Abstract
Despite the striking differences between male and female physiology, female physiology is understudied. In response, the National Institutes of Health is promulgating new policies to increase the use of female organisms in pre-clinical research. Females are commonly believed to have greater variability than males due to the estrous cycle, but recent studies call this belief into question. Effects of estrous cycling on mean arterial pressure were assessed in female Dahl S rats using telemetry and vaginal cytometry, and found that estrous cycling did not affect MAP magnitude or variance. Data from the PhysGen arm of the Program for Genomic Applications was used to compare male and female variance and coefficient of variation in 142 heart, lung, vascular, kidney and blood phenotypes, each measured in hundreds to thousands of individual rats from over 50 inbred strains. 74 of 142 phenotypes from this dataset demonstrated a sex difference in variance; however, 59% of these phenotypes exhibited greater variance in male rats rather than female. Remarkably, a retrospective power analysis demonstrated that only 16 of 74 differentially variable phenotypes would be detected when using an experimental cohort large enough to detect a difference in magnitude. No overall difference in coefficient of variation between male and female rats was detected when analyzing these 142 phenotypes. We conclude that variability of 142 traits in male and female rats is similar, suggesting that differential treatment of males and females for the purposes of experimental design is unnecessary until proven otherwise, rather than the other way around.
Keywords: Sample size, estrous cycle, experimental design, variation, sex, experimental models
Introduction
The National Institutes of Health (NIH) have recently introduced policies to increase the proportion of studies in which both female and male cells and organisms are used1. This ongoing emphasis on the study of female cells and organisms2 directly responds to the overwhelming male bias in pre-clinical scientific research3, 4. This bias limits the translation potential of scientific discovery for the medical care of women, sometimes resulting in poor clinical outcomes5. For example, of the FDA-approved drugs withdrawn from the market between 1997 and 2001, 80% were withdrawn due to greater health risk in women than in men6; not surprising considering the use of male to female animals in pharmacological research is almost 6:17. Moreover, non-harmful drugs are often differentially effective in women and men, further illustrating the consequences of sex bias in clinical and pre-clinical research8.
One of the most prominently believed rationales for male bias in research is that the estrous cycle significantly complicates the study of physiology in females. It is well known that many phenotypic measurements are significantly affected by estrogen and other circulating hormones. It is, therefore, reasonable to suppose that varying hormone levels during the estrous cycle would confound the study of these phenotypes in female organisms by increasing the variance in uncontrolled female experimental groups. If present, this increased variance would mean that greater numbers of female animals than male would be required to detect true experimental differences in the magnitude of a phenotype. Approaches to counteract these effects include precise estrous staging9, 10, incorporation of gonadectomized rat controls11, 12, or increasing the size of experimental groups to counteract the variance added by the estrous cycle.
A more robust understanding of sex differences in variability within specific model systems is clearly needed to guide experimental design. While it is undeniable that specific phenotypes can be profoundly affected by estrous stage9, 10, 13, recent data suggests the effects of estrous cycling upon cohort variance are not as confounding as was assumed. A meta-analysis of 293 publications in which male and female mice were studied found no difference in the distribution of coefficients of variation between the male and female groups14. However, the actual variances and coefficients of variation of individual phenotypes were not compared; rather difference was detected en bloc. Here, we directly compare the variance and coefficient of variation between male and female rats in 142 physiological phenotypes which are most specifically related to physiology and pathophysiology of diseases related to the heart, lung, vasculature, blood and kidney, i.e., tissues of specific relevance to the NHLBI. Unlike a meta-analytical approach, the current study examines a single dataset. This eliminates inter-experimenter noise, as well as avoiding statistical artifacts that can present themselves when compiling many experiments, each with small N.
Methods
Experimental animals
Female rats were obtained at weaning from colonies developed and maintained at the Medical College of Wisconsin under controlled environmental conditions with parents and offspring fed a purified AIN-76A rodent food (Dyets, Bethlehem, PA) containing 0.4% NaCl with water provided ad libitum until the experimental period of 8.0% NaCl diet (high salt; Dyets, Bethlehem, PA). All experimental protocols were approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee.
Measurement of blood pressure
Mean arterial pressure (MAP) was measured by radiotelemetry in adult female rats. At 9 weeks of age, rats were surgically prepared, as we have described previously15, 16, and then given a 5- to 7-day recovery period before recording blood pressure. MAP was recorded for 3 hours in the morning each day. Rats continued to be maintained on the 0.4% NaCl diet for 4 days during baseline MAP measurement, after which diet was switched to an 8% NaCl diet for 13 days. Rats were assessed for estrous stage each day, as described17, and the date of estrus for each rat was noted. Rats were retrospectively split into two groups, indicating those in which estrus was detected 3 or more times during the 16 day period (cycling, N=8) and those in which estrus was detected 2 or fewer times during the 16 day period (non-cycling, N=6). The MAP of these rats was compared using a two-way, repeated measures ANOVA, and the variance of the pressure data was compared using a repeated measures Brown-Forsythe test, each performed using SigmaPlot 12.
PhysGen Program for Genomic Applications Dataset
Data were downloaded from the PhysGen Program for Genomic Applications (PGA) website (pga.mcw.edu) on 2/29/16. Phenotypes were removed from the dataset if they were calculated from two or more other data points within the data set, e.g. ΔMAP after norepinephrine infusion was excluded from further analysis, since both pre-norepinephrine MAP and post-norepinephrine MAP were also included in the dataset. This exclusion reduced the number of phenotypes from 211 to 160. Further exclusions of phenotypes necessary for the performance of specific statistical tests are noted in the sections describing those tests.
Variance testing
Differences in variance between males and females were assessed on a phenotype-by-phenotype basis by using the Brown-Forsythe test, a one-way ANOVA on the set of absolute deviations from the median. Differences in magnitude were assessed on a phenotype-by-phenotype basis by use of heteroscedastic t-test. Those phenotypes which exhibited a difference in both magnitude and variance, or a difference in variance alone, were then subjected to a retrospective power analysis to determine the sample size necessary to detect the observed differences. A one-way ANOVA power analysis was used for both magnitude and variance, most likely resulting in more conservative (i.e. greater) estimates of sample size needed to detect differences in magnitude. The power analyses used α=0.05, β=0.2. Between-group variance was calculated as the variance between the male and female means, and within-group variance was taken as the greater of the male and female variance, again resulting in more conservative estimates for sample size. Phenotypes measured in multiple phenotypic categories were consolidated to one point for the analysis of combined strain data by keeping the measurement with lowest NV and excluding the others. All measurements were used for the analysis of phenotypes by strain, however, an additional exclusion criteria was applied: phenotypes within a single strain that had sample size of two or fewer were removed. Brown-Forsythe and t-tests were performed using functions within the Matlab R2014b Statistics and Machine Learning Toolbox. ANOVA power analyses were performed using R 3.0.1, Stats package.
Coefficient of variation
Coefficients of variation (CVs) were calculated for each phenotype and sex as standard deviation divided by the mean, multiplied by 100%. Because this metric can be heavily skewed by a mean close to 0, any phenotypes whose magnitude did not significantly differ from 0 were excluded from this analysis, resulting in the exclusion of 1 phenotype (Pre to Post Control Δ HR following Angiotensin II (beats/min)). An unweighted Deming linear regression was then performed on the set of all male-female pairs of CVs. 95% confidence intervals were calculated for the slope and intercept of the regression line. If the hypothesis that females are more variable than males were true, the regression line would be deflected towards the female axis. The slope of the regression line was compared to a null hypothesis slope of 1, a slope indicating equal CVs for male and female phenotypes. This analysis was repeated on the specific subsets of data as specified by the phenotypic categories given by the PGA, consisting of Biochemistry, Cardiac, Lung, Respiratory, Renal (consisting of Renal A and Renal B) and Vascular. Phenotypes measured in multiple phenotypic categories were consolidated to one point for the “All Phenotypes” Deming regression by keeping the measurement with the greatest ration of female to male CV (i.e. the most female-variable data) and excluding the others; elimination of these phenotypes reduced the number of phenotypes analyzed from 160 to 141. All measurements were used for the Deming regression on the specific phenotypic categories. Deming regression and slope testing were performed in SigmaPlot 12.
Statistical analysis
Individual statistical analyses are described in their respective sections of the methods. Methods were implemented in R 3.0.1, Matlab R2014b, and SigmaPlot12 as indicated. Unless otherwise noted, data is presented as mean ± SEM.
Results
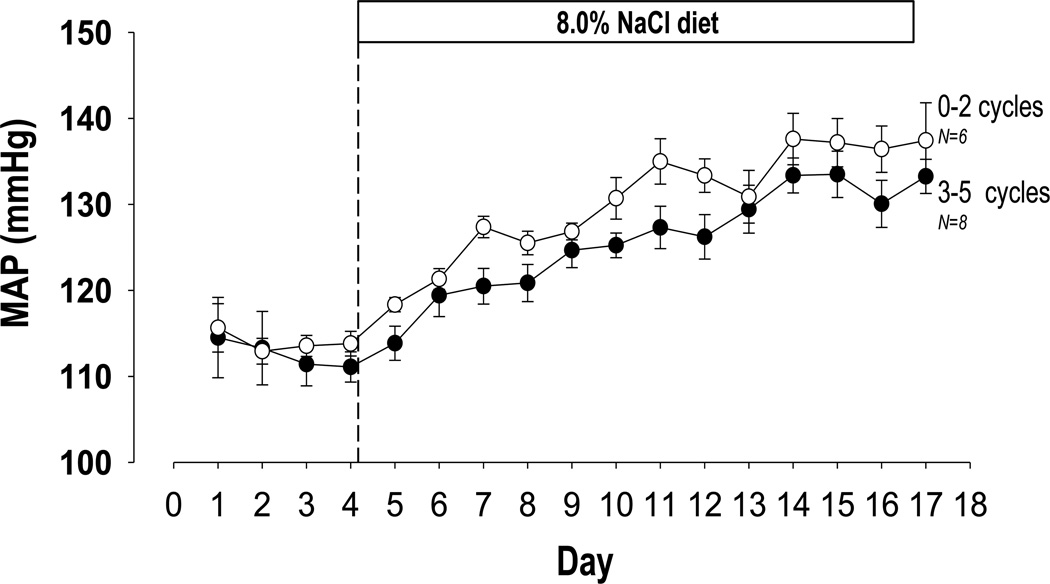
First, we sought to determine whether estrous cycling per se was necessary for maintenance of normal phenotypic outcomes and variance for mean arterial pressure, a phenotype of great clinical interest. To determine whether disruption of the estrous cycle caused by surgical procedures (arterial catheter implantation) affects variability, we examined high salt diet induced changes in mean arterial pressure in female Dahl salt-sensitive rats via implanted telemeters. Using vaginal cytometry to identify the stage in the estrous cycle17, we partitioned a group of 14 rats into those that cycled 3–5 times and rats that cycled 2 or fewer times over 18 days (Figure 1). Using a repeated measures Brown Forsythe test, we found no significant difference in variance between the two groups, nor did we find a significant difference in blood pressure between the two groups using a repeated measures ANOVA, despite the established effects of estrous cycle hormones on these phenotypes18, 19.
Figure 1. Among female Dahl S rats, disruption of the estrous cycle does not alter variance or magnitude of mean arterial pressure measurements.
Mean arterial pressure (MAP) was measured via arterial catheter in female Dahl S rats before and after switch to a high salt (8.0% NaCl) diet. While this procedure is known to disrupt the estrous cycle, no significant differences in variance (p=0.286, repeated measures Brown Forsythe test) or magnitude (p=0.179, 2-way, repeated measure ANOVA) of MAP measurements were detected between rats which cycled 3–5 times and those which cycled 2 or fewer times.
To more thoroughly examine the effects of sex on phenotypic variability, we used publicly available data collected by our department as part of the PhysGen Program for Genomic Applications 20 (pga.mcw.edu). The phenotyping component of PGA provided high throughput, detailed phenotypic data on many consomic and mutant rat strains as well as their respective parental strains. Here, we used data from the consomic panel, which includes 46 consomic strains and 11 commonly used inbred parental strains.
The Brown Forsythe test was used to examine differences in variance for each of the 142 phenotypes remaining after elimination of values mathematically derived from phenotypes otherwise accounted for in the data. Notably, we chose not to correct for multiple testing, to increase the sensitivity of the approach and more accurately represent the potential result of a future investigator who will most likely not be measuring hundreds of phenotypes in a single experiment. We found that 74 phenotypes of the 142 tested had a significant sex difference in variance (p<0.05) (Table S1). Note that variance was greater in in males for 59% (44/74) of these phenotypes despite measurements being taken from freely cycling females (Table S1). Importantly, this trend is maintained within individual strain comparisons, with greater variance in males in 399 of 601 significantly different phenotypes (Table S3).
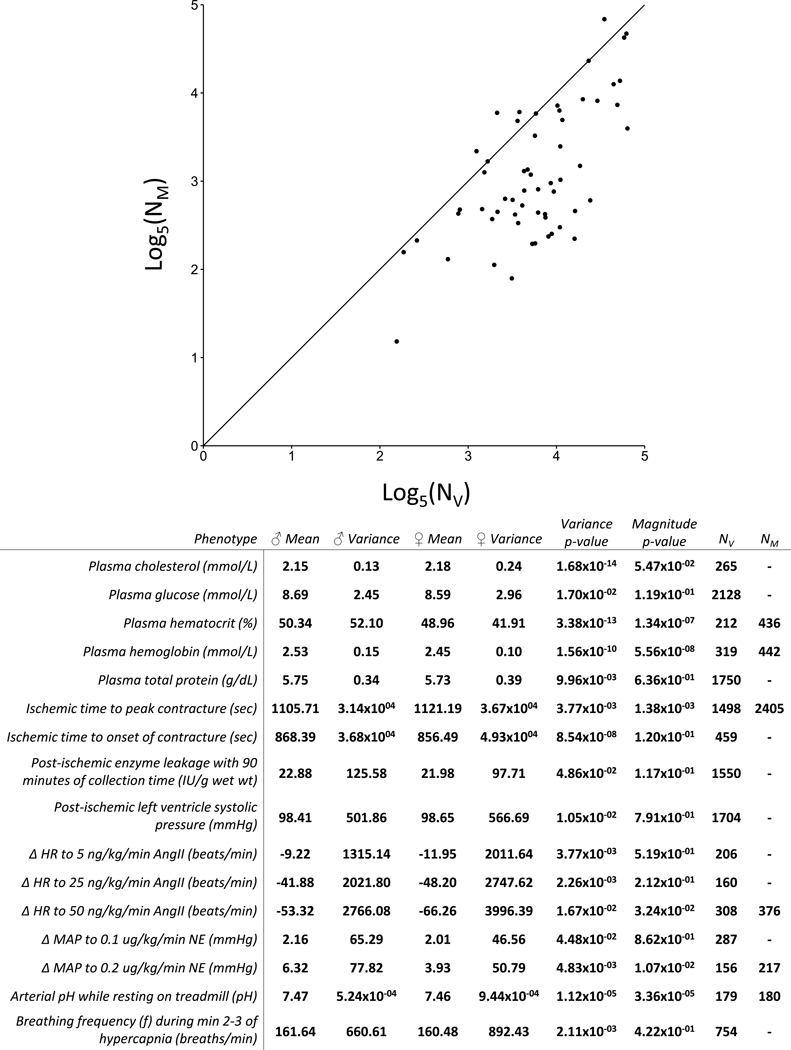
Of the 74 traits with significant differences in variance, 62 also had a significant difference in magnitude. To assess the potential impact of variation on experimental design, we performed retrospective power analyses on these 62 traits with a significant sex difference in both variance and magnitude. For the majority of these phenotypes, the number of rats necessary to detect the sex difference in variance (NV) was much larger than that needed to detect the difference in magnitude (NM) of the trait (Figure 2). For instance, based on data from the respiratory protocol, cohorts of 34 male animals and 34 female animals would be necessary to detect a difference in variance in body weight with an α of 0.05 (corresponding to the standard p<0.05 for significance) and a power of 0.8 (indicating a probability of 0.8 for discovering a true difference when such a difference exists). By contrast, only 7 animals were necessary to detect a significant difference in magnitude of body weight.
Figure 2. Detection of differences in variance between males and females requires greater sample size than detection of corresponding differences in magnitude.
Using a retrospective power analysis, we determined the number of animals needed to detect the sex differences in phenotype magnitude (NM) and phenotype variance (NV) seen in this dataset (power = 0.8, α = 0.05). For the majority of phenotypes with statistically significant sex differences in both variance and magnitude, NM was smaller than NV (56/62). 6 traits had NV greater than NM, and are listed in the table along with the 10 traits which had a difference in variance detected along with no corresponding difference in mean. These traits may form a heretofore understudied scientific field: phenotypes that differ only, or mostly, in terms of variance between the sexes.
In total, we found 16 traits that either displayed differences in variance at lower N than differences in magnitude (6), or differences in variance with no corresponding difference in magnitude detected (10) (Figure 2). Importantly, the relationship between NV and NM is maintained within the individual strain comparisons, with only 81 out of 341 strain-phenotype combinations with significant sex differences in both magnitude and variance having smaller NV than NM (Table S4).
We next sought to determine whether the detected sex differences in phenotypic variance were a consequence of the differences in magnitude of each trait. For example, note that the phenotype of body weight exhibited a significantly different variance between males and females. However, this increase in variance corresponded to an increase in magnitude of the measured trait, i.e. male rats were larger than female. This relationship can be described using the coefficient of variation, defined as the standard deviation for a trait divided by the mean value for that trait, multiplied by 100%. Using this statistic, we found the coefficients of variation were very similar between male and female rats (9.52% vs 9.99%) suggesting that the difference in variance may be explained by the difference in magnitude.
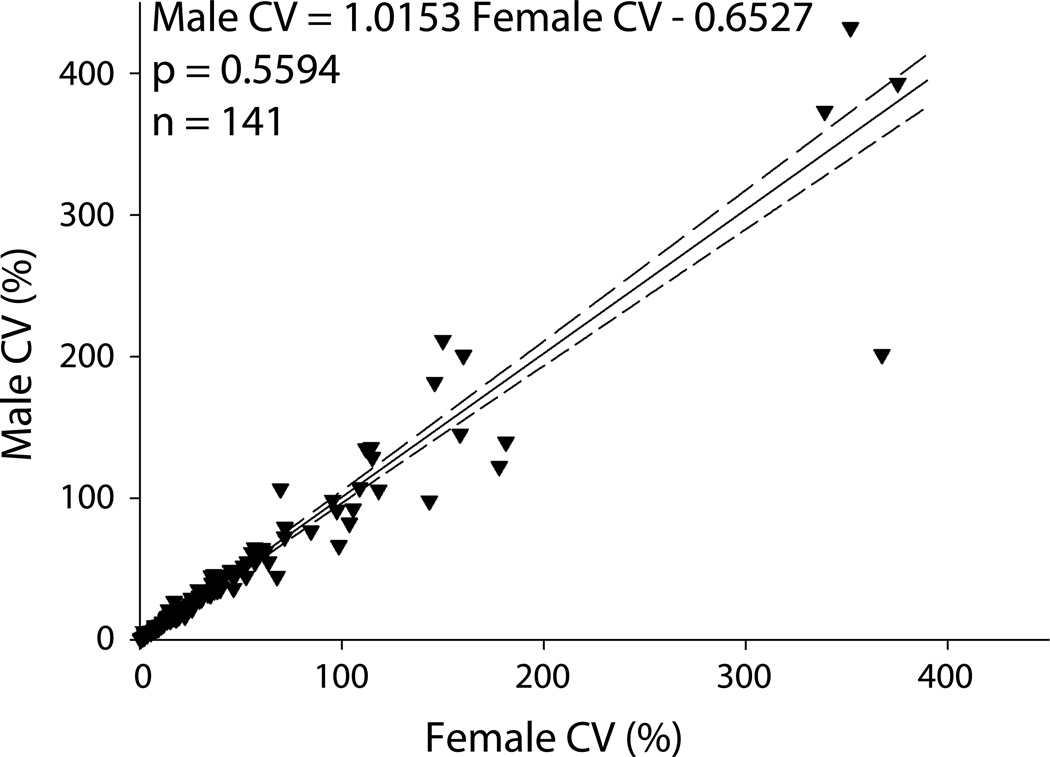
For each phenotype, we calculated the coefficient of variation for both males and females. We then plotted each of these pairs and performed an orthogonal Deming regression on the coefficients of variation (Figure 3). The slope of this regression line between males and females was not significantly different from 1 (p=0.58), which does not support the hypothesis that the female rats display greater variability.
Figure 3. Analysis of coefficients of variation among 167 phenotypes shows no significant difference between males and freely cycling females.
Coefficients of variation (CVs) were calculated for each phenotype and sex as standard deviation divided by the mean, multiplied by 100%. An unweighted Deming linear regression was then performed on the set of all male-female pairs of CVs. Dashed lines represent 95% confidence intervals for the regression line. The slope of the regression line was not significantly different from a slope of 1 (p=0.55), which does not support the hypothesis of a difference in CVs between male and female rats.
To further examine sex differences in coefficient of variation, we examined subgroups of phenotypic data based upon physiological categories from the PhysGen project (Figure S1). We did not find statistically significant evidence that the slopes of the regressions in renal, respiratory, vascular and cardiovascular phenotype categories differed from 1. There was a statistically significant difference of the slope of the regression line from 1 in the lung (p = 0.0374, trending towards female) and biochemistry (p = .0001, trending towards male) phenotype categories.
Discussion
While the scientific community continues to combat male bias in pre-clinical research, representation of women in late-phase clinical trials has improved markedly in recent years21. The NIH initiative to increase the use of female organisms and cells in pre-clinical research ensures that the scientific community as a whole will grow to fully address the health of both men and women. A major obstacle to the incorporation of female animals into studies, however, has been the belief that the rodent estrous cycle would render the study of these animals prohibitively expensive or difficult. Our study shows for the first time in a single large dataset of physiological measurements that this belief is not accurate.
Our findings show that sex differences in coefficient of variation among rats are small despite hormone fluctuations due to the estrous cycle. Moreover, for the majority of phenotypes, cohorts larger than typically used in preclinical research are required to detect the sex differences in variance described here. Taken together, the results of the analyses on coefficient of variation and variance strongly contradict the belief that female animals are inherently more variable than their male counterparts. This is not to say that the estrous cycle does not contribute to the variance of these phenotypes. Indeed, this finding may be due to the fact that male rats are more variable than is commonly appreciated, as pointed out by Prendergast in his excellent meta-analysis14. Clearly, the true nature of this variability is a worthy topic of further study. However, this analysis forms the most comprehensive evidence thus far that study designs currently acceptable for male animals can also be applied to female animals, aside from a few noted exceptions. This finding will allow us to design studies to find true differences in magnitude of traits between the sexes, which undeniably exist.
As for the phenotypes that differ only, or mostly, in terms of variance between the sexes, these may be of particular experimental interest, as they form a heretofore understudied scientific field. This could point to possible differential control systems underlying the sex-specific expression of these phenotypes. As such, they may represent unique opportunities to probe the underlying genetic or molecular systems that control physiological traits. For example, several of the traits related to angiotensin II stimulation showed differences in variance between the sexes. It has been shown that estrogen modifies the expression of both type 1 and type 2 angiotensin II receptors, leading to altered response to AngII and marked differences in physiological measurements22–25. Seltzer and colleagues found that expression of angiotensin receptors changes throughout the estrous cycle, suggesting that changing estrogen levels could lead to the increased variability observed here26. However, the finding that several traits are more variable in males than females suggests that this list is not simply a surrogate for traits affected by the estrous cycle. Clearly, further study is needed to understand the differences in variability observed here.
Perspectives
To contribute to male-female equity in preclinical research, which is both a goal and mandate of the scientific community, we sought to test the hypothesis that freely cycling female rats are more variable than male rats. Historically, female animals have been excluded from large portions of the scientific literature on the assumption that females are more variable than their male counterparts. Here, we show that variability of 142 traits in male and female rats is similar, suggesting that differential treatment of males and females for the purposes of experimental design is unnecessary until proven otherwise, rather than the other way around.
Supplementary Material
Novelty and Significance.
• What is new?
-
◦
This analysis represents the largest study in a single dataset of differences in variance between male and female rats.
-
◦
Variance and coefficient of variation were assessed for 142 phenotypes in over 50 inbred rat strains.
-
◦
Freely cycling female rats were observed to have comparable variance and coefficient of variation to their male counterparts over a broad range of cardiovascular and pulmonary phenotypes.
• What is relevant?
-
◦
The lack of increased variance in the female cohort contradicts the widely held belief that estrous cycling requires increased sample size for the study of female animals.
-
◦
The variance and coefficient of variation data provided in the manuscript and supplement offers a robust resource for the design and implementation of further studies with these rats and phenotypes.
-
◦
Phenotypes which differ primarily in variance between strains provide a novel venue for the study of sex differences.
• Summary
Variance and coefficient of variation were analyzed in 142 phenotypes in over 50 rat strains. No consistent trend towards increased variance in females was observed, indicating that study designs appropriate for males do not require increased sample size for the study of females.
Acknowledgments
Alex Dayton and Eric C Exner performed the statistical design, data analysis and interpretation, and drafted the article. John D Bukowy, Meredith Skelton and Timothy J Stodola contributed to statistical analysis and manuscript editing. Theresa Kurth collected the blood pressure data and performed the estrous cycle cytology. Andrew S Greene performed statistical design and manuscript editing. Allen W Cowley edited the manuscript and participated in data interpretation.
Sources of Funding:
This work was supported by the NIH via the National Heart, Lung, and Blood Institute (grant IDs: HL-116264, HL-082798, HL-122662).
Footnotes
Conflicts of Interest:
None.
References
- 1.Sandberg K, Umans JG Georgetown Consensus Conference Work G. Recommendations concerning the new u.S. National institutes of health initiative to balance the sex of cells and animals in preclinical research. FASEB J. 2015;29:1646–1652. doi: 10.1096/fj.14-269548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maric-Bilkan C, Arnold AP, Taylor DA, Dwinell M, Howlett SE, Wenger N, Reckelhoff JF, Sandberg K, Churchill G, Levin E, Lundberg MS. Report of the national heart, lung, and blood institute working group on sex differences research in cardiovascular disease: Scientific questions and challenges. Hypertension. 2016;67:802–807. doi: 10.1161/HYPERTENSIONAHA.115.06967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zucker I, Beery AK. Males still dominate animal studies. Nature. 2010;465:690. doi: 10.1038/465690a. [DOI] [PubMed] [Google Scholar]
- 4.Yoon DY, Mansukhani NA, Stubbs VC, Helenowski IB, Woodruff TK, Kibbe MR. Sex bias exists in basic science and translational surgical research. Surgery. 2014;156:508–516. doi: 10.1016/j.surg.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Franconi F, Brunelleschi S, Steardo L, Cuomo V. Gender differences in drug responses. Pharmacol Res. 2007;55:81–95. doi: 10.1016/j.phrs.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 6.(GAO) USGAO. Drug safety: Most drugs withdrawn in recent years had greater health risks for women. 2001 [Google Scholar]
- 7.Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2011;35:565–572. doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitley H, Lindsey W. Sex-based differences in drug activity. American Family Physician. 2009;80:1254–1258. [PubMed] [Google Scholar]
- 9.Gomes HL, Graceli JB, Goncalves WL, dos Santos RL, Abreu GR, Bissoli NS, Pires JG, Cicilini MA, Moyses MR. Influence of gender and estrous cycle on plasma and renal catecholamine levels in rats. Can J Physiol Pharmacol. 2012;90:75–82. doi: 10.1139/y11-102. [DOI] [PubMed] [Google Scholar]
- 10.Neves LA, Averill DB, Ferrario CM, Aschner JL, Brosnihan KB. Vascular responses to angiotensin-(1–7) during the estrous cycle. Endocrine. 2004;24:161–165. doi: 10.1385/ENDO:24:2:161. [DOI] [PubMed] [Google Scholar]
- 11.Sasser JM, Brinson KN, Tipton AJ, Crislip GR, Sullivan JC. Blood pressure, sex, and female sex hormones influence renal inner medullary nitric oxide synthase activity and expression in spontaneously hypertensive rats. J Am Heart Assoc. 2015;4:e001738. doi: 10.1161/JAHA.114.001738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sullivan JC, Sasser JM, Pollock JS. Sexual dimorphism in oxidant status in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2007;292:R764–R768. doi: 10.1152/ajpregu.00322.2006. [DOI] [PubMed] [Google Scholar]
- 13.Cheng SB, Dong J, Pang Y, LaRocca J, Hixon M, Thomas P, Filardo EJ. Anatomical location and redistribution of g protein-coupled estrogen receptor-1 during the estrus cycle in mouse kidney and specific binding to estrogens but not aldosterone. Mol Cell Endocrinol. 2014;382:950–959. doi: 10.1016/j.mce.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Prendergast BJ, Onishi KG, Zucker I. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci Biobehav Rev. 2014;40:1–5. doi: 10.1016/j.neubiorev.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Feng D, Yang C, Geurts AM, Kurth T, Liang M, Lazar J, Mattson DL, O'Connor PM, Cowley AW., Jr Increased expression of nad(p)h oxidase subunit p67(phox) in the renal medulla contributes to excess oxidative stress and salt-sensitive hypertension. Cell Metabolism. 2012;15:201–208. doi: 10.1016/j.cmet.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Miguel C, Guo C, Lund H, Feng D, Mattson DL. Infiltrating t lymphocytes in the kidney increase oxidative stress and participate in the development of hypertension and renal disease. American journal of physiology. Renal Physiology. 2011;300:F734–F742. doi: 10.1152/ajprenal.00454.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caligioni CS. Assessing reproductive status/stages in mice. Current protocols in neuroscience. 2009 doi: 10.1002/0471142301.nsa04is48. Appendix 4:Appendix 4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dahl LK, Knudsen KD, Ohanian EV, Muirhead M, Tuthill R. Role of the gonads in hypertension-prone rats. J Exp Med. 1975;142:748–759. doi: 10.1084/jem.142.3.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chappell MC, Westwood BM, Yamaleyeva LM. Differential effects of sex steroids in young and aged female mren2. Lewis rats: A model of estrogen and salt-sensitive hypertension. Gender Medicine. 2008;5(Suppl A):S65–S75. [Google Scholar]
- 20.Kwitek AE, Jacob HJ, Baker JE, Dwinell MR, Forster HV, Greene AS, Kunert MP, Lombard JH, Mattson DL, Pritchard KA, Jr, Roman RJ, Tonellato PJ, Cowley AW., Jr Bn phenome: Detailed characterization of the cardiovascular, renal, and pulmonary systems of the sequenced rat. Physiological Genomics. 2006;25:303–313. doi: 10.1152/physiolgenomics.00288.2005. [DOI] [PubMed] [Google Scholar]
- 21.Poon R, Khanijow K, Umarjee S, Fadiran E, Yu M, Zhang L, Parekh A. Participation of women and sex analyses in late-phase clinical trials of new molecular entity drugs and biologics approved by the fda in 2007–2009. Journal of Women's Health. 2013;22:604–616. doi: 10.1089/jwh.2012.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva-Antonialli MM, Tostes RC, Fernandes L, Fior-Chadi DR, Akamine EH, Carvalho MH, Fortes ZB, Nigro D. A lower ratio of at1/at2 receptors of angiotensin ii is found in female than in male spontaneously hypertensive rats. Cardiovascular Research. 2004;62:587–593. doi: 10.1016/j.cardiores.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 23.Xue Q, Xiao D, Zhang L. Estrogen regulates angiotensin ii receptor expression patterns and protects the heart from ischemic injury in female rats. Biol Reprod. 2015;93:6. doi: 10.1095/biolreprod.115.129619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xue B, Pamidimukkala J, Lubahn DB, Hay M. Estrogen receptor-alpha mediates estrogen protection from angiotensin ii-induced hypertension in conscious female mice. American journal of physiology. Heart and Circulatory Physiology. 2007;292:H1770–H1776. doi: 10.1152/ajpheart.01011.2005. [DOI] [PubMed] [Google Scholar]
- 25.Xue B, Pamidimukkala J, Hay M. Sex differences in the development of angiotensin ii-induced hypertension in conscious mice. American Journal of Physiology. Heart and Circulatory Physiology. 2005;288:H2177–H2184. doi: 10.1152/ajpheart.00969.2004. [DOI] [PubMed] [Google Scholar]
- 26.Seltzer A, Pinto JE, Viglione PN, Correa FM, Libertun C, Tsutsumi K, Steele MK, Saavedra JM. Estrogens regulate angiotensin-converting enzyme and angiotensin receptors in female rat anterior pituitary. Neuroendocrinology. 1992;55:460–467. doi: 10.1159/000126157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.