Abstract
Curcumin, an active nontoxic ingredient of turmeric, possesses potent anti-inflammatory, antioxidant and anti-cancer properties; however, the molecular mechanisms of curcumin are not fully understood. The transcription factor nuclear factor-κB (NF-κB) is key in cellular processes, and the expression/activation of urokinase-type plasminogen activator (uPA) and matrix metalloproteinase-9 (MMP9) are crucial for cell invasion. The present study investigated the hypothesis that curcumin inhibits colon cancer cell invasion by modulating NF-κB-mediated expression and activation of uPA and MMP9. Human colon cancer SW480 and LoVo cells were treated with various concentrations of curcumin. Curcumin was demonstrated to dose-dependently inhibit the adhesion and proliferation ability of LoVo and SW480 cells using Transwell and MTT assays, respectively. In addition, curcumin activated 5′ AMP-activated protein kinase (AMPK) and suppressed p65 NF-κB phosphorylation, as shown by western blot analysis. Compound C, a potent AMPK inhibitor, abolished curcumin-induced inhibition of NF-κB, uPA and MMP9, suggesting that AMPK activation is responsible for curcumin-mediated NF-κB, uPA and MMP9 inhibition. The binding activity of NF-κB to DNA was examined and western blotting and quantitative polymerase reaction was performed to detect the effect of curcumin on the expression of uPA and MMP9. The present results revealed that curcumin significantly decreased the expression of uPA and MMP9 and NF-κB DNA binding activity. Furthermore, curcumin decreased the level of the p65 subunit of NF-κB binding to the promoter of the gene encoding uPA and MMP9, which suppressed transcriptional activation of uPA and MMP9. Overall, the present data suggest that curcumin inhibits colon cancer cell invasion via AMPK activation and subsequent inhibition of p65 NF-κB, uPA and MMP9. The therapeutic potential of curcumin for colon cancer metastasis required additional study.
Keywords: curcumin, colon cancer, nuclear factor-kB, urokinase-type plaminogen activator, matrix metalloproteinases
Introduction
Colorectal cancer remains the third leading cause of cancer-associated mortality in the USA, even though tremendous efforts have been made to improve the effectiveness of treatment (1). Morbidity primarily results from the growth of metastatic tumors in distant organs, and metastasis is one of the major causes of mortality in cancer patients. Multiple steps are involved in the metastatic process. One of the first steps is the invasion of cancer cells through the basement membrane via the adhesion of cancer cells to the extracellular matrix (ECM), followed by degradation of the ECM by proteolytic enzymes (2). Key proteases that degrade the ECM are the serine proteases (plasmins), urokinase plasminogen activator (uPA), cathepsins and matrix metalloproteinases (MMPs). It is well demonstrated that uPA and MMPs are significant in tumor growth, invasion and metastasis (3–5). uPA, a member of the serine proteases, interacts with the uPA receptor, which modulates various biological functions, including cell migration, differentiation and wound healing (6). Expression of uPA is critical in cancer cell metastasis and is involved in cancer cell adhesion and invasion (4,7). MMP9 is also crucial in tumor invasion and angiogenesis, since it mediates the degradation of the ECM (8). Overexpression of MMP9 may be one step in the multi-step process that results in neoplastic cell proliferation and metastasis, and it has been demonstrated that MMP9 is associated with colorectal carcinoma (9). Therefore, inhibition of ECM degradation enzymes and cell adhesion to the ECM should be considered as preventative treatment for cancer metastasis (5,10,11).
Numerous studies have suggested that nuclear factor-κB (NF-κB) is key in regulating a number of cellular processes, including inflammation, cellular proliferation, transformation and tumorigenesis (12,13). NF-κB is a ubiquitous eukaryotic transcription factor, which is identified in the cytoplasm as an inactive heterotrimer consisting of p50, p65 and IκBα subunits. Activation of NF-κB is initiated by the signal-induced degradation of IκB proteins, the most studied of which is IκBα. IκBα undergoes phosphorylation and ubiquitination-dependent degradation via activation of IκB kinase (IKK), which is composed of IKKα, IKKβ and IKKγ (also referred to as NEMO) (14,15). IκBα phosphorylation/degradation leads to NF-κB release, which translocates to the nucleus where it acts as a transcription factor and binds to a specific consensus sequence in DNA, leading to gene transcription (16).
Nontraditional medicine is becoming an increasingly attractive approach for the treatment of various inflammatory disorders among patients unresponsive or unwilling to receive standard medications. Curcumin is the major constituent of turmeric powder extracted from the roots of the East Indian plant Curcuma longa. It has been widely used in therapeutic preparations for centuries owing to its anti-inflammatory and chemotherapeutic properties (17,18). Curcumin presents itself as a pharmacologically safe and effective potential candidate for anti-metastatic therapy, due to the following reasons: Curcumin has been demonstrated to suppress NF-κB activation induced by various inflammatory stimuli (19,20); increasing evidence indicates that curcumin has anticancer effects against various types of human tumor cells, including ovarian, breast and colon cancer and astroglioma (21–25); curcumin has been revealed to downregulate the expression of various NF-κB-regulated genes, including B-cell lymphoma 2, cyclooxygenase 2, tumor necrosis factor and adhesion molecules (19,20,26–29); administration of curcumin in humans is safe (17,18,30).
However, whether curcumin is involved in the regulation of colon cancer cell invasion via the NF-κB signaling pathway is not well elucidated. The present study investigated the human colon cancer LoVo and SW480 cell lines to identify the effects and mechanisms of curcumin on colon cancer cell adhesion and invasion during the metastasis process via the NF-κB signaling pathway. The present results demonstrated that curcumin inhibits the adhesion and invasion of colon cancer cells by suppressing the activation of NF-κB and IKK activity. This led to the downregulation of the expression of uPA and MMP9, which are regulated by NF-κB, and thus suppressed the proliferation of colon cancer cells.
Materials and methods
Materials
Curcumin (diferuloylmethane) was obtained from Sigma-Aldrich (St. Louis, MO, USA), and was dissolved in dimethyl sulfoxide (DMSO) as a 10 mM stock solution and stored at −20°C for further study. Primary antibodies consisted of mouse anti-human monoclonal uPA (catalog no. sc-59727; dilution, 1:1,000) purchased from Santa Cruz Biotechnology, Inc., (Dallas, TX, USA) and rat anti-human polyclonal MMP9 (catalog no. 3852; dilution, 1:1,000), mouse anti-human monoclonal NF-κB p65 (catalog no. 6956; dilution, 1:1,000), rabbit anti-human polyclonal NF-κB p50 (catalog no. 3035; dilution, 1:1,000), rabbit anti-human polyclonal p-p65 (catalog no. 3031; dilution, 1:1,000), mouse anti-human monoclonal IKK (catalog no. 11930; dilution, 1:1,000), rabbit anti-human polyclonal AMPK (catalog no. 2532; dilution, 1:1,000), rabbit anti-human monoclonal p-AMPK (catalog no. 2535; dilution, 1:1,000) and mouse anti-human monoclonal β-actin (catalog no. 3700; dilution, 1:1,000) purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). Compound C was purchased from EMD Millipore (Billerica, MA, USA).
Cell culture
Two human colon cancer cell lines SW480 and LoVo were purchased from the American Type Culture Collection (Manassas, VA, USA). The SW480 cell line was cultured in Dulbecco's modified Eagle's medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing glucose (4.5 g/l) supplemented with 10% fetal calf serum (FCS; Thermo Fisher Scientific, Inc.), 100 µg/ml streptomycin and 100 U/ml penicillin. The LoVo cell line was grown in F-12 medium (Thermo Fisher Scientific, Inc.) containing L-glutamine, supplemented with 10% FCS, 100 µg/ml streptomycin and 100 U/ml penicillin. All cells were cultured in a humidified atmosphere of 5% CO2 at 37°C.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay
MTT proliferation assay was performed to assess the cytotoxicity of curcumin in SW480 and LoVo cells. Briefly, cells were seeded on a 96-well plate at a density of 5,000 cells/well. Following attachment, various concentrations of curcumin (0, 0.01, 0.1, 1, 10, 20 and 50 µM) were added for 24 h. The cells were washed with phosphate-buffered saline (PBS) and incubated with 200 µl MTT (0.5 mg/ml) until formazan had formed. Subsequently, the medium was removed and formazan was dissolved with DMSO. Absorbance was measured at 570 nm using a microplate reader (Bio-Tek Instruments, Inc., Winooski, VT, USA). The experiment was repeated three times.
Invasion assay
A Boyden chamber (BD Biosciences, San Jose, CA, USA) was used to determine the cell invasion ability of SW480 and LoVo cells. Cells were pre-cultured in serum-free medium with or without curcumin (0 and 10 µM) for 24 h. Cells (8×104) suspended in 0.5 ml serum-free medium were loaded into the upper compartment of the invasion chamber, which was coated with Matrigel (30 mg/filter). The lower compartment was loaded with complete medium with or without curcumin. The chamber was incubated at 37°C for 24 h and the filters were removed. Invaded cells were fixed, stained and counted under a microscope. Each experiment was performed three times.
Western blotting
Western blot analysis was performed on 100 µg protein extracts. SW480 and LoVo cells were lysed in lysis buffer [0.5% sodium deoxycholate, 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% Nonidet P-40, 0.1% sodium dodecyl sulfate (SDS)] containing 50 mM NaF, 5 mM EDTA, 1 mM DTT and 10 µg/ml aprotinin. Cell lysates were resolved on SDS-polyacrylamide gel electrophoresis, according to standard protocols (31). The samples were immunoblotted with primary antibodies agaisnt uPA, MMP-9, NF-κB, IKK, p-Thr172 5′ AMP-activated protein kinase (AMPK), AMPK and β-actin, followed by incubation with secondary antibodies: Goat anti-rabbit immunoglobulin G (IgG), horseradish peroxidase (HRP)-linked antibody (catalog no. 7074; dilution, 1:5,000) and horse anti-mouse IgG, HRP-linked antibody (catalog no. 7076; dilution, 1:5,000), purchased from Cell Signaling Technology Inc. Bands were visualized using an Amersham ECL Western Blotting Detection kit (GE Healthcare, Chalfont, UK), according to the manufacturer's protocol.
Quantitative polymerase chain reaction (qPCR)
Total RNA was isolated from SW480 and LoVo cells using RNA Bee RNA Isolation Reagent (Amsbio LLC, Cambridge, MA, USA). cDNA (1 µg) is used as a template for subsequent PCR amplification using primers specific for MMP-9, uPA and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) genes. PCR conditions were: 30 sec at 94°C for denaturation, 30 sec at 54°C for annealing and 30 sec at 65°C for extension, for a total of 30 cycles. qPCR was performed using the SYBR green real-time PCR kit (catalog no. 204141; Qiagen, Valencia, CA, USA) and the QuantStudio® 3 Real-Time PCR System (Applied Biosystems™; Thermo Fisher Scientific, Inc.). PCR products were run on 2% agarose gels to confirm that correct molecular sizes were present. Each sample was tested in triplicate using qPCR. The following primers for qPCR were used: MMP9, forward 5′-AATCTCTTCTAGAGACTGGGAAGGAG-3′ and reverse 5′-AGCTGATTGACTAAAGTAGCTGGA-3′; uPA, forward 5′-CACGCAAGGGGAGATGAA-3′ and reverse 5′-ACAGCATTTTGGTGGTGACTT-3′; GAPDH, forward 5′-AGAGAGAGGCCCTCAGTTGCT-3′ and reverse 5′-TTGTGAGGGAGATGCTCAGTGT-3′, synthesized by BGI Tech (Shenzhen Co., Ltd., Shenzhen, China). GAPDH was used as a loading control. Quantification was performed using the 2−∆∆Cq method, as previously described (32)
Enzyme-linked immunosorbent assay (ELISA)
To investigate whether curcumin affects NF-κB activation, which is critical for transcriptional activity, the DNA binding activity of NF-κB was analyzed by ELISA. The NF-κB p65 Transcription Factor Assay kit (catalog no. ab133112) was purchased from Abcam (Cambridge, MA, USA). NF-κB activation was measured by ELISA according to the manufacturer's protocol.
Luciferase assay
SW480 cells were seeded in 24-well plates at a density of 1×105 cells/ml/well, and the following day they were co-transfected with 100 ng luciferase reporter construct (synthesized by BGI Tech; Shenzhen Co., Ltd.), 20 ng Renilla luciferase pRL-TK reporter (Promega, Madison, WI, USA), and 400 ng HA-tagged p65 NF-κB expression plasmid (Addgene, Cambridge, MA, USA). Subsequent to 24 h, the cells were harvested and the luciferase activity was determined using the Dual-Luciferase® Reporter Assay System Protocol (Promega Corporation, Madison, WI, USA). The relative light units were measured using a GloMax® 20/20 Luminometer (Promega Corporation). Data were normalized by Renilla luciferase. Each experiment was performed at least three times in triplicate wells.
Statistical analysis
Data were expressed as the mean ± standard error of the mean. Differences were analyzed by one-way analysis of variance followed by Fisher's protected least significant difference test using SPSS (version 17.0) software (SPSS Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Curcumin inhibits the proliferation of colon cancer LoVo and SW480 cells
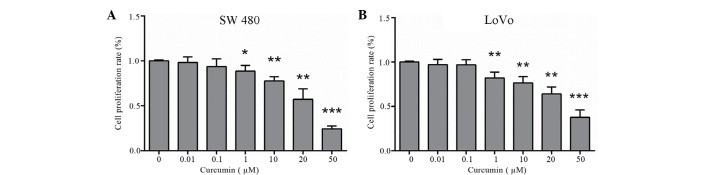
An MTT assay was performed to examine the effect of curcumin on the proliferation of colon cancer LoVo and SW480 cells. LoVo and SW480 cells were treated with various concentrations of curcumin (0–50 µM) for 24 h. Subsequently, curcumin markedly inhibited cell proliferation of colon cancer LoVo and SW480 cells in a dose-dependent manner (P=0.014 and P=0.003 vs. control cells, respectively; Fig. 1A and B). These inhibitory effects were observed following incubation with 1, 10, 20 and 50 µM curcumin. A dose of up to 20 µM curcumin was used in further experiments.
Figure 1.
Cell proliferation is decreased following curcumin treatment in human colon cancer (A) SW480 and (B) LoVo cells. Cells were treated with various concentrations of curcumin (0–50 µM) for 24 h. Cell proliferation was determined using a MTT assay, and the graph shows the results of three independent experiments. The results are presented as folds over control cells (set as 1). *P<0.05; **P<0.01 vs. control cells.
Curcumin inhibits the invasion of colon cancer SW480 and LoVo cells
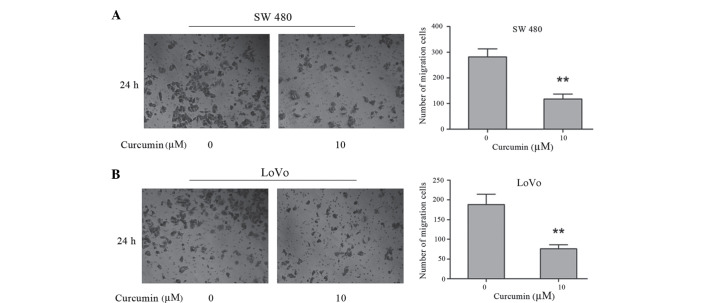
To investigate the effects of curcumin on the invasion of colon cancer SW480 and LoVo cells, a Matrigel-coated polycarbonate filter in a Boyden chamber was used. Following a 24 h incubation, curcumin was demonstrated to significantly decrease cell invasiveness. The number of SW480 cells that penetrated the membrane in 10 µM curcumin-treated cells (117.00±11.36) was significantly lower compared with control cells (281.33±11.22) (P=0.002; Fig. 2A). The number of LoVo cells that penetrated the membrane in 10 µM curcumin-treated cells (188.30±15.03) was significantly lower compared with control cells (76.03±5.77) (P=0.002; Fig. 2B). These results demonstrate that curcumin has a significant inhibitory effect on the invasive activity of human colon cancer SW480 and LoVo cells.
Figure 2.
Effects of curcumin on cell invasion as assessed by Transwell assay. Human colon cancer SW 480 and LoVo cells suspended in serum-free Dulbecco's modified Eagle's medium were overlaid in the upper chamber of a Boyden chamber. Following incubation with curcumin (0 and 10 µM) for 24 h, invaded cells were stained with crystal violet and recorded under a microscope. Photographs depict invasion of (A) SW480 and (B) LoVo cells. Quantified data are expressed as the mean ± standard error of the mean from three independent experiments. **P<0.01 vs. control cells.
Curcumin inhibits the expression of MMP9 and uPA in colon cancer LoVo and SW480 cells
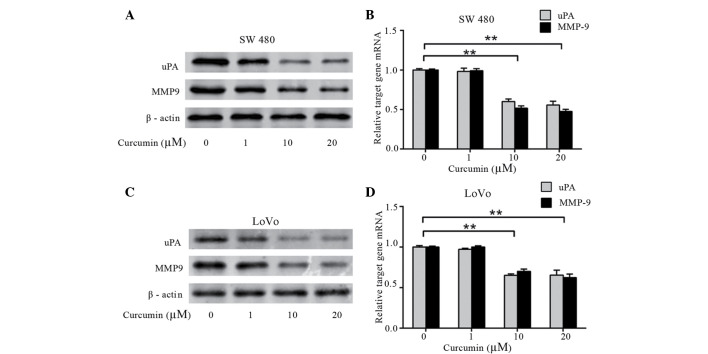
To investigate whether curcumin affects the expression of uPA and MMP9, which are involved in tumor metastasis and invasion, western blotting and qPCR were performed. The protein and mRNA levels of uPA and MMP9 was dose-dependently decreased by curcumin in SW480 (Fig. 3A and B) and LoVo cells (P=0.003; Fig. 3C and D). This data demonstrates that curcumin significantly decreases the expression of uPA and MMP9 in a dose-dependent manner, which results in the inhibitory effect of colon cancer cell adhesion and invasion.
Figure 3.
Effects of curcumin on uPA and MMP9 expression. Human colon cancer (A and B) SW480 and (C and D) LoVo cells were treated with curcumin (0–20 µM) for 24 h. Protein and mRNA expression of uPA and MMP9 were identified by western blotting and quantitative polymerase chain reaction, respectively. uPA and MMP9 mRNA levels in control cells were set to 1 and levels of these transcripts in the curcumin-treated groups were relative following normalization with glyceraldehyde 3-phosphate dehydrogenase. (A and C) Band weights (kDa): uPA, 52 kDa; MMP-9, 92 kDa; β-actin, 45 kDa. **P<0.01 vs. control cells. uPA, urokinase plasminogen activator; MMP9, matrix metalloproteinases.
Curcumin inhibits NF-κB activation in colon cancer LoVo and SW480 cells
The impact of curcumin on the NF-κB pathway has been observed in multiple human carcinomas (33). To investigate whether curcumin affects NF-κB activation, which is critical for transcriptional activity, the DNA binding activity of NF-κB was analyzed by ELISA. Colon cancer LoVo and SW480 cells were pretreated with various concentrations of curcumin (0–20 µM) for 24 h. As shown in Fig. 4, curcumin significantly decreased NF-κB activation in a dose-dependent manner for the two cells lines (P=0.002).
Figure 4.
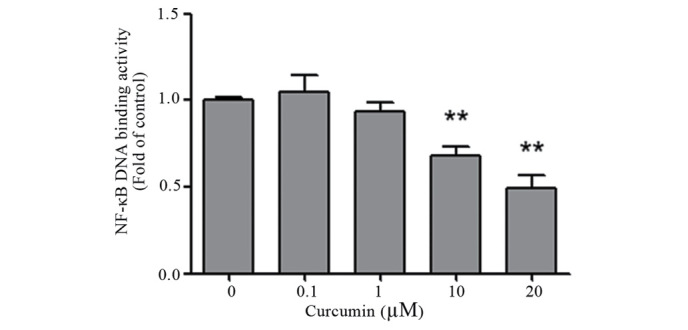
Effects of curcumin on NF-κB activation. Human colon SW480 and LoVo cells were pretreated with various concentrations of curcumin (0–20 µM) for 24 h. Nuclear extracts were prepared and NF-κB activation was measured by enzyme-linked immunosorbent assay. Quantified data are expressed as the mean ± standard error of the mean of three experiments, and are an amalgamation of the two cell lines. **P<0.01 vs. control cells. NF-κB, nuclear factor-κB.
Curcumin inhibits NF-κB, uPA and MMP9 in colon cancer SW480 cells via AMPK activation
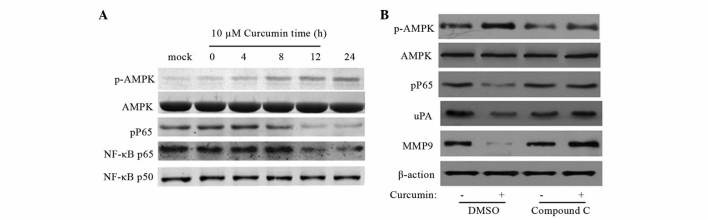
To investigate the effect of curcumin on the signaling pathway that regulates NF-κB activation, the present study investigated the expression of various proteins in SW480 cells using western blot analysis. SW480 cells was treated with 10 µM curcumin at various time points (0, 4, 8, 12 and 24 h). As indicated in Fig. 5A, curcumin increased AMPK phosphorylation at Thr172 and downregulated NF-κB p65 in a time-dependent manner.
Figure 5.
AMPK activation mediates curcumin-induced inhibition of NF-κB, uPA and MMP9. Human colon cancer SW480 cells were cultured until 70% confluence. (A) Cells were treated with curcumin (10 µM) for 0–24 h to investigate the effect on the NF-κB signaling pathway. (B) Cells were treated with curcumin (10 µM) in the absence or presence of 10 µM compound C, an AMPK inhibitor. Data are representative of three experiemnts. Band weights (kDa): uPA, 52 kDa; MMP-9, 92 kDa; β-actin, 45 kDa; p-AMPK, 62 kDa; AMPK, 62 kDa; pP65, 65 kDa; NF-κB p65, 65 kDa; NF-κB p50, 50kDa AMPK, 5′ AMP-activated protein kinase; NF-κB, nuclear factor-κB; uPA, urokinase plasminogen activator; MMP9, matrix metalloproteinases; DMSO, dimethyl sulfoxide.
To further delineate the role of AMPK in curcumin-induced inhibition of uPA and MMP9, pharmacological inhibition of AMPK was performed using compound C. The inhibitory effect of compound C on AMPK activity was confirmed by western blot analysis. As shown in Fig. 5B, compound C abolished curcumin-induced inhibition of NF-κB, uPA and MMP9, suggesting that AMPK activation is responsible for curcumin-mediated NF-κB, uPA and MMP9 inhibition.
Curcumin inhibits p65 NF-κB DNA binding to the uPA and MMP9 promoter and its subsequent transactivation
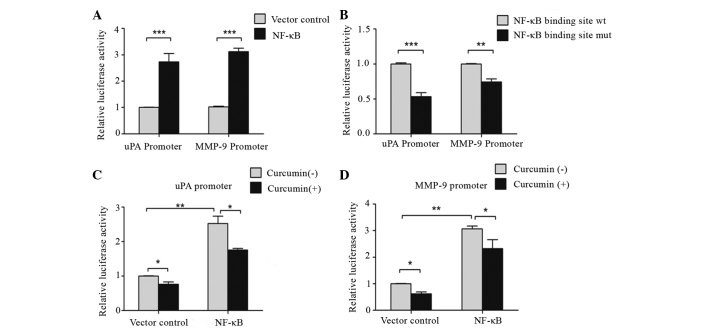
Since several studies have demonstrated that NF-κB is key in the transactivation of uPA and MMP9 (34–36), the present study investigated whether the activation of uPA and MMP9 may be attributed to NF-κB. As shown in Fig. 6A, uPA- and MMP9-specific promoter region activity significantly increased in NF-κB-induced SW480 cells compared with control cells (P=0.0006). Following mutations in the NF-κB binding site TTCC in uPA and MMP9 promoter regions, uPA and MMP9 activity decreased significantly compared with the control. This suggests that NF-κB binds to the uPA and MMP9 promoter sequence TTCC (P=0.0006 and P=0.003; Fig. 6B).
Figure 6.
Curcumin inhibits p65 NF-κB DNA binding to the uPA and MMP9 promoter. (A) Luciferase assay was performed by co-transfection with uPA or MMP9 promoter reporter and HA-tagged p65 NF-κB expression plasmid. The uPA- and MMP9-specific promoter region activity increased dramatically in NF-κB-induced human colon cancer SW480 cells compared with the control. (B) Co-transfection with uPA and MMP9 promoters with mutated NF-κB binding sites and HA-tagged p65 NF-κB expression plasmid led to a significant decrease in activity compared with the control. (C and D) Co-transfection with (C) uPA and (D) MMP9 wild-type promoter reporter and HA-tagged p65 NF-κB expression plasmid in SW480 cells for 8 h and incubation with 10 µM curcumin for 16 h led to a significant decrease in uPA and MMP9-specific promoter region activity. *P<0.05; **P<0.01; ***P<0.001 vs. control. HA, human influenza hemagglutinin; NF-κB, nuclear factor-κB; uPA, urokinase plasminogen activator; MMP9, matrix metalloproteinases.
To further confirm the possibility that downregulation of uPA or MMP9 transactivation, resulting from curcumin treatment, was caused by reduced binding of NF-κB to the uPA and MMP9 promoter sequence, wild-type uPA and MMP9 plasmids were transfected into SW480 cells for 8 h, and the cells were incubated with 10 µM curcumin for 16 h. As shown in Fig. 6C and D, uPA- and MMP9-specific promoter region activity significantly decreased following curcumin treatment (P=0.012; Fig. 6C), and treatment with NF-κB significantly upregulated uPA- and MMP9-specific promoter region activity (P=0.008; Fig. 6D). Overall, these results indicate that curcumin has a significant inhibitory role in NF-κB-mediated binding transactivation of uPA and MMP9.
Discussion
Tumor cell metastasis is known as a complex cascade of events, which involves cell adhesion, ECM component degradation and tumor cell migration. Therefore, blocking one or more of these steps is required for anti-metastatic therapy (2). Curcumin, which has been demonstrated to be anti-tumorigenic, has well-documented chemopreventive properties in a number of cell types, including colon cancer cells (21–25,37–40). The present study provides clear evidence that curcumin is capable of inhibiting the adhesion and invasion of human colon cancer cells through NF-κB-dependent uPA and MMP9 activation and expression in a dose-dependent manner. This suggests that curcumin may possess anti-metastatic potential in human colon cancer.
The present data confirms that curcumin significantly inhibits colon cancer cell growth, particularly at a high concentration (41). Previous studies have revealed that NF-κB activation is critical for the proliferation, survival and metastasis of colon cancer cells (26,41,42); therefore, inhibition of NF-κB activation is a potential antitumor strategy (43–46). The present study observed that curcumin dose-dependently suppresses constitutive NF-κB activation in colon cancer LoVo and SW480 cells. These results are in agreement with previous studies, which demonstrate that curcumin is a potent inhibitor of NF-κB activation (19,20). However, the potential mechanisms concerning NF-κB inhibition by curcumin leading to the suppression of colon cancer cell proliferation requires additional investigation.
NF-κB activation proceeds sequentially through the activation of IKK, phosphorylation of IkBα, degradation of IkBα, release of NF-κB, p65 phosphorylation and p65 nuclear translocation (47). The present study investigated the expression of the different proteins in the NF-κB activation pathway by western blot analysis. Notably, the present study demonstrated that curcumin inhibited NF-κB via the activation of AMPK. The AMPK signaling pathway is important in maintaining cellular survival during stress by regulating metabolic homeostasis (48). Consistent with the present findings, previous studies have suggested that the activation of the AMPK pathway suppresses the function of the NF-κB pathway (49–54). AMPK has certain direct phosphorylation targets (55); however, it is possible that AMPK inhibits NF-κB signaling through its downstream effectors, including peroxisome proliferator-activated receptor γ co-activator 1α and sirtuin (silent mating type information regulation 2 homolog) 1, which suppress inflammatory factors.
Since little is known concerning the function of curcumin in colon cancer metastatic progression, the present study determined whether the colon cancer metastatic process is associated with the suppression of NF-κB. The present cell-adhesion assay revealed that curcumin significantly reduced LoVo and SW480 cell adhesion to Matrigel (reconstituted basement membrane). This result led to additional investigation, which revealed that ECM molecules, including uPA and MMP9, were inhibited by curcumin. Numerous studies indicate that the key proteases involved in ECM degradation are MMP and serine proteases, including uPA and MMP9 (7,11). The present study investigated uPA and MMP9 expression in colon cancer LoVo and SW480 cells. The present results demonstrated that curcumin significantly decreased the expression of uPA and MMP9 in a dose-dependent manner, which led to inhibitory effects on colon cancer cell adhesion and invasion. Since the level of p65 NF-κB phosphorylation was decreased and phosphorylation of p65 is required for NF-κB transcriptional activity (56), the present study further investigated the mechanism by which curcumin controls uPA and MMP9 activation and expression via activation of NF-κB in colon cancer cells. As expected, curcumin suppressed the binding of p65 NF-κB to the uPA and MMP9 promoter. These results clearly indicate that curcumin may inhibit uPA and MMP9 transcription by suppressing NF-κB DNA binding activity to uPA and MMP9 promoter region. Therefore, the anti-metastatic effects of curcumin in human colon cancer may be mediated by inhibition of the NF-κB signaling pathway.
In summary, the present preliminary investigation has revealed that the anti-metastatic effect of curcumin in colon cancer cells may be mediated by the decrease of uPA and MMP9 expression via NF-κB activation. The NF-κB signaling pathway promotes the activation of uPA and MMP9 signaling to regulate colon cancer cell invasion. In addition, curcumin dose-dependently suppresses the activation of NF-κB, and this effect may attribute to a dose-dependent decrease in uPA and MMP9 protein levels by binding to their promoter regions. The present study provides additional evidence that curcumin inhibits metastatic activity in cancer cells and reveals a novel therapeutic potential for curcumin for anti-metastatic therapy.
References
- 1.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-I. [DOI] [PubMed] [Google Scholar]
- 2.Brooks SA, Lomax-Browne HJ, Carter TM, Kinch CE, Hall DM. Molecular interactions in cancer cell metastasis. Acta Histochem. 2010;112:3–25. doi: 10.1016/j.acthis.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 3.Raghu H, Sodadasu PK, Malla RR, Gondi CS, Estes N, Rao JS. Localization of uPAR and MMP-9 in lipid rafts is critical for migration, invasion and angiogenesis in human breast cancer cells. BMC Cancer. 2010;10:647. doi: 10.1186/1471-2407-10-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andreasen PA, Egelund R, Petersen HH. The plasminogen activation system in tumor growth, invasion, and metastasis. Cell Mol Life Sci. 2000;57:25–40. doi: 10.1007/s000180050497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vihinen P, Ala-aho R, Kähäri VM. Matrix metalloproteinases as therapeutic targets in cancer. Curr Cancer Drug Targets. 2005;5:203–220. doi: 10.2174/1568009053765799. [DOI] [PubMed] [Google Scholar]
- 6.Hildenbrand R, Gandhari M, Stroebel P, Marx A, Allgayer H, Arens N. The urokinase-system - role of cell proliferation and apoptosis. Histol Histopathol. 2008;23:227–236. doi: 10.14670/HH-23.227. [DOI] [PubMed] [Google Scholar]
- 7.Andreasen PA, Kjøller L, Christensen L, Duffy MJ. The urokinase-type plasminogen activator system in cancer metastasis: A review. Int J Cancer. 1997;72:1–22. doi: 10.1002/(SICI)1097-0215(19970703)72:1<1::AID-IJC1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 8.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 9.Newell KJ, Witty JP, Rodgers WH, Matrisian LM. Expression and localization of matrix-degrading metalloproteinases during colorectal tumorigenesis. Mol Carcinog. 1994;10:199–206. doi: 10.1002/mc.2940100404. [DOI] [PubMed] [Google Scholar]
- 10.Crowley CW, Cohen RL, Lucas BK, Liu G, Shuman MA, Levinson AD. Prevention of metastasis by inhibition of the urokinase receptor. Proc Natl Acad Sci USA. 1993;90:5021–5025. doi: 10.1073/pnas.90.11.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J Clin Oncol. 2009;27:5287–5297. doi: 10.1200/JCO.2009.23.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baichwal VR. Activate NF-kappa B or die? Curr Biol. 1997;7:R94–R96. doi: 10.1016/S0960-9822(06)00046-7. [DOI] [PubMed] [Google Scholar]
- 13.Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 14.Verma IM, Stevenson J. IkappaB kinase: Beginning, not the end. Proc Natl Acad Sci USA. 1997;94:11758–11760. doi: 10.1073/pnas.94.22.11758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen ZJ, Parent L, Maniatis T. Site-specific phosphorylation of IkappaBalpha by a novel ubiquitination-dependent protein kinase activity. Cell. 1996;84:853–862. doi: 10.1016/S0092-8674(00)81064-8. [DOI] [PubMed] [Google Scholar]
- 16.Shishodia S, Aggarwal BB. Nuclear factor-kappaB activation: A question of life or death. J Biochem Mol Biol. 2002;35:28–40. doi: 10.5483/BMBRep.2002.35.1.028. [DOI] [PubMed] [Google Scholar]
- 17.Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: From ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65:1631–1652. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- 19.Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected] J Biol Chem. 1995;270:24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 20.Kumar A, Dhawan S, Hardegen NJ, Aggarwal BB. Curcumin (Diferuloylmethane) inhibition of tumor necrosis factor (TNF)-mediated adhesion of monocytes to endothelial cells by suppression of cell surface expression of adhesion molecules and of nuclear factor-kappaB activation. Biochem Pharmacol. 1998;55:775–783. doi: 10.1016/S0006-2952(97)00557-1. [DOI] [PubMed] [Google Scholar]
- 21.Shi M, Cai Q, Yao L, Mao Y, Ming Y, Ouyang G. Antiproliferation and apoptosis induced by curcumin in human ovarian cancer cells. Cell Biol Int. 2006;30:221–226. doi: 10.1016/j.cellbi.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 22.Kawamori T, Lubet R, Steele VE, Kelloff GJ, Kaskey RB, Rao CV, Reddy BS. Chemopreventive effect of curcumin, a naturally occurring anti-inflammatory agent, during the promotion/progression stages of colon cancer. Cancer Res. 1999;59:597–601. [PubMed] [Google Scholar]
- 23.Mukhopadhyay A, Bueso-Ramos C, Chatterjee D, Pantazis P, Aggarwal BB. Curcumin downregulates cell survival mechanisms in human prostate cancer cell lines. Oncogene. 2001;20:7597–7609. doi: 10.1038/sj.onc.1204997. [DOI] [PubMed] [Google Scholar]
- 24.Bharti AC, Donato N, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J Immunol. 2003;171:3863–3871. doi: 10.4049/jimmunol.171.7.3863. [DOI] [PubMed] [Google Scholar]
- 25.Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008;269:199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Plummer SM, Holloway KA, Manson MM, Munks RJ, Kaptein A, Farrow S, Howells L. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-kappaB activation via the NIK/IKK signalling complex. Oncogene. 1999;18:6013–6020. doi: 10.1038/sj.onc.1202980. [DOI] [PubMed] [Google Scholar]
- 27.Mohan R, Sivak J, Ashton P, Russo LA, Pham BQ, Kasahara N, Raizman MB, Fini ME. Curcuminoids inhibit the angiogenic response stimulated by fibroblast growth factor-2, including expression of matrix metalloproteinase gelatinase B. J Biol Chem. 2000;275:10405–10412. doi: 10.1074/jbc.275.14.10405. [DOI] [PubMed] [Google Scholar]
- 28.Pan MH, Lin-Shiau SY, Lin JK. Comparative studies on the suppression of nitric oxide synthase by curcumin and its hydrogenated metabolites through down-regulation of IkappaB kinase and NFkappaB activation in macrophages. Biochem Pharmacol. 2000;60:1665–1676. doi: 10.1016/S0006-2952(00)00489-5. [DOI] [PubMed] [Google Scholar]
- 29.Jobin C, Bradham CA, Russo MP, Juma B, Narula AS, Brenner DA, Sartor RB. Curcumin blocks cytokine-mediated NF-kappaB activation and proinflammatory gene expression by inhibiting inhibitory factor I-kappa B kinase activity. J Immunol. 1999;163:3474–3483. [PubMed] [Google Scholar]
- 30.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 31.Al-Tubuly AA. SDS-PAGE and western blotting. Methods Mol Med. 2000;40:391–405. doi: 10.1385/1-59259-076-4:391. [DOI] [PubMed] [Google Scholar]
- 32.Livak KI, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 33.Sandur SK, Deorukhkar A, Pandey MK, Pabón AM, Shentu S, Guha S, Aggarwal BB, Krishnan S. Curcumin modulates the radiosensitivity of colorectal cancer cells by suppressing constitutive and inducible NF-kappaB activity. Int J Radiat Oncol Biol Phys. 2009;75:534–542. doi: 10.1016/j.ijrobp.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Killeen SD, Wang JH, Andrews EJ, Redmond HP. Bacterial endotoxin enhances colorectal cancer cell adhesion and invasion through TLR-4 and NF-kappaB-dependent activation of the urokinase plasminogen activator system. Br J Cancer. 2009;100:1589–1602. doi: 10.1038/sj.bjc.6604942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sliva D, English D, Lyons D, Lloyd FP., Jr Protein kinase C induces motility of breast cancers by upregulating secretion of urokinase-type plasminogen activator through activation of AP-1 and NF-kappaB. Biochem Biophys Res Commun. 2002;290:552–557. doi: 10.1006/bbrc.2001.6225. [DOI] [PubMed] [Google Scholar]
- 36.Tsunoda K, Kitange G, Anda T, Shabani HK, Kaminogo M, Shibata S, Nagata I. Expression of the constitutively activated RelA/NF-kappaB in human astrocytic tumors and the in vitro implication in the regulation of urokinase-type plasminogen activator, migration, and invasion. Brain Tumor Pathol. 2005;22:79–87. doi: 10.1007/s10014-005-0186-1. [DOI] [PubMed] [Google Scholar]
- 37.Bharti AC, Donato N, Singh S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-kappa B and IkappaBalpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood. 2003;101:1053–1062. doi: 10.1182/blood-2002-05-1320. [DOI] [PubMed] [Google Scholar]
- 38.Zong H, Wang F, Fan QX, Wang LX. Curcumin inhibits metastatic progression of breast cancer cell through suppression of urokinase-type plasminogen activator by NF-kappa B signaling pathways. Mol Biol Rep. 2012;39:4803–4808. doi: 10.1007/s11033-011-1273-5. [DOI] [PubMed] [Google Scholar]
- 39.Shishodia S, Amin HM, Lai R, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive NF-kappaB activation, induces G1/S arrest, suppresses proliferation, and induces apoptosis in mantle cell lymphoma. Biochem Pharmacol. 2005;70:700–713. doi: 10.1016/j.bcp.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 40.Shishodia S, Potdar P, Gairola CG, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates cigarette smoke-induced NF-kappaB activation through inhibition of IkappaBalpha kinase in human lung epithelial cells: Correlation with suppression of COX-2, MMP-9 and cyclin D1. Carcinogenesis. 2003;24:1269–1279. doi: 10.1093/carcin/bgg078. [DOI] [PubMed] [Google Scholar]
- 41.Johnson SM, Gulhati P, Arrieta I, Wang X, Uchida T, Gao T, Evers BM. Curcumin inhibits proliferation of colorectal carcinoma by modulating Akt/mTOR signaling. Anticancer Res. 2009;29:3185–3190. [PMC free article] [PubMed] [Google Scholar]
- 42.Chen H, Zhang ZS, Zhang YL, Zhou DY. Curcumin inhibits cell proliferation by interfering with the cell cycle and inducing apoptosis in colon carcinoma cells. Anticancer Res. 1999;19:3675–3680. [PubMed] [Google Scholar]
- 43.Baeuerle PA, Baichwal VR. NF-kappa B as a frequent target for immunosuppressive and anti-inflammatory molecules. Adv Immunol. 1997;65:111–137. doi: 10.1016/S0065-2776(08)60742-7. [DOI] [PubMed] [Google Scholar]
- 44.Garg A, Aggarwal BB. Nuclear transcription factor-kappaB as a target for cancer drug development. Leukemia. 2002;16:1053–1068. doi: 10.1038/sj.leu.2402482. [DOI] [PubMed] [Google Scholar]
- 45.Huang S, Pettaway CA, Uehara H, Bucana CD, Fidler IJ. Blockade of NF-kappaB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene. 2001;20:4188–4197. doi: 10.1038/sj.onc.1204535. [DOI] [PubMed] [Google Scholar]
- 46.Hideshima T, Chauhan D, Richardson P, Mitsiades C, Mitsiades N, Hayashi T, Munshi N, Dang L, Castro A, Palombella V, et al. NF-kappa B as a therapeutic target in multiple myeloma. J Biol Chem. 2002;277:16639–16647. doi: 10.1074/jbc.M200360200. [DOI] [PubMed] [Google Scholar]
- 47.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109 Suppl:S81–S96. doi: 10.1016/S0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 48.Salminen A, Hyttinen JM, Kaarniranta K. AMP-activated protein kinase inhibits NF-kappaB signaling and inflammation: Impact on healthspan and lifespan. J Mol Med (Berl) 2011;89:667–676. doi: 10.1007/s00109-011-0748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bai A, Ma AG, Yong M, Weiss CR, Ma Y, Guan Q, Bernstein CN, Peng Z. AMPK agonist downregulates innate and adaptive immune responses in TNBS-induced murine acute and relapsing colitis. Biochem Pharmacol. 2010;80:1708–1717. doi: 10.1016/j.bcp.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 50.Yang Z, Kahn BB, Shi H, Xue BZ. Macrophage alpha1 AMP-activated protein kinase (alpha1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. J Biol Chem. 2010;285:19051–19059. doi: 10.1074/jbc.M110.123620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sag D, Carling D, Stout RD, Suttles J. Adenosine 5′-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol. 2008;181:8633–8641. doi: 10.4049/jimmunol.181.12.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang S, Zhang M, Liang B, Xu J, Xie Z, Liu C, Viollet B, Yan D, Zou MH. AMPKalpha2 deletion causes aberrant expression and activation of NAD(P)H oxidase and consequent endothelial dysfunction in vivo: Role of 26S proteasomes. Circ Res. 2010;106:1117–1128. doi: 10.1161/CIRCRESAHA.109.212530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu X, Mahadev K, Fuchsel L, Ouedraogo R, Xu SQ, Goldstein BJ. Adiponectin suppresses IkappaB kinase activation induced by tumor necrosis factor-alpha or high glucose in endothelial cells: Role of cAMP and AMP kinase signaling. Am J Physiol Endocrinol Metab. 2007;293:E1836–E1844. doi: 10.1152/ajpendo.00115.2007. [DOI] [PubMed] [Google Scholar]
- 54.Hattori Y, Suzuki K, Hattori S, Kasai K. Metformin inhibits cytokine-induced nuclear factor kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension. 2006;47:1183–1188. doi: 10.1161/01.HYP.0000221429.94591.72. [DOI] [PubMed] [Google Scholar]
- 55.Cantó C, Auwerx J. AMP-activated protein kinase and its downstream transcriptional pathways. Cell Mol Life Sci. 2010;67:3407–3423. doi: 10.1007/s00018-010-0454-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhong H, Voll RE, Ghosh S. Phosphorylation of NF-kappa B p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol Cell. 1998;1:661–671. doi: 10.1016/S1097-2765(00)80066-0. [DOI] [PubMed] [Google Scholar]