Abstract
Vaccines are sensitive biologics that require continuous refrigerated storage to maintain their viability. The vast majority of vaccines are also administered using needles and syringes. The need for cold chain storage and the significant logistics surrounding needle-and-syringe vaccination is constraining the success of immunization programs. Recombinant live viral vectors are a promising platform for the development of vaccines against a number of infectious diseases, however these viruses must retain infectivity to be effective. Microneedles offer an effective and painless method for delivery of vaccines directly into skin that in the future could provide solutions to current vaccination issues. Here we investigated methods of coating live recombinant adenovirus and modified vaccinia virus Ankara (MVA) vectors onto solid microneedle arrays. An effective spray-coating method, using conventional pharmaceutical processes, was developed, in tandem with suitable sugar-based formulations, which produces arrays with a unique coating of viable virus in a dry form around the shaft of each microneedle on the array. Administration of live virus-coated microneedle arrays successfully resulted in virus delivery, transcutaneous infection and induced an antibody or CD8+ T cell response in mice that was comparable to that obtained by needle-and-syringe intradermal immunization. To our knowledge, this is the first report of successful vaccination with recombinant live viral vectored vaccines coated on microneedle delivery devices.
Keywords: Microneedle, Skin, Vaccine, Poxvirus, Adenovirus, Spray-coating
1. Introduction
Recombinant viral vectored vaccines are one of the most rapidly growing fields of vaccine development. Some of the leading vaccine candidates in clinical development for diseases such as malaria, HIV and tuberculosis as well as for cancer treatments are based on recombinant viral vectors. Vectors being used range from measles, lentivirus, sendai, vaccinia virus to perhaps the most widely used adenovirus (AdV) and modified vaccinia virus Ankara (MVA) that have both been developed individually or in prime-boost combinations as vaccine candidates [1]. AdV vectors are made replication-defective by deletion of essential E1, E3 and E4 genes while MVA is a highly attenuated strain of vaccinia virus obtained by multiple passages in chicken embryo fibroblast cells. These viruses differ quite substantially in their physical structure with AdV being a much smaller (hydrodynamic diameter; 120 nm) [2], non-enveloped virus with a protein capsid and MVA being a large (300×250 nm), lipid enveloped virion [3].
Current vaccination programs are limited by cold-chain storage, vaccine wastage, hazardous sharps-waste and the trained personnel requirements. All of these logistic factors add significant and unsustainable financial and logistic costs to immunization programs. Development of needle-free immunization methods and devices aim to overcome these logistic issues thereby reducing the cost of each vaccine dose and preferably resulting in a device that can be self-administered outside of a health-care setting. Dermal vaccine administration using microneedle-based platforms promises to be one such needle-free method that addresses these issues. Furthermore, stabilization of a vaccine on a transcutaneous patch may also overcome the problems associated with reconstitution into a liquid solution for administration.
Microneedle insertion into skin creates transient pores [4,5] in the otherwise impermeable stratum corneum thus enabling delivery of drugs and vaccines directly into the epidermis and/or underlying dermis compartments. Such devices consist of a number of sub-millimeter protrusions piercing the skin and delivering the vaccine. Microneedles must be sharp enough to pierce the stratum corneum (SC) to enable targeted delivery of the formulation to the top layers of the skin but preferably short enough so as not to reach nerve endings deeper within the skin layers resulting in a virtually pain free delivery [5]. Here, we predominantly focus on ‘wet-etched’ solid silicon pyramidal microneedle array patches [6] termed ImmuPatch. By virtue of their smooth surface and ultra-sharp tips, these ImmuPatch devices go cleanlyin and out of tissue at very low insertion forces. We previously demonstrated that our silicon microneedle arrays successfully deliver liquid MVA vaccine resulting in the induction of CD8+ T cell responses to the encoded malaria antigen and protection against disease [7]. Here, we developed a second generation ImmuPatch device where the live vaccine is coated onto the microneedle patch.
Efforts to produce a single delivery device where the drug or vaccine is combined or coated onto the microneedles have demonstrated success for drugs [8,9] or inactivated vaccines or subunit vaccines [10] and with the tuberculosis vaccine Bacille Calmette-Guérin BCG [11]. Current state of the art in the coating of solid microneedles involves the use of specialized coating apparatus for dip coating [12], rolling or brushing on the formulation [13], or pattern coating using, for example, ink-jet coating or microfluidics [14]. These common practices often require the use of wetting agents, surfactants and/or viscosity enhancers which may not be compatible with vaccines. Furthermore, scaling up some of these techniques to a production level is challenging due to the uniqueness of these apparatuses. Our overarching aim is to develop a coating method that stabilizes vaccine, which could be transferred to a scaled-up GMP-compliant process using common pharmaceutical processes that would enable the subsequent clinical application of such a device. Spray-coating is a robust and scalable coating technique that is well established in the pharmaceutical industry. We previously explored and developed this process to filmcoat microneedle arrays [15]. The spray coating process can be divided into three steps, (1) generation of fine droplets (atomisation), (2) deposition on the surface and (3) coalescence of droplets on the substrate. Spray velocity and spray density influence the deposition of droplets on a surface. The spray velocity is influenced by a number of factors, including the atomisation air pressure and the nozzle-to-surface distance. Higher atomisation air pressure and lower nozzle-to-surface distances increase spray velocity. While high spray velocities can facilitate spreading of droplets upon deposition, too high a velocity can result in droplets being blown off the surface by the stream of atomizing air.
To date, spray-coating has not been tested as a method of coating sensitive vaccines onto microneedle arrays. The primary aim of this study was to determine if this process could be successfully used with live recombinant viral vectored vaccines, AdV and MVA. We report on the development of novel methods to coat microneedle arrays with these virus vectors. In this study, excipient compositions and spraying parameters that maintain maximum recombinant AdV and MVA vaccine vector viability are identified. We also demonstrate that combining a spray-coating method and trehalose-based formulations of AdV and MVA produces arrays suitable for the delivery of live vectors into skin resulting in successful virus vector infection and the induction of an antibody or CD8+ T cell response. To our knowledge, this is the first report on microneedle devices designed for delivery of live viral vaccines.
2. Materials and methods
2.1. Microneedle manufacture
Silicon microneedles were prepared using previously reported approaches [6,16]. For this study 5×5 mm arrays containing 25 microneedles (200 µm height, 100 µm base width) and 7×7 mm arrays with 36 microneedles (300 µm height, 150 µm base width) were used. Shorter microneedles were used for visualization, survival and immunization studies while longer microneedles were used for skin infection studies, due to availability of microneedle arrays.
2.2. Viruses
Recombinant MVA and E1/E3-deleted human adenovirus serotype 5 (AdHu5) (AdV) expressing red fluorescent protein (RFP), β-galactosidase (β-gal), mCherry or the malaria antigens Plasmodium yoelii MSP142 (PyMSP142) or Plasmodium berghei circumsporozoite (PbCSP) proteins were grown and purified as previously described [17]. Stock MVA was resuspended in 10 mM Tris (pH 9.0) buffer and stock AdV was resuspended in 10 mM Tris (pH 7.4) buffer. Influenza virus (strain X31 [18]) was grown in MDCK cells and purified by ultrafiltration and ion-exchange chromatography as described (BIA Separations) [19].
2.3. Vaccine formulation optimization
AdV-mCherry or MVA-RFP viruses were formulated in selected water-based formulations consisting of trehalose, maltodextrin, ultra low viscosity carboxymethylcellulose (CMC) sodium salt, Tween 80 (all from Sigma Aldrich) or Lutrol® F68 (BASF). A 5 µl aliquot of formulation was drop-coated over a flat silicon plate and dried at ambient conditions for 18 h. Following drying, formulation was recovered by placing the plate in cell culture medium (‘DMEM/10’; DMEM containing 10% foetal calf serum, L-glutamine, penicillin/streptomycin) for 45 min at ambient temperature and used to infect 293A cells (AdV) or DF1 cells (MVA). Virus survival was then assessed using flow cytometry-based infectivity assay (see below) and compared with non-dried controls.
2.4. Spray-coating
Spray coating was performed using Düsen-Schlick 970 S8 two substance nozzle at the gas spraying flow of 6–16 m/s. Air or N2, filtered on-line through a 0.22 µm membrane was used for aerosolization [15]. Spraying was performed in a laminar flow cabinet in a perspex box to contain virus in an enclosed environment. Unless specified, the spraying rate was 10 µL/min/cm2 for AdV and 600 µL/min/cm2 for MVA, the volume that was sprayed was kept constant and spray rate was precisely controlled using a syringe pump. Spraying distance (nozzle-to-patch) was 7 cm for AdV and 3 cm for MVA to facilitate efficient delivery of vaccine due to different spray velocities and to prevent droplets being blown off the surface by the stream of atomizing air [15]. A known amount of FITC was added to all coating formulations. Coated arrays were dried under vacuum in the presence of desiccant for 2–24 h at 21–24 °C. Coated virus was recovered from arrays in cell culture medium. The volume of formulation (and therefore vaccine concentration) that coated each individual array was determined by determining the volume of FITC from a standard curve of fluorescence compared to volume.
2.5. Assessment of coated microneedle arrays
Coated silicon microneedle arrays were visually assessed by fluorescent light microscopy (10×). FITC was added to the formulation to visualize by fluorescence. Alternatively, coated arrays were Ausputter coated for 20 s prior to imaging by scanning electron microscopy using a JSM 5510 SEM.
2.6. Physical integrity of spray-coated formulation
Purified influenza X31 virus was formulated in 15% trehalose (w/v) + 0.5% Tween 80 (v/v), spray-coated onto microneedle patches and air-dried. These patches containing dried virus were then exposed to a high flow of nitrogen gas (16 m/s) for 10 min. Blown nitrogen gas was washed on-line in RLT buffer (Roche) to collect any virus blown off the patch. The collected rinse was then screened by real time PCR for the presence of influenza virus using a commercially available kit (Primer Design, UK).
2.7. Determination of virus survival
Survival of AdV and MVA expressing fluorescent proteins was measured using flow cytometry. Coated virus was recovered from arrays by placing the array in cell culture medium for 45 min at ambient temperature. Alternatively, a control virus sample was prepared by spraying virus in formulation directly into cell culture medium to avoid drying, termed ‘liquid control’. DF-1 or HEK293A cells grown under standard conditions were infected with MVA-RFP or AdV-mCherry viruses respectively and incubated overnight. Cells were then harvested and infection rate was calculated by measuring fluorescence of infected cells expressing RFP or mCherry proteins using flow cytometry (LSRII Becton-Dickinson). Survival rate was calculated from a standard curve using samples of known titer (in plaque forming units (PFU)/ml units) and is expressed as log PFUeq/ml units (logarithmic value of PFU equivalents per ml). Survival of AdHu5-PyMSP142 (referred to hereafter as AdV-MSP42) virus was measured using commercial adenovirus titer hexon immunoassay (Cell Biolabs Inc.) [20].
2.8. Assessment of skin delivery efficiency
The amount of formulation delivered to the skin was measured using red fluorescent microspheres with 0.1 µm diameter (Invitrogen) that were chosen to resemble AdV shape and size. The use of fluorescent nanospheres is common in the microneedle field [21,22]. Microspheres were formulated in 15% trehalose (w/v) + 0.05% Tween 80 (v/v) solution and spray-coated on silicon microneedle arrays (36 microneedles, 300 µm height) using the same parameters as for spray-coating of AdV. Arrays were fixed onto larger pieces of occlusive adhesive tape (1516 Poly Med, 3M), applied to ears of mice and left for 4 h. Female BALB/c or C57BL/6 mice 4–6 weeks old (Harlan UK) were used in all experiments which were conducted in strict accordance with the terms of licences from the Irish Department of Health and Children, under the Cruelty to Animals Act (licence numbers B100/4034 and B100/3157) and according to the approval of the UCC AECC Animal Ethics Committee. After 4 h, mice were sacrificed. The ears were swabbed with cotton buds, pre-wetted with PBS, to remove excess formulation from the skin surface, similar to other studies [23]. Formulation was recovered from the cotton bud by placing it in 4 ml PBS and vigorously vortexing. Ears were removed and disrupted in PBS/HCl/Tween 80 (7:1:0.5) mixture using MagNA Lyser Green Beads (Roche). Red fluorescence in the supernatant of homogenized ear samples was measured to give the amount of delivered formulation.
2.9. Transcutaneous administration studies
Freshly excised pig skin was used for virus infection as previously described [24]. Briefly, virus-coated or uncoated silicon microneedle arrays were fixed onto occlusive adhesive tape, applied to the skin and left for 18 h in an incubator at 37 °C and 5% CO2 before fixation and staining, using a β-galactosidase detection procedure that was performed as previously described [24].
2.10. immunogenicity
2.10.1. Antibody responses
Microneedle arrays containing an array of 25 microneedles (200 µm height) were coated with 5×109 viral particles (vp) of AdV-MSP42 virus formulated in 15% trehalose (w/v) + 0.05% Tween 80 (v/v) and left for 2 h under vacuum at ambient temperature to dry. Occlusive adhesive tape was applied on the back of each patch. An array was applied to each ear of female 6–8 week old C57BL/6 mice (two arrays per mouse) for 4 h and then removed. Control groups received the same vaccine dose either intradermally, via needle and syringe or coated on flat microneedle plates. On day 17 postimmunization mice were bled and the relative level of anti-PyMSP119 antibodies present in a 1:100 serum dilution was measured by ELISA [17].
2.10.2. CD8+ T cell response
Microneedle arrays with the same design used for antibody responses were coated with 1 × 106pfu MVA-PbCSP formulated in 15% trehalose. Two weeks after a single immunization of BALB/c mice, T cell responses to the dominant MHC class I epitope Pb9 (SYIPSAEKI) [25] were analyzed by intracellular cytokine staining and flow cytometry (ICS) in the same method as previously described [7]. After blocking Fc receptors with anti-CD16/CD32, cells were surface stained for 30 min at 4 °C with Pacific Blue-labeled anti-CD8α and APC-Alexa Fluor 700-labeled anti-CD4. Cells were permeabilized in Cytofix/Cytoperm solution as per manufacturer’s instructions (BD Biosciences). Intracellular cytokines were stained with APC-labeled anti-IFN-γ, FITC-labeled anti-TNF-α, and PE-labeled anti-IL-2. Flow cytometric analyses were performed using an LSRII (BD Biosciences) and data were analyzed with FlowJo (Tree Star) software. One million events per sample were acquired. Analysis of multifunctional T cell responses was performed by using Boolean analysis in FlowJo software and SPICE 4.0 (M. Roederer NIH, Bethesda). Data were analyzed using GraphPad Prism version 5 for Windows (GraphPad Software, San Diego, California, USA). One way ANOVA was performed to compare the responses between groups.
3. Results
3.1. Determination of suitable formulations
Selecting a formulation that permitted successful coating of microneedles and concomitantly retained virus viability onto the patch was the first step to successfully coating live viruses onto microneedle arrays. We screened a number of sugars, surfactants and film-forming agents, which have a safe history of use in humans, to find suitable formulations for coating microneedle arrays with live viruses. Trehalose was assessed as it is well known that this disaccharide preserves bacteria and viruses at ambient temperature for extended periods of time due to its ability to form glass-like structures [26]. Non-ionic surfactants (Tween 80 and Lutrol®) and film-forming agents (CMC) were also assessed as they are routinely included in microneedle coating formulations to facilitate coating cohesion [27–29]. We also examined the polysaccharide maltodextrin as an alternative to CMC as it is also reported to form films [30]. AdV and MVA were mixed in selected formulations, deposited on silicon arrays by pipetting, dried at ambient conditions for 18 h and survival was assessed using flow cytometry-based infectivityassay (see Materials and methods). Results of virus survival testing upon drying in selected formulations (Table 1) showed that for AdV, incorporation of the surfactants Tween 80 or Lutrol® to 15% trehalose does not diminish the stabilizing effect of the sugar. In initial experiments we determined that Tween 80 concentrations from 0.02% to 0.5% did not have a detrimental effect on AdV viability, however the use of a concentration of 0.5% resulted in the most reproducible coating (data not shown). In contrast, addition of CMC to trehalose had a detrimental effect to trehalose effects on AdV viability. Trehalose also resulted in the greatest recovery of viable MVA. Trehalose in combination with sucrose was recently demonstrated as a promising stabilizing excipient for multiple viruses [26], however in this study survival of MVA in trehalose alone was comparable with that of the trehalose/sucrose mixture. Similar to AdV, addition of CMC to trehalose also resulted in a substantial decrease in MVA viability. Unlike AdV, addition of Tween 80, but not Lutrol, to trehalose resulted in significant decreases in MVA viability, compared to formulation with trehalose alone. CMC, a common film-forming agent used in microneedle coating processes, should be particularly avoided for AdV and the enveloped virus MVA. The formulations selected for subsequent use were 15% trehalose in water (w/v) for MVA and 15% trehalose (w/v) + 0.5% Tween 80 (v/v) for AdV.
Table 1.
Survival of AdV and MVA in selected formulations and dried on silicon plates for 18 h. Results are expressed as % survival (± standard deviation of the mean) relative to controls (samples formulated in the same manner and kept in solution at ambient temperature.).
| Formulationa | AdV (% survival ± SD) | MVA (% survival ± SD) |
|---|---|---|
| CMC | 0 ± 0 | ND |
| Trehalose | 70 ± 8 | 45 ± 9 |
| Trehalose + sucrose | ND | 46 ± 4 |
| Trehalose + Tween 80 | 73 ± 11 | 9 ± 6 |
| Trehalose + Lutrol® | 68 ± 5 | 37 ± 12 |
| Trehalose + CMC | 45 ± 4 | 1 ± 1 |
| Maltodextrin | 34 ± 6 | 28 ± 6 |
| Maltodextrin + Tween 80 | 34 ± 11 | 15 ± 4 |
| Maltodextrin + Lutrol® | 15 ± 9 | 32 ± 8 |
Concentrations: trehalose, maltodextrin: 15% (w/v); Lutrol®, Tween 80: 0.5% (v/v); CMC: 1% (w/v) with trehalose, 3% otherwise. ND: not determined.
3.2. Spray-coating of microneedle arrays
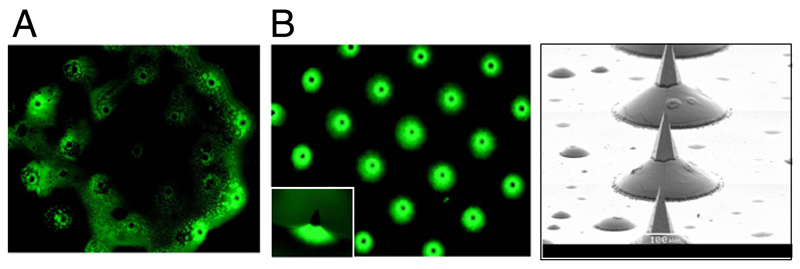
Dip-coating, as described for linear microneedle devices [31], was not applicable for our arrays due to the microneedle dimensions and the density of the array. An alternative, simple method of dropping formulation onto microneedles was initially assessed. However this method did not produce satisfactory results, as the coat did not spread and disperse evenly on the microneedle array (Fig. 1A). Trehalose increases the surface tension of aqueous solutions and therefore would inhibit spreading on the silicon array. Thus it was necessary to identify a suitable coating method to overcome the surface tension effects experienced with the simple drop-coating of arrays and achieve better distribution of the coating on the array. Using FITC in a trehalose/tween 80 formulation, we demonstrate that spray-coating results in the formulation being concentrated predominantly around the shaft and base of each needle (Fig. 1B), compared to the irregular non-uniform pattern resulting from drop-coating formulation. Thus, a targeted coating to just the microneedle shaft and not the array base can be achieved using spray-coating. Coating formulation around the shaft and base of each microneedle will keep the sharp tip exposed and therefore this coating method should not decrease tip sharpness. Furthermore the formulation on the shaft is retained in the immediate vicinity of the opened pore in the skin and has a greater potential of diffusing into these created pores compared to formulation on an array base.
Fig. 1.
Fluorescent microscopy image of silicon microneedle arrays spray-coated or drop-coated with formulation. Silicon microneedle arrays were coated with a formulation consisting of FITC in 15% trehalose (w/v) and 0.5% Tween 80 (v/v). (A) Microneedle array with drop-coated formulation; top view; (B) Microneedle array with (left) spray-coated formulation (top view) with individual microneedle (small frame, side view), and right; SEM of spray-coated microneedles.
3.3. Enhancement of spray-coating parameters
Our next aim was to establish a spray-coating method that results in coating the microneedle shafts and not the array base whilst still maximizing virus viability. As proteins tend to denature when exposed to an air/water interface, exposure to the large surface area during spray-coating may destabilize the protein components of the virus particles. First we determined if the process of spraying selected formulations containing virus damages the live virus. We sprayed AdV formulated in a 15% trehalose solution directly into DMEM/10 medium to avoid drying (termed ‘liquid control’) and compared viability with virus formulations sprayed and dried on a flat silicon surface (Fig. 2). These data showed that there was no decrease in viability after spraying, however, after spraying and drying there was a small but significant reduction. We thus conclude that decreases in virus viability occur during the drying and not during the spraying process.
Fig. 2.
Effect of spraying or spraying and drying on AdV viability. Survival of AdHu5-mCherry sprayed directly into cell culture medium or sprayed onto silicon surface, dried for 2 h in evacuated chamber and recovered in DMEM/10 medium (‘sprayed & dried’) compared to AdV retained in media (‘liquid control’). Mean survival (log PFU equivalent units) with error bars showing SEM. *P<0.05 between the ‘sprayed & dried’ group compared to other groups as measured by unpaired t-tests.
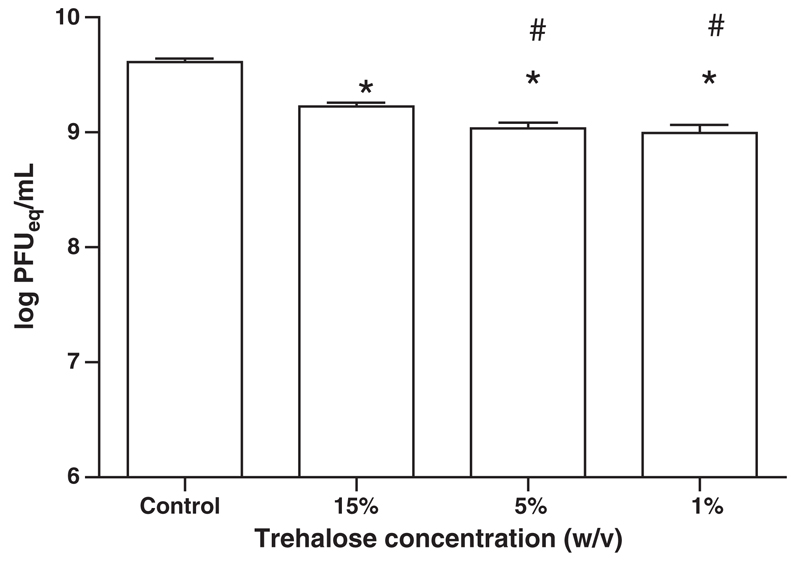
We next examined the effect of trehalose concentration on virus survival when it is spray-coated onto silicon microneedle arrays. Formulations with high trehalose concentrations (15%) were shown to maintain spray-coated AdV viability better than lower trehalose concentration (1% and 5%) (Fig. 3). Although formulations with an even higher trehalose concentration (>50% (w/v)) can be sprayed, such formulations do not form coatings of desired quality (data not shown). Thus, 15% trehalose solution (w/v) resulting in the desired coating pattern and high retention of virus viability was chosen for further studies.
Fig. 3.
Effect of trehalose concentration on survival of AdHu5-mCherry spray-coated and dried on silicon microneedle array. Mean survival (log PFU equivalent units ± SEM) after spraying and drying for 2 h at ambient temperature. Statistically significant differences (P<0.05) in survival rates of sprayed formulations compared to liquid control (*) and viruses sprayed in 15% trehalose formulation compared to 5% and 1% formulations (#) were determined by one-way ANOVA followed by Dunnet’s test.
A potential concern when using spray-coated arrays is that dried formulation may spontaneously aerosolize during application and be inhaled by the user or others. To check the integrity of the coating on the microneedle patch and resistance of dried formulation to aerosolization, rigorous testing was performed on arrays coated with influenza virus. Microneedle arrays coated with influenza virus formulated in 15% trehalose (w/v) + 0.5% Tween 80 (v/v) were exposed to a high flow of nitrogen gas (16 m/s) for 10 min. Blown nitrogen was then washed on-line and screened using qRT-PCR for presence of influenza virus. We determined that under such intensive, prolonged gas flow the amount of virus blown off the array is at the lower measuring range and accounts for less than 0.01% of the virus originally coated on the patch.
Finally, we determined how the liquid-input rate during spraying affected virus viability. A slower liquid-input rate results in a smaller droplet size, higher air/water interface and higher evaporation during spraying compared to a faster liquid input rate. Interestingly, AdV and MVA require different spraying conditions to retain viability upon coating. Survival of AdV dropped substantially with increasing spraying time thus showing that fast spraying is required to maintain AdV viability (Fig. 4A). In contrast, there was a higher survival when MVA-RFP virus was sprayed at low rate (10 µL/min/cm2) compared to a high rate (600 µL/min/cm2) (Fig. 4B). As previously discussed, at high velocity, droplets can be being blown off the surface by the stream of atomizing air. To redress this issue, the spraying distance was increased to 7 cm for AdV coating.
Fig. 4.
Spraying rate differentially affects AdV and MVA viability. Survival of spray coated (A) AdHu5-mCherry formulated in trehalose/Tween 80 (15% w/v and 0.5% v/v respectively) and (B) MVA-RFP formulated in trehalose (15% w/v) after spraying onto silicon microneedle array at various rates and drying for 2 h at ambient temperature. Mean survival (log PFU equivalent units ± standard deviation, n=3) after spraying and drying. Statistically significant differences (P<0.05) in survival between sprayed groups vs. control (*) and “fast sprayed” compared to “slow sprayed” groups (#) were determined by one-way ANOVA followed by Dunnet’s test.
3.4. Dose loading onto silicon microneedle arrays
An optimal coating pattern of microneedle arrays should not only retain maximum vaccine viability, it should also enable maximum formulation to be delivered onto the array. Based on the parameter optimization shown above, a fast spraying rate was selected as the optimal method for coating silicon microneedle arrays with AdV. Using this fast spraying method (600 µL/min/cm2) we determined that on average 6.5 ± 0.6 µL of formulation was coated per cm2 of silicon microneedle patch. In contrast slow spraying permitted higher volumes to be loaded onto silicon microneedle arrays; spray-coating at a rate of 10 µL/min/cm2 delivered 18 ± 3 µL of formulation per cm2 of microneedle patch. Therefore, the rate of spraying, which must be controlled to maximize virus viability, impacts on the volume and therefore the dose that can be loaded onto the microneedles.
3.5. Efficiency of delivery of spray-coated formulation into skin
Based on previous microneedle studies [11,23] it was reasonable to expect that not all of the formulation coated onto microneedle arrays will be effectively delivered across the stratum corneum upon application of a coated array onto skin. We examined the delivery efficiency of spray coated arrays by applying microneedle arrays coated with 100 nm fluorescent beads, approximately the same size as AdV particles, onto mouse ears and measured delivery efficiency after 4 h exposure time by determining fluorescence in homogenized samples. It was shown that on average 40.5 ± 2.5% of formulation is delivered to skin (mean of 50 samples ± standard error of the mean) after 4 h administration to anesthetized mice.We determined that 10.8% ± 2% of the formulation was present on the ear at the time of sacrifice, as evidenced by the amount of material in the cotton swabs.
3.6. Microneedle array surface characteristics
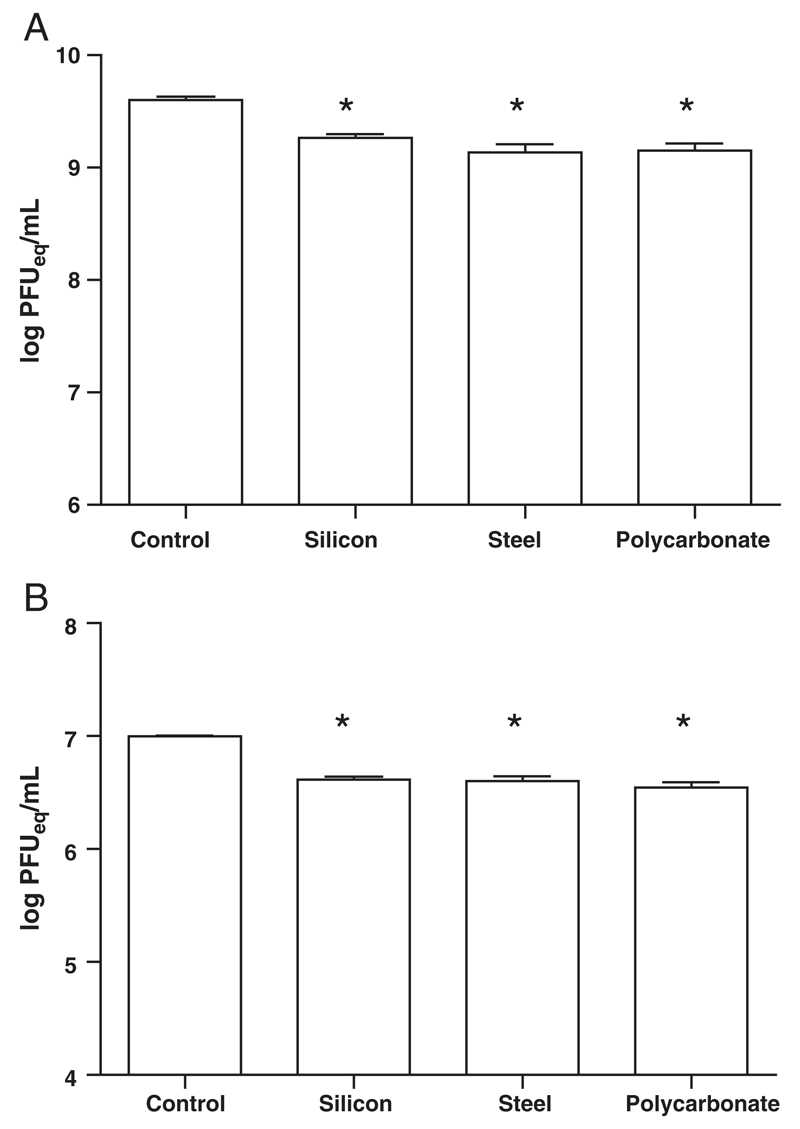
Next we examined if this spray-coating method was effective for the coating of non-silicon surfaces as solid microneedles can also be made of metal, polymer, glass etc. [31–33]. There was no significant difference in survival rate of AdV or MVA coated on stainless steel and polycarbonate surfaces compared to coating on silicon (Fig. 5). Therefore, the spray-coating method developed for coating silicon surfaces is applicable to other materials as well.
Fig. 5.
Spray-coating onto different substrates. Survival of formulated (A) AdHu5-mChery and (B) MVA-RFP viruses spray-coated and dried on silicon, polycarbonate and steel surface, using standard spraying parameters for each virus, compared to liquid control. Mean survival (log PFU equivalent units ± SEM, n = 8) after spray-coating and overnight drying at ambient temperature. Star indicates statistically significant difference between spray coated group and control group (P<0.05) as estimated by oneway ANOVA.
3.7. Transcutaneous vaccine delivery
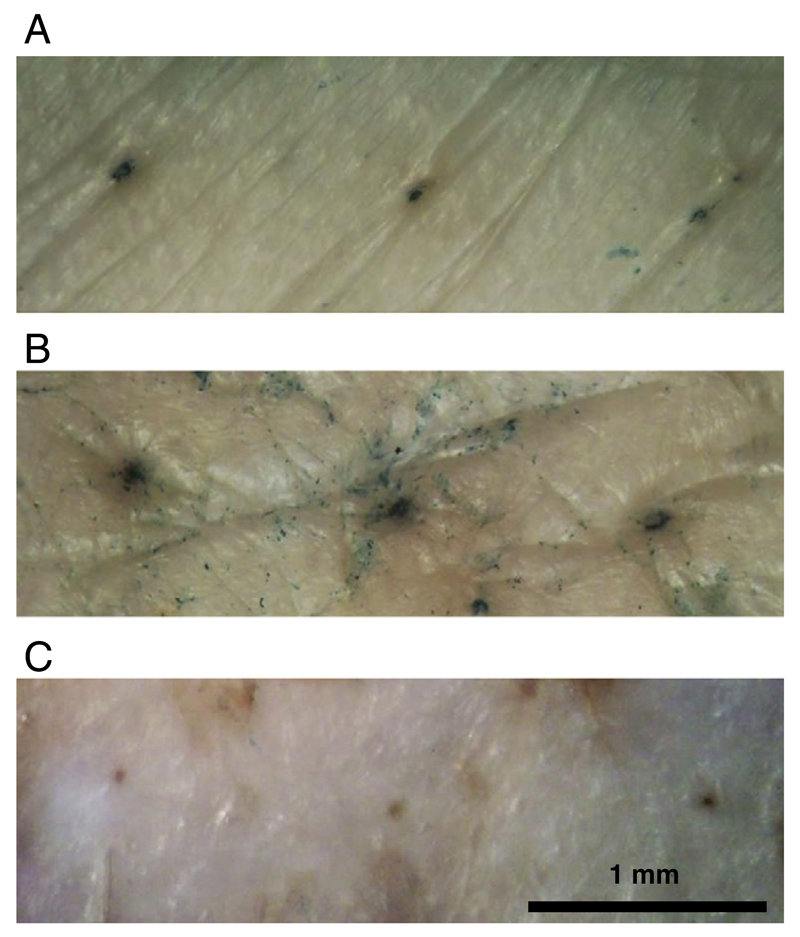
To verify the viral vector delivery potential of spray-coated microneedle arrays, we applied the arrays coated with AdV-β-gal and MVA-β-gal to ex vivo pig skin. Arrays were spray-coated with either AdV- or MVA-β-gal using the standard coating protocol for each virus. The coated microneedle arrays were allowed to dry for 2 h and then applied to freshly excised pig skin. Successful virus infection and β-gal transgene expression by both viruses was observed (Fig. 6).
Fig. 6.
Transcutaneous vaccine delivery. Skin-infection using microneedle arrays spray coated with (A) AdV-β-gal, (B) MVA-β-gal or (C) uncoated microneedle array. Microneedle arrays with 300 µm microneedles containing 1×109 pfu (AdV-β-gal) or 1×106 pfu (MVA-β-gal) spray-coated particles were applied onto freshly excised pigskin. MVA was spray coated at 10 µl/min/cm2 and AdV was spray coated at 600 µl/min/cm2. Infection was visualized by x-gal staining assay [24].
3.8. Immunogenicity
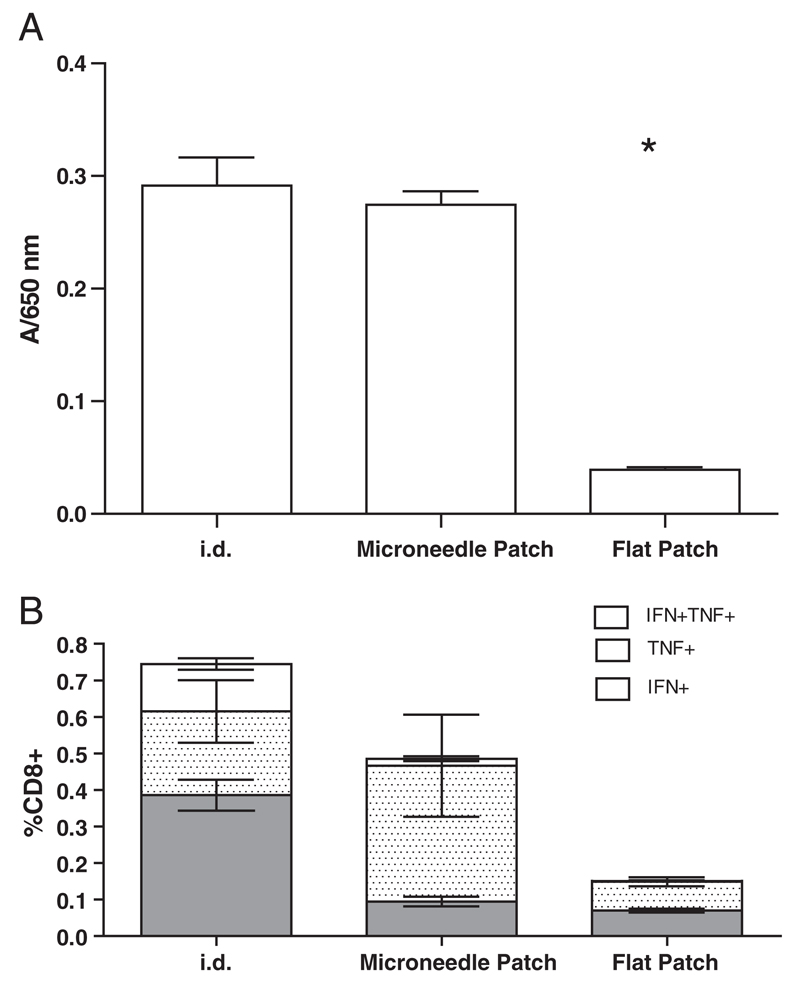
To assess the potency of recombinant virus vaccine coated onto microneedle patches, we immunized C57BL/6 mice with arrays coated with AdV-MSP42 virus [17]. Mice received a single dose by either i.d. injection or by a coated microneedle patch. Antibody responses to the encoded malaria antigen were assessed in serum, diluted 1/100 on day 17 post-immunization by ELISA (Fig. 7A). Results show that antibody response in mice immunized with arrays coated with dried AdV virus are equivalent to responses obtained by i.d. immunization. The absence of an antibody response for the group treated with a flat silicon plate containing no microneedles but coated with virus (“flat patch”) confirms that needle penetration is indeed essential for effective virus delivery.
Fig. 7.
Coated ImmuPatch induces vaccine-induced immunity equivalent to systemic delivery. (A) Serum antibody responses on day 17 after a single immunization with AdV-MSP42 vector were determined by ELISA. Mice received 1×1010 vp in either liquid form via intradermal (i.d.) route or by adhering vaccine-coated microneedle array onto mouse ears. A flat silicon plate with dried virus but without microneedles is used as negative control. Star indicates statistically significant difference (P<0.0001) in antibody titer of “flat patch” group compared to i.d. or coated array immunized groups vs. by one-way ANOVA followed by Dunnet’s test. (B) Magnitude and phenotype of the antigen-specific CD8+ T cell response after a single immunization. Groups of 5 BALB/c mice were immunized with MVA-PbCSP by the intradermal (i.d.) route or using a microneedle array or flat patch coated with MVA-PbCSP. Antigen-specific polyfunctional CD8+ T cell responses in spleen cells were quantified after intracellular cytokine staining (ICS) of IFN-γ, TNF-α, and IL-2 subsequent to epitope stimulation. Data are expressed as the frequencies of single cytokine secreting IFN-γ+ (gray bars), TNF-α+ (stippled bars) and dual-secreting IFN-γ+TNF-α+ CD8+ T cells (white bars), ± standard error of the mean (± SEM), 2 weeks after immunization.
Furthermore, we examined the induction of antigen-specific CD8+ CTL in mice that were immunized with MVA-PbCSP by the intradermal (i.d.) route or by coated microneedle or flat arrays. Two weeks after priming, the frequency and multi-functional quality [34] of CD8+ T cells in the spleen that recognized the dominant MHC class I epitope in PbCSP, termed Pb9, were determined. Flow cytometry combined with Boolean analysis revealed that three populations of poly-functional antigen-specific CD8+ T-cell responses were primed; namely CD8+IFN-γ+ or CD8+TNF-α+ single cytokine-secreting cells and CD8+IFN-γ+TNF-α+, dual cytokine producing T cells (Fig. 7B). No other multi-functional phenotype was observed. The total frequency of cytokine-secreting CD8+ T cells was not significantly different in mice immunized by the i.d. route or using a coated microneedle patch, these responses were significantly greater than responses observed in the flat patch group (P = 0.035). Interestingly, the quality of the CD8+ T cell response depended on the vaccination route. Intradermal immunization induced significantly more CD8+ T cells that expressed IFN-γ+ alone (P = 0.012) and IFN-γ and TNF-α (CD8+IFN-γ+TNF-α+) (P = 0.010) compared to the coated microneedle patch. In contrast, formulating MVA-PbCSP in trehalose and coating onto a microneedle array induced a response that was dominated by antigen-specific CD8+TNF-α+ T cells. Vaccination using a flat silicon patch coated with trehalose-formulated MVA-PbCSP, with no microneedles, resulted in a significantly weaker CD8+ T cell response, demonstrating that breaching the SC significantly enhances the induction of CD8+ CTL by MVA-PbCSP.
4. Discussion
Advances in stabilization of live viral vectors in glass-like structures provide a strategy for the development of microneedle devices coated with vaccines containing dried live viral vectors [26,35]. Microneedles offer several advantages to deliver vaccine safely and effectively and their successful development as needle-free, easy-to-administer, stable and cheap devices would address several logistic obstacles that are currently preventing immunization programs from reaching their full potential. Furthermore vaccine-coated microneedle arrays do not require reconstitution prior to administration. Our over-arching aim is to develop a microneedle-based stabilized vaccine platform that could be manufactured to GMP and clinically translated using conventional processes. Spray-coating is a well established method in the microelectronic and pharmaceutical industries. Potential advantages of spray-coating lie in its scalability and simplicity. However, most surfaces that are sprayed are flat and do not have regularly spaced, micron scale protrusions to coat. For deposition of water-based drugs or vaccines onto microneedle arrays the formulation needs to match certain properties to result in desirable coating and to retain virus viability. We previously optimized spraying parameters for film-coating silicon microneedle arrays [15]. The principal aim of this study was to determine if spray-coating could be adapted for coating sensitive vaccines onto microneedle arrays. A secondary objective was to determine how formulations commonly used for coating microneedle arrays impacted on the viability of these vaccines. Here we found that spraying is superior to dropping formulation onto the array. We identified key formulation and spraying parameters that can be used across diverse viral vector vaccines and microneedle substrates. Our spray approach resulted in a desired coating with formulation concentrated around the microneedles with virtually no material deposited in the inter-needle space. This resulted in a uniform coating pattern and effective retention of viable live viruses that could be successfully delivered into skin, leading to the induction of immune responses that were comparable to conventional needle delivery. This underlies future studies to determine the long-term stability of these vaccines on coated microneedle arrays.
The composition of the coating formulation is a critical factor that must be optimized for coating live vaccines onto microneedle arrays. Suitable formulations need to preserve virus integrity during spraying, upon drying and during subsequent storage while still enabling coating with a desirable distribution pattern. Building on previous results on microneedle coatings [11,27,28,32] we screened a number of formulations for AdV and MVA survival as well as a number of spray-coating process parameters. The film-forming agent CMC, used previously in coating microneedles [11,27,28], was found to substantially decrease the viability of both virus vaccines. Negative effects of CMC on antigen integrity have also been observed with other vaccines such as inactivated flu vaccine and BCG [11,28]. This may limit its future adoption for coating microneedle arrays with vaccines and warrants the identification of more suitable film-forming agents. Thus we evaluated maltodextrin and found it to be superior to CMC alone for virus viability for both vaccines and therefore maltodextrin may provide an alternative film forming agent to CMC. However, maltodextrin was not as effective at preventing vaccine loss during drying compared to the disaccharides, despite the similarity in structure between the polysaccharide and disaccharides. As a large polymer, it is likely that maltodextrin inhibited protein unfolding during dehydration due to steric hindrance of effective hydrogen bonding [36].
It was previously shown that the presence of a sugar lyoprotectant is necessary for stabilizing virus upon drying [26,35]. It is known that sugar hydroxyl groups may effectively mimic water–protein and water–polysaccharide interactions between viral proteins and water molecules in solution. Trehalose was of particular interest due to its superior properties of water immobilization during dehydration which retains protein structure during drying. This has been attributed to amorphous trehalose’s ability to capture moisture into trehalose dehydrate crystallite cages dispersed in its amorphous matrix, and the reversible transition between the anhydrous to trehalose crystalline forms [37]. Trehalose retains relatively low viscosity even at high concentrations thus allowing easy atomization during spray-coating. Both trehalose and sucrose have similar mechanisms of preventing protein destabilization, however, for simplicity, we chose to use a formulation with the least number of excipients and therefore concentrated on the use of trehalose for spray coating live viruses onto microneedle arrays. We determined that concentrations of trehalose lower than 15% resulted in significantly reduced virus viability. It is possible that there is a trehalose concentration between 15% and 50% that is optimum for coating characteristics and virus viability and further study will be required to identify this formulation. During the drying process the initial concentration of trehalose effects drying time, i.e., an initial low concentration of trehalose will take longer to dry. Increased drying time was noted for the 1% and 5% trehalose formulations; however we did not note a substantial difference in drying time between the 15% and 50% trehalose formulations. The increased drying time of the 1% and 5% trahalose formulations is likely responsible for the lower AdV viability observed when these low concentrations of trehalose were used.
Addition of surfactant is known to enhance dip-coating and it had little effect on trehalose-based AdV stabilization and was further used in AdV formulations, however it was incompatible with MVA. Tween 80 was used here above its critical micellar concentration (approximately 0.0013%) and it likely disrupted MVA's lipid membrane resulting in viral inactivation. In contrast, the other non-ionic surfactant tested, Lutrol F68, did not have the same detrimental effect on MVA viability, as it was used below its critical micellar concentration (2–3% w/v) at 0.5% w/v. From this study, we propose that using surfactants below their critical micellar concentration is critical for formulating lipid enveloped viruses but has little impact on viruses that have a protein capsid.
A second critical parameter that we identified that must be optimized for successful coating of viable virus is rate of spraying. Care must be taken when selecting or adjusting spraying parameters as these parameters adversely affected virus viability. We determined that it is stress on drying that is responsible for observed loss in AdV viral titer of dried formulations rather than the act of spraying itself. The rate of spraying impacted differently on the viability of coated AdV and MVA. This is likely due to different susceptibilities of AdV and MVA to the exposure of air–liquid surfaces [38–40] and the rate of solution dehydration. A slower spraying rate results in smaller droplets with a large surface-to-volume ratio, an increased time-exposure of virus to this air–liquid interface and faster dehydration. While rapid dehydration can be advantageous for virus viability, we propose that exposure to the air interface that has a detrimental effect on AdV survival. MVA however was not as susceptible to the air–water interface. The lipid envelope of MVA membrane may provide protection during time-exposure of virus to this air–liquid interface. Additionally, faster dehydration, due to a slower spraying rate, may also enhance MVA survival. Furthermore, the nature of the substrate surface does not seem to influence survival of sprayed AdV and MVA as these were comparable for silicon, stainless steel and polycarbonate surfaces. This highlights that it is the spraying process, not the substrate that is key to successful coating and secondly, it demonstrates the potential range and utility of this spraying method for coating microneedle devices.
While delivery efficiency is normally not an issue with conventional needle-based delivery routes, it is a limiting factor in the use of microneedle devices, partly due to the maximum amount of formulation that can be loaded onto the array and partly due to the delivery efficiency into skin [23]. It is generally accepted that not all of the vaccine or drug-coat is delivered to the skin [11,32]. The amount of formulation which can effectively be delivered using solid coated microneedle arrays is likely to be in the sub-milligram per cm2 range, which may be compensated for by enhanced pharmacokinetics [9]. Clearly, the array design will impact on the dose loading capacity, with arrays containing taller and more densely packed needles being able to carry more formulation. However, it should be noted that increased dose loading does not correlate with delivery efficiency [23]. Here we showed that the spray rate influenced this coating parameter of dose loading. A slow spraying rate allows formulation to dry immediately after getting onto the array surface and hence results in a “layering” of formulation. This slow spraying method resulted in larger amounts of formulation deposited to a maximum level which in turn does not result in microneedle tip occlusion. Therefore, in agreement with other coating methods, the dose of vaccine or drug to be loaded onto an array can be optimized by appropriate selection of coating parameters, in this case, spraying rate. The efficiency of delivery of formulation coated on the microneedle array upon application onto ears of anesthetized mice was 40.5 ± 2.5% after a 4 h exposure time, which is comparable to previously reported arrays made by dip-coating [10].
The optimal coating pattern for the formulation being coated onto solid microneedle devices is the one providing maximum skin-delivery efficiency and vaccine-induced immunity. Formulation being delivered on the microneedle tip might make needles blunt and decrease penetration efficiency [23,41]. Our simple spray-coating method coats microneedle arrays in such a way that most of delivered formulation is concentrated around the base of the needle shaft away from the ultrasharp tip. In this manner, once the tip is inserted into skin, formulation will be in the vicinity to opened pores thus allowing easy diffusion into skin. Successful skin delivery of coated MVA and AdV was verified in ex vivo porcine studies and CD8+ T cell and antibody responses were induced in murine models. It can be seen from the ex vivo pig skin studies that AdV infection was localized to penetration sites only while MVA infection was also dispersed around the pore. Poxviruses, such as MVA, are highly capable of successfully infecting the host through the skin, even superficially injured skin and MVA is known to have a wide cellular host range. These results confirmed the potential of coated microneedle array administration to be used for viral vector delivery and in the case of MVA to perhaps be further developed as an alternative delivery system to skin scarification [42]. Finally, these solid microneedle arrays have been designed to successfully penetrate the skin at low insertion forces and therefore do not require applicator devices devised to deliver a high force to drive the microneedles into the skin [4]. This may have implications on the cost and utility of the final device.
This study provides the first evidence of successful live recombinant viral-vector delivery into skin using a novel coating method of microneedle arrays. This widely used pharmaceutical technology can be adapted to coating microneedle arrays, eliminating the need to develop entirely new systems and processes in a GMP environment. Further studies are required to determine the long-term stability of viruses spray-coated into the microneedle array. Therefore, there is potential to transfer this technology to a scalable environment. In conclusion, this study demonstrates the potential of successfully adapting spraycoating technology for coating sensitive live vaccines onto solid microneedle arrays for transcutaneous immunization.
Acknowledgments
We thank Sarah Gilbert and Alison Turner at The Jenner Institute for providing AdV and MVA for research. The authors would like to thank Enterprise Ireland (Commercialisation Fund), Higher Education Authority (Programme for Research in Third-Level Institutions) and Science Foundation Ireland (National Access Programme) for funding this work. Scanning Electron Microscopy was conducted at the Electron Microscopy facility at the Biosciences Institute, UCC, Ireland.
Contributor Information
Anto Vrdoljak, Email: avrdoljak@gmail.com.
Marie G. McGrath, Email: marie_mc_grath@hotmail.com.
John B. Carey, Email: John.Carey@crl.com.
Simon J. Draper, Email: simon.draper@ndm.ox.ac.uk.
Adrian V.S. Hill, Email: adrian.hill@ndm.ox.ac.uk.
Conor O’Mahony, Email: conor.omahony@tyndall.ie.
Abina M. Crean, Email: a.crean@ucc.ie.
References
- [1].Draper SJ, Heeney JL. Viruses as vaccine vectors for infectious diseases and cancer. Nat Rev Microbiol. 2010;8:62–73. doi: 10.1038/nrmicro2240. [DOI] [PubMed] [Google Scholar]
- [2].Rexroad J, Evans RK, Middaugh CR. Effect of pH and ionic strength on the physical stability of adenovirus type 5. J Pharm Sci. 2006;95:237–247. doi: 10.1002/jps.20496. [DOI] [PubMed] [Google Scholar]
- [3].Moss B. Poxviridae: the viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. Lippincott Williams and Wilkins; Philadelphia: 2001. pp. 2849–2883. [Google Scholar]
- [4].Enfield J, O’Connell ML, Lawlor K, Jonathon E, O’Mahony C, Leahy M. In vivo dynamic characterization of microneedle skin penetration using optical coherence tomography (OCT) J Biomedical Optics. 2010;15:046001. doi: 10.1117/1.3463002. [DOI] [PubMed] [Google Scholar]
- [5].Haq MI, Smith E, John DN, Kalavala M, Edwards C, Anstey A, Morrissey A, Birchall JC. Clinical administration of microneedles: skin puncture, pain and sensation. Biomed Microdevices. 2008;11:35–47. doi: 10.1007/s10544-008-9208-1. [DOI] [PubMed] [Google Scholar]
- [6].Wilke N, Mulcahy A, Ye SR, Morrissey A. Process optimization and characterization of silicon microneedles fabricated by wet etch technology. Microelectron J. 2005;36:650–656. [Google Scholar]
- [7].Carey JB, Pearson FE, Vrdoljak A, McGrath MG, Crean A, Walsh PT, Doody T, O’Mahony C, Hill AV, Moore AC. Microneedle array design determines the induction of protective memory CD8+ T cell responses induced by a recombinant live malaria vaccine in mice. PLoS One. 2011;6:e22442. doi: 10.1371/journal.pone.0022442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ameri M, Daddona PE, Maa YF. Demonstrated solid-state stability of parathyroid hormone PTH(1–34) coated on a novel transdermal microprojection delivery system. Pharm Res. 2009;26:2454–2463. doi: 10.1007/s11095-009-9960-9. [DOI] [PubMed] [Google Scholar]
- [9].Daddona PE, Matriano JA, Mandema J, Maa YF. Parathyroid hormone (1–34)-coated microneedle patch system: clinical pharmacokinetics and pharmacodynamics for treatment of osteoporosis. Pharm Res. 2011;28:159–165. doi: 10.1007/s11095-010-0192-9. [DOI] [PubMed] [Google Scholar]
- [10].Widera G, Johnson J, Kim L, Libiran L, Nyam K, Daddona PE, Cormier M. Effect of delivery parameters on immunization to ovalbumin following intracutaneous administration by a coated microneedle array patch system. Vaccine. 2006;24:1653–1664. doi: 10.1016/j.vaccine.2005.09.049. [DOI] [PubMed] [Google Scholar]
- [11].Hiraishi Y, Nandakumar S, Choi SO, Lee JW, Kim YC, Posey JE, Sable SB, Prausnitz MR. Bacillus Calmette-Guerin vaccination using a microneedle patch. Vaccine. 2011;29:2626–2636. doi: 10.1016/j.vaccine.2011.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gill HS, Prausnitz MR. Coating formulations for microneedles. Pharm Res. 2007;24:1369–1380. doi: 10.1007/s11095-007-9286-4. [DOI] [PubMed] [Google Scholar]
- [13].Ameri M, Fan SC, Maa YF. Parathyroid hormone PTH(1–34) formulation that enables uniform coating on a novel transdermal microprojection delivery system. Pharm Res. 2010;27:303–313. doi: 10.1007/s11095-009-0019-8. [DOI] [PubMed] [Google Scholar]
- [14].Duan DC, Johnson PR. Microneedle Arrays and Methods of Preparing Same. WO2007/059289A1. 2007
- [15].McGrath MG, Vrdoljak A, O’Mahony C, Oliveira JC, Moore AC, Crean AM. Determination of parameters for successful spray coating of silicon microneedle arrays. Int J Pharm. 2011;415:140–149. doi: 10.1016/j.ijpharm.2011.05.064. [DOI] [PubMed] [Google Scholar]
- [16].Ye J, Zentel R, Arpiainen S, Ahopelto J, Jonsson F, Romanov SG, Sotomayor Torres CM. Integration of self-assembled three-dimensional photonic crystals onto structured silicon wafers. Langmuir. 2006;22:7378–7383. doi: 10.1021/la0607611. [DOI] [PubMed] [Google Scholar]
- [17].Draper SJ, Moore AC, Goodman AL, Long CA, Holder AA, Gilbert SC, Hill F, Hill AV. Effective induction of high-titer antibodies by viral vector vaccines. Nat Med. 2008;14:819–821. doi: 10.1038/nm.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Flynn KJ, Belz GT, Altman JD, Ahmed R, Woodland DL, Doherty PC. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity. 1998;8:683–691. doi: 10.1016/s1074-7613(00)80573-7. [DOI] [PubMed] [Google Scholar]
- [19].Peterka M, Strancar A, Kramberger P, Maurer E, Muster T. Method for Influenza Virus Purification. WO 2008/006780A1. 2008
- [20].Bewig B, Schmidt WE. Accelerated titering of adenoviruses. Biotechniques. 2000;28:870–873. doi: 10.2144/00285bm08. [DOI] [PubMed] [Google Scholar]
- [21].Donnelly RF, Morrow DI, Fay F, Scott CJ, Abdelghany S, Singh RR, Garland MJ, Woolfson AD. Microneedle-mediated intradermal nanoparticle delivery: potential for enhanced local administration of hydrophobic pre-formed photosensitisers. Photodiagnosis Photodyn Ther. 2010;7:222–231. doi: 10.1016/j.pdpdt.2010.09.001. [DOI] [PubMed] [Google Scholar]
- [22].Kumar A, Li X, Sandoval MA, Rodriguez BL, Sloat BR, Cui Z. Permeation of antigen protein-conjugated nanoparticles and live bacteria through microneedle-treated mouse skin. Int J Nanomedicine. 2011;6:1253–1264. doi: 10.2147/IJN.S20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cormier M, Johnson B, Ameri M, Nyam K, Libiran L, Zhang DD, Daddona P. Transdermal delivery of desmopressin using a coated microneedle array patch system. J Control Release. 2004;97:503–511. doi: 10.1016/j.jconrel.2004.04.003. [DOI] [PubMed] [Google Scholar]
- [24].Pearton M, Allender C, Brain K, Anstey A, Gateley C, Wilke N, Morrissey A, Birchall J. Gene delivery to the epidermal cells of human skin explants using microfabricated microneedles and hydrogel formulations. Pharm Res. 2008;25:407–416. doi: 10.1007/s11095-007-9360-y. [DOI] [PubMed] [Google Scholar]
- [25].Moore AC, Gallimore A, Draper SJ, Watkins KR, Gilbert SC, Hill AV. Anti-CD25 antibody enhancement of vaccine-induced immunogenicity: increased durable cellular immunity with reduced immunodominance. J Immunol. 2005;175:7264–7273. doi: 10.4049/jimmunol.175.11.7264. [DOI] [PubMed] [Google Scholar]
- [26].Alcock R, Cottingham MG, Rollier CS, Furze J, De Costa SD, Hanlon M, Spencer AJ, Honeycutt JD, Wyllie DH, Gilbert SC, Bregu M, et al. Long-term thermostabilization of live poxviral and adenoviral vaccine vectors at supraphysiological temperatures in carbohydrate glass. Sci Transl Med. 2010;2:19ra12. doi: 10.1126/scitranslmed.3000490. [DOI] [PubMed] [Google Scholar]
- [27].Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J Control Release. 2009 doi: 10.1016/j.jconrel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Stability kinetics of influenza vaccine coated onto microneedles during drying and storage. Pharm Res. 2010;28:135–144. doi: 10.1007/s11095-010-0134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kim YC, Quan FS, Yoo DG, Compans RW, Kang SM, Prausnitz MR. Enhanced memory responses to seasonal H1N1 influenza vaccination of the skin with the use of vaccine-coated microneedles. J Infect Dis. 2010;201:190–198. doi: 10.1086/649228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cilurzo F, Cupone IE, Minghetti P, Selmin F, Montanari L. Fast dissolving films made of maltodextrins. Eur J Pharm Biopharm. 2008;70:895–900. doi: 10.1016/j.ejpb.2008.06.032. [DOI] [PubMed] [Google Scholar]
- [31].Gill HS, Prausnitz MR. Coated microneedles for transdermal delivery. J Control Release. 2007;117:227–237. doi: 10.1016/j.jconrel.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Matriano JA, Cormier M, Johnson J, Young WA, Buttery M, Nyam K, Daddona PE. Macroflux microprojection array patch technology: a new and efficient approach for intracutaneous immunization. Pharm Res. 2002;19:63–70. doi: 10.1023/a:1013607400040. [DOI] [PubMed] [Google Scholar]
- [33].McAllister DV, Wang PM, Davis SP, Park JH, Canatella PJ, Allen MG, Prausnitz MR. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: fabrication methods and transport studies. Proc Natl Acad Sci USA. 2003;100:13755–13760. doi: 10.1073/pnas.2331316100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- [35].Bieganski RM, Fowler A, Morgan JR, Toner M. Stabilization of active recombinant retroviruses in an amorphous dry state with trehalose. Biotechnol Prog. 1998;14:615–620. doi: 10.1021/bp980057d. [DOI] [PubMed] [Google Scholar]
- [36].DePaz RA, Dale DA, Barnett CC, Carpenter JF, Gaertner AL, Randolph TW. Effects of drying methods and additives on the structure, function, and storage stability of subtilisin: role of protein conformation and molecular mobility. Enzyme Microb Tech. 2002;31:765–774. [Google Scholar]
- [37].Kilburn D, Townrow S, Meunier V, Richardson R, Alam A, Ubbink J. Organization and mobility of water in amorphous and crystalline trehalose. Nat Mater. 2006;5:632–635. doi: 10.1038/nmat1681. [DOI] [PubMed] [Google Scholar]
- [38].Brandau DT, Jones LS, Wiethoff CM, Rexroad J, Middaugh CR. Thermal stability of vaccines. J Pharm Sci. 2003;92:218–231. doi: 10.1002/jps.10296. [DOI] [PubMed] [Google Scholar]
- [39].Rexroad J, Wiethoff CM, Green AP, Kierstead TD, Scott MO, Middaugh CR. Structural stability of adenovirus type 5. J Pharm Sci. 2003;92:665–678. doi: 10.1002/jps.10340. [DOI] [PubMed] [Google Scholar]
- [40].Shoyele SA, Cawthorne S. Particle engineering techniques for inhaled biopharmaceuticals. Adv Drug Deliv Rev. 2006;58:1009–1029. doi: 10.1016/j.addr.2006.07.010. [DOI] [PubMed] [Google Scholar]
- [41].Johnson PR. Masking Method for Coating a Microneedle Array. WO 2006/055799 A1. 2006
- [42].Liu L, Zhong Q, Tian T, Dubin K, Athale SK, Kupper TS. Epidermal injury and infection during poxvirus immunization is crucial for the generation of highly protective T cell-mediated immunity. Nat Med. 2009;16:224–227. doi: 10.1038/nm.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]