Abstract
This review describes the perioperative management of patients with suspected or established pulmonary conditions undergoing non-cardiothoracic surgery, with a focus on common pulmonary conditions such as obstructive airway disease, pulmonary hypertension, obstructive sleep apnea, and chronic hypoxic respiratory conditions. Considering that postoperative pulmonary complications are common and given the increasing number of surgical procedures and the size of the aging population, familiarity with current guidelines for preoperative risk assessment and intra- and postoperative patient management is recommended to decrease the morbidity and mortality. In particular, smoking cessation and pulmonary rehabilitation are perioperative strategies for improving patients’ short- and long-term outcomes. Understanding the potential risk for pulmonary complications allows the medical team to appropriately plan the intra- and postoperative care of each patient.
Keywords: postoperative complications, intraoperative care, pulmonary, chronic obstructive pulmonary disease, pulmonary hypertension, obstructive sleep apnea
Introduction
Postoperative pulmonary complications (PPCs) are common complications that increase morbidity and mortality rates after surgery, particularly among patients with pulmonary conditions.1 After a major non-cardiothoracic surgery, pulmonary complications are just as common as cardiac complications.1 Therefore, efforts to stratify the risk for PPCs and implement strategies to reduce these risks will improve patient outcomes. After describing the incidence of and risk factors for PPCs, we review general preoperative evaluation and intra- and postoperative management strategies for patients with pulmonary conditions undergoing non-cardiothoracic surgery. Furthermore, we discuss specific perioperative management strategies appropriate for patients with different pulmonary conditions.
Incidence of PPCs
Among patients undergoing non-cardiothoracic surgery, the overall incidence of PPCs varies from 2% to 19%, in part due to differences in the definition of PPCs.2,3 Approximately 10%–30% of patients who require general anesthesia experience PPCs, which can be more serious than postoperative thromboembolic, cardiovascular, or infectious complications.4,5 PPCs can be classified as major or minor based on their potential for mortality (Table 1). In particular, up to 90% of patients develop some degree of atelectasis during anesthesia due to patient positioning and loss of functional residual capacity.6 Although the overall risk for serious PPCs, such as acute respiratory distress syndrome, is low (0.2%), it is higher in patients with renal failure, chronic obstructive pulmonary disease (COPD), emergency surgery, or those who receive several anesthetics.7
Table 1.
Major and minor PPCs in patients undergoing noncardiothoracic surgery.8
| MAJOR |
|---|
| Acute or worsening respiratory failure |
| Requirement of mechanical ventilation and/or intubation for >48 hours |
| Pneumonia |
| Postoperative arrhythmia/heart failure, especially in patients with pulmonary hypertension |
| Hemodynamic instability in patients with pulmonary vascular disease |
| Worsening of obstructive sleep apnea |
| MINOR |
| Clinically significant atelectasis |
| Purulent tracheobronchitis |
| Bronchospasm/exacerbation of underlying chronic lung disease |
Risk Factors for PPCs
Since the 2000s, multiple evaluation scoring systems have been developed to allow evidence-based risk stratification for the prediction of PPCs.9,10 Because most risk factors are unmodifiable, strategies to reduce complications do not generally attempt to reduce or eliminate particular risk factors.
In 2006, the American College of Physicians (ACP) published the first guidelines on risk assessment for and strategies to reduce PPCs in patients undergoing non-cardiothoracic surgery.11–13 Any patient with dyspnea or cough should be carefully evaluated with a thorough history and physical examination. An increased risk for PPCs is associated with a history of cardiac failure, functional limitation, COPD, current smoker status, an American Society of Anesthesiologist (ASA) category of ≥2, or an age of ≥60 years.14 For elective non-cardiothoracic surgery, preoperative risk assessment can identity patients who should be treated more aggressively to reduce the risk for PPCs.2 However, in patients undergoing emergency surgery, a preoperative pulmonary risk assessment is generally not helpful, as the procedure must be undertaken regardless of the risk.
The most worrisome PPC is respiratory failure, which is characterized by impaired pulmonary gas exchange. Respiratory failure often leads to prolonged mechanical ventilation, lengthy intensive care unit (ICU) stays and associated complications, and increased mortality.15 Several investigators have attempted to predict postoperative respiratory failure.10,16–18 In particular, Canet et al17 identified the following seven independent risk factors for postoperative respiratory failure: (1) low preoperative peripheral capillary oxygen saturation (SpO2), (2) at least one preoperative respiratory symptom, (3) preoperative chronic liver disease, (4) history of congestive heart failure, (5) open intrathoracic or upper abdominal surgery, (6) surgical procedure lasting ≥2 hours, and (7) emergency surgery.
Preoperative evaluation of the risk for PPCs must take into account both patient-related and intraoperative risk factors (Table 2).
Table 2.
Most common risk factors for PPCs.
| PATIENT-RELATED RISK FACTORS |
|---|
| COPD |
| Age |
| Inhaled tobacco use |
| New York Heart Association class II pulmonary hypertension |
| Moderate/severe obstructive sleep apnea |
| Nutrition status |
| INTRAOPERATIVE RISK FACTORS |
| Surgery site (thoracic or abdominal) |
| Duration of surgery |
| General anesthesia |
| Use of long-acting neuromuscular blockers |
| Emergency surgery |
Patient-related risk factors
Classic patient-related risk factors for PPCs include age (with increased risk for each decade of life after 60 years), ASA category of ≥2, functional limitation, hypoalbuminemia, current smoker status, and the presence of COPD or congestive heart failure. According to ACP guidelines, age is the single most powerful predictor among patient-related risk factors.13
As another important risk factor, smoking affects the respiratory tract as well as cardiovascular function and blood clotting factors.19,20 Therefore, an estimate of tobacco use can help clinicians judge a patient’s pulmonary status. An estimate of ≥20 pack-years can predict early stages of small airway involvement and airflow restrictions seen on spirometry, even in asymptomatic patients. Furthermore, a significant smoking history should lead clinicians to assume that COPD and associated pulmonary and cardiac disorders may be an issue, even in the presence of normal spirometry results. As the use of other inhaled tobacco products (eg, e-cigarettes, pipes, cigars, and marijuana) and exposure to second-hand smoke may produce effects similar to those of cigarettes, a careful history must be undertaken.19
Smoking increases hemoglobin concentrations and platelet aggregation, which increases the risk for thrombosis. A recent meta-analysis of 9354 studies concluded that preoperative smoking is associated with an increased risk for general morbidity, wound complications, general infections, pulmonary and neurological complications, and admission to the ICU after surgery.21 Stopping smoking before elective surgery can reduce the risk for PPCs, although the optimal duration of smoking cessation remains unclear. Recent data suggesting that stopping smoking a few weeks prior to surgery might worsen clinical outcomes are unfounded.19 Rather, a systematic review of 25 studies on the optimal timing of smoking cessation concluded that at least 3–4 weeks of abstinence from smoking reduces respiratory and wound-healing complications.20 Preoperative cessation of smoking within 2–4 weeks of surgery decreases airway secretions and hyperresponsiveness and improves mucociliary transport. Furthermore, smoking cessation even closer to the date of surgery decreases carboxyhemoglobin levels and improves tissue O2 utilization.
Intraoperative risk factors
Additional risk factors for PPCs are related to anesthesia and surgical parameters. For instance, sedatives and many drugs used to induce and maintain anesthesia can depress respiration and the autonomic nervous system. Several studies show that patients who receive general anesthesia have higher incidences of postoperative pneumonia, prolonged ventilator dependence, and unplanned postoperative intubation.22 Surgical risk factors for PPCs include the duration of surgery (>3 hours), abdominal or thoracic surgery, neurosurgery, head and neck surgery, vascular surgery including aortic aneurysm repair, emergency operations, use of general anesthesia, and nonselective nasogastric tube placement. The risk for PPCs is also elevated by transfusion of more than four units of packed red blood cells. Abdominal surgery, particularly in the upper abdomen, confers a high risk for PPCs due to the proximity of the surgical incision to the diaphragm and the potential for splinting and shallow breaths after surgery.11,12,23 Furthermore, several studies indicate that laparoscopic techniques confer less of a risk for PPCs than older surgical techniques.13,24–26
General Perioperative Evaluation and Management of Patients
In 2002, the ASA issued a practice advisory on preanesthesia evaluation that stated “preoperative tests should not be ordered routinely [but] may be ordered, required, or performed on a selective basis for purposes of guiding or optimizing perioperative management”.27 Smetana and Macpherson28 found that the incidence of abnormalities identified during preoperative testing that led to changes in patient management ranged from 0% to 3%, and abnormal findings regarding hemoglobin, electrolytes, and kidney function were the only predictors of postoperative complications. For patients with suspected or known pulmonary diseases, however, a meticulous preoperative evaluation is needed because regional or general anesthesia can precipitate several unwanted physiologic events caused by positive pressure ventilation, patient positioning, and drugs used during general anesthesia. Patient positioning, pain, and pharmacologic agents can worsen the reductions in lung volumes, thereby diminishing functional residual capacity and vital capacity. The development of atelectasis can lead to hypoxemia and the translocation of bacteria to the bloodstream, which can increase the risk for ventilator-induced lung injury and sepsis.4
Preoperative evaluation
The most important tools for assessing the risk for PPCs during a preoperative evaluation are a careful and thorough history and physical examination followed by targeted laboratory testing.
History and physical examination
A thorough medical history and physical examination are essential for all patients planned for elective surgery to estimate preoperative pulmonary reserve and the severity of pulmonary compromise. This will permit the identification of signs and symptoms of lung and lung-related conditions and non-pulmonary disorders, such as heart failure, that increase PPCs. A focus on smoking history and exposure to recognized pulmonary toxic medications (eg, bleomycin and amiodarone) or environmental/occupational contamination (eg, coal dust, second-hand smoke, and asbestos) is important. Searching for evidence of respiratory symptoms, limited exercise capacity, preexisting lung disease, and/or recent respiratory infections or exacerbation of lung diseases is also imperative. The physical examination should also include a screen for cardiopulmonary disorders. This information, together with targeted laboratory testing, will allow the estimation of a patient’s risk of developing PPCs. The ASA physical status classification is a useful and widely accepted tool for predicting PPCs.12
Laboratory testing
Chest imaging with radiography or computed tomography (CT) is one of the most commonly used methods of stratifying the risk for PPCs. Findings of routine chest radiography and/or CT are rarely unexpected. Cardiomegaly, vascular congestions, and hyperinflation consistent with COPD generally do not enhance information already available from the patient’s history and examination. Occasionally, CT scans may provide information useful to the anesthesiologist regarding tracheal size and estimation of total lung capacity.29
Pulmonary function tests are another common methods of determining the risk for PPCs. Spirometry with or without the measurement of lung volume and diffusion capacity of the lung for CO can be used to identify patients at higher risk for perioperative morbidity and mortality due to high-risk abdominal and resectional thoracic surgeries.13 However, there is insufficient evidence supporting the use of preoperative spirometry to stratify PPC risk for patients undergoing non-thoracic surgery. In 2006, the ACP recommended that “preoperative spirometry and chest radiography should not be used routinely for predicting risk for PPCs”.11,12 In practice, the value of pulmonary function tests and chest imaging is higher for patients with unexplained dyspnea.12
An estimate of a patient’s exercise capacity before they report shortness of breath is reflective of their cardiopulmonary status. If a patient is able to climb two flights of stairs or walk ~0.4 miles or 350–400 m at a reasonable pace (3.5 miles/hour) without dyspnea, this surpasses the threshold of four metabolic equivalents that are required for elective surgery. Lower levels of exercise capacity increase the risk for both cardiac and pulmonary complications. An informal and simple “exercise test” during a preoperative evaluation consists of walking with the patient during the interview to judge their ability to exercise without dyspnea.30–32
By contrast, formal cardiopulmonary exercise testing is a complicated and sophisticated technique involving electrocardiogram recordings, assessment of breathing patterns, and measurement of O2 uptake during exercise. Cardiopulmonary exercise testing is recommended for high-risk patients undergoing thoracic resection, and a maximal O2 consumption of <15 mL/kg/minute indicates an increased risk for perioperative complications.33
Other laboratory test results indicating an increased risk for PPCs include elevated serum blood urea nitrogen (>30 mg/dL), low serum albumin (<3.5 mg/dL), and low hemoglobin. Although arterial blood gas concentrations do not predict PPCs, their measurement allows better planning of surgery for high-risk patients.12
Preoperative checklist
The physician in charge of the preoperative evaluation works in conjunction with the primary care provider, surgeon, anesthesiologist, pulmonologist, and other specialists as needed. Planning, communication, and coordination are vital for a good outcome. A proposed preoperative checklist includes:
Evaluating functional capacity and physical status.
Choosing elective versus emergency surgery.
Examining previous history of surgery and anesthesia.
Determining the presence of infectious symptoms.
Assessing oxygenation and ventilation status, especially for patients with COPD or restrictive lung disorders.
Controlling underlying conditions and optimizing respiratory status.
Reviewing and optimizing home medications. Continuing home medications before, during, and after surgery.
Evaluating and optimizing treatment of coexisting disorders.
Correcting fluid and electrolyte levels, especially for patients on diuretics or bronchodilators.
Ensuring smoking cessation.
Managing nutrition for obese or malnourished patients.
Avoiding preoperative administration of benzodiazepines for patients with severe asthma or COPD. The administration of sedatives during asthma attacks has been linked to death.34
Using lung expansion maneuvers.
In particular, using lung expansion maneuvers is an effective method of preventing PPCs, as adverse effects of surgery on lung and chest wall mechanics can predispose patients to atelectasis and respiratory infections.12 Lung expansion maneuvers include incentive spirometry, deep breathing exercises, or continuous positive airway pressure (CPAP) therapy. These maneuvers are usually recommended after high-risk surgery, especially abdominal procedures.
Incentive spirometry is the most widely used lung expansion technique, but data regarding its efficacy in preventing PPCs are contradictory. A recent single-center randomized controlled trial by Tyson et al35 including adults who underwent exploratory laparotomy showed that patient education and unmonitored incentive spirometry did not improve pulmonary dynamics or decrease PPCs following laparotomy. An important caveat, however, is that patients in both the control and intervention groups participated in postoperative deep breathing exercises, which reduce PPCs in patients undergoing elective upper abdominal surgery as well as decrease atelectasis and improve pulmonary function in patients undergoing coronary artery bypass grafting surgery.
CPAP therapy has consistently been shown to decrease the rates of reintubation, PPCs, and other complications in patients undergoing thoracic or non-thoracic surgery.36,37
However, CPAP trials have used different methodologies, making it difficult to define the optimal parameters.
Inspiratory muscle training (IMT) is an individualized, highly labor-intensive type of lung expansion maneuver. IMT consists of incentive spirometry, patient education in active breathing techniques, and forced expiration techniques. Preoperative IMT is believed to increase inspiratory muscle strength by applying a resistive load to inspiratory muscles to achieve a training effect. This training can be achieved by isocapnic/normocapnic hyperpnea, inspiratory resistive training, or threshold pressure loading. A meta-analysis by Mans et al38 showed reduced PPCs after cardiac, thoracic, and open abdominal surgery in patients who received IMT.
In summary, all modalities of lung expansion seem to be effective in preventing PPCs, although the contribution of incentive spirometry remains unclear. Elderly patients or those who are unable to perform deep breathing exercises or incentive spirometry should be considered for CPAP therapy.
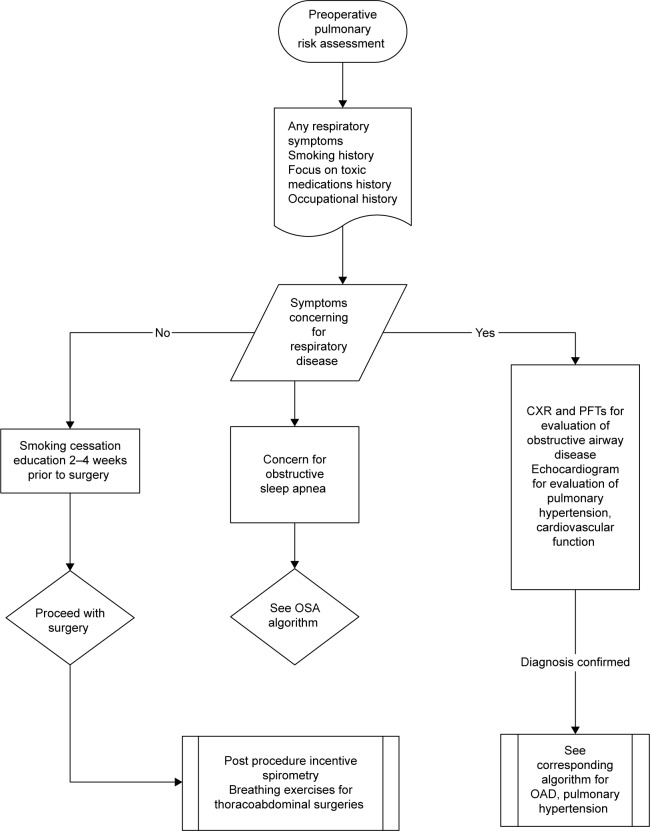
A suggested preoperative management algorithm for patients with respiratory symptoms is shown in Figure 1.
Figure 1.
Algorithm for the perioperative management of patients with respiratory symptoms.
Intra- and postoperative management of patients
Common chronic pulmonary conditions identified during the preoperative evaluation are COPD, pulmonary hypertension (PH), obstructive sleep apnea (OSA), and chronic hypoxic or restrictive lung diseases.1,2,13 Interventions aimed at decreasing PPCs should begin prior to surgery and continue throughout the intra- and postoperative periods regardless of the risk of developing PPCs.
Intraoperative management
The focus during the intraoperative period is on:
Carefully selecting the best surgical approach based on the patient’s risk for PPCs.
Managing medications. Medications used during surgery may contribute to PPCs by decreasing respiratory muscle tone or augmenting airway closure, generating atelectasis. The three primary options during anesthesia are general anesthesia alone, regional anesthesia alone, or a combination of both. Newer inhaled anesthetics, intravenous anesthetics (eg, propofol), and suitable opioid and neuromuscular blocking agents can decrease anesthetic-related risks. Ketamine is the only anesthetic agent that does not cause intraoperative atelectasis.39
Using ventilator graphics to help manage respiratory mechanics and reduce dynamic hyperinflation. Ventilator settings during surgery should provide adequate oxygenation and ventilation and limit lung injury from barotraumas, volutrauma, and atelectrauma. Low tidal volume ventilation and positive end-expiratory pressure (PEEP), which improve survival of patients with acute respiratory distress syndrome, may also reduce PPCs when used intraoperatively with periodic recruitment in patients undergoing abdominal surgery.40–42
Postoperative management
The postoperative period starts as soon as the surgery is completed. A multidisciplinary approach reduces the risk for postoperative complications. The focus during this period is on:
Selectively using nasogastric tube decompression. A fair amount of evidence supports the selective rather than routine use of nasogastric tubes after abdominal surgery. The ACP guidelines define selective use as: (a) no nasogastric tube or (b) insertion of a nasogastric tube during surgery and its withdrawal in the immediate postoperative period (ie, within 24 hours). The decision to insert a nasogastric tube should be based on clinical features such as nausea, vomiting, ileus, or abdominal distension.12
Deciding whether to transfer the patient to the ICU or a monitored setting after surgery. This decision depends on the type of surgery, the severity of the patient’s condition, and the risk for postoperative complications. The decision should be individualized for each patient and planned ahead of time if possible.
Managing ventilation. Patients on invasive mechanical ventilation should be immediately evaluated for weaning protocols and, whenever possible, ventilated in pressure support mode.8
Determining the need for supplemental O2. Patients with underlying pulmonary conditions and spontaneous breathing should be evaluated for the need for supplemental O2 by facemask or catheter.
Using arterial blood gas or pulse oximetry and end tidal carbon dioxide (ETCO2).
Preventing thromboembolism. If possible, this should start during the pre- or intraoperative period for high-risk patients.
Continuing preoperative medications.
Initiating or continuing lung expansion maneuvers. Specific techniques depend on patient condition and institutional availability.
Close monitoring of electrolyte and glucose levels in patients receiving systemic corticosteroids.
Ensuring early mobilization, which is crucial for promoting lung function and limiting/avoiding atelectasis. Maximizing upright posture is key to minimizing aspiration risks.43
Controlling postoperative pain, which may cause splinting and decreased chest and diaphragmatic excursion after high-risk surgery, contributing to decreased lung volume and micro-atelectasis. This may initiate a cascade of events resulting in lobar atelectasis, respiratory failure, or pneumonia. Several randomized controlled trials have addressed the relationship between postoperative analgesic regimens and PPC rates. In general, the use of epidural opioids seems to reduce the risk for atelectasis and pulmonary infection compared with systemic opioids.44
Disease-Specific Perioperative Management of Patients
Obstructive airway diseases
Increased airway hyper-responsiveness is a major concern during the perioperative management of patients with bronchial asthma or COPD. Although asthma and COPD are related disorders with similar perioperative management concerns, it is important to understand specific differences between the disorders. Good preoperative control can reduce the incidence of life-threatening perioperative complications.45
Asthma is a chronic inflammatory disorder of the airways caused by a complex interaction of cells, mediators, and cytokines that results in inflammation.46 This inflammation is associated with airway hyperresponsiveness and variable, often reversible airflow obstruction.46,47
The peak prevalence of asthma is in childhood, when it affects about 10% of the population. This declines to about 5%–6% during adolescence and early adulthood. The prevalence rises again to 7%–9% during later adulthood. Exogenous risk factors for asthma include house dust and house dust mites, and endogenous risk factors include an allergic predisposition and hereditary factors.46 Although previous studies report that surgical patients with asthma have an increased risk for PPCs,48 recent studies suggest that controlled asthma is not a risk for PPCs.3
Like asthma, COPD is an inflammatory disease of the lungs. Lung damage in COPD is caused by proinflammatory mediators, oxidative stress, and proteolytic digestion of lung tissue. An estimated 210 million people worldwide have COPD, which ranks as the 12th largest disease burden in the world. The number of patients with COPD continues to rise dramatically, and it has become the third leading cause of death in the United States. In the developed world, cigarette smoking is the leading cause of COPD. In underdeveloped areas of the world, indoor burning of fossil fuels for cooking and heating is another important cause.49
COPD is mainly a disease of middle-aged and elderly individuals, who are also those most likely to need surgical procedures. The physiologic hallmark of COPD is limited expiratory flow with relatively preserved inspiratory flow, with a characteristic reduction in the proportion of a person’s vital capacity that can be expired in the first second of forced expiration (forced expiratory volume 1/forced vital capacity).50,51 In contrast to asthma, COPD is a well-known independent risk factor for the development of PPCs after thoracic or non-thoracic surgery. Different from patients undergoing thoracic surgery, however, there is no increase in risk with increasing severity of airflow limitation in patients undergoing non-cardiothoracic surgery.52 Gupta et al53 showed that COPD was independently associated with higher postoperative morbidity and mortality, and multivariate analyses show that COPD is associated with an increased risk for postoperative pneumonia, respiratory failure, myocardial infarction, cardiac arrest, sepsis, return to the operating room, renal insufficiency or failure, and wound dehiscence.54 Lawrence et al55 show that abnormal chest examination, including decreased breath sounds, prolonged expiration, or the presence of rales, wheezes, or rhonchi, is the strongest predictor of PPCs.
Therapeutic options during the preoperative period include quick-acting (ie, relievers) and long-acting (ie, controllers) medications. The relievers for acute exacerbations include beta-2 adrenergic agonists. In patients with asthma, either inhaled or systemic corticosteroids can be used for more difficult-to-control attacks. Long-acting medications include long-acting beta-2 adrenergic agonists, inhaled steroids, leukotriene modifiers, inhaled anticholinergics, and immunoglobulin E or anti-interleukin-5 immunotherapy. For patients with asthma who received systemic glucocorticosteroids within the past 6 months, systemic coverage is recommended during the surgical period to avoid the development of adrenal insufficiency (ie, 100 mg hydrocortisone every 8 hours intravenously or equivalent), and medication should be rapidly tapered within 24 hours after surgery.56–59
Life-threatening episodes of asthma can occur during surgery. Bronchospasm can develop in patients with bronchial hyperreactivity after tracheal intubation and during the immediate extubation period. These patients may benefit from short-acting beta-2 adrenergic agonists and systemic corticosteroid pretreatment daily for five days. Silvanus et al60 observed fewer episodes of bronchospasm during intubation in patients with bronchial hyperreactivity who were naive to bronchodilators when they were pretreated with a beta-2 adrenergic agonist and methylprednisolone.45
The mainstay of therapy for COPD patients is short- or long-acting beta-2 adrenergic agonists or anticholinergic inhalers with or without anti-inflammatory medications. Because cholinergic tone mediated through the vagal nerve is the only reversible component in COPD, anticholinergics are the first choice, but using multiple agents may improve efficacy and reduce adverse effects. Preoperative systemic corticosteroid for patients with COPD, if indicated, is probably a safe strategy.61 In addition, Enright62 recommends the correction of fluid and electrolyte imbalance, as high-dose beta-2 adrenergic agonists can cause hypokalemia, hyperglycemia, and hypomagnesemia. In addition to electrolyte imbalance, patients with COPD show a decreased response to beta-2 adrenergic agonists and a predisposition toward cardiac arrhythmias.
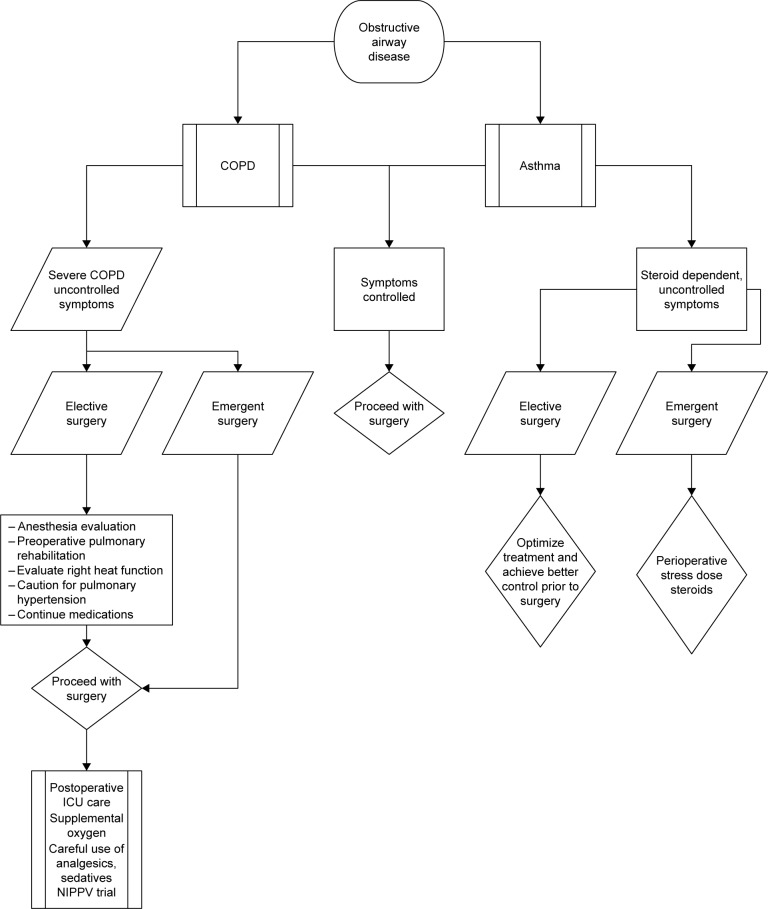
A suggested algorithm for the perioperative management of patients with obstructive airway disease is shown in Figure 2.
Figure 2.
Algorithm for the perioperative management of patients with obstructive airway disease.
Specific management of the asthmatic or COPD patients includes:
Adequately controlling airway hyperresponsiveness and detecting infection before surgery.
Evaluating changes in the quantity and quality of sputum and the presence of allergies and triggering factors for exacerbation.45
Treating infections when identified, although prophylactic use of antibiotics is not recommended.
Following Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines, which state: “To prevent postoperative pulmonary complications, stable COPD patients clinically symptomatic and/or with limited exercise capacity should be treated, before surgery, intensely with all the measures already well-established for stable COPD patients who are not about to have surgery”.61
Evaluating comorbid conditions associated with COPD and the need for pulmonary rehabilitation and medications.
Preoperatively administering steroids to patients who received systemic steroids in the prior six months.
Ensuring adequate hydration to decrease the viscosity of secretions.61 The usefulness of oral mucolytic drugs to decrease secretions has not been confirmed.
Considering preoperative pulmonary rehabilitation including respiratory muscle training and abdominal breathing for patients with severe COPD undergoing elective surgery.
Providing nutritional supplementation to patients with anorexia or pulmonary cachexia to promote weight gain and improve bodily function before elective surgery.
Obtaining an electrocardiogram for most COPD patients. Evidence of right heart strain may prompt the need for additional testing. Coexisting coronary artery disease or PH is common in these patients.63
Choosing an appropriate anesthetic agent. Some anesthetics (ie, isoflurane and ketamine) have bronchodilator properties, and other anesthetics protect against increased airway resistance during tracheal intubation (ie, propofol).45
Carefully considering the mode of anesthesia. Epidural block is effective for postoperative analgesia, but concerns regarding decreased respiratory function due to diaphragmatic or respiratory muscle paralysis exist. Spinal and epidural anesthesia are options for patients with advanced COPD in whom intubation should be avoided.45 Using data from the National Surgical Quality Improvement Program database, Hausman et al24 evaluated COPD patients undergoing surgery under general, spinal, epidural, or peripheral nerve block anesthesia. Compared with patients who received regional anesthesia, patients who received general anesthesia had higher incidences of postoperative pneumonia, prolonged ventilator dependence, and unplanned postoperative intubation. Morbidity was also increased in the general anesthesia group, whereas 30-day mortality was similar between groups.
Using bronchodilators, steroids, and lidocaine, as needed, for asthma or COPD patients at risk of developing increased airway resistance or postoperative respiratory complications due to tracheal intubation. Inhaled beta-2 adrenergic agonists before tracheal intubation can prevent increased airway resistance.64,65 Controversy remains as to whether extubation should be performed when patients are fully awake or still under anesthesia, as risk for aspiration exists if patients are extubated before complete recovery from anesthesia.61,66
Providing oxygenation and avoiding air trapping and hyperinflation for patients on mechanical ventilation. In a conventional mode of ventilation, this is achieved by prolonged expiratory time and a low respiratory rate. Permissive hypercapnia with close monitoring of pH and partial pressure of CO2 (PaCO2) in the arterial blood is acceptable. SpO2 and ETCO2 should be closely monitored, especially if the patient has chronic hypercapnia.
Carefully monitoring postoperative supplemental O2 for patients with severe COPD. A goal SpO2 of 88%–91% is acceptable. Initiation of noninvasive ventilation is recommended in cases of exacerbation of COPD.
Transferring patients with severe COPD to the ICU or a monitored setting during the early postoperative period, especially those with a high risk for cardiac complications.
Carefully using analgesics and sedatives, as they can precipitate bronchospasm or hypercapnia respiratory failure.
Pulmonary hypertension
PH is defined as a mean pulmonary artery pressure of ≥25 mmHg at rest.67 It is classified into the following five groups: (1) pulmonary arterial hypertension, (2) PH due to left heart disease, (3) PH due to chronic lung disease and/or hypoxia, (4) chronic thromboembolic PH, and (5) PH due to unclear multifactorial mechanisms. PH has recently been recognized as a significant risk factor for PPCs. Patients with PH undergoing noncardiac surgery are more likely to develop chronic heart failure, hemodynamic instability, sepsis, and respiratory failure. In addition, these patients require prolonged ventilatory support and longer ICU stays, have higher readmission rates within 30 days, and have higher mortality.68,69 This increased risk reflects the unique pathophysiology of pulmonary pressures involving differences between the right ventricle (RV), left ventricle (LV), and interventricular interdependence.
The RV differs substantially from the LV in shape and design due to differences in the functional requirements of adjacent pumps. Compared with the systemic circulation, the pulmonary circulation is a low-pressure circuit. In patients with PH, there is a gradual increase in pulmonary pressure, resulting in RV hypertrophy. In the early stages of the disease, the systolic function of the RV is preserved; however, with the progression of PH, the RV further enlarges, wall tension increases, and the RV free wall thins, leading to dilatation and reduced RV function. Tricuspid regurgitation resulting from the widening of the tricuspid annulus worsens RV function.70 Elevated RV pressure results in the bulging of the interventricular septum into the LV during diastole, which impairs LV diastolic filling. The development of specific therapies for pulmonary arterial hypertension, such as endothelin receptor antagonists, prostanoids, and phosphodiesterase 5 inhibitors, has improved the prognosis of patients with PH. The Pulmonary Hypertension Connection Registry and the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management show 1- and 3-year survival rates of approximately 85% and 69%, respectively.71,72 Thus, the perioperative management of these patients remains a challenge.
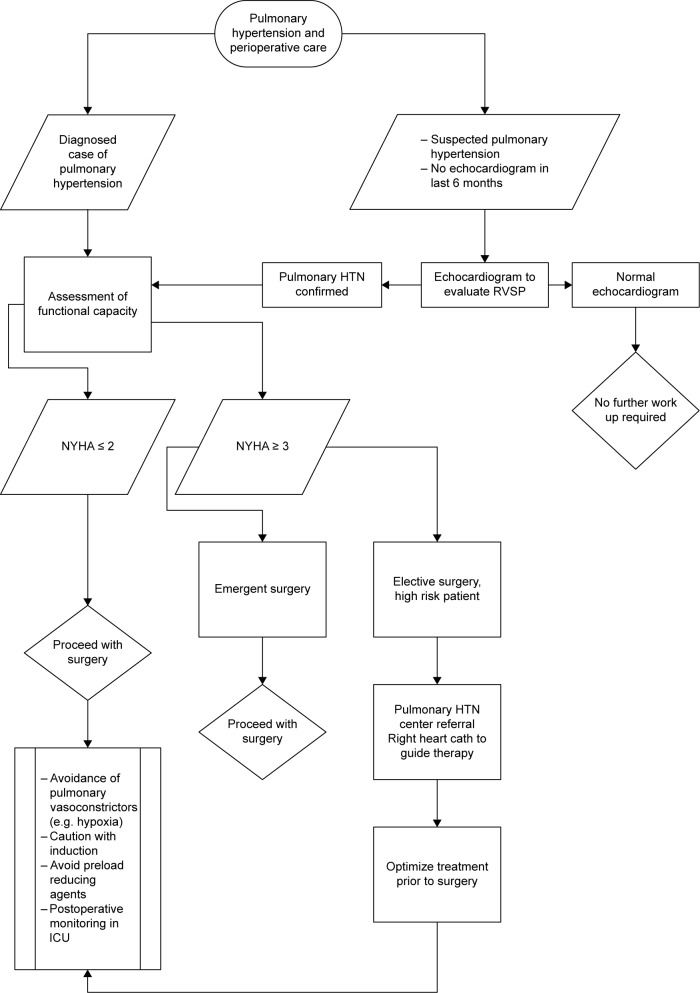
A suggested algorithm for the perioperative management of patients with PH is shown in Figure 3.
Figure 3.
Algorithm for the perioperative management of patients with pulmonary hypertension.
In addition to general recommendations, the evaluation and management of patients with PH undergoing non-cardiothoracic surgery includes:
Carefully considering the patient’s functional status if the etiology of PH is known.
Assessing the severity of PH. The clinical severity of pulmonary arterial hypertension was initially classified according to the New York Heart Association and then modified by the World Health Organization (Table 3).73 The gold standard for the diagnosis of PH and assessment of its severity is right heart catheterization. Echocardiogram is the preferred initial noninvasive test. Arbitrarily suggested criteria for detecting the presence of PH are based on tricuspid regurgitation peak velocity, Doppler-calculated pulmonary artery systolic pressure (assuming a normal right atrial pressure of 5 mmHg), and additional echocardiographic variables.73
Evaluating exercise capacity. Echocardiogram should be performed to assess the severity of PH if the last test was performed over six months ago. Exercise capacity can be assessed by the 6-minute walk test, which measures functional limitation and correlates with peak aerobic exercise capacity.74 The 6-minute walk test is a simple, inexpensive, reproducible, and well-standardized test.75
Coordinating a multidisciplinary team consisting of the primary care physician, nurses, anesthesiologist, cardiologist, and pulmonary and critical care physicians prior to surgery. Owing to the complexity of most PH treatment protocols, a decision to proceed with surgery requires a detailed discussion about the risks based on the severity of PH, functional classification, and exercise capacity. If risk is deemed very excessive (ie, New York Heart Association Class III/IV, severe PH, and poor exercise tolerance), alternative procedures or palliative care may be considered. Acute decompensation during the intra- or early postoperative period is common and can result in acute RV failure.76 For very high-risk patients, it may be reasonable to consider right heart catheterization.
Completing the preoperative evaluation and initiating specific treatments to optimize the condition of the patient if PH is newly diagnosed and the surgery is elective.
Employing a cardiovascular or critical care physician with skills in the management of PH, complex hemodynamics, and transesophageal echocardiography. Medications that can decrease blood pressure (eg, propofol) should be avoided. Airway management and ventilation is critical to avoid hypoxia. Perioperative management should include avoidance of pulmonary vasoconstrictors such as hypoxia, inspiratory pressure of >30 mmHg, high PEEP (>15 mmHg), hypercarbia, and acidosis. Strategies to promote pulmonary vasodilatation such as improving oxygenation, permissive hypocapnea (PaCO2 ≤ 30–35 mmHg), alkalosis (pH > 7.4), and optimal ventilator volume are encouraged.77
Deciding whether to postoperatively extubate and transfer the patient to the ICU depending on the recommendation of the anesthesiologist.
Closely monitoring the patient both invasively and noninvasively during the postoperative period. Central venous pressure, lactate, and cardiac output monitoring can initially be performed using LiDCO. Volume management is crucial, as both hypovolemia and hypervolemia can have deleterious effects on cardiac output. Right coronary perfusion occurs at both systole and diastole. If aortic root pressure falls in patients with PH, systolic perfusion is impaired. Any further drop in pressure will impair diastolic right coronary perfusion with catastrophic outcomes, resulting in RV infarct, worsening shock, and further drops in systolic blood pressure and aortic root pressure. Mean arterial pressure must be maintained with pressors if needed to avoid impaired right coronary perfusion. Inotropic support with dobutamine is required after RV preload is optimized. Right heart catheterization is an option for managing shock, but cardiac output measurements can be misleading owing to tricuspid regurgitation. Mixed venous saturation from pulmonary artery catheterization and lactate can be used as surrogates.
Optimizing mechanical ventilation settings. Conventional ventilator strategies include optimal oxygenation (which acts as a pulmonary venodilator), avoiding high PEEP (which decreases RV preload) and maintaining adequate minute ventilation for permissive hypocapnea and alkalosis. In instances when conventional mechanical ventilation fails, RV assist devices and venoarterial extracorporeal membrane oxygenation are options. In patients on maximal resuscitative measures, palliative care and advance directive discussions are appropriate, as the risk for mortality after cardiac arrest is very high.78,79
Table 3.
Functional classification of PH.
| Class I | Patients with PH but without limitation of physical activity. Ordinary physical activity does not cause undue dyspnea or fatigue, chest pain, or near syncope. |
| Class II | Patients with PH resulting in slight limitation of physical activity. Patients are comfortable at rest, but ordinary physical activity causes undue dyspnea or fatigue, chest pain, or near syncope. |
| Class III | Patients with PH resulting in marked limitation of physical activity. Patients are comfortable at rest, but less than ordinary activity causes undue dyspnea or fatigue, chest pain, or near syncope. |
| Class IV | Patients with PH and inability to carry out any physical activity without symptoms. Dyspnea and/or fatigue may be present at rest, and discomfort is increased by any physical activity. These patients manifest signs of right heart failure. |
Note: Criteria Committee, New York Heart Association, Inc. Diseases of the Heart and Blood Vessels. Nomenclature and Criteria for diagnosis, 6th edition Boston, Little, Brown and Co. 1964, p. 114.
Obstructive sleep apnea
Sleep apnea is a disorder characterized by recurrent episodes of complete (ie, apnea) or partial (ie, hypopnea) upper airway obstruction during sleep, resulting in awakening and oxygen desaturation. The prevalence of OSA accompanied by daytime sleepiness is 3%–7% for adult men and 2%–5% for adult women in the general population.80 However, most patients with OSA remain undiagnosed.81,82 Patients with sleep apnea are at increased risk for perioperative complications, including hypoxemia, pneumonia, difficult intubation, myocardial infarction, pulmonary embolism, atelectasis, cardiac arrhythmias, and unanticipated admission to the ICU.83–85 Comorbidities associated with OSA include obesity, hypertension, depression, gastroesophageal reflux disease, diabetes mellitus, hypercholesterolemia, and asthma.86 The administration of sedatives, anesthetics, and opioids during the perioperative period increases pharyngeal collapse and impairs ventilation and arousal responses, which worsens sleep apnea. As obesity is associated with decreased residual volume, functional residual capacity, and expiratory reserve volume,87 obese patients with OSA may have difficult airways, causing intubation, oxygenation, and ventilation problems.
During the preoperative evaluation, patients may present with diagnosed OSA undergoing noninvasive positive pressure ventilation therapy or undiagnosed OSA, especially those planned for bariatric surgery. In addition to general recommendations, the preoperative evaluation of patients with known or suspected sleep disorders includes a careful history and physical examination. Many questionnaires have been developed to identify surgical patients at high risk for OSA, including the Berlin questionnaire, ASA checklist, and the STOP BANG questionnaire (Table 4).
Table 4.
STOP BANG sleep apnea questionnaire.
| Do you snore loudly (ie, louder than talking or loud enough to be heard through closed doors)? |
| Do you often feel tired, fatigued, or sleepy during the day? |
| Has anyone observed you stop breathing during your sleep? |
| Do you have or are you being treated for high blood pressure? |
| Do you have a body mass index more than 35 kg/m2? |
| Age over 50 years old? |
| Neck circumference > 40 cm? |
| Are you male? |
Note: High risk of OSA; answering Yes to three or more questions. Low risk of OSA, answering yes to less than three items. Adapted from Chung, F et al. Anesthesiology. 2008;108:812–821.
The STOP BANG questionnaire has high sensitivity, specificity, and negative predictive value to detect moderate and severe sleep apnea. The additional presence of a serum bicarbonate level ≥28 mmol/L improves the specificity for all OSA, moderate/severe OSA, and severe OSA to 85.2%, 81.7%, and 79.7%, respectively.88
The evaluation and management of patients with OSA undergoing non-cardiothoracic surgery include:
Conducting sleep studies and initiating treatment before surgery if the preoperative evaluation detects possible OSA based on a STOP BANG score of ≥3 with a serum bicarbonate level of ≥28 mmol/L. For patients for whom surgery cannot be delayed, it may be acceptable to employ CPAP therapy during the postoperative period.
Coordinating a multidisciplinary team including a primary care physician, pulmonologist, anesthesiologist, and surgeon. Discussions should include sleep apnea severity, potential airway difficulties, type of anesthesia, and postoperative discharge versus in-patient management.
Performing a preoperative echocardiogram for patients with signs or symptoms of OSA. Patients should be counseled to be adherent to CPAP during the preoperative period, which has been shown to reduce postoperative complications.89
Extubating patients undergoing general anesthesia while awake unless contraindicated. Full reversal of neuromuscular block must be verified before extubation. When possible, extubation and recovery should be carried out in a lateral, semi-upright, or other non-supine position.90
Postoperatively providing supplemental O2 and initiating CPAP therapy.
Carefully considering the effects of sedating analgesics on upper airway collapse and depression of the respiratory drive during the postoperative period.
Closely monitoring patients with moderate to severe OSA in an ASA Class III or higher and patients with suspected OSA and elevated serum bicarbonate level in the ICU or other monitored setting, especially when use of opioids or benzodiazepines is planned. Continuous pulse oximetry and ETCO2 monitoring is desirable. Arterial blood gas analysis is recommended if evidence of hypercarbia is found.
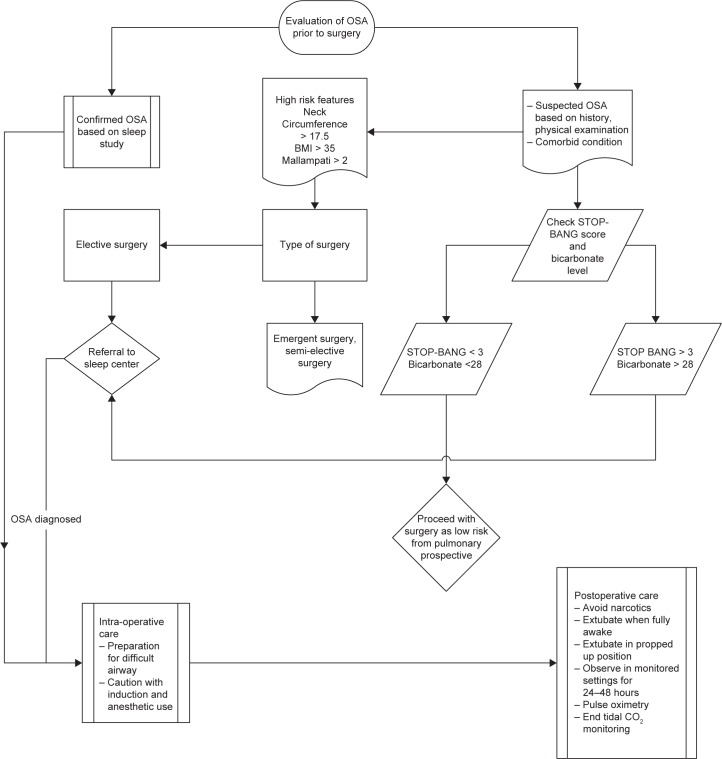
A suggested algorithm for the perioperative management of patients with OSA is shown in Figure 4.
Figure 4.
Algorithm for the perioperative management of patients with obstructive sleep apnea.
Restrictive lung disease
Numerous pathophysiological conditions and diseases may be classified as restrictive lung disease, which is characterized by decreased lung volume and reduced total and vital capacity. Patients with restrictive lung disease are at risk for increased PPCs. Causes of restrictive lung disease can be classified as intrapulmonary (ie, parenchymal) or extrapulmonary. The most common intrapulmonary causes include sarcoidosis, occupational lung diseases (eg, silicosis and asbestosis), idiopathic interstitial lung diseases, hypersensitivity pneumonitis, eosinophilic pneumonias, pulmonary alveolar proteinosis, lung resection/atelectasis, acute respiratory distress syndrome, and pulmonary edema. Extrapulmonary causes include obesity, skeletal/costovertebral deformities (eg, scoliosis), sternal deformities (eg, pectus excavatum), neuromuscular disorders, and pneumothorax/pleural effusion.
Literature on evidence-based recommendations for the perioperative management of patients with restrictive lung disease is sparse. The risks for PPCs in patients with chronic restrictive lung disease or restrictive physiology due to extrapulmonary causes have not been evaluated.13 General anesthesia and mechanical ventilation may exacerbate the inflammatory process of parenchymal fibrotic diseases and promote adult respiratory distress syndrome.91 There is an up to 60% decrease in spirometric variables in patients with scoliosis, many of whom have severe restrictive lung diseases, which contributes to prolonged postoperative mechanical ventilation. The peak fall in lung volumes occurs on the third day after surgery, and recovery to baseline levels may take up to two months.92
The perioperative evaluation and management of patients with restrictive lung diseases depends on the underlying cause. In addition to general recommendations, the focus is on:
Conducting pulmonary function tests to quantify restriction and allow better planning for anesthesia.
Evaluating exercise tolerance, which is related to the risk for PPCs.
Performing preoperative arterial blood gas analysis to estimate baseline oxygenation and ventilation.
Considering undiagnosed PH in patients with advanced interstitial lung disease. The presence of moderate/severe dyspnea at rest and a PaCO2/PaO2 ratio of >0.72 are predictive of PPCs in patients undergoing non-thoracic surgery.93
Maintaining oxygenation and ventilation during surgery.
Conclusion
In this review, we highlight the most influential studies published since the early 2000s on the preoperative evaluation of patients with respiratory conditions undergoing non-cardiothoracic surgery. A careful preoperative evaluation is highly recommended for patients with respiratory conditions who are candidates for surgery, as this can identify ways of reducing the risk for PPCs. The initial assessment is clinical and can be complemented by focused laboratory testing, whereas the general use of pulmonary function testing or chest radiography is not advocated. For patients undergoing elective surgery, the goals of the preoperative evaluation are stabilizing and controlling the underlying lung condition, maximizing lung function, ensuring smoking cessation, and instituting preoperative lung expansion maneuvers. The presence of comorbidities as well as the cardiovascular, metabolic, renal, and venous thromboembolism risks involved in anesthesia and the surgical procedure should also be evaluated.
Concerning intra- and postoperative patient management, surgical advances including minimally invasive techniques have been welcome, particular for older, more vulnerable patients with multiple comorbidities who are more likely to undergo complicated surgeries. Recent studies show that intraoperative lung protective strategies during general anesthesia decrease the rates of PPCs, improve clinical outcomes, and decrease health care utilization.40,42 Advances in postoperative care to minimize the risk for delirium include lung expansion maneuvers, early mobilization, fluid and ventilator management, thromboembolic disease prevention, and careful management of medications.
Additional research to validate or improve our current knowledge and practice of the perioperative management of patients with pulmonary conditions is needed. Areas for future research include identifying the most effective lung expansion techniques, the role of high flow oxygen during the postoperative period, the role of a dedicated multidisciplinary team to manage high-risk patients throughout the perioperative period, and the most beneficial ventilator strategies to employ during the operation. Restrictive lung diseases are common and patients are living longer. Hence studies evaluating the best evaluation and management of patients with restrictive lung diseases are needed.
Footnotes
ACADEMIC EDITOR: Charles Phillips, Editor in Chief
PEER REVIEW: Two peer reviewers contributed to the peer review report. Reviewers’ reports totaled 470 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert single-blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: GDF, SV. Analyzed the data: GDF, SV. Wrote the first draft of the manuscript: GDF, SV. Contributed to the writing of the manuscript: HRTH. Agree with manuscript results and conclusions: GDF, SV, HRTH. Jointly developed the structure and arguments for the paper: GDF, SV, HRTH. Made critical revisions and approved final version: GDF, SV. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Smetana GW, Conde MV. Preoperative pulmonary update. Clin Geriatr Med. 2008;24(4):607–624. vii. doi: 10.1016/j.cger.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Bapoje SR, Whitaker JF, Schulz T, Chu ES, Albert RK. Preoperative evaluation of the patient with pulmonary disease. Chest. 2007;132(5):1637–1645. doi: 10.1378/chest.07-0347. [DOI] [PubMed] [Google Scholar]
- 3.Fisher BW, Majumdar SR, McAlister FA. Predicting pulmonary complications after nonthoracic surgery: a systematic review of blinded studies. Am J Med. 2002;112(3):219–225. doi: 10.1016/s0002-9343(01)01082-8. [DOI] [PubMed] [Google Scholar]
- 4.Bruells CS, Rossaint R. Physiology of gas exchange during anaesthesia. Eur J Anaesthesiol. 2011;28(8):570–579. doi: 10.1097/EJA.0b013e32834942a3. [DOI] [PubMed] [Google Scholar]
- 5.Sweitzer BJ, Smetana GW. Identification and evaluation of the patient with lung disease. Anesthesiol Clin. 2009;27(4):673–686. doi: 10.1016/j.anclin.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Malbouisson LM, Humberto F, Rodrigues Rdos R, Carmona MJ, Auler JO. Atelectasis during anesthesia: pathophysiology and treatment. Rev Bras Anestesiol. 2008;58(1):73–83. doi: 10.1590/s0034-70942008000100011. [DOI] [PubMed] [Google Scholar]
- 7.Blum JM, Stentz MJ, Dechert R, et al. Preoperative and intraoperative predictors of postoperative acute respiratory distress syndrome in a general surgical population. Anesthesiology. 2013;118(1):19–29. doi: 10.1097/ALN.0b013e3182794975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Degani-Costa LH, Faresin SM, dos Reis Falcao LF. Preoperative evaluation of the patient with pulmonary disease. Braz J Anesthesiol. 2014;64(1):22–34. doi: 10.1016/j.bjane.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Arozullah AM, Daley J, Henderson WG, Khuri SF. Multifactorial risk index for predicting postoperative respiratory failure in men after major noncardiac surgery. The National Veterans Administration Surgical Quality Improvement Program. Ann Surg. 2000;232(2):242–253. doi: 10.1097/00000658-200008000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson RG, Arozullah AM, Neumayer L, Henderson WG, Hosokawa P, Khuri SF. Multivariable predictors of postoperative respiratory failure after general and vascular surgery: results from the patient safety in surgery study. J Am Coll Surg. 2007;204(6):1188–1198. doi: 10.1016/j.jamcollsurg.2007.02.070. [DOI] [PubMed] [Google Scholar]
- 11.Lawrence VA, Cornell JE, Smetana GW, American College of Physicians Strategies to reduce postoperative pulmonary complications after noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med. 2006;144(8):596–608. doi: 10.7326/0003-4819-144-8-200604180-00011. [DOI] [PubMed] [Google Scholar]
- 12.Qaseem A, Snow V, Fitterman N, et al. Risk assessment for and strategies to reduce perioperative pulmonary complications for patients undergoing noncardiothoracic surgery: a guideline from the American College of Physicians. Ann Intern Med. 2006;144(8):575–580. doi: 10.7326/0003-4819-144-8-200604180-00008. [DOI] [PubMed] [Google Scholar]
- 13.Smetana GW, Lawrence VA, Cornell JE, American College of Physicians Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med. 2006;144(8):581–595. doi: 10.7326/0003-4819-144-8-200604180-00009. [DOI] [PubMed] [Google Scholar]
- 14.Canet J, Mazo V. Postoperative pulmonary complications. Minerva Anestesiol. 2010;76(2):138–143. [PubMed] [Google Scholar]
- 15.Kor DJ, Lingineni RK, Gajic O, et al. Predicting risk of postoperative lung injury in high-risk surgical patients: a multicenter cohort study. Anesthesiology. 2014;120(5):1168–1181. doi: 10.1097/ALN.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta H, Gupta PK, Fang X, et al. Development and validation of a risk calculator predicting postoperative respiratory failure. Chest. 2011;140(5):1207–1215. doi: 10.1378/chest.11-0466. [DOI] [PubMed] [Google Scholar]
- 17.Canet J, Sabaté S, Mazo V, et al. Development and validation of a score to predict postoperative respiratory failure in a multicentre European cohort: a prospective, observational study. Eur J Anaesthesiol. 2015;32(7):458–470. doi: 10.1097/EJA.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 18.Staehr-Rye AK, Eikermann M. Eliminate postoperative respiratory complications: preoperative screening opens the door to clinical pathways that individualise perioperative treatment. Eur J Anaesthesiol. 2015;32(7):455–457. doi: 10.1097/EJA.0000000000000210. [DOI] [PubMed] [Google Scholar]
- 19.Myers K, Hajek P, Hinds C, McRobbie H. Stopping smoking shortly before surgery and postoperative complications: a systematic review and meta-analysis. Arch Intern Med. 2011;171(11):983–989. doi: 10.1001/archinternmed.2011.97. [DOI] [PubMed] [Google Scholar]
- 20.Wong J, Lam DP, Abrishami A, Chan MT, Chung F. Short-term preoperative smoking cessation and postoperative complications: a systematic review and meta-analysis. Can J Anaesth. 2012;59(3):268–279. doi: 10.1007/s12630-011-9652-x. [DOI] [PubMed] [Google Scholar]
- 21.Grønkjær M, Eliasen M, Skov-Ettrup LS, et al. Preoperative smoking status and postoperative complications: a systematic review and meta-analysis. Ann Surg. 2014;259(1):52–71. doi: 10.1097/SLA.0b013e3182911913. [DOI] [PubMed] [Google Scholar]
- 22.Bovill JG. Inhalation anaesthesia: from diethyl ether to xenon. Handb Exp Pharmacol. 2008;182:121–142. doi: 10.1007/978-3-540-74806-9_6. [DOI] [PubMed] [Google Scholar]
- 23.McAlister FA, Khan NA, Straus SE, et al. Accuracy of the preoperative assessment in predicting pulmonary risk after nonthoracic surgery. Am J Respir Crit Care Med. 2003;167(5):741–744. doi: 10.1164/rccm.200209-985BC. [DOI] [PubMed] [Google Scholar]
- 24.Hausman MS, Jr, Jewell ES, Engoren M. Regional versus general anesthesia in surgical patients with chronic obstructive pulmonary disease: does avoiding general anesthesia reduce the risk of postoperative complications? Anesth Analg. 2015;120(6):1405–1412. doi: 10.1213/ANE.0000000000000574. [DOI] [PubMed] [Google Scholar]
- 25.McAlister FA, Bertsch K, Man J, Bradley J, Jacka M. Incidence of and risk factors for pulmonary complications after nonthoracic surgery. Am J Respir Crit Care Med. 2005;171(5):514–517. doi: 10.1164/rccm.200408-1069OC. [DOI] [PubMed] [Google Scholar]
- 26.Owens WD, Felts JA, Spitznagel EL., Jr ASA physical status classifications: a study of consistency of ratings. Anesthesiology. 1978;49(4):239–243. doi: 10.1097/00000542-197810000-00003. [DOI] [PubMed] [Google Scholar]
- 27.American Society of Anesthesiologists Task Force on Preanesthesia Evaluation Practice advisory for preanesthesia evaluation: a report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology. 2002;96(2):485–496. doi: 10.1097/00000542-200202000-00037. [DOI] [PubMed] [Google Scholar]
- 28.Smetana GW, Macpherson DS. The case against routine preoperative laboratory testing. Med Clin North Am. 2003;87(1):7–40. doi: 10.1016/s0025-7125(02)00147-5. [DOI] [PubMed] [Google Scholar]
- 29.Regan EA, Lynch DA, Curran-Everett D, et al. Clinical and radiologic disease in smokers with normal spirometry. JAMA Intern Med. 2015;175(9):1539–1549. doi: 10.1001/jamainternmed.2015.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Badgett RG, Tanaka DJ, Hunt DK, et al. Can moderate chronic obstructive pulmonary disease be diagnosed by historical and physical findings alone? Am J Med. 1993;94(2):188–196. doi: 10.1016/0002-9343(93)90182-o. [DOI] [PubMed] [Google Scholar]
- 31.Reilly DF, McNeely MJ, Doerner D, et al. Self-reported exercise tolerance and the risk of serious perioperative complications. Arch Intern Med. 1999;159(18):2185–2192. doi: 10.1001/archinte.159.18.2185. [DOI] [PubMed] [Google Scholar]
- 32.Karpman C, Benzo R. Gait speed as a measure of functional status in COPD patients. Int J Chron Obstruct Pulmon Dis. 2014;9:1315–1320. doi: 10.2147/COPD.S54481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colice GL, Shafazand S, Griffin JP, Keenan R, Bolliger CT, American College of Chest Physicians Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: ACCP evidenced-based clinical practice guidelines (2nd edition) Chest. 2007;132(3 suppl):161S–177S. doi: 10.1378/chest.07-1359. [DOI] [PubMed] [Google Scholar]
- 34.Forster A, Gardaz JP, Suter PM, Gemperle M. Respiratory depression by midazolam and diazepam. Anesthesiology. 1980;53(6):494–497. doi: 10.1097/00000542-198012000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Tyson AF, Kendig CE, Mabedi C, Cairns BA, Charles AG. The effect of incentive spirometry on postoperative pulmonary function following laparotomy: a randomized clinical trial. JAMA Surg. 2015;150(3):229–236. doi: 10.1001/jamasurg.2014.1846. [DOI] [PubMed] [Google Scholar]
- 36.Tusman G, Böhm SH, Warner DO, Sprung J. Atelectasis and perioperative pulmonary complications in high-risk patients. Curr Opin Anaesthesiol. 2012;25(1):1–10. doi: 10.1097/ACO.0b013e32834dd1eb. [DOI] [PubMed] [Google Scholar]
- 37.Kindgen-Milles D, Müller E, Buhl R, et al. Nasal-continuous positive airway pressure reduces pulmonary morbidity and length of hospital stay following thoracoabdominal aortic surgery. Chest. 2005;128(2):821–828. doi: 10.1378/chest.128.2.821. [DOI] [PubMed] [Google Scholar]
- 38.Mans CM, Reeve JC, Elkins MR. Postoperative outcomes following preoperative inspiratory muscle training in patients undergoing cardiothoracic or upper abdominal surgery: a systematic review and meta analysis. Clin Rehabil. 2015;29(5):426–438. doi: 10.1177/0269215514545350. [DOI] [PubMed] [Google Scholar]
- 39.Hedenstierna G, Edmark L. The effects of anesthesia and muscle paralysis on the respiratory system. Intensive Care Med. 2005;31(10):1327–1335. doi: 10.1007/s00134-005-2761-7. [DOI] [PubMed] [Google Scholar]
- 40.Ladha K, Vidal Melo MF, McLean DJ, et al. Intraoperative protective mechanical ventilation and risk of postoperative respiratory complications: hospital based registry study. BMJ. 2015;351:h3646. doi: 10.1136/bmj.h3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guay J, Ochroch EA. Intraoperative use of low volume ventilation to decrease postoperative mortality, mechanical ventilation, lengths of stay and lung injury in patients without acute lung injury. Cochrane Database Syst Rev. 2015;12:CD011151. doi: 10.1002/14651858.CD011151.pub2. [DOI] [PubMed] [Google Scholar]
- 42.Futier E, Constantin JM, Paugam-Burtz C, et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med. 2013;369(5):428–437. doi: 10.1056/NEJMoa1301082. [DOI] [PubMed] [Google Scholar]
- 43.Haines KJ, Skinner EH, Berney S, Austin Health POST Study Investigators Association of postoperative pulmonary complications with delayed mobilisation following major abdominal surgery: an observational cohort study. Physiotherapy. 2013;99(2):119–125. doi: 10.1016/j.physio.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 44.Liu SS, Wu CL. Effect of postoperative analgesia on major postoperative complications: a systematic update of the evidence. Anesth Analg. 2007;104(3):689–702. doi: 10.1213/01.ane.0000255040.71600.41. [DOI] [PubMed] [Google Scholar]
- 45.Yamakage M, Iwasaki S, Namiki A. Guideline-oriented perioperative management of patients with bronchial asthma and chronic obstructive pulmonary disease. J Anesth. 2008;22(4):412–428. doi: 10.1007/s00540-008-0650-2. [DOI] [PubMed] [Google Scholar]
- 46.Global Strategy for Asthma Management and Prevention Global Initiative for Asthma (GINA) 2011. Available at: http://www.ginasthma.org.
- 47.Holgate ST, Davies DE, Puddicombe S, et al. Mechanisms of airway epithelial damage: epithelial-mesenchymal interactions in the pathogenesis of asthma. Eur Respir J Suppl. 2003;44:24s–29s. doi: 10.1183/09031936.03.00000803. [DOI] [PubMed] [Google Scholar]
- 48.Shnider SM, Papper EM. Anesthesia for the asthmatic patient. Anesthesiology. 1961;22:886–892. doi: 10.1097/00000542-196111000-00003. [DOI] [PubMed] [Google Scholar]
- 49.Snider GL. Chronic obstructive pulmonary disease: risk factors, pathophysiology and pathogenesis. Annu Rev Med. 1989;40:411–429. doi: 10.1146/annurev.me.40.020189.002211. [DOI] [PubMed] [Google Scholar]
- 50.Mannino DM, Gagnon RC, Petty TL, Lydick E. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2000;160(11):1683–1689. doi: 10.1001/archinte.160.11.1683. [DOI] [PubMed] [Google Scholar]
- 51.Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. [Accessed April 16, 2012]. http://www.goldcopd.org/uploads/users/files/GOLD2011_Summary.pdf.
- 52.Wong DH, Weber EC, Schell MJ, Wong AB, Anderson CT, Barker SJ. Factors associated with postoperative pulmonary complications in patients with severe chronic obstructive pulmonary disease. Anesth Analg. 1995;80(2):276–284. doi: 10.1097/00000539-199502000-00013. [DOI] [PubMed] [Google Scholar]
- 53.Gupta H, Ramanan B, Gupta PK, et al. Impact of COPD on postoperative outcomes: results from a national database. Chest. 2013;143(6):1599–1606. doi: 10.1378/chest.12-1499. [DOI] [PubMed] [Google Scholar]
- 54.van Ramshorst GH, Nieuwenhuizen J, Hop WC, et al. Abdominal wound dehiscence in adults: development and validation of a risk model. World J Surg. 2010;34(1):20–27. doi: 10.1007/s00268-009-0277-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lawrence VA, Dhanda R, Hilsenbeck SG, Page CP. Risk of pulmonary complications after elective abdominal surgery. Chest. 1996;110(3):744–750. doi: 10.1378/chest.110.3.744. [DOI] [PubMed] [Google Scholar]
- 56.Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 57.Hong CM, Galvagno SM., Jr Patients with chronic pulmonary disease. Med Clin North Am. 2013;97(6):1095–1107. doi: 10.1016/j.mcna.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 58.Kingston HG, Hirshman CA. Perioperative management of the patient with asthma. Anesth Analg. 1984;63(9):844–855. [PubMed] [Google Scholar]
- 59.Oh SH, Patterson R. Surgery in corticosteroid-dependent asthmatics. J Allergy Clin Immunol. 1974;53(6):345–351. doi: 10.1016/0091-6749(74)90118-3. [DOI] [PubMed] [Google Scholar]
- 60.Silvanus MT, Groeben H, Peters J. Corticosteroids and inhaled salbutamol in patients with reversible airway obstruction markedly decrease the incidence of bronchospasm after tracheal intubation. Anesthesiology. 2004;100(5):1052–1057. doi: 10.1097/00000542-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 61.Bousquet J, Clark TJ, Hurd S, et al. GINA guidelines on asthma and beyond. Allergy. 2007;62(2):102–112. doi: 10.1111/j.1398-9995.2006.01305.x. [DOI] [PubMed] [Google Scholar]
- 62.Enright A. Bronchospastic disease and emergency surgery. Middle East J Anaesthesiol. 2004;17(5):927–938. [PubMed] [Google Scholar]
- 63.Mandra A, Simić D, Stevanović V, Ugrinović D, Skodrić V, Kalezić N. Preoperative considerations for patients with chronic obstructive pulmonary disease. Acta Chir Iugosl. 2011;58(2):71–75. doi: 10.2298/aci1102071m. [DOI] [PubMed] [Google Scholar]
- 64.Edrich T, Sadovnikoff N. Anesthesia for patients with severe chronic obstructive pulmonary disease. Curr Opin Anaesthesiol. 2010;23(1):18–24. doi: 10.1097/ACO.0b013e328331ea5b. [DOI] [PubMed] [Google Scholar]
- 65.Wu RS, Wu KC, Wong TK, et al. Effects of fenoterol and ipratropium on respiratory resistance of asthmatics after tracheal intubation. Br J Anaesth. 2000;84(3):358–362. doi: 10.1093/oxfordjournals.bja.a013440. [DOI] [PubMed] [Google Scholar]
- 66.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 67.Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 suppl):D42–D50. doi: 10.1016/j.jacc.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 68.Kaw R, Pasupuleti V, Deshpande A, Hamieh T, Walker E, Minai OA. Pulmonary hypertension: an important predictor of outcomes in patients undergoing non-cardiac surgery. Respir Med. 2011;105(4):619–624. doi: 10.1016/j.rmed.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 69.Lai HC, Lai HC, Wang KY, Lee WL, Ting CT, Liu TJ. Severe pulmonary hypertension complicates postoperative outcome of non-cardiac surgery. Br J Anaesth. 2007;99(2):184–190. doi: 10.1093/bja/aem126. [DOI] [PubMed] [Google Scholar]
- 70.Hinderliter AL, Willis PW, IV, Long WA, et al. Frequency and severity of tricuspid regurgitation determined by Doppler echocardiography in primary pulmonary hypertension. Am J Cardiol. 2003;91(8):1033–1037. A9. doi: 10.1016/s0002-9149(03)00136-x. [DOI] [PubMed] [Google Scholar]
- 71.Benza RL, Miller DP, Barst RJ, Badesch DB, Frost AE, McGoon MD. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest. 2012;142(2):448–456. doi: 10.1378/chest.11-1460. [DOI] [PubMed] [Google Scholar]
- 72.Thenappan T, Shah SJ, Rich S, Tian L, Archer SL, Gomberg-Maitland M. Survival in pulmonary arterial hypertension: a reappraisal of the NIH risk stratification equation. Eur Respir J. 2010;35(5):1079–1087. doi: 10.1183/09031936.00072709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Galie N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Heart J. 2009;30(20):2493–2537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 74.Ross RM, Murthy JN, Wollak ID, Jackson AS. The six minute walk test accurately estimates mean peak oxygen uptake. BMC Pulm Med. 2010;10:31. doi: 10.1186/1471-2466-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 76.Ramakrishna G, Sprung J, Ravi BS, Chandrasekaran K, McGoon MD. Impact of pulmonary hypertension on the outcomes of noncardiac surgery: predictors of perioperative morbidity and mortality. J Am Coll Cardiol. 2005;45(10):1691–1699. doi: 10.1016/j.jacc.2005.02.055. [DOI] [PubMed] [Google Scholar]
- 77.McGlothlin D, Ivascu N, Heerdt PM. Anesthesia and pulmonary hypertension. Prog Cardiovasc Dis. 2012;55(2):199–217. doi: 10.1016/j.pcad.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 78.Hoeper MM, Galié N, Murali S, et al. Outcome after cardiopulmonary resuscitation in patients with pulmonary arterial hypertension. Am J Respir Crit Care Med. 2002;165(3):341–344. doi: 10.1164/ajrccm.165.3.200109-0130c. [DOI] [PubMed] [Google Scholar]
- 79.Huynh TN, Weigt SS, Sugar CA, Shapiro S, Kleerup EC. Prognostic factors and outcomes of patients with pulmonary hypertension admitted to the intensive care unit. J Crit Care. 2012;27(6):e7–e13. doi: 10.1016/j.jcrc.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 80.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20(9):705–706. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- 82.Kapur V, Strohl KP, Redline S, Iber C, O’Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath. 2002;6(2):49–54. doi: 10.1007/s11325-002-0049-5. [DOI] [PubMed] [Google Scholar]
- 83.Vasu TS, Grewal R, Doghramji K. Obstructive sleep apnea syndrome and perioperative complications: a systematic review of the literature. J Clin Sleep Med. 2012;8(2):199–207. doi: 10.5664/jcsm.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Memtsoudis S, Liu SS, Ma Y, et al. Perioperative pulmonary outcomes in patients with sleep apnea after noncardiac surgery. Anesth Analg. 2011;112(1):113–121. doi: 10.1213/ANE.0b013e3182009abf. [DOI] [PubMed] [Google Scholar]
- 85.Kaw R, Pasupuleti V, Walker E, Ramaswamy A, Foldvary-Schafer N. Postoperative complications in patients with obstructive sleep apnea. Chest. 2012;141(2):436–441. doi: 10.1378/chest.11-0283. [DOI] [PubMed] [Google Scholar]
- 86.Pinto JA, Ribeiro DK, Cavallini AF, Duarte C, Freitas GS. Comorbidities Associated with Obstructive Sleep Apnea: a Retrospective Study. Int Arch Otorhinolaryngol. 2016;20(2):145–150. doi: 10.1055/s-0036-1579546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jones RL, Nzekwu MM. The effects of body mass index on lung volumes. Chest. 2006;130(3):827–833. doi: 10.1378/chest.130.3.827. [DOI] [PubMed] [Google Scholar]
- 88.Chung F, Chau E, Yang Y, Liao P, Hall R, Mokhlesi B. Serum bicarbonate level improves specificity of STOP-Bang screening for obstructive sleep apnea. Chest. 2013;143(5):1284–1293. doi: 10.1378/chest.12-1132. [DOI] [PubMed] [Google Scholar]
- 89.Gupta RM, Parvizi J, Hanssen AD, Gay PC. Postoperative complications in patients with obstructive sleep apnea syndrome undergoing hip or knee replacement: a case-control study. Mayo Clin Proc. 2001;76(9):897–905. doi: 10.4065/76.9.897. [DOI] [PubMed] [Google Scholar]
- 90.American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Obstructive Sleep Apnea Practice guidelines for the perioperative management of patients with obstructive sleep apnea: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology. 2014;120(2):268–286. doi: 10.1097/ALN.0000000000000053. [DOI] [PubMed] [Google Scholar]
- 91.Honma K, Tango Y, Honma K, Isomoto H. Perioperative management of severe interstitial pneumonia for rectal surgery: a case report. Kurume Med J. 2007;54(3–4):85–88. doi: 10.2739/kurumemedj.54.85. [DOI] [PubMed] [Google Scholar]
- 92.Yuan N, Fraire JA, Margetis MM, Skaggs DL, Tolo VT, Keens TG. The effect of scoliosis surgery on lung function in the immediate postoperative period. Spine (Phila Pa 1976) 2005;30(19):2182–2185. doi: 10.1097/01.brs.0000181060.49993.4a. [DOI] [PubMed] [Google Scholar]
- 93.Carrillo G, Estrada A, Pedroza J, et al. Preoperative risk factors associated with mortality in lung biopsy patients with interstitial lung disease. J Invest Surg. 2005;18(1):39–45. doi: 10.1080/08941930590905206. [DOI] [PubMed] [Google Scholar]