Abstract
The present study aimed to investigate the reversal effect of resveratrol on the phenomenon of multidrug resistance in U2OS/adriamycin (ADR) cells and to clarify the molecular mechanisms. To examine the cell survival and half-inhibitory concentration (IC50) of ADR in U2OS and U2OS/ADR cells, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay was used. The accumulation of ADR in U2OS and U2OS/ADR cells was investigated by flow cytometry. Reverse transcription-quantitative polymerase chain reaction and western blot analysis were used to detect the expression of multidrug resistance protein 1 (MDR1), P-glycoprotein (P-gp), p65 and p38. Compared with U2OS cells, the IC50 value of ADR was significantly increased in U2OS/ADR cells, which exhibited high levels of MDR1/P-gp. However, resveratrol could drastically reduce the IC50 value of ADR and the expression of MDR1/P-gp, and increased the accumulation of ADR in U2OS/ADR cells. In addition, the expression levels of p38 (phosphorylated) and p65 (acetylated and total) in U2OS/ADR cells were also significantly suppressed by resveratrol. These results suggested that the nuclear factor (NF)-κB and p38 mitogen-activated protein kinase (MAPK) signaling pathways are correlated with ADR-induced drug resistance in U2OS/ADR cells. Furthermore, resveratrol could downregulate the expression of MDR1/P-gp and reverse the drug resistance phenomenon in U2OS/ADR cells partly at least by suppressing the activation of the NF-κB and p38 MAPK signaling pathways.
Keywords: resveratrol, transporter, P-glycoprotein, multidrug resistance, natural product
Introduction
Osteosarcoma, a high-grade malignant tumor associated with a 5-year survival rate of 37% (1), is the most frequent primary bone tumor, and occurs mainly in children and adolescents (2,3). It is a highly malignant tumor that is often transferred via the blood stream to the lung, liver and other vital organs. The majority of current protocols for the treatment of osteosarcoma include a period of preoperative (neoadjuvant) chemotherapy (4). Chemotherapy drugs, including adriamycin (ADR), methotrexate and cyclophosphamide, are also applied in patients with osteosarcoma to further kill osteosarcoma cells following amputation surgery (1–3). However, upon long-term exposure of the tumor cells to chemotherapy drugs, the surface of the tumor cells may overexpress P-glycoprotein (P-gp), an adenosine triphosphate-dependent drug efflux pump encoded by the multidrug resistance protein 1 (MDR1) gene (5,6). Overexpressed P-gp can mediate the efflux of a large number of intracellular chemotherapy drugs, thus leading to a significant reduction in the intracellular drug concentration (7,8), which causes drug resistance of tumor cells. Therefore, a reduction in the efflux of chemotherapy drugs can increase the concentration of chemotherapeutic agents in tumor cells and reverse the phenomenon of tumor drug resistance by inhibiting the function of P-gp or reducing P-gp expression (9–11).
It is well known that P-gp expression is closely associated with the nuclear factor (NF)-κB signaling pathway (12), the mitogen-activated protein kinase (MAPK) signaling pathway (13), cylooxygenases-2 (14) and phosphoinositide 3-kinase (15). NF-κB can bind to the NF-κB binding sites in the MDR1 promoter region, which results in the activation of the transcription of the MDR1 gene (16). In addition, p38 MAPK may regulate P-gp expression through the activation of NF-κB expression (17). Thereby, the NF-κB and MAPK signaling pathways play significant roles in the molecular mechanisms of P-gp-mediated multidrug resistance.
Resveratrol (trans-3,4,5-trihydroxystilbene) is a plant polyphenol present in grapes, peanuts and various other plants, and has potent effects in reversing multidrug resistance (18). Quan et al has reported that resveratrol successfully reversed multidrug resistance in KBv200 cells by downregulation of MDR1/P-gp (19). However, the reversal mechanism of multidrug resistance is still unknown. The present study aimed to investigate whether resveratrol could reverse the phenomenon of multidrug resistance in U2OS/ADR cells, an ADR-resistant human osteosarcoma cell line, and to investigate the molecular mechanisms.
Materials and methods
Chemicals
Resveratrol of >99% purity was purchased from Dalian Meilun Biotech Co., Ltd. (Dalian, China). ADR was purchased from Shenzhen Main Luck Pharmaceuticals, Inc. (Shenzhen, China), while 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was obtained from USB Corporation (Cleveland, OH, USA). Anti-p38 (phosphorylated and total; catalog nos. sc-7972 and sc-7973, respectively) and anti-p65 (total; catalog no. sc-8008) antibodies were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Anti-p65 (acetylate; catalog no. A16567) was purchased from Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Antibodies against β-actin (catalog no. ab8226) and MDR1 (catalog no. ab3366) were purchased from Abcam (Cambridge, MA, USA). High glucose Dulbecco's modified Eagle (DMEM) medium and fetal bovine serum (FBS) were provided by Gibco (Thermo Fisher Scientific, Inc.). All other analytical grade chemicals used in the present study were readily available from commercial sources.
Cell culture
U2OS cells were purchased from Nanjing KeyGen Biotech Co., Ltd. (Nanjing, China) and were cultured in high glucose DMEM supplemented with 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin. Upon culture of U2OS cells in DMEM with 0.01, 0.04, 0.1, 0.4, 1.0 and 4.0 µg/ml ADR for 6 months, U2OS/ADR cells were successfully induced. Then, U2OS/ADR cells steadily grew in high DMEM containing ADR (4.0 µg/ml). All cells were kept in an incubator at 37°C with 95% humidity and 5% CO2.
Cytotoxicity assay and multidrug resistance reversal assay
Chemosensitivity in vitro was measured by means of MTT colorimetric assay performed in 96-well plates. U2OS and U2OS/ADR cells (1×104 cells/ml) were inoculated into each well with 90 µl culture medium. Following overnight incubation, various concentrations of ADR (10 µl) with or without resveratrol were added to the cultures. Upon incubation for 48 h, 10 µl of MTT reagent [5 mg/ml in phosphate-buffered saline (PBS)] was added to each well, and left to incubate for an additional 4 h. A 100 µl aliquot of sodium dodecyl sulfate (SDS)-isobutanol-HCl solution (5% isobutanol, 10% SDS and 12 µM HCl) was added and left to incubate overnight. Relative cell viability was obtained on a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA) with a 570-nm filter.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. RNA pellets were resuspended in diethyl pyrocarbonate-treated deionized water. RNA samples were analyzed by 15% agarose gel electrophoresis, and integrity was examined by visualization of intact 18S and 28S ribosomal RNA under ultraviolet light. Total RNA (1 µg) was used to prepare complementary (c)DNA by RT using a PrimeScript™ RT Reagent kit (Takara Biotechnology Co., Ltd., Dalian, China). The primer sequences were as follows: MDR1, forward (F) 5′-GGAGCCTACTTGGTGGCACATAA-3′ and reverse (R) 5′-TGGCATAGTCAGGAGCAAATGAAC-3′ (20); and β-actin, F 5′-ATTGAACACGGCATTGTCAC-3′ and R 5′-CATCGGAACCGCTCATTG-3′. The cDNA was amplified using SYBR® Premix Ex Taq kit (Takara Biotechnology Co., Ltd.) in a M×3000P instrument (Agilent Technologies, Inc., Santa Clara, CA, USA). The PCR conditions were as follows: 1 cycle of denaturation at 95°C for 30 sec, followed by 40 cycles of denaturation at 95°C for 5 sec and annealing at 60°C for 34 sec. The PCR products were analyzed using the ΔΔCq method by ABI PRISM® 7500 Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.), and compared to the housekeeping gene β-actin (20).
Western blotting
Upon incubation with or without resveratrol solutions for 48 h, U2OS and U2OS/ADR cells were collected and washed with PBS. Proteins were extracted using a total protein extraction kit (Nanjing KeyGen Biotech Co., Ltd.), according to the manufacturer's protocol. The proteins were separated by centrifugation at 12,000 × g for 30 min. Protein concentrations were measured using a bicinchoninic acid protein assay kit (Nanjing KeyGen Biotech Co., Ltd.). Proteins (60 µg) were resuspended in electrophoresis sample buffer containing β-mercaptoethanol and subjected to 10% SDS-polyacrylamide gel electrophoresis. Proteins were transferred onto a polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA, USA), and then membranes were blocked using 5% non-fat milk in Tris-buffered saline with 0.1% Tween 20 (TBST) for 2 h at 37°C. β-actin served as a loading control. Membranes were incubated overnight at 4°C with a 1:1,500 dilution of polyclonal antibodies against MDR1 and β-actin (Abcam), and with a 1:500 dilution of monoclonal antibodies against p38 (phosphorylated and total) and p65 (acetylated and total) (Santa Cruz Biotechnology, Inc.). Upon incubation with the primary antibody, membranes were rinsed three times with TBST and incubated for 2 h at 37°C with a 1:1,500 dilution of anti-mouse horseradish peroxidase-conjugated secondary antibody (catalog no. sc-8008; Invitrogen, Thermo Fisher Scientific, Inc.). According to the manufacturer's protocol, membranes were exposed to Enhanced Chemiluminescence Plus reagent from Beyotime Institute of Biotechnology (Haimen, China), following extensive washing with TBST. The emitted light was documented with a BioSpectrum 410 multispectral imaging system with a Chemi HR 410 camera (UVP, LLC, Upland, CA, USA). Protein bands were visualized and photographed under transmitted ultraviolet light. Images were used for semiquantitative measurements based on band densitometry.
Accumulation of ADR
U2OS and U2OS/ADR cells (5×105 cells/ml) were seeded in 6-well plates. Following incubation in DMEM containing resveratrol (100 µM) at 37°C for 48 h, U2OS and U2OS/ADR cells were incubated with 10 µM ADR for 1 h at 37°C, and then washed three times with ice-cold PBS. The fluorescence intensity of intracellular ADR was determined by flow cytometry with an excitation wavelength of 488 nm and an emission wavelength of 575 nm (BD Biosciences, Franklin Lakes, NJ, USA) (21).
Small interfering (si)RNA transfection
According to the manufacturer's protocol, U2OS/ADR cells (5×105 cells/ml) were seeded in 6-well plates and transfected with specific siRNAs against p65 and p38 (Shanghai GenePharma Co., Ltd., Shanghai, China) at a concentration of 100 nM using Lipofectamine™ 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Cells transfected with control siRNA were pooled and used as a negative control. The sequences of the siRNA targeting human p65 were: Sense, 5′-GAUUGAGGAGAAACGUAAA-3′ and antisense, 5′-UUUACGUUUCUCCUCAAUC-3′ (22). The siRNA sequences used to target human p38 (23) were: Sense, 5′-CCCUGUAAAGCUUUCAGAA-3′ and antisense, 5′-UUCUGAAAGCUUUACAGGG-3′. The transfected cells were incubated at 37°C in serum-free DMEM. After transfection for 6 h, cells were cultured in DMEM with 10% FBS. After growing for additional 48 h, cells were collected for western blot analysis to determine the levels of the indicated proteins.
Statistical analysis
Statistical analysis was performed using SPSS version 11.0 software (SPSS, Inc., Chicago, IL, USA). Group data were expressed as the mean ± standard deviation. Statistically significant differences of data from two sets were compared using one-way analysis of variance. P<0.05 was considered to indicate a statistically significant difference.
Results
Multidrug resistance of U2OS/ADR cells
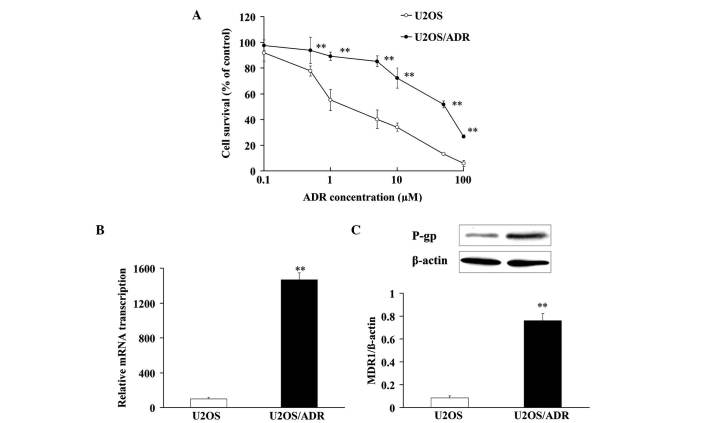
In order to verify the drug resistance of U2OS/ADR cells, MTT assay was applied to analyze the cell viability, once U2OS and U2OS/ADR cells had been incubated with various concentrations of ADR. ADR exerted cytotoxicity against U2OS and U2OS/ADR cells with IC50 values of 2.7±0.4 and 31.7±2.9 µM, respectively (Fig. 1A). These results indicated that there was a remarkable drug resistance to ADR in U2OS/ADR cells.
Figure 1.
Characterization of U2OS and U2OS/ADR cells. (A) Effects of ADR on the viability of U2OS and U2OS/ADR cells. (B) Expression of MDR1 messenger RNA by reverse transcription-quantitative polymerase chain reaction analysis in U2OS and U2OS/ADR cells. (C) P-gp expression was detected in U2OS and U2OS/ADR cells by western blotting. MDR1/P-gp expression was normalized to β-actin level. Data are expressed as the mean ± standard deviation. (**P<0.01 vs. U2OS cells group; n=6). ADR, adriamycin; P-gp, P-glycoprotein; mRNA, messenger RNA; MDR1, multidrug resistance protein 1.
In addition, the messenger (m)RNA expression of MDR1 in U2OS/ADR cells was ~14.7 times higher than that in U2OS cells (Fig. 1B). Compared with U2OS cells, the protein expression of P-gp in U2OS/ADR cells was notably upregulated (~8.94-fold) (Fig. 1C). These findings demonstrated that the phenomenon of drug resistance was at least partly associated with the overexpression of P-gp in U2OS/ADR cells.
Reversal effect of resveratrol on U2OS/ADR cells
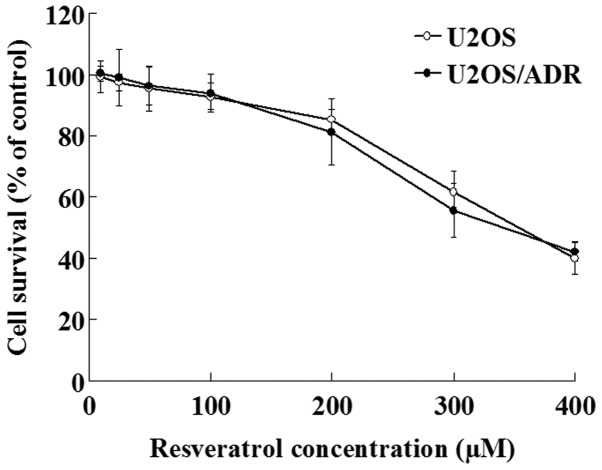
The effect of resveratrol on U2OS/ADR cells growth was determined with MTT assay. The results demonstrated that concentrations of resveratrol ranging from 10 to 100 µmol/l did not exert inhibitory effects on the growth of U2OS/ADR cells, since the cell survival rate was >90% (Fig. 2). However, higher concentrations of resveratrol (200–400 µmol/l) exhibited significant anti-proliferative effects on these cells (P<0.01; Fig. 2). Therefore, 100 µmol/l was selected as the concentration of resveratrol required to reverse multidrug resistance in U2OS/ADR cells.
Figure 2.
Effects of resveratrol on the survival of U2OS/adriamycin cells. Data are expressed as the mean ± standard deviation (n=6). ADR, adriamycin.
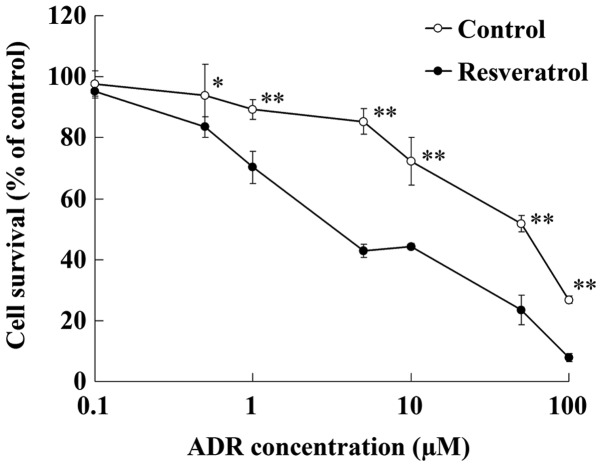
After being incubated with resveratrol (100 µmol/l) for 48 h, U2OS/ADR cells displayed increased sensitivity to ADR (Fig. 3). The IC50 value of ADR in U2OS/ADR cells was reduced to 4.7±0.5 µM by resveratrol (100 µmol/l). This result indicated that resveratrol could reverse the drug resistance of U2OS/ADR cells towards ADR.
Figure 3.
Resveratrol increased the sensitivity of U2OS/ADR cells to ADR. Data are expressed as the mean ± standard deviation (*P<0.05; **P<0.01 vs. control group; n=6). ADR, adriamycin.
Accumulation of ADR in U2OS and U2OS/ADR cells
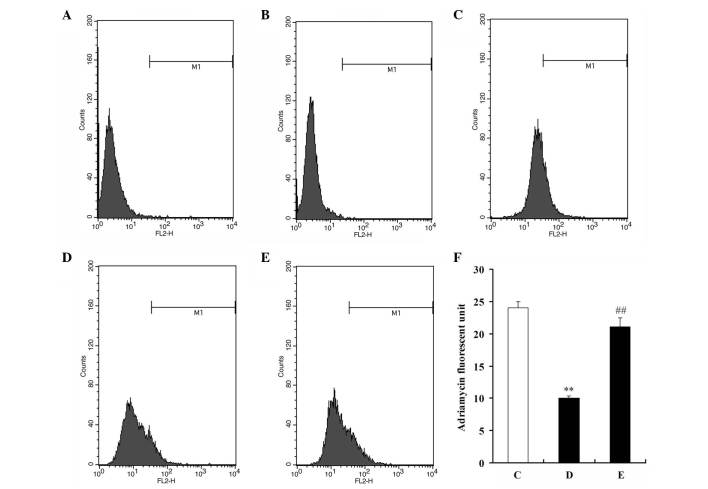
Due to the autofluorescence capacity of ADR, fluorescence intensity was used as an indicator of ADR intracellular accumulation. After U2OS and U2OS/ADR cells had been pre-incubated with 100 µM resveratrol for 48 h, cells were incubated with 10 µM ADR for 1 h, and flow cytometry was then performed to detect the fluorescence intensity of ADR in the cells. The mean fluorescence intensity of ADR in U2OS/ADR cells was 58.1% lower than that in U2OS cells (Fig. 4). However, the intracellular accumulation of ADR in U2OS/ADR cells was significantly increased by resveratrol (P<0.01), and the fluorescence intensity of ADR in resveratrol-treated U2OS/ADR cells increased by 1.99-fold compared with untreated U2OS/ADR cells (P<0.01; Fig. 4). These findings indicated that resveratrol could significantly increase the intracellular accumulation of ADR in U2OS/ADR cells.
Figure 4.
Resveratrol increased the accumulation of ADR in U2OS/ADR cells. (A) U2OS control cells. (B) U2OS/ADR control cells. Untreated (C) U2OS and (D) U2OS/ADR cells were incubated with 10 µM ADR for 1 h. (E) U2OS/ADR cells were treated with 100 µM resveratrol for 48 h and then incubated with 10 µM ADR for 1 h. (F) Following incubation with 10 µM ADR for 1 h at 37°C, the mean ADR fluorescence intensity comparison in U2OS, U2OS/ADR and U2OS/ADR cells treated with 100 µM resveratrol for 48 h was evaluated. Data are expressed as the mean ± standard deviation (**P<0.01 vs. untreated U2OS cells group; ##P<0.01 vs. untreated U2OS/ADR cells group; n=6). ADR, adriamycin.
Resveratrol decreases the expression of MDR1/P-gp in U2OS/ADR cells
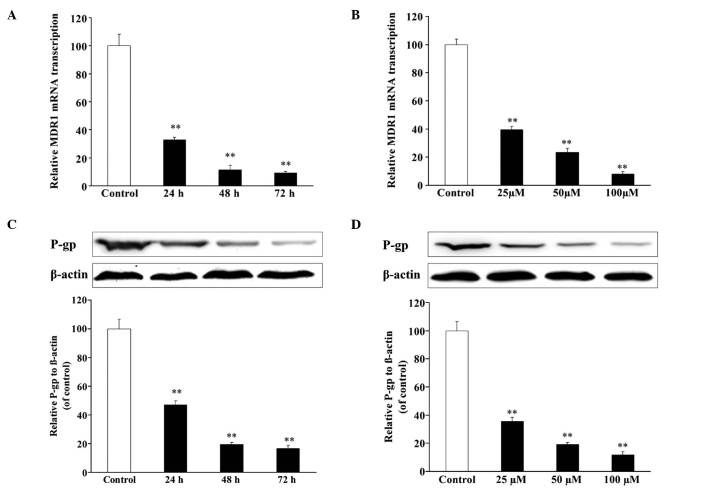
In the reversal experiment, resveratrol could reverse the drug resistance of U2OS/ADR cells towards ADR. To explore the molecular mechanisms, the expression levels of MDR1/P-gp were detected by RT-qPCR and western blotting. After incubation with resveratrol (100 µmol/l) for 24, 48 and 72 h, the mRNA expression level of MDR1 in U2OS/ADR cells decreased to 32.8, 11.1 and 9.1%, respectively, in comparison with untreated U2OS/ADR cells (Fig. 5A). In addition, compared with untreated U2OS/ADR cells, treatment with 25, 50 and 100 µmol/l of resveratrol for 48 h led to a reduction in the mRNA expression level of MDR1 (40.0, 23.4 and 9.2%, respectively) in U2OS/ADR cells (Fig. 5B). The protein expression of P-gp was consistent with the results of RT-qPCR (Fig. 5C and D). The findings indicated that resveratrol could downregulate the mRNA and protein expression of MDR1/P-gp.
Figure 5.
Effects of resveratrol on MDR1/P-gp expression in U2OS/adriamycin cells. Cells were incubated with (A) 100 µM resveratrol for 0–72 h or (B) with various concentrations of resveratrol for 48 h. MDR1 messenger RNA expression was analyzed by reverse transcription-quantitative polymerase chain reaction. Cells were incubated with (C) 100 µM resveratrol for 0–72 h or (D) with various concentrations of resveratrol for 48 h. P-gp expression was analyzed by western blotting and normalized to β-actin level. Data are expressed as the mean ± standard deviation (**P<0.01 vs. untreated U2OS cells group; n=6). P-gp, P-glycoprotein; mRNA, messenger RNA; MDR1, multidrug resistance protein 1.
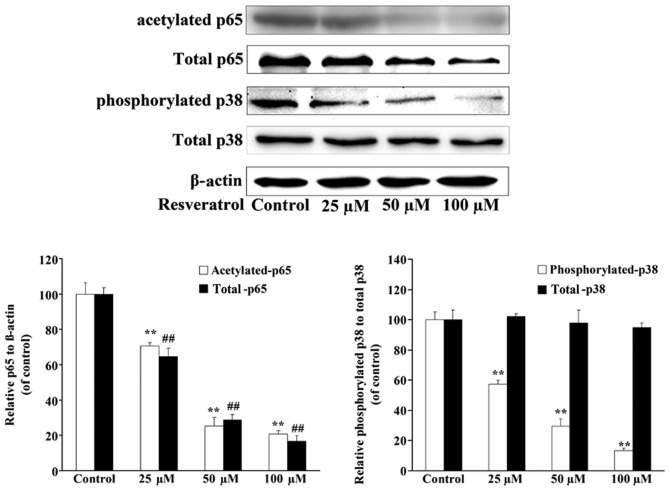
Resveratrol decreases the expression of p38 and p65 in U2OS/ADR cells
In order to investigate whether the downregulation of the expression of P-gp by resveratrol in U2OS/ADR cells was associated with p38 and p65, the expression levels of p38 and p65 in U2OS/ADR cells were investigated. Although the results of western blotting analysis demonstrated that the expression levels of total p38 were not changed, the expression levels of p38 (phosphorylated) and p65 (acetylated and total) in U2OS/ADR cells were significantly suppressed in comparison with untreated U2OS/ADR cells after 48-h incubation with 25, 50 and 100 µmol/l of resveratrol (Fig. 6). These results indicated that resveratrol downregulated the expression of P-gp at least partly by suppressing the activation of the NF-κB and p38 MAPK signaling pathways in U2OS/ADR cells.
Figure 6.
Resveratrol downregulated the expression of P-glycoprotein by inhibiting the nuclear factor-κB p65 and p38 mitogen-activated protein kinase signaling pathways. The protein levels of p65 (acetylated and total), p38 (phosphorylated and total) and β-actin in U2OS/adriamycin cells incubated without or with resveratrol (100 µM) for 48 h were determined using western blotting. The expression level of p65 (acetylated and total) was normalized to that of β-actin. Phosphorylated p38 expression was normalized to p38 level. Data are expressed as the mean ± standard deviation (**P<0.01, expression of acetylated p65 and phosphorylated p38 in treated U2OS cells vs. untreated cells; ##P<0.01, expression of total p65 in treated U2OS cells vs. untreated cells; n=6).
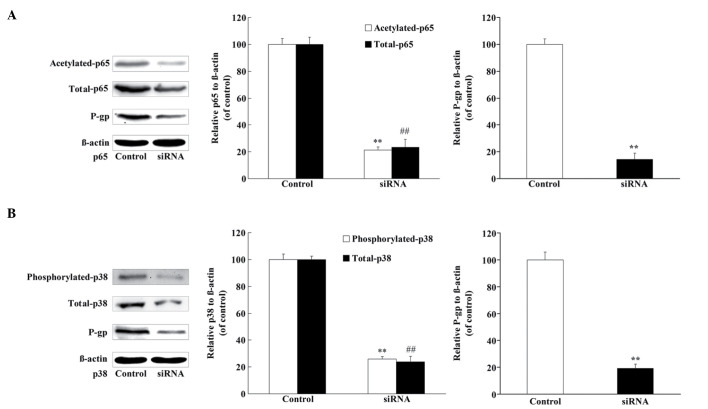
p38 and p65 regulate the expression of P-gp in U2OS/ADR cells
In order to explore whether p38 and p65 could regulate the expression of P-gp in U2OS/ADR cells, siRNAs specific for p38 and p65 were applied to knock down p38 and p65, respectively. U2OS/ADR cells were transfected with siRNA for p38 or p65, and the protein level of p38 (phosphorylated and total) or p65 (acetylated and total) was significantly reduced (Fig. 7). In addition, the protein level of P-gp was also significantly reduced (Fig. 7). These findings revealed that the expression of P-gp was at least partly associated with p38 and p65 in U2OS/ADR cells.
Figure 7.
P-gp protein expression was regulated by NF-κB p65 and p38 MAPK. (A) The protein levels of p65 (acetylated and total), P-gp and β-actin were determined using western blotting, following the knockdown of NF-κB p65 in U2OS/ADR cells using siRNA. (B) The protein levels of p38 (phosphorylated and total), P-gp and β-actin were determined using western blotting, following the knockdown of p38 MAPK in U2OS/ADR cells using siRNA. Data are expressed as the mean ± standard deviation (**P<0.01, expression of acetylated p65 and phosphorylated p38 in treated U2OS cells vs. untreated cells; ##P<0.01, expression of total p65 and p38 in treated U2OS cells vs. untreated cells; n=6). siRNA, small interfering RNA; P-gp, P-glycoprotein; NF-κB, nuclear factor-κB; MAPK, mitogen-activated protein kinase; ADR, adriamycin.
Discussion
Natural medicines have been widely used for anti-oxidative stress, anti-inflammation and reversal of multidrug resistance of tumor cells in recent years (15,19). Resveratrol has an excellent effect on anti-oxidative stress and anti-inflammation (24–26), and it has the potential to ameliorate local inflammation by suppressing tumor necrosis factor-α-induced interleukin-8 release (19,24–26). In addition, resveratrol can effectively inhibit NF-κB and MAPK pathways (27–29). It is widely reported that the NF-κB and MAPK signaling pathways play a major role in the molecular mechanisms of P-gp-mediated multidrug resistance, and overexpression of P-gp is one of the important causes of multidrug resistance of tumor cells (12,13). Therefore, the present study investigated whether resveratrol could significantly reduce the levels of P-gp expression and reverse the drug resistance of U2OS/ADR cells by inhibiting the NF-κB and MAPK signaling pathways.
In order to verify the drug resistance of U2OS/ADR cells, the viabilities of U2OS and U2OS/ADR cells incubated with various concentrations of ADR were analyzed by MTT assay. The results indicated that the IC50 of U2OS/ADR cells was increased by 11.7-fold (Fig. 1A). Overexpression of P-gp has been reported as one of the important causes of multidrug resistance; thus, the expression of MDR1/P-gp was examined by RT-qPCR and western blotting. Compared with U2OS cells, the expression levels of MDR1/P-gp were significantly increased in U2OS/ADR cells (Fig. 1B and C). These results suggested that U2OS/ADR cells had a remarkable drug resistance to ADR and that overexpression of P-gp was an important cause of multidrug resistance in U2OS/ADR cells.
To investigate the effect of resveratrol on the reversal of multidrug resistance, the inhibition rate of various concentrations of resveratrol on U2OS/ADR cells was evaluated. The results indicated that concentrations of resveratrol ranging from 10 to 100 µmol/l had no inhibitory effects on the growth of U2OS/ADR cells (Fig. 2). Therefore, a concentration of resveratrol of 100 µmol/l was selected to reverse multidrug resistance in U2OS/ADR cells. After U2OS/ADR cells had been incubated with resveratrol for 48 h, the IC50 value of ADR that exerted cytotoxicity against U2OS/ADR cells was remarkably reduced, and the intracellular accumulation of ADR was significantly increased (Figs. 3 and 4). These results indicated that resveratrol can reverse the drug resistance of U2OS/ADR cells to ADR. To explore the reversal mechanism of drug resistance, the levels of MDR1/P-gp expression were investigated. The results indicated that resveratrol could decrease the expression of MDR1/P-gp in U2OS/ADR cells in a time– and concentration-dependent manner (Fig. 5). Therefore, resveratrol can reverse drug resistance partly at least by reducing the expression of MDR1/P-gp in U2OS/ADR cells.
It was previously reported that resveratrol could inhibit cell growth and proliferation, and induce apoptosis and arrest in the G0/G1 phase of the cell cycle by reducing the activity of NF-κB in tumor cells (29,30). Furthermore, resveratrol could also effectively inhibit the activation of the p38 MAPK signaling pathway (27). Therefore, the present study focused on the effect of resveratrol on the regulation of the activation of the NF-κB and p38 MAPK signaling pathways. It was observed that the expression of p65 (acetylated and total) and p38 (phosphorylated) was suppressed upon incubation with resveratrol (Fig. 6). The NF-κB signaling pathway is highly correlated with P-gp-mediated drug resistance (16). When the NF-κB signaling pathway was activated, the expression of MDR1 was dramatically upregulated (12). In addition, the p38 MAPK signaling pathway is associated with multidrug resistance events (17). In order to clearly verify the phenomenon of NF-κB and MAPK signaling-mediated multidrug resistance in U2OS/ADR cells, the expression of NF-κB p65 subunit (acetylated and total), p38 (phosphorylated and total) and P-gp were examined following siRNA knockdown of p65 or p38. The results revealed that the expression of p65 (acetylated and total) and p38 (phosphorylated and total) was significantly reduced (Fig. 7). Furthermore, the expression P-gp was consistent with that of p65 and p38 (Fig. 7). These results confirmed that resveratrol reverses P-gp-mediated multidrug resistance of U2OS/ADR cells by suppressing the activation of the NF-κB and p38 MAPK signaling pathways.
Numerous studies have reported that p38 MAPK can activate NF-κB expression (31), and thus regulate P-gp expression. The present study could not clarify whether the expression of p65 was directly downregulated by resveratrol or was correlated with resveratrol-induced suppression of the p38 MAPK signaling pathway in U2OS/ADR cells. Mulakayala et al confirmed that resveratrol may prevent the translocation of NF-κB by interacting with it, and may also prevent the binding of NF-κB to DNA by interacting with the residues involved in DNA binding, according to the results of the analysis with AutoDock 4.2 software (32). The interaction of resveratrol and p38 MAPK would be investigated in further studies.
In conclusion, the NF-κB and p38 MAPK signaling pathways are correlated with ADR-induced drug resistance in U2OS/ADR cells. Furthermore, resveratrol can downregulate the expression of P-gp and reverse the drug resistance of U2OS/ADR cells at least partly by suppressing the activation of the NF-κB and p38 MAPK signaling pathways.
Acknowledgements
The present study was supported by a grant from the National Natural Science Foundation of China (Beijing, China; grant no. 81270052) and and the Liaoning Natural Science Foundation (Liaoning, China; grant no. L2015159).
References
- 1.Lee JS, Fetsch JF, Wasdhal DA, Lee BP, Pritchard DJ, Nascimento AG. A review of 40 patients with extraskeletal osteosarcoma. Cancer. 1995;76:2253–2259. doi: 10.1002/1097-0142(19951201)76:11<2253::AID-CNCR2820761112>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 2.Lamoureux F, Trichet V, Chipoy C, Blanchard F, Gouin F, Redini F. Recent advances in the management of osteosarcoma and forthcoming therapeutic strategies. Expert Rev Anticancer Ther. 2007;7:169–181. doi: 10.1586/14737140.7.2.169. [DOI] [PubMed] [Google Scholar]
- 3.Young G, Toretsky JA, Campbell AB, Eskenazi AE. Recognition of common childhood malignancies. Am Fam Physician. 2000;61:2144–2154. [PubMed] [Google Scholar]
- 4.Ritter J, Bielack SS. Osteosarcoma. Ann Oncol. 2010;21(Suppl 7):vii320–325. doi: 10.1093/annonc/mdq276. [DOI] [PubMed] [Google Scholar]
- 5.Fung KL, Pan J, Ohnuma S, Lund PE, Pixley JN, Kimchi-Sarfaty C, Ambudkar SV, Gottesman MM. MDR1 synonymous polymorphisms alter transporter specificity and protein stability in a stable epithelial monolayer. Cancer Res. 2014;74:598–608. doi: 10.1158/0008-5472.CAN-13-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gromnicova R, Romero I, Male D. Transcriptional control of the multi-drug transporter ABCB1 by transcription factor Sp3 in different human tissues. PLoS One. 2012;7:e48189. doi: 10.1371/journal.pone.0048189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson WW. P-glycoprotein-mediated efflux as a major factor in the variance of absorption and distribution of drugs: Modulation of chemotherapy resistance. Methods Find Exp Clin Pharmacol. 2002;24:501–514. doi: 10.1358/mf.2002.24.8.705071. [DOI] [PubMed] [Google Scholar]
- 8.Sorokin A. Cyclooxygenase-2: Potential role in regulation of drug efflux and multidrug resistance phenotype. Curr Pharm Des. 2004;10:647–657. doi: 10.2174/1381612043453117. [DOI] [PubMed] [Google Scholar]
- 9.Patel VA, Dunn MJ, Sorokin A. Regulation of MDR-1 (P-glycoprotein) by cyclooxygenase-2. J Biol Chem. 2002;277:38915–38920. doi: 10.1074/jbc.M206855200. [DOI] [PubMed] [Google Scholar]
- 10.Thomas H, Coley HM. Overcoming multidrug resistance in cancer: An update on the clinical strategy of inhibiting p-glycoprotein. Cancer Control. 2003;10:159–165. doi: 10.1177/107327480301000207. [DOI] [PubMed] [Google Scholar]
- 11.Zhu L, Zhao L, Wang H, Wang Y, Pan D, Yao J, Li Z, Wu G, Guo Q. Oroxylin A reverses P-glycoprotein-mediated multidrug resistance of MCF7/ADR cells by G2/M arrest. Toxicol Lett. 2013;219:107–115. doi: 10.1016/j.toxlet.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 12.Ronaldson PT, Ashraf T, Bendayan R. Regulation of multidrug resistance protein 1 by tumor necrosis factor alpha in cultured glial cells: Involvement of nuclear factor-kappaB and c-Jun N-terminal kinase signaling pathways. Mol Pharmacol. 2010;77:644–659. doi: 10.1124/mol.109.059410. [DOI] [PubMed] [Google Scholar]
- 13.Barancík M, Bohácová V, Kvackajová J, Hudecová S, Krizanová O, Breier A. SB203580, a specific inhibitor of p38-MAPK pathway, is a new reversal agent of P-glycoprotein-mediated multidrug resistance. Eur J Pharm Sci. 2001;14:29–36. doi: 10.1016/S0928-0987(01)00139-7. [DOI] [PubMed] [Google Scholar]
- 14.Lee JY, Tanabe S, Shimohira H, Kobayashi Y, Oomachi T, Azuma S, Ogihara K, Inokuma H. Expression of cyclooxygenase-2, P-glycoprotein and multi-drug resistance-associated protein in canine transitional cell carcinoma. Res Vet Sci. 2007;83:210–216. doi: 10.1016/j.rvsc.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 15.Choi BH, Kim CG, Lim Y, Shin SY, Lee YH. Curcumin down-regulates the multidrug-resistance mdr1b gene by inhibiting the PI3K/Akt/NF kappa B pathway. Cancer Lett. 2008;259:111–118. doi: 10.1016/j.canlet.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Bentires-Alj M, Barbu V, Fillet M, Chariot A, Relic B, Jacobs N, Gielen J, Merville MP, Bours V. NF-kappaB transcription factor induces drug resistance through MDR1 expression in cancer cells. Oncogene. 2003;22:90–97. doi: 10.1038/sj.onc.1206056. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Zhao Y, Wang C, Xiao X, Zhou X, Xu G. Inhibition of p38 MAPK diminishes doxorubicin-induced drug resistance associated with P-glycoprotein in human leukemia K562 cells. Med Sci Monit. 2012;18:BR383–BR388. doi: 10.12659/MSM.883477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torres P, Poveda A, Jimenez-Barbero J, Ballesteros A, Plou FJ. Regioselective lipase-catalyzed synthesis of 3-o-acyl derivatives of resveratrol and study of their antioxidant properties. J Agric Food Chem. 2010;58:807–813. doi: 10.1021/jf903210q. [DOI] [PubMed] [Google Scholar]
- 19.Quan F, Pan C, Ma Q, Zhang S, Yan L. Reversal effect of resveratrol on multidrug resistance in KBv200 cell line. Biomed Pharmacother. 2008;62:622–629. doi: 10.1016/j.biopha.2008.07.089. [DOI] [PubMed] [Google Scholar]
- 20.Huo X, Liu Q, Wang C, Meng Q, Sun H, Peng J, Ma X, Liu K. Enhancement effect of P-gp inhibitors on the intestinal absorption and antiproliferative activity of bestatin. Eur J Pharm Sci. 2013;50:420–428. doi: 10.1016/j.ejps.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Du J, Pan Y, Shi Y, Guo C, Jin X, Sun L, Liu N, Qiao T, Fan D. Overexpression and significance of prion protein in gastric cancer and multidrug-resistant gastric carcinoma cell line SGC7901/ADR. Int J Cancer. 2005;113:213–220. doi: 10.1002/ijc.20570. [DOI] [PubMed] [Google Scholar]
- 22.Kim K, Kim KH, Kim HY, Cho HK, Sakamoto N, Cheong J. Curcumin inhibits hepatitis C virus replication via suppressing the Akt-SREBP-1 pathway. FEBS Lett. 2010;584:707–712. doi: 10.1016/j.febslet.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 23.Bae CH, Kim JW, Ye SB, Song SY, Kim YW, Park SY, Kim YD. AMPK induces MUC5B expression via p38 MAPK in NCI-H292 airway epithelial cells. Biochem Biophys Res Commun. 2011;409:669–674. doi: 10.1016/j.bbrc.2011.05.062. [DOI] [PubMed] [Google Scholar]
- 24.Sinha K, Chaudhary G, Gupta YK. Protective effect of resveratrol against oxidative stress in middle cerebral artery occlusion model of stroke in rats. Life Sci. 2002;71:655–665. doi: 10.1016/S0024-3205(02)01691-0. [DOI] [PubMed] [Google Scholar]
- 25.Gupta YK, Briyal S, Chaudhary G. Protective effect of trans-resveratrol against kainic acid-induced seizures and oxidative stress in rats. Pharmacol Biochem Behav. 2002;71:245–249. doi: 10.1016/S0091-3057(01)00663-3. [DOI] [PubMed] [Google Scholar]
- 26.Bishayee A, Barnes KF, Bhatia D, Darvesh AS, Carroll RT. Resveratrol suppresses oxidative stress and inflammatory response in diethylnitrosamine-initiated rat hepatocarcinogenesis. Cancer Prev Res (Phila) 2010;3:753–763. doi: 10.1158/1940-6207.CAPR-09-0171. [DOI] [PubMed] [Google Scholar]
- 27.Huang Z, Wang C, Wei L, Wang J, Fan Y, Wang L, Wang Y, Chen T. Resveratrol inhibits EMMPRIN expression via P38 and ERK1/2 pathways in PMA-induced THP-1 cells. Biochem Biophys Res Commun. 2008;374:517–521. doi: 10.1016/j.bbrc.2008.07.058. [DOI] [PubMed] [Google Scholar]
- 28.El-Mowafy AM, White RE. Resveratrol inhibits MAPK activity and nuclear translocation in coronary artery smooth muscle: Reversal of endothelin-1 stimulatory effects. FEBS Lett. 1999;451:63–67. doi: 10.1016/S0014-5793(99)00541-4. [DOI] [PubMed] [Google Scholar]
- 29.Sun C, Hu Y, Liu X, Wu T, Wang Y, He W, Wei W. Resveratrol downregulates the constitutional activation of nuclear factor-kappaB in multiple myeloma cells, leading to suppression of proliferation and invasion, arrest of cell cycle, and induction of apoptosis. Cancer Genet Cytogenet. 2006;165:9–19. doi: 10.1016/j.cancergencyto.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 30.Yu H, Pan C, Zhao S, Wang Z, Zhang H, Wu W. Resveratrol inhibits tumor necrosis factor-alpha-mediated matrix metalloproteinase-9 expression and invasion of human hepatocellular carcinoma cells. Biomed Pharmacother. 2008;62:366–372. doi: 10.1016/j.biopha.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Berghe W Vanden, Plaisance S, Boone E, De Bosscher K, Schmitz ML, Fiers W, Haegeman G. p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways are required for nuclear factor-kappaB p65 transactivation mediated by tumor necrosis factor. J Biol Chem. 1998;273:3285–3290. doi: 10.1074/jbc.273.6.3285. [DOI] [PubMed] [Google Scholar]
- 32.Mulakayala C, Babajan B, Madhusudana P, Anuradha CM, Rao RM, Nune RP, Manna SK, Mulakayala N, Kumar CS. Synthesis and evaluation of resveratrol derivatives as new chemical entities for cancer. J Mol Graph Model. 2013;41:43–54. doi: 10.1016/j.jmgm.2013.01.005. [DOI] [PubMed] [Google Scholar]