Abstract
Background
Early detection of fetal alcohol spectrum disorders (FASDs) is desirable to allow earlier and more comprehensive interventions to be initiated for the mother and infant. We examined prenatal ultrasound as an early method of detecting markers of the physical features and neurobehavioral deficits characteristic of FASD.
Methods
A longitudinal cohort of pregnant women in Ukraine was recruited as part of the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD). Women were enrolled into a moderately to heavy-alcohol-exposed group or a low- or no-alcohol exposure group and were followed to pregnancy outcome. In the second trimester, a fetal ultrasound was performed to measure Trans Cerebellar Diameter (TCD), Occipital Frontal Diameter (OFD), Caval-Calvarial Distance (CCD), Frontothalamic Distance (FTD), Inter-orbital Distance (IOD), Outer-orbital Distance (OOD), and Orbital Diameter (OD). Live born infants received a dysmorphological exam and a neurobehavioral evaluation using the Bayley Scales of Infant Development. These data were used to classify infants with respect to FASD. Comparisons were made on the ultrasound measures between those with and without features of FASD, adjusting for gestational age at ultrasound and maternal smoking.
Results
233 mother/child dyads were included. Children classified as FASD had significantly longer IOD, and lower FTD/IOD, OFD/IOD and FTD/OD ratios (p <0.05). Children with a Bayley score <85 had significantly shorter FTD, longer IOD, lower OFD/IOD and FTD/IOD ratios (p <0.05). In general mean differences were small. Ultrasound variables alone predicted <10% of the variance in the FASD outcome.
Conclusions
Some ultrasound measurements were associated with FASD, selected facial features of the disorder, and lower neurobehavioral scores. However, mean differences were relatively small, making it difficult to predict affected children based solely on these measures. It may be advantageous to combine these easily obtained ultrasound measures with other data to aid in identifying high risk for an FASD outcome.
Keywords: fetal alcohol spectrum disorders, alcohol, ultrasound, brain measurement, prenatal
Introduction
Fetal Alcohol Spectrum Disorders (FASDs) are estimated to affect approximately 1-5% of the population in the United States and fetal alcohol syndrome (FAS), the most severe form of FASD, affects at least 0.1% (May et al., 2009a, Sampson et al., 1997). Despite ample evidence of the association between prenatal alcohol consumption and FASDs, pregnant women still consume alcohol. A national study based on self-reported data collected between 2011 and 2013 found that 10.2% of pregnant women in the United States consume alcohol and 3.1% have consumed four or more drinks on at least one occasion within the last month (Tan et al., 2015). In addition, 53.6% of non-pregnant women of childbearing age drink alcohol, 18.2% drink in a heavy episodic fashion, and half of all pregnancies are unplanned indicating there is a high likelihood that a woman may continue to consume alcohol before she is aware that she is pregnant (Finer and Zolna, 2011, Goldsmith et al., 2008, May et al., 2009b).
FAS can be diagnosed at birth but often remains undiagnosed until learning or behavioral problems become evident (Chasnoff et al., 2015, May et al., 2009b). Early diagnosis is crucial if the child and the child's family are to receive the full benefit of treatments available and if interventions are to be tailored to specific needs to optimize management strategies. Data have shown that the later the diagnosis, the greater the chances for more adverse life outcomes (Streissguth et al., 2004).
There are a number of ways to detect “at risk” pregnancies based on a measure of quantity and frequency of maternal alcohol consumption. Prior to birth, maternal self-report is the primary source of information, with varying reliability and validity (Lange et al., 2014, Chang, 2001). Appropriately sensitive and specific maternal biomarkers for recent alcohol consumption are being developed. At birth, it is possible to detect some patterns of prenatal alcohol exposure by infant biomarkers (Bakhireva et al., 2014, Kwak et al., 2014). It is also possible to identify children with physical features of FASD in the newborn period through a targeted dysmorphology examination (Jones and Smith, 1973).
Prenatal ultrasound, currently used routinely to monitor fetal growth and to detect structural anomalies, has previously been explored as a method for early detection of abnormal fetal brain development in pregnancies exposed to alcohol. Previous studies using prenatal ultrasound have demonstrated associations of alcohol exposure with reduced frontal cortex size (Wass et al., 2001), reduced ratio of head circumference to abdominal circumference, and reduced cerebellar growth (Handmaker et al., 2006).
A pilot study performed by our group involving 66 alcohol-exposed and 64 comparison pregnancies with second trimester ultrasound measures found associations between prenatal alcohol exposure and shorter femur length as well as selected brain measurements including shorter caval-calvarial and frontothalamic distances, after adjusting for gestational age at ultrasound and controlling for maternal smoking (ps<0.05)(Kfir et al., 2009). In this same sample, 47 alcohol-exposed and 31 unexposed also had a third trimester ultrasound. The finding of significantly shorter frontothalamic distance in the alcohol exposed group persisted. In addition, orbital diameter was significantly shorter in those who were alcohol exposed, a finding which was not evident in the second trimester scans.
However, to our knowledge, no previously published study has examined associations between prenatal ultrasound brain measures and characteristic physical features of FASD or neurobehavioral deficits in those infants after birth. The purpose of this study was two-fold. First, we wished to expand on our previous investigation with a larger sample size focused on second trimester ultrasound to examine fetal brain measures in association with maternal alcohol exposure. Second, we sought to examine these same second-trimester measures as predictors of infant outcome, including the physical features of FASD, and neurobehavioral deficits detectable in infancy. We approached the second objective to address two clinically relevant scenarios in which the clinician may or may not have access to information about maternal drinking at the time of the ultrasound: a) in an overall sample of women for whom we assume no prior knowledge regarding prenatal exposure to alcohol, and b) in a selected sample of women who specifically reported moderate to heavy alcohol exposure in pregnancy.
Methods
The study sample was drawn from a longitudinal prospective cohort study conducted among pregnant women in Ukraine between 2008 and 2012. The study was part of the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), an international research consortium funded by NIH-NIAAA (www.cifasd.org). Pregnant women were enrolled at two different sites in Western Ukraine: the Rivne Provincial Medical Diagnostic Center in the Rivne oblast and the Khmelnytsky City Perinatal Center in the Khmelnytsky oblast. This study was approved by the institutional review board at the University of California, San Diego and by the institutional review board of Lviv Medical University in Ukraine.
Recruitment and Maternal Interviews
Recruitment and study measures have been previously described (Kfir et al., 2009, Chambers et al., 2014). In brief, trained study nurses screened pregnant women for alcohol consumption at a routine clinic visit. Women reporting that they had ever consumed alcohol were asked questions regarding the quantity and frequency of alcohol use in the month around conception and in the most recent month of pregnancy. In addition, women completed standard screening questions to identify risky drinking: the TWEAK (tolerance, worried, eye-opener, amnesia, cut-down) and the Alcohol Use Disorders Identification Test (AUDIT) (Russell et al., 1994, Bush et al., 1998). Women met eligibility for enrollment in the alcohol-exposed group if they reported ≥4 episodes of ≥5 standard drinks or ≥5 episodes of 3-4 standard drinks or ≥10 episodes of 1-2 standard drinks in either the month around conception or the most recent month in pregnancy or both. Eligibility for enrollment into the comparison group defined as those with no or low-alcohol exposure required that women report no more than 2 drinks in any week, fewer than 2 drinks in any single day in the month around conception, and no drinking in the most recent month of pregnancy. For each woman who qualified and consented in the alcohol-exposed group, the next pregnant woman reporting no or low exposure was invited to enroll for a 1:1 recruitment ratio.
All participants who consented completed a subsequent detailed study interview which included day-by-day questions using the timeline follow-back procedure (Sobell and Sobell, 1992, Sobell et al., 2001) about the amount and type of alcohol consumed in a week around conception and in the most recent two weeks before the interview. Interview data was also collected on demographic, lifestyle, and reproductive health characteristics. Socioeconomic status based on maternal and paternal occupation and education was calculated on a 1-5 point Hollingshead scale (Hollingshead, 1975) where lower scores indicated higher socioeconomic strata.
Ultrasound Examinations
Ultrasonographers at each location trained by one of us (ADH) performed routine prenatal ultrasounds, blinded to the participant's prenatal alcohol exposure status, using Aloka SSD-650CL (Tokyo, Japan) machines with convex array transabdominal transducers 3.5/5.0 MHz. Each scan included specific study-related measures related to brain and facial development. These measurements included the following (see Figure 1):
Transverse Cerebellar Diameter (TCD) measured as the maximum diameter of the cerebellum in the standard posterior fossa view
Occipital Frontal Diameter (OFD)
Caval-Calvarial Distance (CCD) measured as the distance between the inner surface of the frontal calvarium and the posterior margin of the cavum pellucidum
Frontothalamic Distance (FTD) measured as the distance between the inner surface of the frontal calvarium and the posterior margin of the thalami
Interorbital Distance (IOD)
Orbital Diameter (OD)
Outer Orbital Diameter (OOD)
Figure 1.
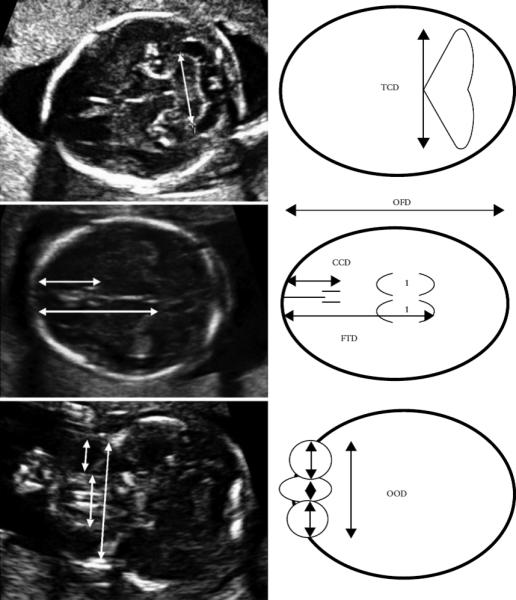
Axial ultrasound images with accompanying schematic diagrams illustrating the measured fetal brain parameters. CCD, caval–calvarial distance; FTD, frontothalamic distance; OFD, occipitofrontal diameter; OOD, outer orbital diameter; TCD, transverse cerebellar diameter (Kfir et al., 2009).
Some measures (TCD, OFD, CCD, FTD) were selected as markers of brain development that were consistent with known effects of prenatal alcohol and as previously reported in the literature as possibly associated with prenatal exposure (Wass et al., 2001, Handmaker et al., 2006, Kfir et al., 2009). Additional measures were selected for their potential correlation with known facial features of FASD, (e.g., orbital diameter and short palpebral fissure length). In addition to single measures, three ratio measures were created (FTD/OD, OFD/IOD, FTD/IOD). Ultrasound examinations were obtained from gestational week 14 through 24.
Infant Dysmorphological and Neurobehavioral Examinations
Infants were examined by Ukrainian geneticists trained by one of us (KLJ) using a standard checklist for physical features of FASD including key facial features and additional features seen more frequently with FASD, e.g, hockey stick palmar crease. Infants were also evaluated at approximately 6 and/or 12 months of age by trained study psychologists who were blinded to the child's prenatal alcohol exposure status using the Bayley Scales of Infant Development, 2nd Ed. (BSID-II) (Bayley, 1993, Aylward and Verhulst, 2000). The BSID-II yielded two standardized scores: the Mental Development Index (MDI) which measured problem solving and prelinguistic development, and the Psychomotor Development Index (PDI) which measured fine and gross motor skills. Both the dysmorphologists/geneticists and the neurobehavioral examiners were blinded to ultrasound examination results.
Using data from the standard study physical examination, cardinal facial features of FASD were classified as follows: short palpebral fissure length was defined as ≤10th centile using the unpublished Thomas chart (Thomas et al., 1987); smooth philtrum was defined as lipometer code of 4 or 5 using the Astley scale (Astley and Clarren, 2000); thin vermilion border was defined as lipometer code of 4 or 5 using the Astley scale (Astley and Clarren, 1996). Microcephaly was defined as ≤10th centile for head circumference; growth deficiency was defined as ≤10th centile for height or weight using U.S. National Center for Health Statistics curves (Kuczmarski et al., 2000) specific to sex and age of the child.
Developmental scores were classified as delayed if the MDI or PDI was <85 (1 standard deviation below the standardized mean of 100). Classification as FASD was made using the following criteria: Children were classified as FAS if they had prenatal alcohol exposure and at least two key facial features plus growth deficiency or microcephaly or both. Additional infants were classified as FASD if they had prenatal alcohol exposure and only 1 key facial feature plus growth deficiency and microcephaly; or 1 key facial feature, 1 growth abnormality plus at least 1 additional alcohol-related feature if they also had a BSID-II score <85.
If more than one physical or neurobehavioral assessment was conducted, infants were classified as exhibiting that feature or having delayed developmental scores if the feature or score met criteria on at least one of the examinations.
Statistical Analysis
Comparisons of continuous, dichotomous, or categorical maternal characteristic variables were conducted using t-tests or ANOVA (continuous), χ2 (dichotomous), and Fisher's exact test (dichotomous with small cell sizes). All comparisons by exposure or outcome were adjusted for gestational age at the time of the scan. Additional covariates were considered including vitamin use, maternal age, marital status, SES, child sex, whether the pregnancy was planned, and maternal smoking (never, ever but quit before pregnancy, ever but quit in pregnancy, continued in pregnancy). Maternal smoking modified the relationship between measured variables and outcome, and was therefore selected as a confounder. Adjusted comparisons for gestational age at ultrasound and maternal smoking were made using ANCOVA. Ultrasound measures were entered into a logistic regression model with FASD as the outcome to determine the proportion of variance in FASD accounted for by combined and individual measures. Maternal alcohol variables (pattern of consumption and score on the AUDIT) were entered into a second logistic regression model with FASD as the outcome. Missing values were excluded list-wise, analysis by analysis. Statistical significance was defined as 2-sided p-value of <0.05. Statistical analyses were carried out using SPSS (PASW 18, SPSS Inc., Chicago, IL).
The sample selected for the present analysis consisted of mothers who completed the maternal enrollment interview, had completed a second trimester ultrasound examination, had given birth to a live born infant, and that infant had at least one study dysmorphology exam and the BSID-II neurobehavioral evaluation.
Results
Data were available for 233 mother-child pairs who enrolled in the study between 2008 and 2012. There were 15 children in the sample classified as having an FASD: 4 who met criteria for full FAS and 11 who met criteria for the broader spectrum of FASD.
Maternal characteristics are shown in Table 1. Participants whose pregnancies were exposed to higher levels of alcohol were less likely to have planned the pregnancy or to be married. They were more likely to be current smokers and to be from a lower socioeconomic group. Characteristics of maternal alcohol consumption, including the amount of alcohol consumed per day at the time of conception and in the most recent two weeks of pregnancy prior to enrollment, are also presented in Table 1.
Table 1.
Maternal demographic and alcohol use characteristics by pregnancy alcohol exposure status, N= 233, Ukraine, 2008-2012
| Parameter | Alcohol Exposed Mean ± SD or n (%) (n=98) |
Low or No Alcohol Mean ± SD or n (%) (n=135) |
P-value |
|---|---|---|---|
| Maternal age (years) | 26.0 ± 5.7 | 26.3 ± 4.9 | 0.677 |
| Paternal age (years) | 29.4 ± 6.7 | 28.2 ± 5.3 | 0.145 |
| Marital status (%) | 0.001 | ||
| Single, never married | 12 (12.2) | 4 (3.0) | |
| Married, living with husband | 72 (73.5) | 122 (91.0) | |
| Not married, living w partner | 14 (14.3) | 8 (6.0) | |
| Employed (% yes) | 71 (72.4) | 86 (64.2) | 0.183 |
| SES (Hollingshead range 1-5) | 3.05 ± 1.03 | 2.70 ± 0.87 | 0.006 |
| Maternal vitamin use (% yes) | 55 (56.1) | 75 (56.0) | 0.982 |
| Planned pregnancy (% yes) | 41 (41.8) | 91 (67.9) | <0.001 |
| Smoking status (%) | <0.001 | ||
| Never | 37 (37.8) | 120 (89.6) | |
| Past smoker, quit before pregnancy | 8 (8.2) | 10 (7.5) | |
| Past smoker, quit after realized pregnant | 29 (29.6) | 3 (2.2) | |
| Current smoker | 24 (24.5) | 1 (0.7) | |
| Scores on Symptoms of Alcohol Abusea | |||
| Tolerance | 14.8 ± 7.8 | 8.9 ± 5.9 | <0.001 |
| AUDIT score | 6.36 ± 4.15 | 1.11 ± 1.20 | <0.001 |
| TWEAK score | 2.03 ± 1.60 | 0.05 ± 0.28 | <0.001 |
| Quantity/Frequency Drinkingb | |||
| Periconceptional period | |||
| AA per day | 0.58 ± 0.52 | 0.00 ± 0.02 | <0.001 |
| AA per drinking day | 1.70 ± 1.12 | 0.02 ± 0.14 | <0.001 |
| Pregnancy – last 2 weeks at enrollment | |||
| AA per day | 0.07 ± 0.20 | 0.00 ± 0.00 | <0.001 |
| AA per drinking day | 0.36 ± 0.60 | 0.01 ± 0.05 | <0.001 |
Tolerance>=6, self-reported ability to consume six or more standard alcoholic drinks on a single occasion without passing out or feeling too ill to continue; AUDIT, Alcohol Use Disorders Identification Test; TWEAK, Tolerance, Worried, Eye opener, Amnesia, Cut-down; missing values for Tolerance 8 exposed, 20 unexposed; AUDIT 2 exposed, 5 unexposed; TWEAK 2 exposed, 6 unexposed
AA, absolute ounces of alcohol
As shown in Table 2, FTD adjusted for gestational age differed by alcohol group. Once adjusted for maternal smoking, the difference was no longer statistically significant.
Table 2.
Selected ultrasound measures by exposure, N=233, Ukraine, 2008-2012a
| Parameter | Exposed Mean ± SE (n=98) |
Comparison Mean ± SE (n=135) |
p (adjusted for gestational age only) | P (+ smoking) |
|---|---|---|---|---|
| Transverse Cerebellar Diameter (mm) (TCD) | 20.66 ± 0.15 | 20.53 ± 0.12 | 0.959 | 0.530 |
| Occipital Frontal Diameter (mm) (OFD) | 63.15 ± 0.62 | 61.61 ± 0.52 | 0.494 | 0.086 |
| Caval-Calvarial Distance (mm) (CCD) | 23.82 ± 0.30 | 23.97 ± 0.24 | 0.693 | 0.726 |
| Frontothalamic Distance (mm) (FTD) | 38.50 ± 0.46 | 39.18 ± 0.38 | 0.018 | 0.297 |
| Outer Orbital Diameter (mm) (OOD) | 32.68 ± 0.27 | 32.89 ± 0.22 | 0.252 | 0.590 |
| Interorbital Distance (mm) (IOD) | 9.41 ± 0.24 | 9.50 ± 0.20 | 0.383 | 0.791 |
| Orbital Diameter (mm) (OD) | 9.81 ± 0.15 | 9.56 ± 0.13 | 0.285 | 0.241 |
| FTD/OD | 4.02 ± 0.09 | 4.16 ± 0.07 | 0.057 | 0.258 |
| OFD/IOD | 6.98 ± 0.20 | 6.86 ± 0.17 | 0.603 | 0.655 |
| FTD/IOD | 4.29 ± 0.14 | 4.39 ± 0.12 | 0.136 | 0.608 |
Estimated marginal means +/−SE; adjusted for gestational age at ultrasound and maternal smoking
As shown in Table 3, in the total sample, OD and OFD were significantly associated with selected cardinal facial features of FASD. After adjustment for gestational age at scan and maternal smoking, mean OD was longer in those infants with thin vermillion border (p=0.010) or short palpebral fissures (p=0.041). Mean OFD was longer in those with smooth philtrum (p=0.009) or thin vermillion border (p=0.040). Similarly, mean IOD length was longer among infants exhibiting characteristics of FASD (p=0.010). However, all of the ratio measures were significantly lower in infants with FASD compared to those without: FTD/IOD, p=0.018; FTD/OD, p=0.026; OFD/IOD, p=0.016).
Table 3.
Selected ultrasound measures by three cardinal features of FASD, FASD, and delayed neurobehavioral development, N=233, Ukraine, 2008-2012a
| Ultrasound Measurement |
Short Palpebral Fissure Lengthb |
Smooth Philtrumc | Thin Vermiliond | FASDe | Delayed Neurobehavioral Development |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Present N=23 |
Absent N=204 |
p | Present N=28 |
Absent N=202 |
p | Present N=52 |
Absent N=178 |
p | Present N=15 |
Absent N=215 |
p | Present N=90 |
Absent N=129 |
p | |
| TCD | 20.5±0.3 | 20.6±0.1 | 0.744 | 21.0±0.2 | 20.5±0.1 | 0.099 | 20.7±0.2 | 20.5±0.1 | 0.314 | 20.1±0.3 | 20.6±0.1 | 0.160 | 20.5±0.1 | 20.7±0.1 | 0.176 |
| OFD | 61.6±1.1 | 62.3±0.4 | 0.572 | 64.8±1.0 | 61.9±0.4 | 0.009 | 63.6±0.8 | 61.9±0.4 | 0.040 | 61.2±1.5 | 62.3±0.4 | 0.471 | 61.9±0.6 | 62.6±0.5 | 0.346 |
| CCD | 24.8±0.5 | 23.8±0.2 | 0.080 | 23.6±0.5 | 23.9±0.2 | 0.507 | 23.7±0.4 | 24.0±0.2 | 0.594 | 24.8±0.7 | 23.8±0.2 | 0.168 | 23.7±0.3 | 24.1±0.2 | 0.335 |
| FTD | 38.5±0.8 | 38.9±0.3 | 0.608 | 39.0±0.7 | 38.9±0.3 | 0.899 | 38.7±0.5 | 38.9±0.3 | 0.723 | 37.2±1.0 | 39.0±0.3 | 0.089 | 38.1±0.4 | 39.6±0.3 | 0.006 |
| OOD | 32.9±0.5 | 32.8±0.2 | 0.849 | 32.5±0.4 | 32.8±0.2 | 0.506 | 33.0±0.3 | 32.7±0.2 | 0.452 | 32.9±0.6 | 32.8±0.2 | 0.815 | 32.8±0.2 | 32.9±0.2 | 0.817 |
| IOD | 9.84±0.43 | 9.45±0.14 | 0.381 | 9.41±0.39 | 9.47±0.15 | 0.900 | 9.71±0.28 | 9.38±0.15 | 0.321 | 10.82±0.54 | 9.36±0.14 | 0.010 | 10.12±0.21 | 9.05±0.18 | <0.001 |
| OD | 10.20±0.27 | 9.62±0.09 | 0.041 | 9.97±0.25 | 9.62±0.09 | 0.186 | 10.06±0.18 | 9.54±0.10 | 0.011 | 10.28±0.35 | 9.62±0.09 | 0.067 | 9.67±0.14 | 9.72±0.12 | 0.796 |
| FTD/OD | 3.84±0.15 | 4.11±0.05 | 0.085 | 3.98±0.14 | 4.12±0.05 | 0.376 | 3.93±0.11 | 4.15±0.06 | 0.060 | 3.67±0.20 | 4.13±0.05 | 0.026 | 4.00±0.08 | 4.16±0.07 | 0.144 |
| OFD/IOD | 6.51±0.33 | 6.90±0.11 | 0.256 | 7.15±0.33 | 6.88±0.12 | 0.436 | 6.80±0.24 | 6.94±0.13 | 0.590 | 5.85±0.45 | 6.99±0.12 | 0.016 | 6.40±0.18 | 7.26±0.15 | <0.001 |
| FTD/IOD | 4.12±0.25 | 4.35±0.08 | 0.390 | 4.36±0.23 | 4.35±0.09 | 0.968 | 4.18±0.17 | 4.40±0.09 | 0.250 | 3.60±0.33 | 4.40±0.08 | 0.018 | 3.98±0.13 | 4.61±0.11 | <0.001 |
Estimated marginal means +/−SE; adjusted for gestational age at ultrasound and maternal smoking
≤10th percentile
4 or 5 on Lipometer scale
4 or 5 on Vermilion Border Lipometer scale
Fetal Alcohol Spectrum Disorder
Infants displaying neurodevelopmental delay on the BSID-II had significantly longer mean IOD (p=0.001) and two of the ratio measures were significantly lower (OFD/IOD, p=0.001; FTD/IOD, p=0.001). Shorter FTD was associated with alcohol exposure (Table 2) prior to adjustment for smoking, and on average non-significantly shorter in infants with any of the cardinal features or FASD (p=0.089). Neither of these relationships are statistically significant after controlling for maternal smoking. However, FTD was significantly shorter in those with neurodevelopmental delay on the BSID-II (p=0.006).
When the sample was restricted to the 98 pregnancies in the alcohol-exposed group, findings were similar despite the smaller sample size with reduced statistical power. As shown in Table 4, after adjustment for gestational age at scan and maternal smoking, longer OD was associated with thin vermillion (p=0.039) and longer OFD was associated with smooth philtrum (p=0.002). In relation to FASD as the outcome, ratio measures for FTD/IOD were lower (p=0.020) and OFD/IOD (p=0.006). With respect to the outcome of neurodevelopmental delay, IOD was longer on average (p=0.006), FTD was shorter (p=0.023), and the ratio measures OFD/IOD and FTD/IOD were lower (p-0.005, 0.013 respectively). Consistency in the specific significant associations in the restricted sample compared to the overall sample was due in general to larger adjusted mean differences within the alcohol-exposed only group.
Table 4.
Selected ultrasound measures by three cardinal features of FASD, FASD, and delayed neurobehavioral development in Alcohol Exposed Group, N=98, Ukraine, 2008-2012a
| Ultrasound Measurement |
Short Palpebral Fissure Lengthb |
p | Smooth Philtrumc | p | Thin Vermiliond | p | FASDe | p | Delayed Neurobehavioral Development |
p | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Present N=12 |
Absent N=84 |
Present N=14 |
Absent N=84 |
Present N=27 |
Absent N=71 |
Present N=15 |
Absent N=83 |
Present N=48 |
Absent N=42 |
||||||
| TCD | 20.2±0.4 | 20.6±0.1 | 0.230 | 20.6±0.3 | 20.6±0.1 | 0.871 | 20.5±0.2 | 20.6±0.1 | 0.735 | 20.1±0.3 | 20.7±0.1 | 0.089 | 20.5±0.2 | 20.8±0.2 | 0.337 |
| OFD | 61.6±2.3 | 62.5±0.9 | 0.724 | 68.7±2.0 | 61.5±0.8 | 0.002 | 64.8±1.4 | 61.7±0.9 | 0.079 | 61.3±2.0 | 62.7±0.8 | 0.504 | 61.5±1.2 | 64.1±1.2 | 0.131 |
| CCD | 25.0±0.8 | 23.9±0.3 | 0.168 | 23.9±0.8 | 24.0±0.3 | 0.859 | 23.9±0.6 | 24.0±0.4 | 0.825 | 24.9±0.7 | 23.8±0.3 | 0.208 | 24.2±0.4 | 23.9±0.4 | 0.564 |
| FTD | 37.0±1.2 | 38.3±0.4 | 0.294 | 38.5±1.2 | 38.1±0.5 | 0.769 | 38.1±0.8 | 38.2±0.5 | 0.898 | 36.8±1.1 | 38.5±0.5 | 0.176 | 37.5±0.6 | 39.5±0.6 | 0.023 |
| OOD | 32.8±0.5 | 32.5±0.2 | 0.626 | 33.1±0.5 | 32.5±0.2 | 0.204 | 33.0±0.3 | 32.4±0.2 | 0.144 | 32.8±0.5 | 32.5±0.2 | 0.606 | 32.6±0.3 | 32.6±0.3 | 0.989 |
| IOD | 10.48±0.62 | 9.47±0.24 | 0.136 | 9.76±0.59 | 9.56±0.24 | 0.754 | 9.91±0.41 | 9.46±0.26 | 0.362 | 10.88±0.54 | 9.35±0.23 | 0.010 | 10.24±0.30 | 8.98±0.33 | 0.006 |
| OD | 10.46±0.43 | 9.66±0.16 | 0.085 | 9.82±0.41 | 9.75±0.17 | 0.878 | 10.25±0.28 | 9.56±0.18 | 0.039 | 10.24±0.38 | 9.67±0.17 | 0.171 | 9.57±0.22 | 10.11±0.24 | 0.095 |
| FTD/OD | 3.59±0.22 | 4.04±0.09 | 0.060 | 3.96±0.22 | 4.00±0.09 | 0.881 | 3.79±0.15 | 4.07±0.10 | 0.122 | 3.64±0.21 | 4.06±0.09 | 0.070 | 3.99±0.11 | 3.96±0.13 | 0.841 |
| OFD/IOD | 6.12±0.50 | 6.93±0.19 | 0.136 | 7.39±0.46 | 6.74±0.19 | 0.202 | 6.87±0.33 | 6.83±0.21 | 0.916 | 5.85±0.43 | 7.03±0.18 | 0.013 | 6.34±0.24 | 7.37±0.26 | 0.005 |
| FTD/IOD | 3.71±0.34 | 4.27±0.13 | 0.131 | 4.16±0.33 | 4.21±0.13 | 0.871 | 4.07±0.23 | 4.26±0.15 | 0.484 | 3.55±0.30 | 4.33±0.13 | 0.020 | 3.91±0.17 | 4.54±0.19 | 0.013 |
Estimated marginal means +/− SE; adjusted for gestational age at ultrasound and maternal smoking
≤10th percentile
4 or 5 on Lipometer scale
4 or 5 on Vermilion Border Lipometer scale
Fetal Alcohol Spectrum Disorder
In regression analysis, the proportion of the variance in FASD accounted for by the ultrasound variables was a maximum of 5.8% among the total sample and 9.6% for the sample restricted to the alcohol exposed group only. When alcohol consumption and AUDIT data were added to the ultrasound variables in the overall sample, 35.6% of the variation was explained.
Discussion
An early indication of developmental difficulties caused by prenatal alcohol exposure obtained using routinely employed technology such as ultrasound would be helpful in reducing harm and allocating resources. In this study of alcohol-exposed and no or low-alcohol exposed comparison pregnancies incorporating second trimester ultrasound measurements and data regarding known confounders, we show that, while some measurements are associated with FASD outcome and specific dysmorphology measures, it is difficult to predict which children will be classified with an FASD. As in other applications of fetal biometry such as the use of transverse cerebellar diameter/abdominal circumference ratio to detect fetal growth restriction, calculated ratio measures may potentially be more indicative of developmental abnormalities than single measures.
The main contribution of the current study is to assess the predictive value of prenatal ultrasound measures of brain and face to FASD-related outcome data. We were able to explore the relationships among ultrasound brain measures and prenatal alcohol-specific dysmorphology as well as the classification of FASD itself. The similarity of study-specific brain ultrasound measures’ associations with FASD and neurodevelopmental delay is of interest as this is an area where interventions may prove particularly beneficial regardless of dysmorphology. The incidence of neurodevelopmental delay following prenatal alcohol exposure is thought to be much higher than the incidence of alcohol-related facial dysmorphology. (Jones, 2011, Russell et al., 1994, Roozen et al., 2016, Astley and Clarren, 2000, Sampson et al., 1997)
Our finding of longer OFD in children later exhibiting specific cardinal features is consistent with the suggestion that a longer, thinner face is characteristic of FASD(Naidoo et al., 2006). A wider IOD associated with FASD is consistent with hypertelorism which has been noted as a characteristic feature of alcohol-related dysmorphology (Hoyme et al., 2005). However, wider OD in the second trimester in infants subsequently identified as having short palpebral fissure lengths is somewhat counterintuitive. One explanation for this finding is that longer orbital size at this time in mid-gestation may actually reflect delayed eye development. Eye growth is not linear across gestation, with the relative size of the eye becoming smaller as the fetus develops. Thus, a second trimester fetus that will go on to exhibit short palpebral fissures after birth may lag in their position on the eye growth curve earlier in gestation (Fledelius and Christensen, 1996, Denis et al., 1998).
Despite the associations noted between our ultrasound variables and FASD classification, these variables were not strong predictors of outcome by themselves. They explained less than 10% of the variability in the data whether looking at the entire sample or only those in the alcohol exposed group. When ultrasound measures were combined with AUDIT screen and alcohol consumption data, the ability to identify risk of FASD classification was enhanced with more than a third of the variation in FASD explained.
Even if the predictive value of our study ultrasound measures is not 100%, there are noteworthy advantages to this approach. Second trimester ultrasound measures are standard procedures and easily accessible for nearly all pregnancies. Prenatal ultrasound is considered generally safe and acceptable to pregnant women. The specific ultrasound measures in this study were obtained using existing standard ultrasound equipment and standard views. Collection of these measures required minimal additional time or effort. Detection of at-risk pregnancies, such as alcohol-exposed pregnancies, during the second trimester would allow time to implement interventions. As data regarding prenatal alcohol exposure is not always available, the ability to identify infants at risk of neurodevelopmental delay without such data might be advantageous. There also may be an advantage to using multiple screening tools, including ultrasound measures, to provide information to help target interventions.
This study had limitations including the small sample size in some groups, the reliance upon self-reported exposure data which could have led to misclassification of exposure group, the variability of outcome due to the specific dose and timing of alcohol exposure, and the somewhat subjective nature of some of the outcome measurements. Strengths include that the study was a prospective cohort study, as well as the standardized methods used to obtain exposure information, ultrasound data and to measure the outcomes.
This area may be explored further by incorporating fetal behavioral data, biomarkers of alcohol exposure and fetal and placental health, and by increasing the precision of ultrasound measures with 3D ultrasound.
Acknowledgements
This research was supported by NIH Research Grant #U01AA014835 funded by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the NIH Office of Dietary Supplements (ODS)-Christina Chambers, PI. All or part of this work was done in conjunction with the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), which is funded by grants from the National Institute on Alcohol Abuse and Alcoholism (NIAAA). Additional information about CIFASD can be found at www.cifasd.org. The authors would like to thank the Ukrainian families who participated in this research and the wonderful staff of the Rivne Provincial Medical Diagnostic Center, the Khmelnytsky City Perinatal Center and OMNI-Net Ukraine.
Footnotes
Conflict of interest statement: The authors declare that there are no conflicts of interest.
References
- Astley SJ, Clarren SK. A case definition and photographic screening tool for the facial phenotype of fetal alcohol syndrome. The Journal of pediatrics. 1996;129:33–41. doi: 10.1016/s0022-3476(96)70187-7. [DOI] [PubMed] [Google Scholar]
- Astley SJ, Clarren SK. Diagnosing the full spectrum of fetal alcohol-exposed individuals: introducing the 4-digit diagnostic code. Alcohol and alcoholism (Oxford, Oxfordshire) 2000;35:400–410. doi: 10.1093/alcalc/35.4.400. [DOI] [PubMed] [Google Scholar]
- Aylward GP, Verhulst SJ. Predictive utility of the Bayley Infant Neurodevelopmental Screener (BINS) risk status classifications: clinical interpretation and application. Developmental Medicine & Child Neurology. 2000;42:25–31. doi: 10.1017/s0012162200000062. [DOI] [PubMed] [Google Scholar]
- Bakhireva LN, Leeman L, Savich RD, Cano S, Gutierrez H, Savage DD, Rayburn WF. The validity of phosphatidylethanol in dried blood spots of newborns for the identification of prenatal alcohol exposure. Alcoholism: Clinical and Experimental Research. 2014;38:1078–1085. doi: 10.1111/acer.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley N. Bayley scales of infant development: manual, Psychological Corporation. 1993.
- Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Archives of Internal Medicine. 1998;158:1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Yevtushok L, Zymak-Zakutnya N, Korzhynskyy Y, Ostapchuk L, Akhmedzhanova D, Chan PH, Xu R, Wertelecki W. Prevalence and predictors of maternal alcohol consumption in 2 regions of Ukraine. Alcoholism: Clinical and Experimental Research. 2014;38:1012–1019. doi: 10.1111/acer.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang G. Alcohol screening instruments for pregnant women. Alcohol Research and Health. 2001;25:204–209. [PMC free article] [PubMed] [Google Scholar]
- Chasnoff IJ, Wells AM, King L. Misdiagnosis and missed diagnoses in foster and adopted children with prenatal alcohol exposure. Pediatrics. 2015;135:264–270. doi: 10.1542/peds.2014-2171. [DOI] [PubMed] [Google Scholar]
- Denis D, Burguiere O, Burillon C. A biometric study of the eye, orbit, and face in 205 normal human fetuses. Investigative ophthalmology & visual science. 1998;39:2232–2238. [PubMed] [Google Scholar]
- Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception. 2011;84:478–485. doi: 10.1016/j.contraception.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fledelius HC, Christensen AC. Reappraisal of the human ocular growth curve in fetal life, infancy, and early childhood. British Journal of Ophthalmology. 1996;80:918–921. doi: 10.1136/bjo.80.10.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith KA, Kasehagen LJ, Rosenberg KD, Sandoval AP, Lapidus JA. Unintended childbearing and knowledge of emergency contraception in a population-based survey of postpartum women. Maternal and child health journal. 2008;12:332–341. doi: 10.1007/s10995-007-0252-x. [DOI] [PubMed] [Google Scholar]
- Handmaker NS, Rayburn WF, Meng C, Bell JB, Rayburn BB, Rappaport VJ. Impact of Alcohol Exposure After Pregnancy Recognition on Ultrasonographic Fetal Growth Measures. Alcoholism: Clinical and Experimental Research. 2006;30:892–898. doi: 10.1111/j.1530-0277.2006.00104.x. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. New Haven, CT.: 1975. [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N, Viljoen DL, Jones KL, Robinson LK. A Practical Clinical Approach to Diagnosis of Fetal Alcohol Spectrum Disorders: Clarification of the 1996 Institute of Medicine Criteria. Pediatrics. 2005;115:39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K, Smith D. RECOGNITION OF THE FETAL ALCOHOL SYNDROME IN EARLY INFANCY. The Lancet. 1973;302:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Jones KL. The effects of alcohol on fetal development. Birth Defects Research Part C: Embryo Today: Reviews. 2011;93:3–11. doi: 10.1002/bdrc.20200. [DOI] [PubMed] [Google Scholar]
- Kfir M, Yevtushok L, Onishchenko S, Wertelecki W, Bakhireva L, Chambers C, Jones K, Hull A. Can prenatal ultrasound detect the effects of in-utero alcohol exposure? A pilot study. Ultrasound in Obstetrics & Gynecology. 2009;33:683–689. doi: 10.1002/uog.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States. Advance data. 2000:1–27. [PubMed] [Google Scholar]
- Kwak HS, Han JY, Choi JS, Ahn HK, Kwak DW, Lee YK, Koh SY, Jeong GU, Velázquez-Armenta EY, Nava-Ocampo AA. Dose-response and time-response analysis of total fatty acid ethyl esters in meconium as a biomarker of prenatal alcohol exposure. Prenatal diagnosis. 2014;34:831–838. doi: 10.1002/pd.4374. [DOI] [PubMed] [Google Scholar]
- Lange S, Shield K, Koren G, Rehm J, Popova S. A comparison of the prevalence of prenatal alcohol exposure obtained via maternal self-reports versus meconium testing: a systematic literature review and meta-analysis. BMC pregnancy and childbirth. 2014;14:127. doi: 10.1186/1471-2393-14-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May P, Gossage J, Kalberg W, Robinson L, Buckley D, Manning M, Hoyme H. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in school studies. Developmental Disabilities Research Reviews. 2009a;15:176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in school studies. Developmental Disabilities Research Reviews. 2009b;15:176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- Naidoo S, Harris A, Swanevelder S, Lombard C. Foetal alcohol syndrome: a cephalometric analysis of patients and controls. The European Journal of Orthodontics. 2006;28:254–261. doi: 10.1093/ejo/cji110. [DOI] [PubMed] [Google Scholar]
- Roozen S, Peters G-JY, Kok G, Townend D, Nijhuis J, Curfs L. Worldwide Prevalence of Fetal Alcohol Spectrum Disorders: A Systematic Literature Review Including Meta-Analysis. Alcoholism: Clinical and Experimental Research. 2016;40:18–32. doi: 10.1111/acer.12939. [DOI] [PubMed] [Google Scholar]
- Russell M, Martier SS, Sokol RJ, Mudar P, Bottoms S, Jacobson S, Jacobson J. Screening for Pregnancy Risk-Drinking. Alcoholism: Clinical and Experimental Research. 1994;18:1156–1161. doi: 10.1111/j.1530-0277.1994.tb00097.x. [DOI] [PubMed] [Google Scholar]
- Sampson PD, Streissguth AP, Bookstein FL, Little RE, Clarren SK, Dehaene P, Hanson JW, Graham JM. Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology. 1997;56:317–326. doi: 10.1002/(SICI)1096-9926(199711)56:5<317::AID-TERA5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Agrawal S, Annis H, Ayala-Velazquez H, Echeverria L, Leo GI, Rybakowski JK, Sandahl C, Saunders B, Thomas S. Cross-cultural evaluation of two drinking assessment instruments: alcohol timeline followback and inventory of drinking situations. Substance Use & Misuse. 2001;36:313–331. doi: 10.1081/ja-100102628. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Measuring alcohol consumption, Measuring alcohol consumption. Springer; 1992. Timeline follow-back; pp. 41–72. [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O'MALLEY K, Young JK. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. Journal of Developmental & Behavioral Pediatrics. 2004;25:228–238. doi: 10.1097/00004703-200408000-00002. [DOI] [PubMed] [Google Scholar]
- Tan CH, Denny CH, Cheal NE, Sniezek JE, Kanny D. Alcohol use and binge drinking among women of childbearing age - United States, 2011-2013. MMWR Morb Mortal Wkly Rep. 2015;64:1042–1046. doi: 10.15585/mmwr.mm6437a3. [DOI] [PubMed] [Google Scholar]
- Thomas IT, Gaitantzis YA, Frias JL. Palpebral fissure length from 29 weeks gestation to 14 years. J Pediatr. 1987;111:267–268. doi: 10.1016/s0022-3476(87)80085-9. [DOI] [PubMed] [Google Scholar]
- Wass TS, Persutte WH, Hobbins JC. The impact of prenatal alcohol exposure on frontal cortex development in utero. American Journal of Obstetrics and Gynecology. 2001;185:737–742. doi: 10.1067/mob.2001.117656. [DOI] [PubMed] [Google Scholar]


