Indian J Med Paediatr Oncol. 2010;31:132–136. doi: 10.4103/0971-5851.76197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol. 2010;28:1075–1083. doi: 10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the PI3K/Akt/mTOR pathway: Effective combinations and clinical considerations. Drug Resist Updat. 2008;11:32–50. doi: 10.1016/j.drup.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diaz-Padilla I, Duran I, Clarke BA, Oza AM. Biologic rationale and clinical activity of mTOR inhibitors in gynecological cancer. Cancer Treat Rev. 2012;38:767–775. doi: 10.1016/j.ctrv.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Zhou H, Luo Y, Huang S. Updates of mTOR inhibitors. Anticancer Agents Med Chem. 2010;10:571–581. doi: 10.2174/187152010793498663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutson TE, Escudier B, Esteban E, Bjarnason GA, Lim HY, Pittman KB, Senico P, Niethammer A, Lu DR, Hariharan S, Motzer RJ. Randomized phase III trial of temsirolimus versus sorafenib as second-line therapy after sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2014;32:760–767. doi: 10.1200/JCO.2013.50.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, Grünwald V, Thompson JA, Figlin RA, Hollaender N, et al. Efficacy of everolimus in advanced renal cell carcinoma: A double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 16.Baselga J, Campone M, Piccart M, Burris HA, III, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun F, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and Elaboration. Ann Intern Med. 2009;151:W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 18.The University of York, corp. http://www.crd.york.ac.uk/PROSPERO/ Centre for Reviews and Dissemination. [cited 02/23/2015]. Available at.
- 19.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 20.Bae-Jump VL, Zhou C, Gehrig PA, Whang YE, Boggess JF. Rapamycin inhibits hTERT telomerase mRNA expression, independent of cell cycle arrest. Gynecol Oncol. 2006;100:487–494. doi: 10.1016/j.ygyno.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 21.Brana I, Berger R, Golan T, Haluska P, Edenfield J, Fiorica J, Stephenson J, Martin LP, Westin S, Hanjani P, et al. A parallel-arm phase I trial of the humanised anti-IGF-1R antibody dalotuzumab in combination with the AKT inhibitor MK-2206, the mTOR inhibitor ridaforolimus, or the NOTCH inhibitor MK-0752, in patients with advanced solid tumours. Br J Cancer. 2014;111:1932–1944. doi: 10.1038/bjc.2014.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brüning A, Rahmeh M, Friese K. Nelfinavir and bortezomib inhibit mTOR activity via ATF4-mediated sestrin-2 regulation. Mol Oncol. 2013;7:1012–1018. doi: 10.1016/j.molonc.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen YJ, Kay N, Yang JM, Lin CT, Chang HL, Wu YC, Fu CF, Chang Y, Lo S, Hou MF, et al. Total synthetic protoapigenone WYC02 inhibits cervical cancer cell proliferation and tumour growth through PIK3 signalling pathway. Basic Clin Pharmacol Toxicol. 2013;113:8–18. doi: 10.1111/bcpt.12057. [DOI] [PubMed] [Google Scholar]
- 24.Chi EY, Viriyapak B, Kwack HS, Lee YK, Kim SI, Lee KH, Park TC. Regulation of paclitaxel-induced programmed cell death by autophagic induction: A model for cervical cancer. Obstet Gynecol Sci. 2013;56:84–92. doi: 10.5468/OGS.2013.56.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi Y, Lee JH. The combination of tephrosin with 2-deoxy-D-glucose enhances the cytotoxicity via accelerating ATP depletion and blunting autophagy in human cancer cells. Cancer Biol Ther. 2011;12:989–996. doi: 10.4161/cbt.12.11.18364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen EE, Sharma MR, Janisch L, Llobrera M, House L, Wu K, Ramirez J, Fleming GF, Stadler WM, Ratain MJ. A phase I study of sirolimus and bevacizumab in patients with advanced malignancies. Eur J Cancer. 2011;47:1484–1489. doi: 10.1016/j.ejca.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cui F, Li X, Zhu X, Huang L, Huang Y, Mao C, Yan Q, Zhu J, Zhao W, Shi H. MiR-125b inhibits tumor growth and promotes apoptosis of cervical cancer cells by targeting phosphoinositide 3-kinase catalytic subunit delta. Cell Physiol Biochem. 2012;30:1310–1308. doi: 10.1159/000343320. [DOI] [PubMed] [Google Scholar]
- 28.Dan S, Okamura M, Mukai Y, Yoshimi H, Inoue Y, Hanyu A, Sakaue-Sawano A, Imamura T, Miyawaki A, Yamori T. ZSTK474, a specific phosphatidylinositol 3-kinase inhibitor, induces G1 arrest of the cell cycle in vivo. Eur J Cancer. 2012;48:936–943. doi: 10.1016/j.ejca.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 29.Farha AK, Dhanya SR, Mangalam SN, Geetha BS, Latha PG, Remani P. Deoxyelephantopin impairs growth of cervical carcinoma SiHa cells and induces apoptosis by targeting multiple molecular signaling pathways. Cell Biol Toxicol. 2014;30:331–343. doi: 10.1007/s10565-014-9288-z. [DOI] [PubMed] [Google Scholar]
- 30.Faried LS, Faried A, Kanuma T, Nakazato T, Tamura T, Kuwano H, Minegishi T. Inhibition of the mammalian target of rapamycin (mTOR) by rapamycin increases chemosensitivity of CaSki cells to paclitaxel. Eur J Cancer. 2006;42:934–947. doi: 10.1016/j.ejca.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 31.Guan TJ, Qin FJ, Du JH, Geng L, Zhang YY, Li M. AICAR inhibits proliferation and induced S-phase arrest, and promotes apoptosis in CaSki cells. Acta Pharmacol Sin. 2007;28:1984–1990. doi: 10.1111/j.1745-7254.2007.00675.x. [DOI] [PubMed] [Google Scholar]
- 32.Hou LL, Gao C, Chen L, Hu GQ, Xie SQ. Essential role of autophagy in fucoxanthin-induced cytotoxicity to human epithelial cervical cancer HeLa cells. Acta Pharmacol Sin. 2013;34:1403–1410. doi: 10.1038/aps.2013.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hou MM, Liu X, Wheler J, Naing A, Hong D, Coleman RL, Tsimberidou A, Janku F, Zinner R, Lu K, Kurzrock R, Fu S. Targeted PI3K/AKT/mTOR therapy for metastatic carcinomas of the cervix: A phase I clinical experience. Oncotarget. 2014;5:11168–11179. doi: 10.18632/oncotarget.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janku F, Tsimberidou AM, Garrido-Laguna I, Wang X, Luthra R, Hong DS, Naing A, Falchook GS, Moroney JW, Piha-Paul SA, et al. PIK3CA mutations in patients with advanced cancers treated with PI3K/AKT/mTOR axis inhibitors. Mol Cancer Ther. 2011;10:558–565. doi: 10.1158/1535-7163.MCT-10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janku F, Wheler JJ, Naing A, Falchook GS, Hong DS, Stepanek VM, Fu S, Piha-Paul SA, Lee JJ, Luthra R, et al. PIK3CA mutation H1047R is associated with response to PI3K/AKT/mTOR signaling pathway inhibitors in early phase clinical trials. Cancer Res. 2013;73:276–284. doi: 10.1158/0008-5472.CAN-12-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janku F, Wheler JJ, Naing A, Stepanek VM, Falchook GS, Fu S, Garrido-Laguna I, Tsimberidou AM, Piha-Paul SA, Moulder SL, et al. PIK3CA mutations in advanced cancers: Characteristics and outcomes. Oncotarget. 2012;3:1566–1575. doi: 10.18632/oncotarget.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ji J, Zheng P-S. Activation of mTOR signaling pathway contributes to survival of cervical cancer cells. Gynecol Oncol. 2010;117:103–108. doi: 10.1016/j.ygyno.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 38.Kang S, Dong SM, Kim BR, Park MS, Trink B, Byun HJ, Rho SB. Thioridazine induces apoptosis by targeting the PI3K/Akt/mTOR pathway in cervical and endometrial cancer cells. Apoptosis. 2012;17:989–997. doi: 10.1007/s10495-012-0717-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim J, Park M, Ryu BJ, Kim SH. The protein kinase 2 inhibitor CX-4945 induces autophagy in human cancer cell lines. Bulletin of the Korean Chemical Society. 2014;35:2985–2989. doi: 10.5012/bkcs.2014.35.10.2985. [DOI] [Google Scholar]
- 40.Kwan HT, Chan DW, Cai PC, Mak CS, Yung MM, Leung TH, Wong OG, Cheung AN, Ngan HY. AMPK activators suppress cervical cancer cell growth through inhibition of DVL3 mediated Wnt/β-catenin signaling activity. PloS One. 2013;8:e53597. doi: 10.1371/journal.pone.0053597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li J, Ping Z, Ning H. MiR-218 impairs tumor growth and increases chemo-sensitivity to cisplatin in cervical cancer. Int J Mol Sci. 2012;13:16053–16064. doi: 10.3390/ijms131216053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li SR, Li Y, Hu R, Li W, Qiu H, Cai H, Wang S. The mTOR inhibitor AZD8055 inhibits proliferation and glycolysis in cervical cancer cells. Oncol Lett. 2013;5:717–721. doi: 10.3892/ol.2012.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma J, Zi Jiang Y, Shi H, Mi C, Li J, Nan J Xing, Wu X, Lee J Joon, Jin X. Cucurbitacin B inhibits the translational expression of hypoxia-inducible factor-1α. Eur J Pharmacol. 2014;723:46–54. doi: 10.1016/j.ejphar.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 44.Martin-Liberal J, Gil-Martín M, Sáinz-Jaspeado M, Gonzalo N, Rigo R, Colom H, Muñoz C, Tirado OM, García del Muro X. Phase I study and preclinical efficacy evaluation of the mTOR inhibitor sirolimus plus gemcitabine in patients with advanced solid tumours. Br J Cancer. 2014;111:858–865. doi: 10.1038/bjc.2014.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Molinolo AA, Marsh C, El Dinali M, Gangane N, Jennison K, Hewitt S, Patel V, Seiwert TY, Gutkind JS. mTOR as a molecular target in HPV-associated oral and cervical squamous carcinomas. Clin Cancer Res. 2012;18:2558–2568. doi: 10.1158/1078-0432.CCR-11-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O'Donnell A, Faivre S, Burris HA, III, Rea D, Papadimitrakopoulou V, Shand N, Lane HA, Hazell K, Zoellner U, Kovarik JM, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol. 2008;26:1588–1595. doi: 10.1200/JCO.2007.14.0988. [DOI] [PubMed] [Google Scholar]
- 47.Papadopoulos KP, Tabernero J, Markman B, Patnaik A, Tolcher AW, Baselga J, Shi W, Egile C, Ruiz-Soto R, Laird AD, et al. Phase I safety, pharmacokinetic, and pharmacodynamic study of SAR245409 (XL765), a novel, orally administered PI3K/mTOR inhibitor in patients with advanced solid tumors. Clin Cancer Res. 2014;20:2445–2456. doi: 10.1158/1078-0432.CCR-13-2403. [DOI] [PubMed] [Google Scholar]
- 48.Rashmi R, DeSelm C, Helms C, Bowcock A, Rogers BE, Rader J, Grigsby PW, Schwarz JK. AKT inhibitors promote cell death in cervical cancer through disruption of mTOR signaling and glucose uptake. PloS One. 2014;9:e92948. doi: 10.1371/journal.pone.0092948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reddy GL, Guru SK, Srinivas M, Pathania AS, Mahajan P, Nargotra A, Bhushan S, Vishwakarma RA, Sawant SD. Synthesis of 5-substituted-1H-pyrazolo[4,3-d]pyrimidin-7(6H)-one analogs and their biological evaluation as anticancer agents: mTOR inhibitors. Eur J Med Chem. 2014;80:201–208. doi: 10.1016/j.ejmech.2014.04.051. [DOI] [PubMed] [Google Scholar]
- 50.Roy B, Pattanaik AK, Das J, Bhutia SK, Behera B, Singh P, Maiti TK. Role of PI3K/Akt/mTOR and MEK/ERK pathway in Concanavalin A induced autophagy in HeLa cells. Chem Biol Interact. 2014;210:96–102. doi: 10.1016/j.cbi.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 51.Sahin K, Tuzcu M, Basak N, Caglayan B, Kilic U, Sahin F, Kucuk O. Sensitization of cervical cancer cells to cisplatin by genistein: The role of NFκB and Akt/mTOR signaling pathways. J Oncol. 2012;2012:461562. doi: 10.1155/2012/461562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shin JM, Jeong YJ, Cho HJ, Park KK, Chung IK, Lee IK, Kwak JY, Chang HW, Kim CH, Moon SK, et al. Melittin suppresses HIF-1α/VEGF expression through inhibition of ERK and mTOR/p70S6K pathway in human cervical carcinoma cells. PloS One. 2013;8:e69380. doi: 10.1371/journal.pone.0069380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Temkin SM, Yamada SD, Fleming GF. A phase I study of weekly temsirolimus and topotecan in the treatment of advanced and/or recurrent gynecologic malignancies. Gynecol Oncol. 2010;117:473–476. doi: 10.1016/j.ygyno.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 54.Wan G, Xie W, Liu Z, Xu W, Lao Y, Huang N, Cui K, Liao M, He J, Jiang Y, et al. Hypoxia-induced MIR155 is a potent autophagy inducer by targeting multiple players in the MTOR pathway. Autophagy. 2014;10:70–79. doi: 10.4161/auto.26534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang L, Chang L, Li Z, Gao Q, Cai D, Tian Y, Zeng L, Li M. miR-99a and −99b inhibit cervical cancer cell proliferation and invasion by targeting mTOR signaling pathway. Med Oncol. 2014;31:934. doi: 10.1007/s12032-014-0934-3. [DOI] [PubMed] [Google Scholar]
- 56.Xiao X, He Q, Lu C, Werle KD, Zhao RX, Chen J, Davis BC, Cui R, Liang J, Xu ZX. Metformin impairs the growth of liver kinase B1-intact cervical cancer cells. Gynecol Oncol. 2012;127:249–255. doi: 10.1016/j.ygyno.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu QJ, Hou LL, Hu GQ, Xie SQ. Molecular mechanism of ophiopogonin B induced cellular autophagy of human cervical cancer HeLa cells. Yao Xue Xue Bao. 2013;48:855–859. (In Chinese) [PubMed] [Google Scholar]
- 58.Yu SY, Chan DW, Liu VW, Ngan HY. Inhibition of cervical cancer cell growth through activation of upstream kinases of AMP-activated protein kinase. Tumor Biol. 2009;30:80–85. doi: 10.1159/000216843. [DOI] [PubMed] [Google Scholar]
- 59.Zhang C, Yang N, Yang CH, Ding HS, Luo C, Zhang Y, Wu MJ, Zhang XW, Shen X, Jiang HL, et al. S9, a novel anticancer agent, exerts its anti-proliferative activity by interfering with both PI3K-Akt-mTOR signaling and microtubule cytoskeleton. PloS One. 2009;4:e4881. doi: 10.1371/journal.pone.0004881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moroney JW, Schlumbrecht MP, Helgason T, Coleman RL, Moulder S, Naing A, Bodurka DC, Janku F, Hong DS, Kurzrock R. A Phase I trial of liposomal doxorubicin, bevacizumab, and temsirolimus in patients with advanced gynecologic and breast malignancies. Clin Cancer Res. 2011;17:6840–6846. doi: 10.1158/1078-0432.CCR-11-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Piha-Paul SA, Wheler JJ, Fu S, Levenback C, Lu K, Falchook GS, Naing A, Hong DS, Tsimberidou AM, Kurzrock R. Advanced gynecologic malignancies treated with a combination of the VEGF inhibitor bevacizumab and the mTOR inhibitor temsirolimus. Oncotarget. 2014;5:1846–1855. doi: 10.18632/oncotarget.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tinker AV, Ellard S, Welch S, Moens F, Allo G, Tsao MS, Squire J, Tu D, Eisenhauer EA, MacKay H. Phase II study of temsirolimus (CCI-779) in women with recurrent, unresectable, locally advanced or metastatic carcinoma of the cervix. A trial of the NCIC clinical trials group (NCIC CTG IND 199) Gynecol Oncol. 2013;130:269–274. doi: 10.1016/j.ygyno.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 63.Serrano-Olvera A, Cetina L, Coronel J, Dueñas-González A. Emerging drugs for the treatment of cervical cancer. Expert Opin Emerg Drugs. 2015;20:165–182. doi: 10.1517/14728214.2015.1002768. [DOI] [PubMed] [Google Scholar]
- 64.Chan S. Targeting the mammalian target of rapamycin (mTOR): A new approach to treating cancer. Br J Cancer. 2004;91:1420–1424. doi: 10.1038/sj.bjc.6602162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Molinolo AA, Marsh C, El Dinali ME, Gangane N, Jennison K, Hewitt S, Patel V, Seiwert TY, Gutkind JS. mTOR as a molecular target in HPV-associated oral and cervical squamous carcinomas. Clin Cancer Res. 2012;18:2558–2568. doi: 10.1158/1078-0432.CCR-11-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ferreira CG, Alves FVG, Grazziotin R, Erlich F, Moralez G, Carneiro MP, et al. Abstract CT403: A Phase I study of oral administration of mTOR inhibitor everolimus (E) in association with cisplatin (C) and radiotherapy (R) for the treatment of locally advanced cervix cancer (LACC)-PHOENIX I. Cancer Res. 2014;74(Suppl 19):CT403. doi: 10.1158/1538-7445.AM2014-CT403. [DOI] [Google Scholar]
- 67.Moroney J, Wheler J, Hong D, Naing A, Falchook G, Bodurka D, Coleman R, Lu K, Xiao L, Kurzrock R. Phase I clinical trials in 85 patients with gynecologic cancer: The M. D. Anderson Cancer Center experience. Gynecol Oncol. 2010;117:467–472. doi: 10.1016/j.ygyno.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]