Abstract
Acute lymphoblastic leukemia (ALL) accounts for 30% of all pediatric cancers. Currently available treatments exhibit toxicity and certain patients may develop resistance. Thus, less toxic and chemoresistance-reversal agents are required. In the present study, the potential effect of curcumin, a component of Curcuma longa, as a pharmacological co-adjuvant of several chemotherapeutic agents against ALL, including prednisone, 6-mercaptopurine, dexamethasone, cyclophosphamide, l-asparaginase, vincristine, daunorubicin, doxorubicin, methotrexate and cytarabine, was investigated in the REH ALL cell line cultures treated in combination with chemotherapeutic agents and curcumin. The results of cell viability, gene expression and activation of NF-κB and caspase 3 indicated that curcumin potentiates the anticancer effects of the aforementioned chemotherapeutic agents in the REH ALL cell line. Following treatment with the above chemotherapeutic agents, curcumin enhanced caspase-3 activation and downregulated nuclear factor-kappa B (NF-κB) activation. Curcumin also downregulated the oxidative stress induced by certain chemotherapies. Notably, curcumin did not affect the gene expression of cell survival proteins such as B-cell lymphoma (Bcl)-2, Bcl-extra large, survivin, c-Myc and cyclin D1, which are regulated by the NF-κB transcription factor. In conclusion, curcumin has the potential to improve the effect of chemotherapeutic agents against ALL.
Keywords: chemotherapy, curcumin, leukemia, NF-κB
Introduction
Acute lymphoblastic leukemia (ALL) is one of the most malignant types of hematological disease, which accounts for 30% of all pediatric cancers (1). Chemotherapeutic drugs commonly used for ALL treatment include prednisone, 6-mercaptopurine, dexamethasone, cyclophosphamide, l-asparaginase, vincristine, daunorubicin or doxorubicin, methotrexate and cytarabine; with overall cure rates of 80% (Fig. 1) (2). However, a significant number of patients develop resistance to these drugs and outcome is poor among patients who relapse (2). The pathogenesis of ALL includes changes in gene expression, which are regulated by diverse transcription factors (3) such as nuclear factor-kappa B (NF-κB), which has been associated with cell proliferation and survival (4). Additionally, in solid tumors, NF-κB exhibits an important function in invasion, angiogenesis, aggressive tumor growth and chemoresistance (4–6). Constitutive activation of NF-κB is observed in ~92% of pediatric ALL patients (7) and thus is one of the targets for chemosensitization.
Figure 1.
Chemical structures of curcumin and the 10 chemotherapeutic agents used in the present study.
Since ~80% of clinical drugs are derived from natural products, numerous compounds have been identified that downmodulate NF-κB (8). Curcumin (diferuloylmethane), a polyphenol derived from the plant Curcuma longa, has been demonstrated to inhibit NF-κB activation, which is induced by a wide variety of carcinogens and chemotherapeutic agents (9). The use of curcumin was classified by the USA Food and Drug Administration as ‘generally recognized as safe’ (10). Furthermore, various clinical trials indicate that curcumin may be administered at oral doses as high as 8 g/day with no side effects (11).
The aim of the present study was to investigate the potential anticancer effect of curcumin on the human REH ALL cell line, when administered alone and in combination with currently used therapies. The results indicate that curcumin potentiates the effect of chemotherapeutic agents against ALL cells by activation of caspase-3 through downregulation of oxidative stress, NF-κB activation and various NF-κB-regulated cell survival gene products.
Materials and methods
Reagents
Curcumin and sodium dodecyl sulfate were obtained from Sigma-Aldrich (St. Louis, MO, USA). Penicillin, streptomycin, RPMI-1640 medium, phosphate-buffered saline (PBS), fetal bovine serum (FBS) and TaqMan Assays for B-cell lymphoma (Bcl)-extra large (xL), Bcl-2, cyclin D1, survivin, c-Myc and beta-glucuronidase (GUSB) were obtained from Life Technologies (Thermo Fisher Scientific, Inc., Waltham, MA, USA). DNA and RNA extraction was performed using QIAzol Lysis reagent and QIAmp Circulating Nucleic Acid kit, which were obtained from Qiagen GmbH (Hilden, Germany). Monoclonal anti-NF-κB (dilution, 1:50; cat. no. A88940), anti-cluster of differentiation (CD)45 (dilution, 1:10; cat. nos. IM2652U and IM0782U) and anti-caspase-3 (dilution, 1:50; cat. no. A88950) antibodies, 7-aminoactinomycin D (7-AAD) viability dye, PerFix EXPOSE Phospho-Epitopes Exposure kit and IntraPrep™ permeabilization reagent were obtained from Beckman Coulter, Inc. (Brea, CA, USA). Anti-NF-κB p65 antibody (dilution, 1 µg; cat. no. sc-8008) was obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Highly sensitive 8-hydroxy-2′-deoxyguanosine (8-OHdG) was supplied by the Japanese Institute for the Control of Aging (Fukuroi, Japan). Prednisone, 6-mercaptopurine, dexamethasone, cyclophosphamide, l-asparaginase, vincristine, daunorubicin, doxorubicin, methotrexate and cytarabine were provided by PiSA Farmacéutica (Guadalajara, México).
Cell culture
The REH cell line (St. Jude Children's Research Hospital, Memphis, TN, USA) was cultured in RMPI-1640 medium supplemented with 100 U/ml penicillin and 100 g/ml streptomycin with 10% FBS in a humidified incubator at 37°C with an atmosphere of 5% CO2. Next, 5×105 REH cells were treated for 48 h in triplicate with 125 µg/ml prednisone, 250 µg/ml 6-mercaptopurine, 0.4 µg/ml dexamethasone, 50 µg/ml cyclophosphamide, 5 U l-asparaginase, 25 µg/ml vincristine, 1 µg/ml daunorubicin, 0.5 µg/ml doxorubicin, 7.5 µg/ml methotrexate and 1.25 µg/ml cytarabine, with or without 20 µM curcumin. Untreated cells served as the control group.
Viability assay
Cell viability was determined by flow cytometry using a 7-AAD dye exclusion test. After treatment with the various drugs, cells were harvested, washed once in PBS and centrifuged at 100 × g at room temperature for 1 min, and incubated with 100 µl PBS, 20 µl anti-CD45-FITC (dilution, 1:10; cat. no. IM0782U; Beckman Coulter, Inc.) and 100 µl 7-AAD for 20 min at room temperature in the dark. Following incubation, the cells were suspended in PBS and 20,000 events were analyzed using a Gallios Flow Cytometer (Beckman Coulter, Inc.). Gating was set to exclude cell debris and autofluorescence.
DNA oxidation
To determine if curcumin prevents oxidative damage to DNA caused by chemotherapy treatment, oxidative DNA adducts were measured using the highly sensitive 8-OHdG. DNA was isolated from the cells using QIAamp DNA Mini kit (Qiagen GmbH) and mixed with 50 µl nuclease-free water. Later, the cells were digested with Mung Bean Nuclease (6 U; Promega, Madison, WI, USA) at 37°C for 45 min, followed by treatment with alkaline phosphatase (2 U) for an additional 45 min. DNA was precipitated with absolute ethanol and centrifuged at 2,370 × g for 2 min, followed by hydration with 50 µl nuclease-free water. The digested DNA was added to the 8-OHdG (Highly Sensitive 8-OHdG Check ELISA kit; cat. no. KOG-HS10E; Japan Institute for the Control of Aging, Fukuroi, Japan.) well strip and incubated with 50 µl primary monoclonal antibody (dilution, 1 µg/50 µl) specific for 8-OHdG at 4°C overnight. Following incubation, 3 washes were performed with 250 µl washing solution (Japanese Institute for the Control of Aging) at room temperature, with agitation of the plate from side to side for 20 seconds, disposing washing solution each time. The samples were then incubated with 50 µl horseradish peroxidase-conjugated anti-mouse secondary antibody (dilution, 1 µg/5 µl; cat. no. 405310) for 1 h at room temperature in the dark. After 3 washes, 50 µl chromatic solution (Japanese Institute for the Control of Aging) was added and incubated for 15 min at 4°C. The reaction was terminated following addition of 100 µl termination solution (Japanese Institute for the Control of Aging), and the samples were analyzed at a wavelength of 450 nm in a plate spectrophotometer.
NF-κB detection
To assess the involvement of curcumin in NF-κB activation, flow cytometry was performed using anti-human-phospho-NF-κB p65 antibody (Beckman Coulter, Inc.). After the treatment the cells were fixed using PerFix Fixative reagent (Beckman Coulter, Inc.) for 10 min at room temperature and permeabilized using PerFix Permeabilizing reagent for 5 min at 37°C in a water bath (PerFix EXPOSE Phospho-Epitopes Exposure kit; Beckman Coulter, Inc.). A total of 50 µl staining reagent pre-mixed with 2 µl conjugated anti-NF-κB-AlexaFluor 647 (dilution, 1:50; cat. no. A88940; Beckman Coulter, Inc.) and 10 µl anti-CD45-FITC antibodies were added to each tube immediately and incubated at room temperature for 30 min in the dark. Cells were then washed with 3 ml 1X wash reagent (PerFix EXPOSE; Beckman Coulter, Inc.) at room temperature, centrifuged at 300 × g for 5 min. The washing solution was removed and the cells were suspended in 500 µl final 1X reagent (PerFix EXPOSE; Beckman Coulter, Inc.), and 20,000 events were analyzed using Gallios software version 10 (Beckman Coulter, Inc.). Gating was set to exclude cell debris and autofluorescence.
Gene expression
To determine changes in the expression of various genes that are downregulated by NF-κB, RNA extraction was performed using QIAzol Lysis reagent. The cells were washed with PBS at room temperature, centrifuged at 100 × g for 2 min at room temperature and incubated with 1 ml QIAzol Lysis reagent for 5 min, followed by the addition of 200 µl chloroform and centrifugation at 12,350 × g at 4°C for 10 min. The aqueous phase was recovered in 500 µl isopropanol and incubated at −20°C overnight. Next, samples were centrifuged at 12,350 × g at 4°C for 10 min and washed twice with 70% ethanol, with centrifugation performed at 9,680 × g at 4°C for 5 min between each wash, and then air dried for 30 min. The samples were reconstituted in 30 µl diethylpyrocarbonate-treated water (Invitrogen; Thermo Fisher Scientific, Inc.). Reverse transcription was performed using 1 µg total RNA and a High-Capacity cDNA Reverse Transcription kit (Applied Biosystems; Thermo Fisher Scientific, Inc.) in a GeneAmp PCR System 9700 thermal cycler (Applied Biosystems; Thermo Fisher Scientific, Inc.). The reaction conditions were as follows: 25°C for 25 min, 37°C for 120 min, 85°C for 5 min and infinite hold at 4°C. Quantitative polymerase chain reaction (PCR) was performed using TaqMan Assays for the different genes and the TaqMan Universal PCR Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) in a 7900HT Fast Real-Time PCR System using SDS 2.4 software (Applied Biosystems; Thermo Fisher Scientific, Inc.). PCR was performed according to the manufacturer's protocol, and the cycling conditions were as follows: 50°C for 2 min, 95°C for 10 min, 95°C for 15 sec and 60°C for 1 min (40 cycles). Data was quantified according to the relative quantitation 2−∆∆Cq method (12), using the GUSB gene as an endogenous control and the chemotherapy treatment groups without curcumin as a calibrator (13).
Caspase-3 detection
To determine whether curcumin potentiates caspase-3 activity, flow cytometry was performed using polyclonal anti-human cleaved caspase-3 (Asp-175). The cells (5×105) were mixed with 20 µl anti-CD45-PC5 (dilution, 1:10; cat. no. IM2653U; Beckman Coulter, Inc.) and incubated for 20 min at room temperature in the dark. Cells were fixed using 100 µl IntraPrep Fixation reagent (Beckman Coulter, Inc.) for 15 min at room temperature, washed in 4 ml PBS and centrifuged at 300 × g for 5 min. Subsequent to washing, the cells were permeabilized for 5 min at room temperature using IntraPrep™ Permeabilization reagent (Beckman Coulter, Inc.). Next, the cells were incubated with 2 µl polyclonal anti-human cleaved caspase-3 (Asp-175) for 45 min at room temperature in the dark. The cells were then washed and suspended in 500 µl PBS and 20,000 events were analyzed using Gallios software version 10 (Beckman Coulter, Inc.). Gating was set to exclude cell debris and autofluorescence.
Statistical analysis
Differences in various parameters were compared in the control and treatment groups using the PASW 18.0 Software (SPSS, Inc., Chicago, IL, USA). The data were firstly analyzed using the Kolmogorov-Smirnov test. When the data had a normal distribution, the groups were compared using the Student's t-test. When the distribution did not have a normal distribution, the data were analyzed using Mann-Whitney U test. P<0.05 was considered to indicate a statistically significant difference.
Results
The antitumor properties of curcumin have been evaluated in a large number of solid tumors (14), however, less is known regarding hematological neoplasias.
Curcumin decreases cell viability of REH cells
To evaluate the effect of curcumin alone on cell viability, flow cytometry was performed using 7-AAD. To determine the effect of curcumin on the viability of REH cells, six different concentrations were investigated (10, 20, 25, 30, 40 and 50 µM). Curcumin decreased cell viability in a dose-dependent manner in REH cells, and following treatment with 25, 30, 40 and 50 µM curcumin, the cell viability was significantly decreased when compared with the control (P<0.001). A dose of 20 µM curcumin was selected for further experiments, as the next tested dose of 25 µM curcumin had statistical differences in cell viability compared with the control group from a pilot study.
Combined treatment with chemotherapeutic agents and curcumin decreases cell viability in REH cells
7-AAD flow cytometry was performed to investigate whether curcumin potentiates the effect of chemotherapeutic agents and decreases the cell viability of REH cells. The results revealed that treatment with all chemotherapeutic agents reduced cell viability when compared with the control. Furthermore, combined treatment with curcumin resulted in a further decrease in cell viability for all chemotherapeutic drugs (Fig. 2A). The group treated with curcumin alone exhibited a cell viability of 86.3%, whereas the group treated with l-asparaginase alone exhibited a cell viability of 89.7%. Notably, combined treatment with l-asparaginase and curcumin decreased cell viability to 59.9% (Fig. 2B). The same potentiating effect of curcumin was observed with prednisone, cyclophosphamide, 6-mercaptopurine, dexamethasone, vincristine and methotrexate (P<0.05).
Figure 2.
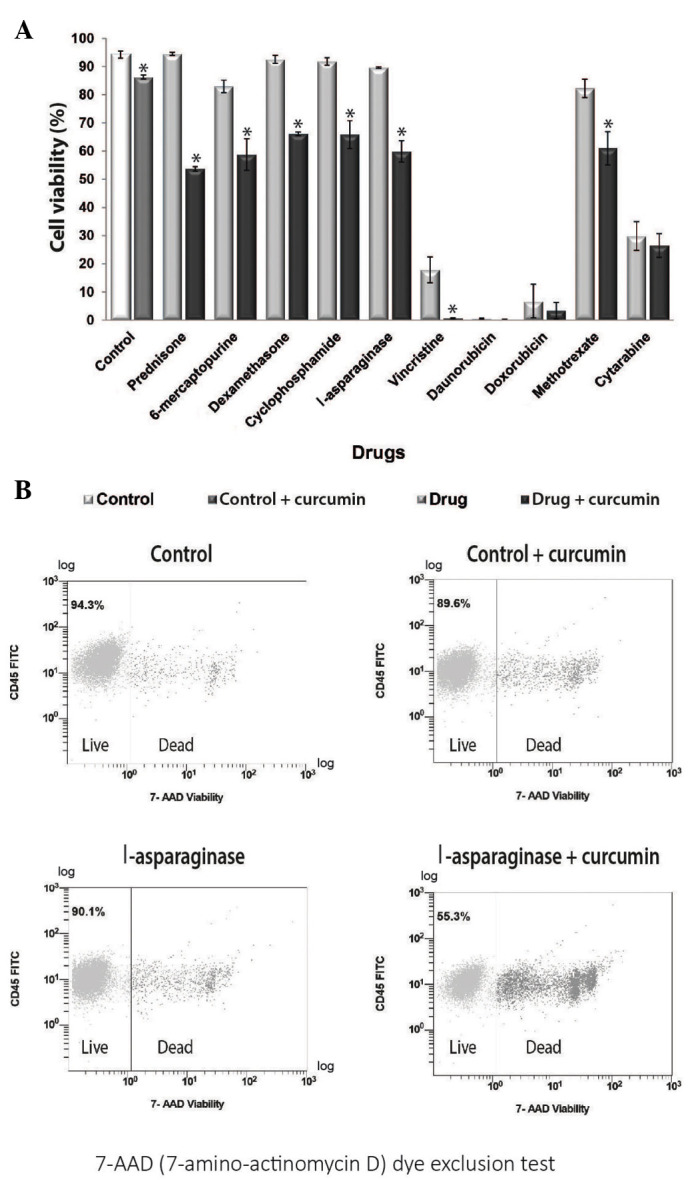
Curcumin decreases the viability of the REH cell line following treatment with 10 therapeutic agents. (A) Curcumin decreased cell viability of tumor cells in the 10 groups co-treated with curcumin and chemotherapeutic agents. *P<0.05 vs. control. (B) Treatment with 20 µM curcumin decreased cell viability in REH cells, when compared with the l-asparaginase treatment group. However, cell viability was markedly decreased following combined treatment with curcumin and l-asparaginase. CD, cluster of differentiation; FITC, fluorescein isothiocyanate; 7-AAD, 7-aminoactinomycin D.
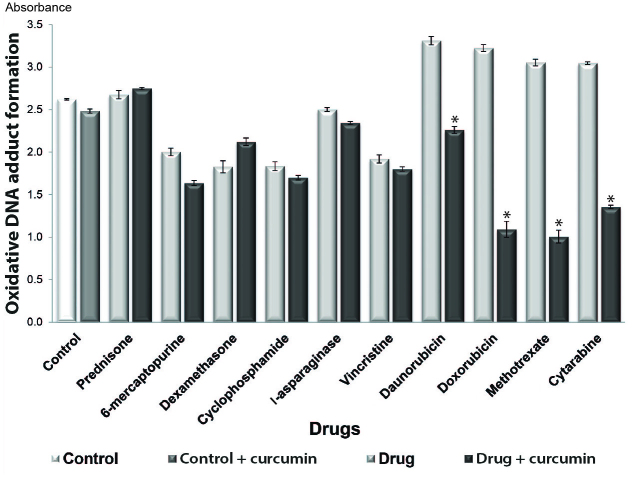
Curcumin prevents DNA oxidation
It has been demonstrated that chemotherapeutic agents induce DNA damage in normal and abnormal cells (15). To determine whether curcumin acts as an antioxidant when combined with chemotherapeutic drugs (16), in the present study, oxidative DNA adduct formation was analyzed in cultures treated with or without curcumin. The results revealed that oxidative DNA adduct formation was decreased in all combined treatment groups, with the exception of the prednisone + curcumin and dexamethasone + curcumin treatment groups. Significant decreases in DNA adduct formation were observed in the groups treated with curcumin and daunorubicin, doxorubicin, methotrexate and cytarabine (P<0.05) (Fig. 3).
Figure 3.
Effect of curcumin on DNA oxidation. Comparison of DNA oxidation in the groups treated with the chemotherapeutic agents alone and in those co-treated with 20 µM curcumin. *P<0.05 vs. control.
Curcumin decreases NF-κB activation in cells treated with chemotherapeutic agents
It has been reported that constitutive NF-κB activation occurs in ALL (7,17), and a previous study has suggested that chemotherapy alone may increase this activation (18). To determine whether curcumin decreases the levels of active NF-κB in the REH cell line, the levels of NF-κB phosphorylated at Ser536 were evaluated by flow cytometry. The results demonstrated that treatment with 8/10 of the therapeutic agents led to increased NF-κB activity when compared with the control group (P<0.05), whereas all of the combined treatment groups (chemotherapeutic agent + curcumin) exhibited decreased NF-κB activation (Fig. 4A). The control and curcumin alone treatment groups exhibited an NF-κB activation rate of 24.8 and 11.7%, respectively. The cyclophosphamide treatment group exhibited an NF-κB activation rate of 81%, while combined treatment with cyclophosphamide and curcumin decreased the NF-κB activation rate to 55.3% (P<0.05) (Fig. 4B). The most significant NF-κB inactivation was observed following combined treatment with curcumin and 6-mercaptopurine, cyclophosphamide, vincristine, daunorubicin, doxorubicin, methotrexate and cytarabine (P<0.05).
Figure 4.
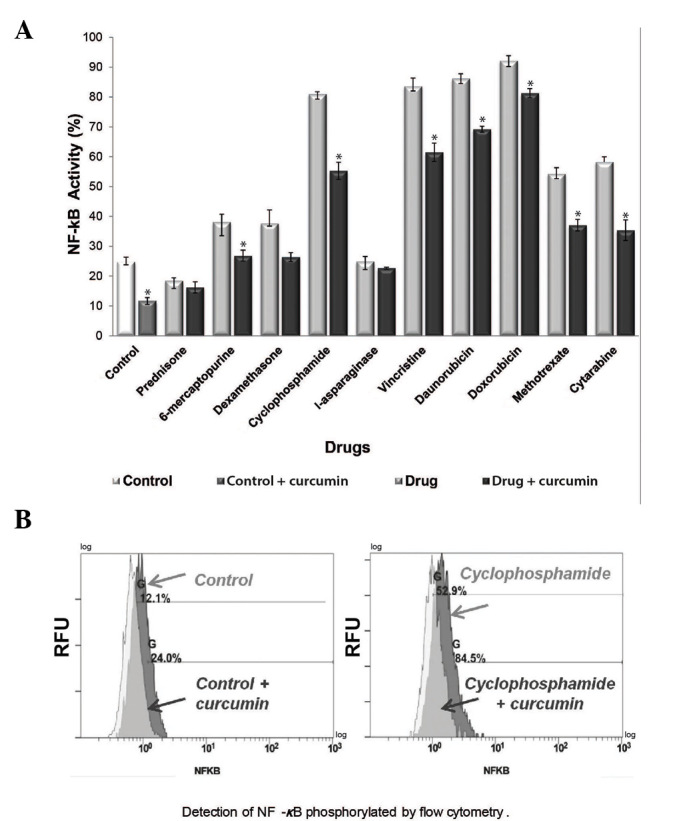
Effect of curcumin and chemotherapy on NF-κB activity. (A) Comparison of NF-κB activity in the groups treated with chemotherapeutic agents alone and in those co-treated with 20 µM curcumin. *P<0.05 vs. control. (B) Treatment with 20 µM curcumin increased NF-κB activity in REH cells, and this increase was most evident in the cyclophosphamide-treated group compared with the cyclophosphamide + curcumin-treated group. NF-κB, nuclear factor-kappa B.
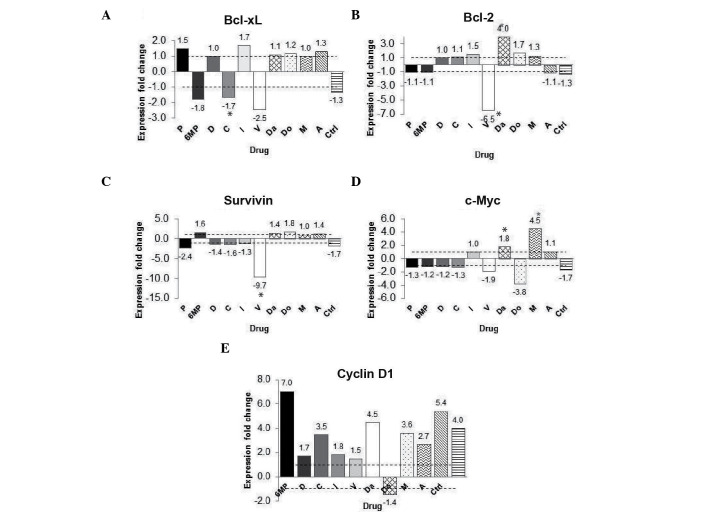
Curcumin affects the expression of NF-κB target genes
Since apoptosis and proliferation-related genes Bcl-2, Bcl-xl, survivin, cyclin D1 and c-Myc, have all been demonstrated to be regulated by NF-κB (19), in the present study, the expression of these genes was analyzed. Gene expression was analyzed in the chemotherapy-treated groups with and without curcumin. The gene expression fold change was calculated using the group with chemotherapy without curcumin as calibrator and the chemotherapy with curcumin group as a target group. The gene expression of the anti-apoptosis gene Bcl-xL was decreased by 2.5 fold in the vincristine group; however, this difference was not significant (P=0.513) (Fig. 5A). Furthermore, Bcl-2 gene expression was decreased by 6.5 fold in the vincristine + curcumin group (P<0.05) (Fig. 5B). The expression of the survivin gene was decreased by 9.7 fold in the vincristine + curcumin group (P<0.05), when compared with the vincristine group (Fig. 5C). The expression of the proliferative gene c-Myc increased in the methotrexate + curcumin group and decreased by 3.8 fold in the doxorubicin + curcumin group when compared with their calibrator (same chemotherapy without curcumin); however, these changes were not significant (P=0.827 and P=0.275, respectively) (Fig. 5D). Notably, the expression of the cyclin D1 gene was increased in 9/10 of the co-treated groups (with the exception of the doxorubicin group) compared with their calibrator; however, no significant differences were identified (Fig. 5E). These findings indicate that curcumin did not result in a downregulation pattern in the c-Myc and cyclin D1 groups.
Figure 5.
Effect of curcumin on NF-κB-regulated gene expression. (A) Changes in fold expression of the (A) Bcl-extra large, (B) Bcl-2, (C) survivin, (D) c-Myc and (E) cyclin D1 genes. The data are presented as the fold-change in gene expression between the groups treated with chemotherapy alone and the groups treated with chemotherapy and curcumin.*P<0.05 vs. control. P, prednisone; 6MP, 6-mercaptopurine; D, dexamethasone; C, cyclophosphamide; L, l-asparaginase; V, vincristine; Da, daunorubicin; Do, doxorubicin; M, methotrexate; A, cytarabine; Ctrl, control; Bcl, B-cell lymphoma; xL, extra large; NF-κB, nuclear factor-kappa B.
Curcumin activates caspase-3
As NF-κB has been demonstrated to exhibit an anti-apoptosis effect (20), in the present study, the effect of curcumin on apoptosis was investigated. The activity of cleaved caspase-3, the effector protein of the receptor-mediated and chemical-induced apoptosis pathways (21), was evaluated. To determine if curcumin induces caspase-3 activation, flow cytometry was performed to analyze the percentage of active caspase-3 in the chemotherapy-treated cultures with or without curcumin. The results demonstrated that curcumin increased caspase-3 activity following treatment with all the chemotherapeutic agents tested (Fig. 6A). The most significant increases in caspase-3 activity were identified in the prednisone + curcumin (Fig. 6B), l-asparaginase + curcumin and methotrexate + curcumin groups. However, treatment with curcumin alone also significantly increased caspase-3 activity (P<0.05).
Figure 6.
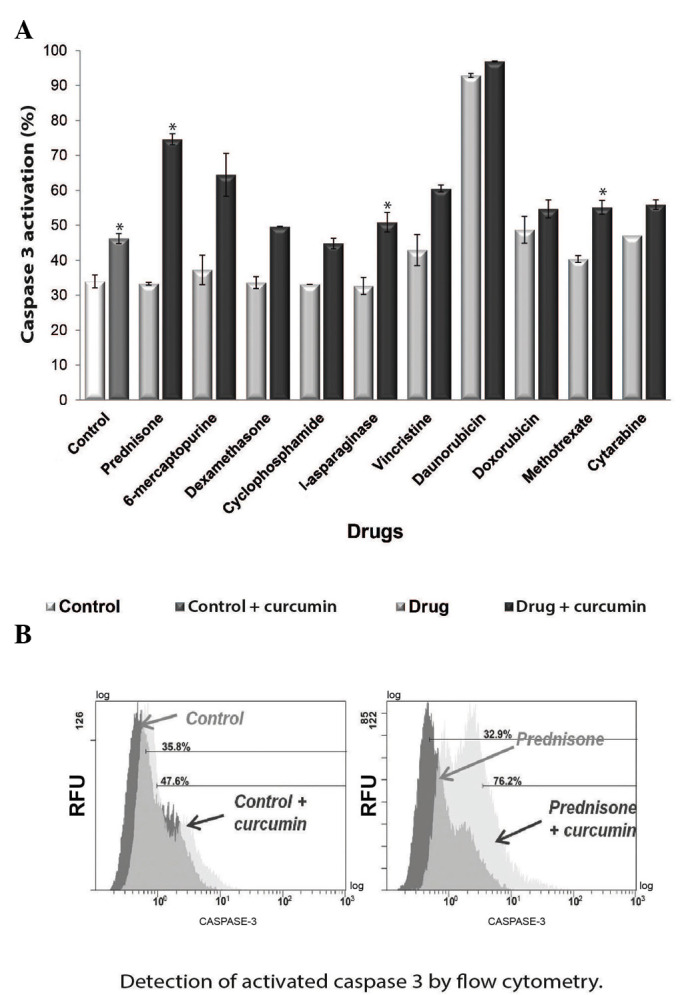
Curcumin induces apoptosis via caspase-3 activation, leading to apoptosis. (A) Caspase-3 activity in the groups treated with chemotherapy alone and co-treated with 20 µM curcumin. (B) Curcumin treatment increased caspase-3 activity in REH cells, which was most evident in the prednisone + curcumin group. *P<0.05 vs. control.
Discussion
In the present study, the effect of curcumin as a phytochemical with chemopreventive and antitumor properties was investigated, as curcumin has been previously used in herbal medicine and as a dietary compound with non-toxic effects (22). The aim of the present study was to evaluate the effect of curcumin in the human ALL REH cell line in combination with a variety of therapeutic agents used to treat ALL. The results revealed that NF-κB activation was decreased in all chemotherapeutic agent + curcumin groups, and a subsequent increase in apoptosis was also observed.
Curcumin was demonstrated to decrease the chemotherapeutic activation of NF-κB. Previous studies have revealed that chemotherapeutic agents increase NF-κB activation (18), while curcumin is able to reduce it (23). In the present study, with the exception of prednisone, treatment with all the chemotherapeutic agents tested resulted in increased NF-κB activation when compared with the control groups. However, treatment with 20 µM curcumin downregulated the constitutively active NF-κB in the REH cell line, both alone and in combination with all the therapeutic agents tested. It is postulated that this downregulation occurs via activation of the inhibitor of kappa B α (24).
The results of the present study indicate that curcumin functions as a sensitizer in tumor cells and potentiates the antitumor effect of the tested chemotherapeutic agents, as shown by the decreased cell viability observed. Previous evidence that curcumin may potentiate the antitumor effect of chemotherapeutic agents in ALL, was initially observed in ALL-derived Jurkat, REH and RS4;11 cell lines exposed to l-asparaginase and curcumin via inhibition of protein kinase B (AKT) and AKT-regulated gene products (25). In the present study, a decrease in cell viability and an increase in apoptosis was observed following treatment with cyclophosphamide, which was potentiated by curcumin. By contrast, curcumin has been demonstrated to inhibit cyclophosphamide-induced tumor regression in a breast cancer murine model (26). The results of the present study are in agreement with previous studies that have demonstrated a decrease in cell viability following curcumin treatment and the synergistic effect of curcumin following co-treatment with l-asparaginase in leukemia Jurkat, REH and RS4;11 cell lines (25), and vincristine in multiple myeloma cells (27). In the present study, the observed decrease in cell viability following co-treatment with curcumin and vincristine, daunorubicin or doxorubicin was not statistically significant, which may be due to cytotoxicity. Notably, the chemotherapeutic agents in which the decrease in cell viability was most evident in the presence of curcumin exhibited the highest levels of cell viability following treatment with the chemotherapeutic agents alone. Thus, the decrease in cell survival may be attributed to curcumin.
It was also reported that the downregulation of NF-κB led to apoptosis of ALL cells, as indicated by the increased expression of the apoptosis effector protein caspase-3 (27). Previously, the antitumoral effect of curcumin was associated with caspase-3 activation (28), and in the present study, all co-treatment groups exhibited an increase in caspase-3 activity. In contrast to a previous study (29), in the present study, treatment with vincristine alone activated caspase-3, and the reported increase in caspase-3 activity following combined treatment with l-asparaginase and curcumin was confirmed (30). Notably, in the present study, in the 6-mercaptopurine + curcumin group, the marked increase in caspase-3 activity was not significant, despite the significant decrease in cell viability observed. Therefore, it can be hypothesized that cell death may be activated via an alternative pathway. In the present study, curcumin treatment did not lead to the downregulation of anti-apoptosis and proliferative genes, as previously described (31), indicating that an alternative pathway may be activated, instead of that involving NF-κB. A previous study revealed that, in AML daunorubicin-resistant cell lines, apoptosis increased following combined treatment with daunorubicin and curcumin (32), and in the present study, an increase in caspase-3 activity was observed in the daunorubicin and curcumin co-treated cell cultures, although this increase was not statistically significant.
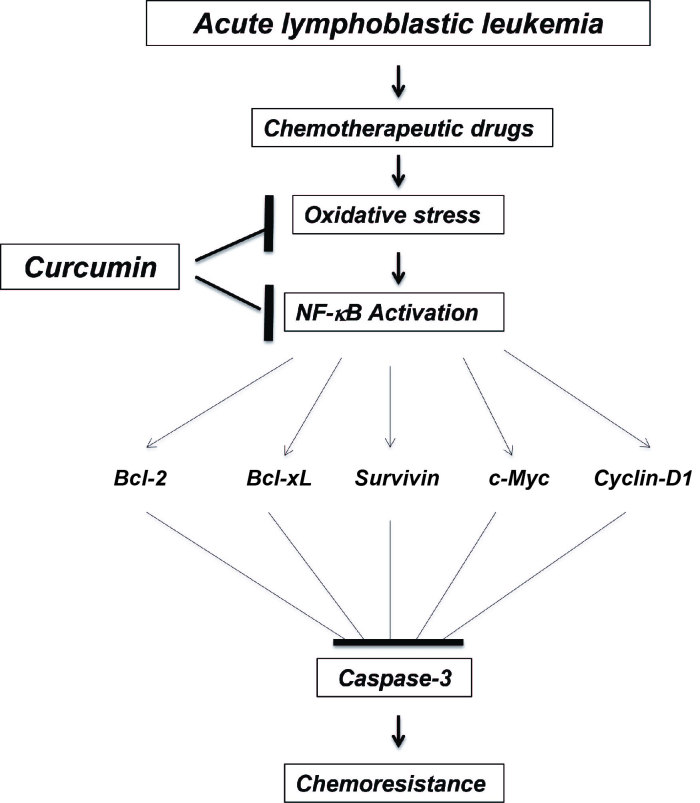
Oxidative stress caused by therapeutic agents used for the treatment of ALL cause damage in non-cancerous tissues (33) leading to the formation of oxidative DNA adducts (34). Curcumin may act as a scavenger of the free radicals caused by the therapy, subsequently reducing these molecules (35). Notably, a previous study revealed that in the NG108-15 (glioblastoma/neuroblastoma hybrid) cell line, curcumin protected the cells from oxidative damage when administered in combination with hydrogen peroxide, but not following pre-treatment (36). In the present study, cell cultures were treated simultaneously with the chemotherapeutic agents and curcumin, which may explain the free radical scavenging properties of curcumin. Notably, not all of the chemotherapeutic agents tested in the present study exhibited increased levels of 8-OHdG when compared with the control group. However, all co-treated groups exhibited a reduction in 8-OHdG molecules compared with the control group, indicating that curcumin controlled the free radicals produced by the therapeutic agents, resulting in less DNA damage. A proposed model that demonstrates the possible mechanism of action of combined treatment with curcumin and chemotherapeutic agents for ALL is shown in Fig. 7.
Figure 7.
Proposed model of the mechanism of action of curcumin in ALL treatment. In ALL cells, the chemotherapeutic agents cause oxidative stress, which activates the transcription factor NF-κB and subsequently leads to the activation of anti-apoptotic and proliferative genes. Consequently, apoptosis is inhibited and chemoresistance is increased. Curcumin decreases the oxidative stress caused by the chemotherapeutic agents and blocks NF-κB activation, consequently increasing apoptosis via caspase-3 activation, which prevents chemoresistance. NF-κB, nuclear factor-kappa B; Bcl, B-cell lymphoma; xL, extra large; ALL, acute lymphoblastic leukemia.
Clinical studies investigating the efficacy of curcumin for the treatment of pancreatic (37) and colorectal cancer (38) have yielded positive results. However, to the best of our knowledge, no studies have investigated pediatric hematological neoplasias to date. These promising clinical trials in solid tumors, considered together with the decrease in multi-drug resistance gene expression over curcumin in primary ALL cell cultures (39) and potentiation through curcumin of l-asparaginase (25) correspond to the only studies in vitro, highlighting the importance of the present study. Additional in vitro and mice models to assess the effect of combined treatment with chemotherapeutic agents and curcumin are required. Possible interactions between curcumin at various concentrations and chemotherapeutic agents cannot be excluded and thus, more studies that investigate the possible interactions between curcumin and chemotherapeutic agents are also required.
In conclusion, the present study revealed that curcumin inhibits survival, increases apoptosis and decreases DNA oxidation of REH leukemia cells in an NF-κB-dependent manner, both alone and in combination with all the therapeutic agents tested (prednisone, 6-mercaptopurine, dexamethasone, cyclophosphamide, l-asparaginase, vincristine, daunorubicin, doxorubicin, methotrexate and cytarabine). The application of this compound in the treatment of pediatric lymphoblastic leukemia may improve the outcome of patients. Overall, the present results indicate that curcumin may improve the efficacy of chemotherapeutic agents against ALL.
Acknowledgements
The authors would like to thank Dr Bharat B. Aggarwal (Cytokine Research Section Department of Molecular Oncology, The University of Texas, M. D. Anderson Cancer Center, Houston, TX, USA) for assistance with the present study, the St. Jude Children's Research Hospital (Memphis, TN, USA) for providing the REH cell line and all the staff members of the Cytogenetics Unit (Pediatric Hematology and Oncology Service, Pediatric Division, Civil Hospital from Guadalajara, Guadalajara, Jalisco, México). The present study was partially supported by the State Council of Science and Technology of Jalisco (Consejo Estatal de Ciencia y Tecnología de Jalisco; Guadalajara, México; grant no. 5-2010-1-1083).
References
- 1.Pui CH, editor. Childhood Leukemias. 3rd. Cambridge University Press; Cambridge: 2012. [DOI] [Google Scholar]
- 2.Pui CH, Jeha S. New therapeutic strategies for the treatment of acute lymphoblastic leukaemia. Nat Rev Drug Discov. 2007;6:149–165. doi: 10.1038/nrd2240. [DOI] [PubMed] [Google Scholar]
- 3.Libermann TA, Zerbini LF. Targeting transcription factors for cancer gene therapy. Curr Gene Ther. 2006;6:17–33. doi: 10.2174/156652306775515501. [DOI] [PubMed] [Google Scholar]
- 4.Brown M, Cohen J, Arun P, Chen Z, Van Waes C. NF-kappaB in carcinoma therapy and prevention. Expert Opin Ther Targets. 2008;12:1109–1122. doi: 10.1517/14728222.12.9.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montagut C, Tusquets I, Ferrer B, Corominas JM, Bellosillo B, Campas C, Suarez M, Fabregat X, Campo E, Gascon P, et al. Activation of nuclear factor-kappaB is linked to resistance to neoadjuvant chemotherapy in breast cancer patients. Endocr Relat Cancer. 2006;13:607–616. doi: 10.1677/erc.1.01171. [DOI] [PubMed] [Google Scholar]
- 6.Li F, Sethi G. Targeting transcription factor NF-kappaB to overcome chemoresistance and radioresistance in cancer therapy. Biochim Biophys Acta. 2010;1805:167–180. doi: 10.1016/j.bbcan.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Kordes U, Krappmann D, Heissmeyer V, Ludwig WD, Scheidereit C. Transcription factor NF-kappaB is constitutively activated in acute lymphoblastic leukemia cells. Leukemia. 2000;14:399–402. doi: 10.1038/sj.leu.2401705. [DOI] [PubMed] [Google Scholar]
- 8.Sethi G, Sung B, Aggarwal BB. Nuclear factor-kappaB activation: From bench to bedside. Exp Biol Med (Maywood) 2008;233:21–31. doi: 10.3181/0707-MR-196. [DOI] [PubMed] [Google Scholar]
- 9.Ravindran J, Prasad S, Aggarwal BB. Curcumin and cancer cells: How many ways can curry kill tumor cells selectively? AAPS J. 2009;11:495–510. doi: 10.1208/s12248-009-9128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lao CD, Ruffin MT, IV, Normolle D, Heath DD, Murray SI, Bailey JM, Boggs ME, Crowell J, Rock CL, Brenner DE. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006;6:10. doi: 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta SC, Kismali G, Aggarwal BB. Curcumin, a component of turmeric: From farm to pharmacy. Biofactors. 2013;39:2–13. doi: 10.1002/biof.1079. [DOI] [PubMed] [Google Scholar]
- 12.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 13.Beillard E, Pallisgaard N, van der Velden VH, Bi W, Dee R, van der Schoot E, Delabesse E, Macintyre E, Gottardi E, Saglio G, et al. Evaluation of candidate control genes for diagnosis and residual disease detection in leukemic patients using 'real-time' quantitative reverse-transcriptase polymerase chain reaction (RQ-PCR) - a Europe against cancer program. Leukemia. 2003;17:2474–2486. doi: 10.1038/sj.leu.2403136. [DOI] [PubMed] [Google Scholar]
- 14.Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Curcumin and cancer: An ‘old-age’ disease with an ‘age-old’ solution. Cancer Lett. 2008;267:133–164. doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Jungsuwadee P, Vore M, Butterfield DA, St Clair DK. Collateral damage in cancer chemotherapy: Oxidative stress in nontargeted tissues. Mol Interv. 2007;7:147–156. doi: 10.1124/mi.7.3.6. [DOI] [PubMed] [Google Scholar]
- 16.Blakemore LM, Boes C, Cordell R, Manson MM. Curcumin-induced mitotic arrest is characterized by spindle abnormalities, defects in chromosomal congression and DNA damage. Carcinogenesis. 2013;34:351–360. doi: 10.1093/carcin/bgs345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xue TY, Xu W, An Q, Wu Y, Xu CP, Zhang XY. Expression of nuclear transcription factor kappaB in childhood acute lymphoblastic leukemia and its significance. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2007;15:767–771. (In Chinese) [PubMed] [Google Scholar]
- 18.Bottero V, Busuttil V, Loubat A, Magné N, Fischel JL, Milano G, Peyron JF. Activation of nuclear factor kappaB through the IKK complex by the topoisomerase poisons SN38 and doxorubicin: A brake to apoptosis in HeLa human carcinoma cells. Cancer Res. 2001;61:7785–7791. [PubMed] [Google Scholar]
- 19.Aggarwal BB, Gehlot P. Inflammation and cancer: How friendly is the relationship for cancer patients? Curr Opin Pharmacol. 2009;9:351–369. doi: 10.1016/j.coph.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karin M. NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol. 2009;1:a000141. doi: 10.1101/cshperspect.a000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elmore S. Apoptosis: A review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anto RJ, Mukhopadhyay A, Denning K, Aggarwal BB. Curcumin (diferuloylmethane) induces apoptosis through activation of caspase-8, BID cleavage and cytochrome c release: Its suppression by ectopic expression of Bcl-2 and Bcl-xl. Carcinogenesis. 2002;23:143–150. doi: 10.1093/carcin/23.1.143. [DOI] [PubMed] [Google Scholar]
- 23.Shishodia S, Amin HM, Lai R, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive NF-kappaB activation, induces G1/S arrest, suppresses proliferation, and induces apoptosis in mantle cell lymphoma. Biochem Pharmacol. 2005;70:700–713. doi: 10.1016/j.bcp.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 24.Kasinski AL, Du Y, Thomas SL, Zhao J, Sun SY, Khuri FR, Wang CY, Shoji M, Sun A, Snyder JP, et al. Inhibition of IkappaB kinase-nuclear factor-kappaB signaling pathway by 3,5-bis(2-flurobenzylidene)piperidin-4-one (EF24), a novel monoketone analog of curcumin. Mol Pharmacol. 2008;74:654–661. doi: 10.1124/mol.108.046201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang H, Geng QR, Wang L, Lu Y. Curcumin potentiates antitumor activity of L-asparaginase via inhibition of the AKT signaling pathway in acute lymphoblastic leukemia. Leuk Lymphoma. 2012;57:1376–1382. doi: 10.3109/10428194.2011.649478. [DOI] [PubMed] [Google Scholar]
- 26.Somasundaram S, Edmund NA, Moore DT, Small GW, Shi YY, Orlowski RZ. Dietary curcumin inhibits chemotherapy-induced apoptosis in models of human breast cancer. Cancer Res. 2002;62:3868–3875. [PubMed] [Google Scholar]
- 27.Bharti AC, Donato N, Singh S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-kappa B and IkappaBalpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood. 2003;101:1053–1062. doi: 10.1182/blood-2002-05-1320. [DOI] [PubMed] [Google Scholar]
- 28.Chan WH, Wu HY, Chang WH. Dosage effects of curcumin on cell death types in a human osteoblast cell line. Food Chem Toxicol. 2006;44:1362–1371. doi: 10.1016/j.fct.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Al-Katib A, Arnold AA, Aboukameel A, Sosin A, Smith P, Mohamed AN, Beck FW, Mohammad RM. I-kappa-kinase-2 (IKK-2) inhibition potentiates vincristine cytotoxicity in non-Hodgkin's lymphoma. Mol Cancer. 2010;9:228. doi: 10.1186/1476-4598-9-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H, Geng QR, Wang L, Lu Y. Curcumin potentiates antitumor activity of L-asparaginase via inhibition of the AKT signaling pathway in acute lymphoblastic leukemia. Leuk Lymphoma. 2012;53:1376–1382. doi: 10.3109/10428194.2011.649478. [DOI] [PubMed] [Google Scholar]
- 31.Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008;269:199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 32.Rao J, Xu DR, Zheng FM, Long ZJ, Huang SS, Wu X, Zhou WH, Huang RW, Liu Q. Curcumin reduces expression of Bcl-2, leading to apoptosis in daunorubicin-insensitive CD34+ acute myeloid leukemia cell lines and primary sorted CD34+ acute myeloid leukemia cells. J Transl Med. 2011;9:71. doi: 10.1186/1479-5876-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y, Jungsuwadee P, Vore M, Butterfield DA, St Clair DK. Collateral damage in cancer chemotherapy: Oxidative stress in nontargeted tissues. Mol Interv. 2007;7:147–156. doi: 10.1124/mi.7.3.6. [DOI] [PubMed] [Google Scholar]
- 34.Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27:120–139. doi: 10.1080/10590500902885684. [DOI] [PubMed] [Google Scholar]
- 35.Thangapazham RL, Sharma A, Maheshwari RK. Multiple molecular targets in cancer chemoprevention by curcumin. AAPS J. 2006;8:E443–E449. doi: 10.1208/aapsj080352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahakunakorn P, Tohda M, Murakami Y, Matsumoto K, Watanabe H, Vajaragupta O. Cytoprotective and cytotoxic effects of curcumin: Dual action on H2O2-induced oxidative cell damage in NG108-15 cells. Biol Pharm Bull. 2003;26:725–728. doi: 10.1248/bpb.26.725. [DOI] [PubMed] [Google Scholar]
- 37.Dhillion N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, Ng CS, Badmaev V, Kurzrock R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14:4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 38.He ZY, Shi CH, Wen H, Li FL, Wang BL, Wang J. Upregulation of p53 expression in patients with colorectal cancer by administration of curcumin. Cancer Invest. 2011;29:208–213. doi: 10.3109/07357907.2010.550592. [DOI] [PubMed] [Google Scholar]
- 39.Anuchapreeda S, Thanarattanakorn P, Sittipreechacharn S, Tima S, Chanarat P, Limtrakul P. Inhibitory effect of curcumin on MDR1 gene expression in patient leukemic cells. Arch Pharm Res. 2006;29:866–873. doi: 10.1007/BF02973907. [DOI] [PubMed] [Google Scholar]