Abstract
Objective
To examine the effect of acupressure on Raynaud's phenomenon (RP) in a randomized controlled clinical trial (RCT) and to evaluate the difficulties of conducting a RP RCT.
Methods
A pilot single center RCT of acupressure vs. targeted patient education was conducted for the treatment of RP. Patients with either primary (N = 15) or secondary (N = 8) RP were randomized in an 8-week study. The primary endpoints included a decrease in the frequency and duration of RP. Secondary endpoints included several serum biomarkers including endothelial dysfunction, Raynaud's attack symptoms, Raynaud's Condition Score, and patient and physician global assessments of RP. Primary data analysis was conducted using the last observation carried forward and t-tests or a Wilcoxon rank test was used to compare the two groups.
Results
23 patients were randomized and 7 discontinued prematurely. 78% of patients were female, 96% were Caucasian, and the mean age was 49.8 (SD=16) years. No statistically significant differences were detected between the acupressure vs. education groups in primary and secondary outcomes (p> 0.05). Frequency of attacks decreased by 6.7 attacks (SD=8.8) in the acupressure group vs. 7.2 (SD=12.8) in the education group (p=0.96), and the duration of attacks decreased by 11.4 (SD=19.9) minutes in the acupressure group vs. an increase of 0.8 minutes (SD=11.2) in the education group (p=0.14). There were no adverse events noted in the RCT.
Conclusion
This pilot study does not support efficacy of acupressure for RP.
Keywords: Raynaud's Phenomenon, Composite Index, Acupressure, Clinical Trial
INTRODUCTION
Raynaud's phenomenon (RP) is a condition caused by an intermittent vasoconstriction of blood vessels in the extremities in response to cold or emotional stress. In the US, it affects from 2% to 11% of women and 1.5% to 8% of men (1-4). The burden of RP in the general population is quite high and can lead to loss of productivity and a reduced quality of life (5). Currently used pharmacologic therapies for RP have only modest efficacy and include calcium channel blockers, angiotensin II antagonists, alpha-1 adrenergic blockers, selective serotonin reuptake inhibitors, prostacyclins, nitrates, statins, botulin toxin, phosphodiesterase inhibitors, endothelin receptor antagonists and antioxidants (6, 7). Side effects of these therapies are common and efficacy is limited with the exception of calcium channel blockers and prostacyclins, which decrease the frequency and duration of attacks (6, 8, 9). The high cost, lack of efficacy, and side effects of traditional medical therapies (10) make other complementary treatments such as acupressure an attractive alternative (11).
Chinese acupuncture has been reported in a randomized controlled study of 33 primary Raynaud's syndrome patients to decrease the frequency of RP attacks (N = 17) when compared to placebo (N=16, p<0.01) (12). However, acupuncture needs to be administered by trained professionals, and usually requires an out of pocket expense. Alternatively, acupressure uses pressure to stimulate key areas based upon ancient Chinese medicine meridian theory. It has been used for several conditions such as back pain, and nausea (13, 14). The advantage of acupressure is that it can be taught to patients and be self-administered at home. As such, acupressure may provide beneficial effects for the treatment of RP(15).
The purpose of this pilot single center trial was to evaluate the feasibility and potential efficacy of standardized acupressure for improving circulation in RP. Among the outcome measures, we included published patient-reported measures such as frequency of RP, duration of RP attacks, patient assessment, attack symptoms [tingling, numbness and pain], and the Raynaud's Condition Score, physician global assessment on a visual analogue score (VAS), serum biomarkers, and a measure of endothelial function (EndoPAT). We also explored a recently reported composite index(16) in this trial.
METHODS
Study Design
This was a single-center, un-blinded, randomized controlled trial that was conducted over a period of 8 weeks and was approved by the University of Michigan Institutional Review Board. Two acupressure protocols were examined in this study: one focusing on vasodilation (thus counteracting the vasospasm in RP), and the second focusing on relaxation (taking advantage of the calming effect of acupressure and the potential for stress as a trigger of RP). At the baseline visit, patients were randomized to vasodilation acupressure, relaxation acupressure or targeted education by use of a computer-generated randomization schedule with a block size of 3.
The education (control) group received an education packet provided by the Raynaud's Association (http://www.raynauds.org/frequently-asked-questions/). The acupressure groups were instructed on how to perform their treatment protocol by H.G and were also provided a video to take home. Each patient had one pre-screening phone visit, two visits, a baseline (day1), and a follow-up visit at 8 weeks. In addition, patients completed a daily diary for the entire study period. Endpoints included clinical assessments, serum biomarkers, and EndoPAT, all of which were assessed at baseline and at the follow-up visit. The primary outcome was comparing the two acupressure groups vs. the education group.
Patients
Patients were eligible if they were > 18 years old, had a self-reported history of RP with at least biphasic color change, reported at least three attacks per week, had been on stable vasodilator medications for the previous 2 weeks and were willing to comply with study visits and treatment plans. Patients with both primary and secondary Raynaud's were included. Exclusion criteria included a history of stroke, myocardial infarction or life-threatening arrhythmia within the previous six months, uncontrolled hypertension (SBP >140 mm Hg, DBP >90 mmHg), significant digital ulcers or difficulty with hand dexterity limiting their ability to perform acupressure.
Efficacy Assessments
The primary outcomes were a decrease in the frequency and duration of RP attacks during week eight compared to attacks during week one (17, 18). Secondary assessments included Raynaud's Condition Score, RP pain, tingling and numbness which collectively were combined into the parameter “attack symptoms”, physician and patient global assessment on a visual analog scale, and EndoPAT. Serum markers included basic fibroblast growth factor (bFGF), vascular cell adhesion molecule-1 (VCAM-1), soluble-intercellular cell adhesion molecule-1 (sICAM-1), soluble-E-selectin (sE-selectin), tissue type plasminogen activator (tPA), procollagen type I N-terminal propeptide (PINP), and vascular endothelial growth factor (VEGF) were also assessed.
Clinical Evaluation
Patients were asked to keep a daily paper diary for the 8-week treatment period noting the frequency and duration (in minutes) of attacks, Raynaud's Condition Score (0-10 mm VAS), and attack symptoms (pain, tingling and numbness) on a scale of 0-100 mm VAS. Patients were contacted weekly by a study member to identify any adverse event, difficulty performing acupressure, or difficulty with diary recording. At baseline and week 8 visits, the patient and physician visual analog scale (0-10 mm on VAS) rating the overall severity of their Raynaud's was recorded.
Serum Parameters
Venous blood was obtained at baseline (0 week visit) and at 8 weeks. Patient plasma was immediately collected and stored at −80°C until use. Parameters measured included: VEGF, tPA, sE- Selectin, bFGF, VCAM-1, and sICAM-1 in patient plasma by ELISA using commercially available Kits (bFGF, VCAM-1, and sICAM-1: RayBiotech Inc, Norcross, GA;. tPA: Abcam, Cambridge, MA; .sE-selectin and VEGF: R&D Systems Inc, Minneapolis, MN; PINP: MyBioSource, San Diego, CA). Proper dilutions were made to ensure that the concentration fell within the standard curve. Samples were all above the detection limit of the assay. Samples were run in duplicate. Anti-human primary antibodies were coated on a 96-well plate before the addition of samples and standards. Sandwich ELISA was completed by adding biotinylated anti-human antibodies, followed by streptavidine-HRP and substrate solution. The optical density of each well was measured using an ELISA plate reader (BioTeK, Winooski, VT).
Measurement of Arterial Tone
Endo-PAT 2000 is a device for assessing endothelial function. Endothelial dysfunction is thought to occur when an artery fails to dilate appropriately in response to stimuli, and it can be assessed by measuring the arterial pulse wave at a finger artery (19). Endo-PAT is performed by placing a sensor on the patient's bilateral index fingers, while one arm has a blood pressure cuff inflated. The software automatically analyzes the post-occlusion (arm with blood pressure cuff) to the pre-occlusion ratio which provides an endo-score. This serves as a non-invasive way to measure arterial tone changes. In this study, Endo-Pat 2000 was performed at the baseline and 8-week visits by a trained vascular technician.
Acupressure protocols
Acupressure protocols were developed based upon experience from three local acupressure specialists who have been studying and practicing Chinese medicine for over 10 years.
The vasodilation acupressure group (Group A) was instructed on the following acupressure points:
- Ba Xia Hands and feet- 4 points per hand/foot- 1 minute per point (16 minutes)
- Si 3 bilaterally 1.5 minutes per point (3 minutes)
- Li 4 bilaterally 1.5 minutes per point (3 minutes)
- Lu 9 bilaterally 1.5 minutes per point (3 minutes)
- Liv 3 bilaterally 1.5 minutes per point (3 minutes)
- Total of 28 minutes performed twice daily
The Relaxation acupressure group (Group B) was instructed on the following acupressure points:
- Sp 6 bilaterally 3 minutes per point (6 minutes)
- GB 34 bilaterally 3 minutes per point (6 minutes)
- Liv 3 bilaterally 3 minutes per point (6 minutes)
- An Men bilaterally 3 minutes per point (6 minutes)
- Shen Mien unilateral (3 minutes)
- Total 27 minutes twice daily
Patients in the acupressure groups were taught how to perform acupressure by HG at the beginning of the trial, and were given a DVD instructional video to take home.
Statistical analysis
To assess if daily acupressure (vasodilation acupressure and relaxation acupressure) had an effect on the frequency, and duration of RP attacks as compared to targeted patient education, for each endpoint of interest, we performed a standard t-test (if the outcome was normally distributed) or a Wilcoxon rank test (if the outcome was not normally distributed). Using measurements collected on patients at baseline and week 8 for each of the outcomes (Raynaud's Condition Score, patient and physician VAS assessment of RP, pain, numbness and tingling during an RP attack, average number of attacks per day and duration of attacks), we derived the change in each outcome from baseline to week 8 and compared the changes in each of the response variables among two groups of patients, those receiving targeted education, and those receiving acupressure (either vasodilation or relaxation acupressure).
For patients who dropped out of the study before week 8, or who did not have measurements at week 8, changes in any of the outcome variables were obtained by carrying the last observation forwards (LOCF). To determine whether the results obtained are robust to the method we used to account for drop-out and missing data (e.g. LOCF), we repeated the analyses and performed the hypothesis tests using data from completers.
We assessed any difference among the two groups using either a two-sample t-test or a Wilcoxon rank test, depending on the distributional characteristic of the outcome in consideration. We also assessed if within each group, there were statistical differences between the outcome variables at week 8 vs. week 1. For these latter groups of tests, we used a Wilcoxon rank test or a paired t-test, again depending on the distribution of the outcome variable in consideration.
Finally, we compared the acupressure groups vs. the education group with respect to the proportion of patients within the two groups that showed improvement of more than 10%, 20%, 30%, 40%, 50% and 60% in one, two, three, four, five or all six core variables using Fisher's exact test. Following Gladue et al, (16) we chose six core variables: number of attacks, duration (in minutes) of attacks, Raynaud's Condition Score, symptoms of attacks (pain, numbness and tingling), patient assessment and physician assessment. For symptoms of attacks, we defined a patient improved if at least one of the three variables (pain, numbness and tingling) showed an improvement from baseline to week 8. Similarly, we deemed patient improvement by 10, 20, 30, or 40% in symptoms of attacks if at least one of the variables improved by 10, 20, 30, 40%, respectively, from baseline to week 8. To assess variability among individual parameters, we computed the intraclass correlation coefficient for the first week after randomization. An ICC of > 0.70 was considered satisfactory for group comparisons (20).
RESULTS
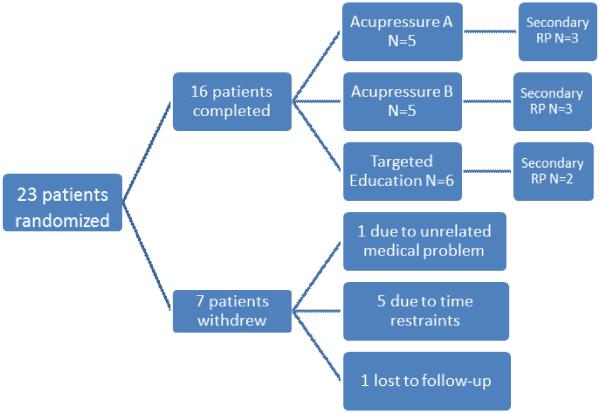
Twenty-three patients were randomized between January and April 2013 (Figure 1). Seventy-eight percent of patients were female, 96% were Caucasian, the mean age was 49.8 (SD=16) years. N=15 (65%) had primary RP and N=8 (35%) had secondary RP (4 limited cutaneous SSc, 1 Sjogren's, 2 mixed connective tissue disease, 1 systemic lupus erythematosus). Of 23 patients, 7 discontinued prematurely (5 patients withdrew due to time restraints, 1 for unrelated medical problems, and 1 was lost to follow-up). Seven (29%) patients were on stable doses of vasodilators and 2 (8%) were on warfarin at randomization. There were no statistical differences in the baseline values for any of the clinical and biomarker parameters between the two groups except for tPA which was statistically higher at 3.8 ng/ml in the acupressure group as compared to 1.6 ng/ml in the education group (p=0.03) (Table 1 and Appendix Table 1).
Figure 1.
Schematic showing patient enrollment and randomization. The number of patients assigned to each group is indicated.
Table 1.
Baseline Demographics
| Variable | All Patients | Acupressure Group Aα | Acupressure Group Bβ | Acupressure Group A+B | Education | P-value A+B vs C |
|---|---|---|---|---|---|---|
| No of patients | 23 | 8 | 8 | 16 | 7 | |
| Female, N(%) | 18 (78.3%) | 6 (75.0%) | 6 (75.0%) | 12 (75.0%) | 6 (85.7%) | 0.57 |
| Age: mean (SD) | 49.9 (16.0) | 56.3 (20.6) | 48.4 (9.7) | 52.3 (16.1) | 44.3 (15.5) | 0.28 |
| Caucasian, N (%) | 22 (95.7%) | 8 (100%) | 8 (100%) | 15 (93.8%) | 7 (100%) | 0.50 |
| (N)% on vasodilators/warfarin | 7 (30%)/2(8.7%) | 3 (38%)/1(12.5%) | 2(25%)/1(12.5%) | 5 (31%)/2 (12.5%) | 2 (28%), 0 (0%) | |
| Secondary RP, N (%) | 8 (34.8%) | 3 (37.5%) | 3 (37.5%) | 6 (37.5%) | 2 (28.6) | 0.68 |
| No. attacks previous week, mean (SD) | 15.3 (15.0) | 10.4 (5.6) | 16.4 (11.5) | 13.41 (9.3) | 19.6 (24.0) | 0.53 |
| Patient VAS * for RP, mean (SD) | 6.2 (2.6) | 6.8 (2.6) | 6.1 (2.5) | 6.4 (2.5) | 5.8 (3.1) | 0.63 |
| Physician VAS* for RP, mean (SD) | 6.4 (2.3) | 6.7 (2.1) | 6.5 (2.1) | 6.6 (2.0) | 6 (2.9) | 0.66 |
| Pain VAS**, mean (SD) | 30.1 (25.3) | 37.9 (27.7) | 17.9 (16.6) | 30.5 (23.9) | 33.7 (29.4) | 0.69 |
| Tingling VAS **, mean (SD) | 17.0 (19.4) | 14.4 (13.4) | 11.9 (13.5) | 14.5 (12.8) | 23.3 (27.6) | 0.30 |
| Numbness VAS **, mean (SD) | 33.3 (31.7) | 38.7 (40.4) | 14.5 (12.5) | 27.9 (32.5) | 44.4 (32.5) | 0.43 |
| EndoPAT, mean (SD) | 1.6 (0.7) | 1.7 (0.6) | 1.1 (0.2) | 1.5 (0.6) | 1.7 (0.9) | 0.71 |
| EndoPAT for primary RP, mean (SD) | 2.0 (0.8) | 2.3 (0.4) | 1.5 (NA) | 2.0 (0.6) | 2.1 (0.9) | 0.90 |
| EndoPAT for secondary RP, mean (SD) | 1.2 (0.3) | 1.4 (0.5) | 1.0 (NA) | 1.2 (0.4) | 1 (0) | 0.22 |
0-10
0-100
Acupressure Group A= vasodilation
Acupressure Group B= relaxation
Primary Results
At the end of the study, there were no statistical differences between the acupressure and the education groups with respect to the 2 primary outcome measures—frequency of attacks decreased by 6.7 attacks (SD=8.8) in the acupressure group compared to a decrease of 7.2 (SD=12.8) in the education group (p=0.96) while the duration of attacks decreased by 11.4 (SD=19.9) minutes in the acupressure group vs. an increase of 0.8 ( SD=11.2) minutes in the education group (p=0.14). However, there were trends in the patient-reported pain and duration of RP as well as in patient and physician VAS for improvement in the acupressure group (Table 2; p= 0.17 and p=0.12). There were also no significant differences in serum biomarkers of vasculopathy or EndoPAT (Table 2 and Appendix Table 2). Sensitivity analyses carried out using only the completers yielded similar results (results not shown).
Table 2.
Change from baseline to week 8 in the RCT*
| Variable | Acupressure groups N=10 | Control N=6 | P value |
|---|---|---|---|
| No of attacks Mean (SD) | −6.7 (8.8) p=0.06 |
−7.2 (12.8) p=0.21 |
0.96 |
| Pain** Mean (SD) | −15.2(32.9) p=0.23 |
9.6 (27.6) p=0.58 |
0.13 |
| Tingling** Mean (SD) | −6.7 (14.8) p=0.19 |
−1.2 (7.5) p=1.0 |
0.35 |
| Numbness** Mean (SD) | −13.7 (40.1) p= 0.56 |
1.1 (22.1) p= 1.00 |
0.36 |
| Attack Symptoms Mean (SD) | 1.49 (21.2) (p=1.00) |
12.60 (23.8) (p=0.42) |
0.51 |
| Average duration of attacksα Mean (SD) | −11.4 (19.9) p= 0.12 |
0.8 (11.2) p=0.44 |
0.14 |
| RCS Mean (SD) | −1.7(2.3) p=0.05 |
−0.6 (2.9) (N=5) p=0.44 |
0.62 |
| Patient VAS ∂ Mean (SD) | −2.5 (2.3) p=0.02 |
−0.8(2.1) p=0.42 |
0.17 |
| Physician VAS ∂ Mean (SD) | −3.3 (1.6) p=0.006 |
−2.2 (1.2) p=0.04 |
0.12 |
| EndoPAT | −0.1 (0.6) p=1.0 |
−0.3 (0.8) p=0.79 |
0.74 |
| Primary RP EndoPAT | −0.4 (1.0) (N=3) p=1.0 |
−0.5 (0.9) (N=4) p=0.37 |
0.37 |
| Secondary RP EndoPAT | 0.02 (0.2) (N=6) p=1.0 |
0.3 (0.4) (N=2) p=1.0 |
0.39 |
Using LOCF
0-100 scale
In minutes
0-10 scale
When we compared within each group, there were also trends within the acupressure groups for number of attacks, RCS, Patient and physician VAS that showed a significant change within the acupressure group from baseline to week 8. In the education group, there was significant change in the physician VAS from baseline to week 8 (Table 2).
Variability in individual parameters and composite end point
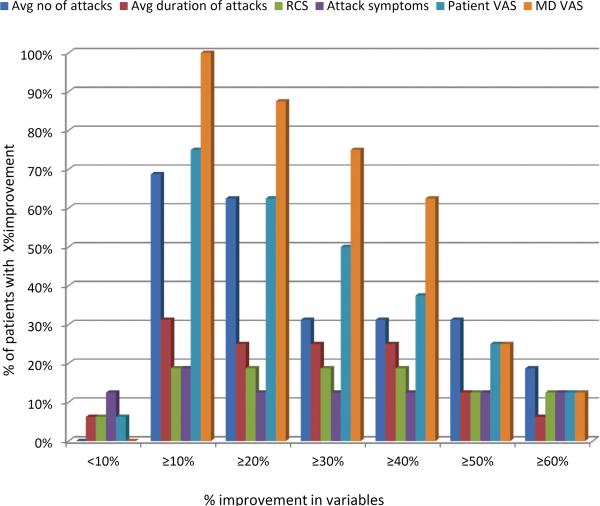
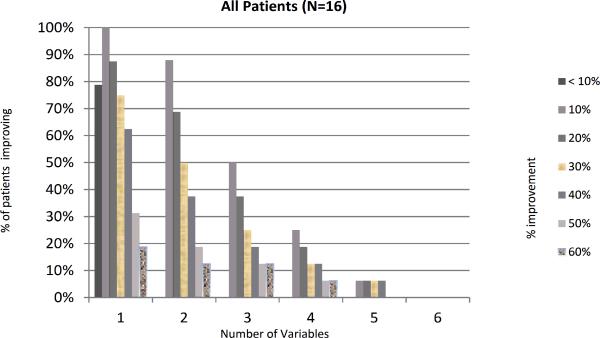
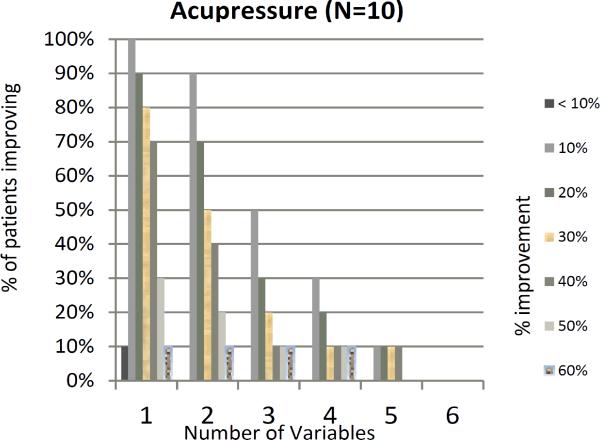
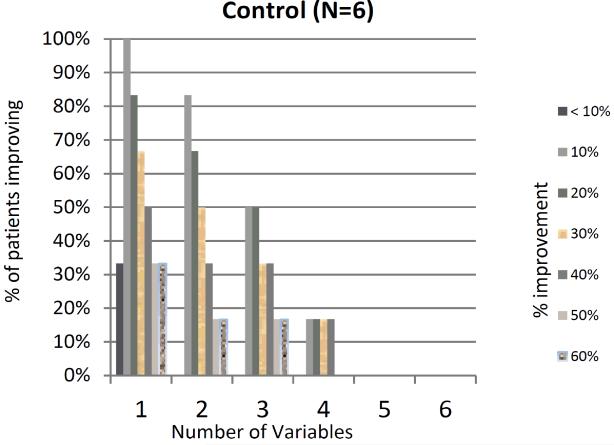
Our previous work has shown a marked variability in individual parameters used to measure RP(16). We explored this in our current trial and found ICC< 0.70 for all parameters at baseline except for number of attacks and secondary EndoPAT (Appendix Table 3). In addition, a great deal of variability was noted during the trial with response rates ranging from a 0% improvement to 100% improvement (Figure 2). We assessed whether patients with at least 10%, 20% or 30% improvement in 3-4 variables (as proposed by Gladue et al) were significantly different from patients who did not have such improvements. This analysis revealed no statistical difference between the acupressure and education groups (Figure 3). The placebo response rate for an individual parameter was as high as 37.5%, however when combining 4 variables with at least a >10% improvement up to a >40% improvement, the placebo response rate decreased to 6.3%, providing some support for the composite index. Within the acupressure groups, the percent of patients with a ≥ 20% improvement in 4 variables was 12.5%.
Figure 2.
Percentage of patients showing improvement in the 6 core set measures. The graph shows the % of the patients showing the level of improvement indicated on the X axis for the following parameters: (1) average number of attacks; (2) Average duration of attacks; (3) RCS; (4) attack symptoms; (5) Patients VAS; (6) MD VAS
Figure 3.
Percentage of patients showing X% improvement in X number of variables. The graphs show the percentage of patients showing improvement on the x-axis, and the number of variables that have an improvement on the y-axis. (P value comparing acupressure groups vs. control >0.10). a- all patients, b- acupressure group, c control group.
Adverse events
Patients did not experience any adverse effects from acupressure with no bruising or pain reported. There was a high drop out rate approaching 30% which is hypothesized to be due to the difficulty in patients keeping up the daily acupressure treatment or due to lack of efficacy.
DISCUSSION
Our pilot study assessed the efficacy of acupressure for the treatment of primary and secondary RP as compared to an education only group by using several parameters. The results did not demonstrate statistically significant differences in patient-reported or objective outcome measures. In addition, as shown in our previous study (16), we found a high degree of variability in each of the individual measures used to evaluate efficacy in RP highlighting the need for new outcome measure(s) with acceptable reliability or well-defined composite index to assess efficacy in clinical trials.
The rationale to assess acupressure in RP was in part based on a study by Appiah et al. who conducted a 23-week study using Chinese acupuncture in patients with primary Raynaud's syndrome and reported a decrease in the frequency of RP attacks (N = 17; P<0.01) when compared to placebo (N=16) (12). This study was performed in Germany during the winter months and the mean age was 45.5 years (SD 10.7) for treatment group and 41.5 (SD 11.5) for placebo group and the baseline number of attacks was 1.2 (SD 1.1) vs 1.6 (SD 1.3) per day in the placebo group. Our study was a randomized, controlled, prospective study conducted during the winter months that included both patients with primary and secondary RP. Our mean age was slightly higher 52.3 (SD 6.1) for the acupressure group vs. 44.3 (SD 15.5) for the education group. Our higher age may have contributed to worse underlying vasculature and less response to treatment as compared to the acupuncture study. In addition, our study had a higher mean number of attacks; weekly average in the acupressure group was 13.41 (SD 9.3) vs. 19.6 (SD 24) in the education group. As outcome measures, we considered both subjective outcome measures and objective outcome a capillary flow-stop reaction that has been shown to be sensitive and specific for RP, although it has not been routinely used in Raynaud's clinical trials (21). We divided treatment groups into two acupressure groups in order to better explore the benefit of 2 different acupressure protocols.
Identifying effective new treatments for RP patients has been hampered by the variability in clinical trial outcome measures used to assess efficacy. The variability and lack of standardized outcome measures has also made it difficult to compare different clinical trials as RCTs in RP often use different clinical outcome measures, resulting in conflicting outcomes in different trials (17, 18). For example with iloprost, one study showed improvement in duration and severity whereas another study showed no statistical significance in duration, average number of attacks or RCS (22, 23). Tadalafil studies also showed mixed outcomes with one study showing an improvement in RCS, duration and frequency and another study showing no improvement(17, 18) Further, assessing efficacy using different individual outcome measures has led to difficulty in obtaining statistical significance due to the high placebo response rate. This is also highlighted by the change in the outcome measures within each group in this trial where there was marked variability in each outcome measure from baseline to week 8. In an analysis of 3 multicenter RCTs, Gladue et al (16) showed a placebo response rate for an individual outcome that ranged from 92.8% for the group of patients with more than 10% improvement in at least one core set measure to 38.5% for the group of patients with an improvement of more than 60% in one parameter. This variation in placebo response rates has made clinical trials inconclusive rendering the approval of new therapies for RP difficult despite anecdotal evidence of improvement. In this study, by combining 4 variables the placebo response rate decreased to 6.3%, providing some support for the composite index. In our study, within the acupressure groups, the percent of patients with a ≥ 20% improvement in 4 variables was 12.5%.
The lack of reliable objective biomarkers or endpoints to predict efficacy has also pushed investigators to utilize subjective endpoints that have high placebo response rates. Objective measures have been shown to be reliable and valid but are not always available for trials and there is a need for better outcome measures that can be incorporated into multicenter trials. In a small, double blinded trial of sildenafil, blood flow increased significantly after treatment as measured by laser flux Doppler (24). While laser flux Doppler is sensitive to change in vasoreactivity at the surface of the skin, circulation is very heterogeneous and one area of perfusion does not necessarily correlate with another area (25). EndoPAT noninvasively evaluates vasoreactivity without the limitations of laser doppler(26). EndoPAT has been used in one Raynaud's RCT, but no significant difference was detected between the treatment and placebo groups (27). In our study we were unable to show a significant difference in objective measures (EndoPAT and serum markers). EndoPAT baseline values were worse with a mean right heart index of 1.2 (SD=0.3) in secondary RP as compared to a mean of 2.0 (SD=0.8) in the group with primary RP, thus showing decreased endothelial functions in secondary RP. It is unclear whether this lack of significance is due to a lack of efficacy or due to lack of sensitivity to change in these tests that are used to detect the vascular changes attributed to RP.
The serum markers ICAM-1, VCAM-1, soluble E-selectin, VEGF, t-PA and endothelin-1 have been shown to be associated with RP (28-31) and were chosen based on an ongoing systemic review and previous published data (28, 32) which revealed that these markers did change in longitudinal studies. Few small studies have shown a decrease in these parameters in association with treatment, but larger studies are needed to evaluate their sensitivity to change (28).
Our pilot study was designed to explore the established acupressure protocols developed by experts in Chinese medicine. Our study may have been underpowered to show statistical differences in patient reported outcomes since it was designed as a proof of concept study. The lack of any trends of improvement in objective outcomes makes it highly unlikely that acupressure has a meaningful effect on RP. This trial further illustrates the variability that can be observed in RP RCT's, such that conclusive significant results are difficult to achieve with new treatments despite trends in improvement, highlighting the need for new outcome measures with better reliability or development and validation of a composite index for RP.
Supplementary Material
KEY MESSAGE.
Acupressure is not effective for the treatment of Raynaud's phenomenon.
Outcome measures in Raynaud's phenomenon have a high level of variability, making conclusive results difficult.
Acknowledgements
We would like to thank Heather Sloan and Brodie Burris both experienced Chinese Acupuncturists for their help in designing the acupressure protocols
Funding
Dr. Khanna and Dr. Berrocal were supported by a grant from NIH/ NIAMS K24AR063120. Dr. Tsou was supported by the Arthritis Foundation and Scleroderma Foundation New Investigator Award.
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000433
Footnotes
Registration number of Clinical Trial: clinical trials.gov NCT01784354, https://clinicaltrials.gov/ct2/show/NCT01784354?term=acupressure&rank=15
Conflict of Interest
The authors declare no conflict of interest.
References:
- 1.Suter LG, Murabito JM, Felson DT, Fraenkel L. The incidence and natural history of Raynaud's phenomenon in the community. Arthritis Rheum. 2005;52:1259–63. doi: 10.1002/art.20988. [DOI] [PubMed] [Google Scholar]
- 2.Herrick AL. The pathogenesis, diagnosis and treatment of Raynaud phenomenon. Nat Rev Rheumatol. 2012;8:469–79. doi: 10.1038/nrrheum.2012.96. [DOI] [PubMed] [Google Scholar]
- 3.Brand FN, Larson MG, Kannel WB, McGuirk JM. The occurrence of Raynaud's phenomenon in a general population: the Framingham Study. Vasc Med. 1997;2:296–301. doi: 10.1177/1358863X9700200404. [DOI] [PubMed] [Google Scholar]
- 4.Weinrich MC, Maricq HR, Keil JE, McGregor AR, Diat F. Prevalence of Raynaud phenomenon in the adult population of South Carolina. J Clin Epidemiol. 1990;43:1343–9. doi: 10.1016/0895-4356(90)90101-t. [DOI] [PubMed] [Google Scholar]
- 5.De Angelis R, Salaffi F, Grassi W. Health-related quality of life in primary Raynaud phenomenon. J Clin Rheumatol. 2008;14:206–10. doi: 10.1097/RHU.0b013e31817a2485. [DOI] [PubMed] [Google Scholar]
- 6.Pope JE. The diagnosis and treatment of Raynaud's phenomenon: a practical approach. Drugs. 2007;67:517–25. doi: 10.2165/00003495-200767040-00003. [DOI] [PubMed] [Google Scholar]
- 7.Levien TL. Advances in the treatment of Raynaud's phenomenon. Vasc Health Risk Manag. 2010;6:167–77. doi: 10.2147/vhrm.s4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vinjar B, Stewart M. Oral vasodilators for primary Raynaud's phenomenon. Cochrane Database Syst Rev. 2008:CD006687. doi: 10.1002/14651858.CD006687.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Henness S, Wigley FM. Current drug therapy for scleroderma and secondary Raynaud's phenomenon: evidence-based review. Curr Opin Rheumatol. 2007;19:611–8. doi: 10.1097/BOR.0b013e3282f13137. [DOI] [PubMed] [Google Scholar]
- 10.Goundry B, Bell L, Langtree M, Moorthy A. Diagnosis and management of Raynaud's phenomenon. BMJ. 2012;344:e289. doi: 10.1136/bmj.e289. [DOI] [PubMed] [Google Scholar]
- 11.Malenfant D, Catton M, Pope JE. The efficacy of complementary and alternative medicine in the treatment of Raynaud's phenomenon: a literature review and meta-analysis. Rheumatology (Oxford) 2009;48:791–5. doi: 10.1093/rheumatology/kep039. [DOI] [PubMed] [Google Scholar]
- 12.Appiah R, Hiller S, Caspary L, Alexander K, Creutzig A. Treatment of primary Raynaud's syndrome with traditional Chinese acupuncture. J Intern Med. 1997;241:119–24. doi: 10.1046/j.1365-2796.1997.91105000.x. [DOI] [PubMed] [Google Scholar]
- 13.Genc F, Tan M. The effect of acupressure application on chemotherapy-induced nausea, vomiting, and anxiety in patients with breast cancer. Palliat Support Care. 2014:1–10. doi: 10.1017/S1478951514000248. [DOI] [PubMed] [Google Scholar]
- 14.Hsieh LL, Kuo CH, Yen MF, Chen TH. A randomized controlled clinical trial for low back pain treated by acupressure and physical therapy. Prev Med. 2004;39:168–76. doi: 10.1016/j.ypmed.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 15.Chatterjee S. Pulmonary hypertension in systemic sclerosis. Semin Arthritis Rheum. 2011;41:19–37. doi: 10.1016/j.semarthrit.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Gladue H, Maranian P, Paulus HE, Khanna D. Evaluation of test characteristics for outcome measures used in Raynaud's phenomenon clinical trials. Arthritis Care Res (Hoboken) 2013;65:630–6. doi: 10.1002/acr.21858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shenoy PD, Kumar S, Jha LK, Choudhary SK, Singh U, Misra R, et al. Efficacy of tadalafil in secondary Raynaud's phenomenon resistant to vasodilator therapy: a double-blind randomized crossover trial. Rheumatology (Oxford) 2010;49:2420–8. doi: 10.1093/rheumatology/keq291. [DOI] [PubMed] [Google Scholar]
- 18.Schiopu E, Hsu VM, Impens AJ, Rothman JA, McCloskey DA, Wilson JE, et al. Randomized placebo-controlled crossover trial of tadalafil in Raynaud's phenomenon secondary to systemic sclerosis. J Rheumatol. 2009;36:2264–8. doi: 10.3899/jrheum.090270. [DOI] [PubMed] [Google Scholar]
- 19.Betik AC, Luckham VB, Hughson RL. Flow-mediated dilation in human brachial artery after different circulatory occlusion conditions. Am J Physiol Heart Circ Physiol. 2004;286:H442–8. doi: 10.1152/ajpheart.00314.2003. [DOI] [PubMed] [Google Scholar]
- 20.Hays RD RD. Reliability and validity (including responsiveness). In: Fayers PHR, editor. Assessing quality of life in clinical trials. 2nd ed. Oxford University Press; New York: 2005. pp. 25–39. [Google Scholar]
- 21.Mahler F, Saner H, Boss C, Annaheim M. Local cold exposure test for capillaroscopic examination of patients with Raynaud's syndrome. Microvasc Res. 1987;33:422–7. doi: 10.1016/0026-2862(87)90033-1. [DOI] [PubMed] [Google Scholar]
- 22.Wigley FM, Korn JH, Csuka ME, Medsger TA, Jr., Rothfield NF, Ellman M, et al. Oral iloprost treatment in patients with Raynaud's phenomenon secondary to systemic sclerosis: a multicenter, placebo-controlled, double-blind study. Arthritis Rheum. 1998;41:670–7. doi: 10.1002/1529-0131(199804)41:4<670::AID-ART14>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 23.Black CM, Halkier-Sorensen L, Belch JJ, Ullman S, Madhok R, Smit AJ, et al. Oral iloprost in Raynaud's phenomenon secondary to systemic sclerosis: a multicentre, placebo-controlled, dose-comparison study. Br J Rheumatol. 1998;37:952–60. doi: 10.1093/rheumatology/37.9.952. [DOI] [PubMed] [Google Scholar]
- 24.Fries R, Shariat K, von Wilmowsky H, Bohm M. Sildenafil in the treatment of Raynaud's phenomenon resistant to vasodilatory therapy. Circulation. 2005;112:2980–5. doi: 10.1161/CIRCULATIONAHA.104.523324. [DOI] [PubMed] [Google Scholar]
- 25.Tenland T, Salerud EG, Nilsson GE, Oberg PA. Spatial and temporal variations in human skin blood flow. Int J Microcirc Clin Exp. 1983;2:81–90. [PubMed] [Google Scholar]
- 26.Moerland M, Kales AJ, Schrier L, van Dongen MG, Bradnock D, Burggraaf J. Evaluation of the EndoPAT as a Tool to Assess Endothelial Function. Int J Vasc Med. 2012;2012:904141. doi: 10.1155/2012/904141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herrick AL, van den Hoogen F, Gabrielli A, Tamimi N, Reid C, O'Connell D, et al. Modified-release sildenafil reduces Raynaud's phenomenon attack frequency in limited cutaneous systemic sclerosis. Arthritis Rheum. 2011;63:775–82. doi: 10.1002/art.30195. [DOI] [PubMed] [Google Scholar]
- 28.Mittag M, Beckheinrich P, Haustein UF. Systemic sclerosis-related Raynaud's phenomenon: effects of iloprost infusion therapy on serum cytokine, growth factor and soluble adhesion molecule levels. Acta Derm Venereol. 2001;81:294–7. doi: 10.1080/00015550152572976. [DOI] [PubMed] [Google Scholar]
- 29.Malenfant D, Summers K, Seney S, McBain D, Petrlich L, Watson S, et al. Results of a Pilot Randomized Placebo-Controlled Trial in Primary and Secondary Raynaud's Phenomenon with St. John's Wort: Detecting Changes in Angiogenic Cytokines When RP Improves. ISRN Rheumatol. 2011;2011:580704. doi: 10.5402/2011/580704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silveri F, De Angelis R, Poggi A, Muti S, Bonapace G, Argentati F, et al. Relative roles of endothelial cell damage and platelet activation in primary Raynaud's phenomenon (RP) and RP secondary to systemic sclerosis. Scand J Rheumatol. 2001;30:290–6. doi: 10.1080/030097401753180372. [DOI] [PubMed] [Google Scholar]
- 31.Dziadzio M, Denton CP, Smith R, Howell K, Blann A, Bowers E, et al. Losartan therapy for Raynaud's phenomenon and scleroderma: clinical and biochemical findings in a fifteen-week, randomized, parallel-group, controlled trial. Arthritis Rheum. 1999;42:2646–55. doi: 10.1002/1529-0131(199912)42:12<2646::AID-ANR21>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 32.Denton CP, Howell K, Stratton RJ, Black CM. Long-term low molecular weight heparin therapy for severe Raynaud's phenomenon: a pilot study. Clin Exp Rheumatol. 2000;18:499–502. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.