Abstract
Objective: Assess efficacy and safety of once-daily topical dapsone gel, 7.5% compared with vehicle for treating acne vulgaris (acne). Design: A pooled analysis of data from two identically designed, randomized, double-blind, vehicle-controlled, multicenter, 12-week clinical trials. Setting: Study sites in the United States and Canada. Participants: overall, 4,340 patients were randomized 1:1 to dapsone and vehicle. Criteria included age 12 years or older with acne diagnosis, 20 to 50 facial inflammatory lesions (papules and pustules), 30 to 100 facial noninflammatory lesions (open and closed comedones), and acne grade of 3 (moderate) on the Global Acne Assessment Score scale. Measurements: Efficacy assessments included the Global Acne Assessment Score success rate (proportion of patients with Global Acne Assessment Score of 0 [none] or 1 [minimal]) and percentage change from baseline in inflammatory and noninflammatory lesions at Week 12. Results: Global Acne Assessment Score success rates were 29.8 percent and 21.1 percent for patients who received dapsone gel, 7.5% and vehicle, respectively (p<0.001). Patients receiving dapsone gel, 7.5% had greater percentage change in lesion counts than patients receiving vehicle (inflammatory lesions: -54.6% vs. -48.1%; p<0.001; -45.1 %; noninflammatory lesions: -39.4%; p<0.001). Most adverse events were mild to moderate in severity. Mean dermal tolerability scores for stinging/burning, dryness, scaling, and erythema were similarly low with dapsone gel, 7.5% and vehicle. Conclusion: Dapsone gel, 7.5%, with a 50-percent greater dapsone concentration than twice-daily dapsone gel, 5% formulation, is applied topically once daily for acne, is effective, safe, and well-tolerated over 12 weeks, and has local tolerability similar to that of vehicle. www.clinicaltrials.gov identifiers: NCT01974141 and NCT01974323
FOR BOTH ADOLESCENTS AND adult acne vulgaris (acne) is a common dermatologic disorder that may have a negative impact on an individual’s quality of life (Qol) and psychological well-being.1-4 effective treatment has been found to improve Qol and to reduce symptoms of depression and anxiety.2,5
Poor adherence to long-term management of acne is common and may raise the risk for treatment failure.6-9 Factors that influence adherence include disease severity, the types and severity of treatment side effects, and the individual’s satisfaction with the treatment and its outcomes.7,8,10 common reasons for nonadherence to acne medications include forgetting to use them and having inadequate time to apply topical medications.11 By minimizing the dosing frequency— optimally, to once-daily—it may be possible to improve patient adherence to acne treatment regimens.9,11–13
A topical gel formulation of the anti-inflammatory sulfone compound dapsone (Aczone Gel, 5%, Allergan plc, Dublin, Ireland) has been in use for the treatment of acne for several years.14,15 Clinical trials in patients with acne demonstrated that dapsone gel, 5% was effective,16 was well-tolerated,16,17 had a safety profile similar to vehicle,16 and provided sustained effectiveness through one year of open-label treatment.17 However, dapsone gel, 5% is applied twice daily,15 which can be inconvenient for some patients.
A once-daily formulation of dapsone gel may enhance patient adherence. Data from two identically designed pivotal studies of a new, once-daily formulation of topical dapsone gel, 7.5% demonstrated safety and efficacy versus vehicle.18,19 The new formulation of dapsone gel, 7.5% recently gained approval from the United States Food and Drug Administration for the once-daily treatment of acne vulgaris.20 The current analysis aimed to assess the efficacy and safety of once-daily topical dapsone gel, 7.5% compared with vehicle for the treatment of acne, using pooled data from the two pivotal registration trials.
METHODS
Study design. Two identically designed, randomized, double-blind, vehicle-controlled, multicenter clinical trials were conducted in the United states and canada (www.clinicaltrials.gov identifiers NCT01974141 and NCT01974323). Details of the procedures for institutional review, Good clinical Practices (GcP) compliance, and obtaining written, informed consent were previously reported for both studies.18,19
Patients. In both studies, patients were included if they were age 12 years or older, had a diagnosis of acne with 20 to 50 facial inflammatory lesions (papules and pustules) and 30 to 100 facial noninflammatory lesions (open and closed comedones), and had an acne grade of 3 (indicating moderate severity) on the Global Acne Assessment score (GAAs) scale at screening and at baseline.
Key exclusion criteria included a diagnosis of severe cystic acne, acne conglobata, acne fulminans, or secondary acne; at least one nodule or cyst above the mandibular line; existence of skin abnormalities, excessive hair, or other physical characteristics in or around the test sites that could confound study results; or other clinically significant findings or conditions that could confound the study results or interfere with study participation, per the investigator’s opinion; use of oral contraceptives solely for the control of acne; use of systemic immunosuppressive drugs within four weeks prior to screening or plans to use any systemic therapy that could potentially affect acne during the study; and topical procedures within one week of screening or use of topical acne treatments, including anti-inflammatory drugs, salicylic acid, corticosteroids, and retinoids, within two weeks of screening.
Treatments. Patients were randomized in a 1:1 ratio to receive dapsone gel, 7.5% or vehicle to be applied topically once daily for 12 weeks and were stratified by sex. Details of instructions given to patients regarding the application of the assigned study product were previously reported for both studies. Patients applied the first dose of study product at the investigational center under the supervision of study staff. The remaining study doses were to be applied by the patients at home. Patients were encouraged to apply the study product at about the same time every day. They were to gently wash and dry their skin, then apply an approximately pea-sized amount of study medication to their entire face in a thin layer; other acne-affected areas were also to receive a thin layer of study medication.
Efficacy assessments. All patients underwent a dermatologic evaluation at screening, baseline, and Weeks 1, 2, 4, 8, and 12 of treatment or at early exit. Facial acne severity was assessed using the 5- point Gaas (grade 0=none, grade 1=minimal, grade 2=mild, grade 3=moderate, and grade 4=severe).The investigator or a trained evaluator counted the inflammatory and noninflammatory lesions separately; a total lesion count of both types was also noted.
Both studies used the following co-primary assessments at Week 12: Gaas success rate, defined as the proportion of patients with a Gaas of 0 (none) or 1 (minimal), and inflammatory and noninflammatory lesion counts. Secondary endpoints included change from baseline in total lesion counts and percentage change from baseline for inflammatory, noninflammatory, and total lesion counts.
Safety and tolerability. Assessments included adverse events and local dermal tolerability. Local dermal tolerability (face only) was based on the patient rating of stinging/burning and on the investigator/trained evaluator ratings of dryness, scaling, and erythema; all ratings used a scale of 0 (none), 1 (mild), 2 (moderate), and 3 (severe).
Statistical analysis. Efficacy analyses were performed using the intent-to-treat (ITT) population, which consisted of all randomized patients (excluding patients from a discontinued study site in one of the trials). Safety analyses used data from the safety population, which comprised all patients who received at least one application of study product (excluding patients from the discontinued site).
GAAS findings were analyzed using a Cochran-Mantel-Haenszel (CMH) test stratified by sex. Two-sided Wald-type confidence intervals (CIs) with CMH weights were used to determine treatment differences in response rates. Between-group comparisons of mean change from baseline in inflammatory and noninflammatory lesion counts at Week 12 were performed using an analysis of covariance model with treatment group, corresponding count at baseline, and sex as covariates. All statistical analyses were performed using SAS version 9.3 (SAS rnstitute; Cary, North Carolina).
RESULTS
Patient disposition and demographics. A total of 4,340 patients were enrolled in the two studies(Table 1), and 3,977 (91.6%) completed their respective study. The most common reason for discontinuation was patients lost to follow-up.
TABLE 1.
Patient disposition (intent-to-treat population; N=4,340)
| PATIENTS | DAPSONE GEL, 7.5% (N=2,162) | VEHICLE (N=2,178) | TOTAL (N=4,340) |
|---|---|---|---|
| Total, N* | 2,162 | 2,178 | 4,340 |
| Completed, N (%)† | 1,974 (91.3) | 2,003 (92.0) | 3,977 (91.6) |
| Discontinued, N (%)‡ | 188 (8.7) | 175 (8.0) | 363 (8.4) |
| Adverse event | 6(0.3) | 7 (0.3) | 13 (0.3) |
| Lack of efficacy | 1 (0.0) | 2 (0.1) | 3 (0.1) |
| Pregnancy | 5 (0.2) | 4 (0.2) | 9 (0.2) |
| Lost to follow-up | 83 (3.8) | 69 (3.2) | 152 (3.5) |
| Personal reasons§ | 36 (1.7) | 39 (1.8) | 75 (1.7) |
| Protocol violation | 4 (0.2) | 8 (0.4) | 12 (0.3) |
| Other§ | 53 (2.5) | 46 (2.1) | 99 (2.3) |
Includes all randomized patients in the pooled intent-to-treat population.
Includes patients who completed the study through Week 12.
Only the primary reasons for discontinuation are summarized.
Personal reasons were not otherwise identified. “Other” included moves or scheduling conflicts, lack of improvement, patient decision not otherwise specified, and closure of one investigational site that was discontinued from participation in this clinical trial because of serious noncompliance with Good Clinical Practice guidelines (protocol adherence and clinical study management).
In the overall ITT group, the mean age of patients who received dapsone gel, 7.5% and vehicle was 20 years (Table 2). Similar proportions of patients were aged 12 to 17 years and 18 years and older. A slight majority of patients were female. caucasian patients comprised more than 50 percent of the patient population; the remaining patients were predominantly black and Hispanic. All skin phototypes were represented; the most common skin phototypes were II, III, and IV. All but one patient had moderate acne, according to GAAs findings; that one patient had severe acne.
TABLE 2.
Patient demographics and baseline characteristics (intent-to-treat population; N=4,340)
| CHARACTERISTIC | DAPSONE GEL, 7.5% (N=2,162) | VEHICLE (N=2,178) |
|---|---|---|
| AGE, YEARS | ||
| Mean (SD) | 20.3 (7.8) | 20.2 (7.5) |
| Median (range) | 18.0 (12–63) | 18.0 (12–54) |
| AGE GROUP, n(%), YEARS | ||
| 12–17 | 1,066 (49.3) | 1,084 (49.8) |
| ≥18 | 1,096 (50.7) | 1,094 (50.2) |
| SEX, n(%) | ||
| Male | 953 (44.1) | 965 (44.3) |
| Female | 1,209 (55.9) | 1,213 (55.7) |
| RACE/ETHNICITY, n(%) | ||
| Caucasian | 1,248 (57.7) | 1,242 (57.0) |
| Black | 403 (18.6) | 409 (18.8) |
| Asian | 81 (3.7) | 87 (4.0) |
| Hispanic | 347 (16.0) | 347 (15.9) |
| Other | 83 (3.8) | 93 (4.3) |
| SKIN PHOTOTYPE, n(%) | ||
| N | 2,157 | 2,170 |
| I | 68 (3.2) | 80 (3.7) |
| II | 467(21.7) | 436 (20.1) |
| III | 588 (27.3) | 577 (26.6) |
| IV | 504 (23.4) | 540 (24.9) |
| V | 307 (14.2) | 315 (14.5) |
| VI | 223 (10.3) | 222 (10.2) |
| GAAS, n (%) | ||
| N | 2,162 | 2,177 |
| 3 (moderate) | 2,161 (100.0) | 2,177 (100.0) |
| 4(severe) | 1 (0.0) | 0(0.0) |
| INFLAMMATORY LESION COUNT | ||
| N | 2,162 | 2,177 |
| Mean(SD) | 29.2 (7.8) | 29.7 (8.0) |
| Median (range) | 27.0 (11–62) | 27.0(20–57) |
| NONINFLAMMATORY LESION COUNT | ||
| N | 2,162 | 2,177 |
| Mean(SD) | 46.8 (16.0) | 47.6 (16.3) |
| Median (range) | 41.0 (4–112) | 43.0 (30–106) |
| TOTAL LESION COuNT | ||
| N | 2,162 | 2,177 |
| Mean(SD) | 76.0 (19.7) | 77.3 (19.9) |
| Median (range) | 71.0 (15–150) | 72.0 (50–148) |
GAAS=Global Acne Assessment Score; SD=standard deviation
Efficacy. The primary efficacy endpoints were met. GAAs success was achieved at Week 12 by 29.8 percent of patients who received dapsone gel, 7.5% and 21.1 percent of patients who received vehicle in the pooled analysis (p<0.001; Table 3). The dapsone gel, 7.5% group had significantly greater least squares (LS) mean changes at Week 12 in inflammatory lesion and noninflammatory lesion counts from baseline than did the vehicle group (p<0.001; Table 3).
TABLE 3.
Efficacy analyses at Week 12 (intent-to-treat population; N=4,340)
| PARAMETER | DAPSONE GEL, 7.5% (N=2,162) | VEHICLE (N=2,178) | p VALUE |
|---|---|---|---|
| PRIMARY END POINTS | |||
| GAAS SUCCESS* | |||
| Percent of patients (95% CI) | 29.8 (27.9, 31.8) | 21.1 (19.3, 22.8) | <0.001 |
| INFLAMMATORY LESION COUNT, CHANGE FROM BASELINE | |||
| LS mean (SE) | -15.8 (0.24) | -13.9 (0.24) | <0.001 |
| NONINFLAMMATORY LESION COUNT, CHANGE FROM BASELINE | |||
| LS mean (SE) | -20.7 (0.39) | -18.0 (0.39) | <0.001 |
| SECONDARY END POINTS | |||
| TOTAL LESION COUNT | |||
| LS mean absolute change from baseline (SE) | -36.5 (0.54) | -32.0 (0.53) | <0.001 |
| LS mean percentage change from baseline (SE) | -48.8 (0.69) | -42.8 (0.67) | <0.001 |
| INFLAMMATORY LESION COUNT | |||
| LS mean percentage change from baseline (SE) | -54.6 (0.79) | -48.1 (0.79) | <0.001 |
| NONINFLAMMATORY LESION COUNT | |||
| LS mean percentage change from baseline (SE) | -45.1 (0.83) | -39.4 (0.81) | <0.001 |
GAAS success was defined as a score of 0 (none) or 1 (minimal).
CI=confidence interval; GAAS=Global Acne Assessment Score; LS=least squares; SE=standard error
The Ls mean change at Week 12 in total lesion count from baseline was significantly greater for the dapsone gel, 7.5% group compared with the vehicle group (-36.5 vs. -32.0; p<0.001; Table 3). The LS mean percentage change from baseline for total lesion count was significantly higher with the dapsone gel, 7.5% group compared with the vehicle group at Week 12 (p<0.001). The Ls mean percentage changes at Week 12 from baseline for inflammatory and noninflammatory lesion counts were significantly greater for patients who received dapsone gel, 7.5% than for those who received vehicle (p<0.001, both comparisons).
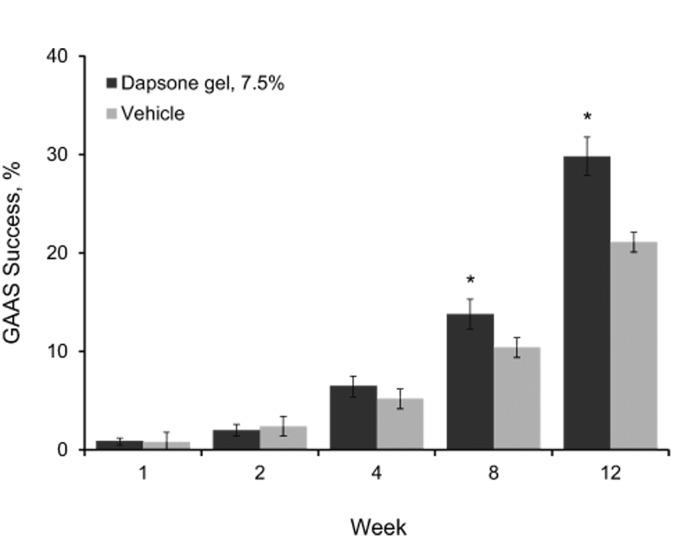
The proportion of patients who achieved GAAS success rose over the 12 weeks of treatment. For the patients who received dapsone gel, 7.5%, the GAAS success rate was significantly higher than that for the patients who received vehicle starting at Week 8, and it remained significant at Week 12 (Figure 1).
Figure 1.
Proportion of patients with GAAS success (intent-to-treat population; N=4,340). GAAS success was defined as a score of 0 (none) or 1 (minimal) on the gAAS. Error bars denote 95% confidence intervals.
*p<0.001 vs. vehicle; GAAS=Global Acne Assessment Score
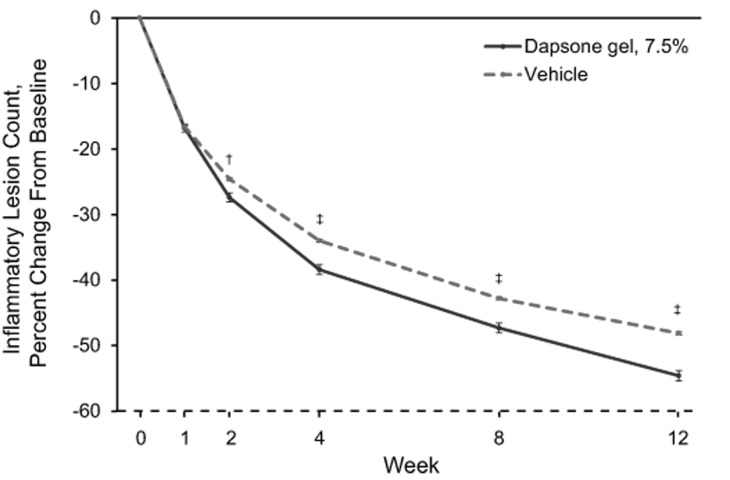
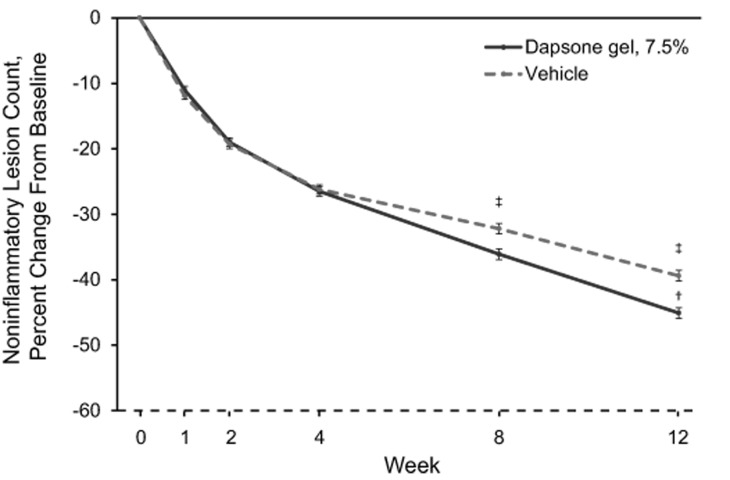
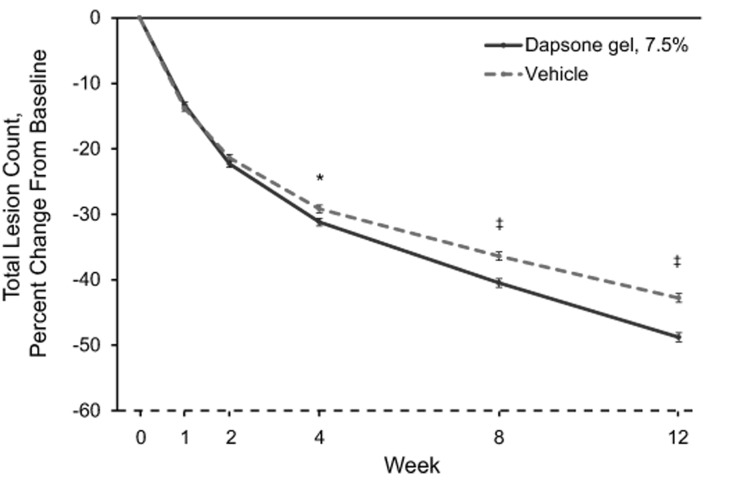
Mean percentage changes in inflammatory, noninflammatory, and total lesion counts are shown in Figure 2.The mean percentage change from baseline in inflammatory lesion counts was significantly greater for the dapsone gel, 7.5% group compared with the vehicle group at Week 2, and it remained significantly greater through Week 12 (Figure 2A).The difference between the dapsone gel, 7.5% and vehicle groups for percentage change from baseline in noninflammatory lesion counts became significant at Week 8 in favor of dapsone gel, 7.5% and remained significant at Week 12 (Figure 2B). The decrease in the mean total lesion count for patients who received dapsone gel, 7.5% was significantly greater than that for patients who received vehicle starting at Week 4, and the difference between the groups remained significant through Week 12 (Figure 2C).
Figures 2A-2C.
Percentage change from baseline in lesion counts (intent-to-treat population; N=4,340) for (A) inflammatory lesion counts, (B) noninflammatory lesion counts, and (C ) total lesion counts. Error bars denote standard error.*p<0.05;†p<0.01;‡p<0.001
Safety. Adverse events were reported for 18.3 percent (396/2161) of patients who received dapsone gel, 7.5% and 18.8 percent (409/2175) of patients who received vehicle. Most adverse events were mild to moderate in severity. The most commonly reported adverse events (≥1% of patients in any treatment group) are listed in Table 4.
TABLE 4.
Commonly reported adverse events occurring in at least one percent of patients in any treatment group (safety population; N=4,336)
| EVENT, N (%) | DAPSONE GEL, 7.5% (N=2,161) | VEHICLE (N=2,175) |
|---|---|---|
| Nasopharyngitis | 40 (1.9) | 48 (2.2) |
| Headache | 34 (1.6) | 26 (1.2) |
| Upper respiratory tract infection | 32 (1.5) | 34 (1.6) |
| Application site dryness | 26 (1.2) | 22 (1.0) |
| Application site pruritus | 23 (1.1) | 14 (0.6) |
| Application site pain | 11 (0.5) | 33 (1.5) |
The most common application site events (≥1% of patients in any treatment group) are included in Table 4. These events consisted of application site dryness, application site pruritus, and application site pain. The incidence of these events for patients who received dapsone gel, 7.5% was similar to that for patients who received vehicle. Seven patients (0.3%) in the dapsone gel, 7.5% group and nine patients in the vehicle group experienced serious adverse events. One of these events (depression in a patient who received vehicle) was considered to be related to treatment, and the event was ongoing at study exit.
Six patients (0.3%) who received dapsone gel, 7.5% and seven (0.3%) who received vehicle discontinued because of an adverse event. Three of the discontinued patients in the dapsone gel, 7.5% group experienced adverse events considered to be related to treatment by the investigator (application site acne and dermatitis in 1 patient, application site vesicles, swelling, and pruritus in 1 patient, and application site discomfort in 1 patient). Three patients in the vehicle group reported adverse events deemed to be treatment related by the investigator (application site pain in 2 patients and application site acne in the other). All of these events resolved without sequelae, with the exception of application site dermatitis in the patient in the dapsone gel, 7.5% group, which was ongoing at study exit.
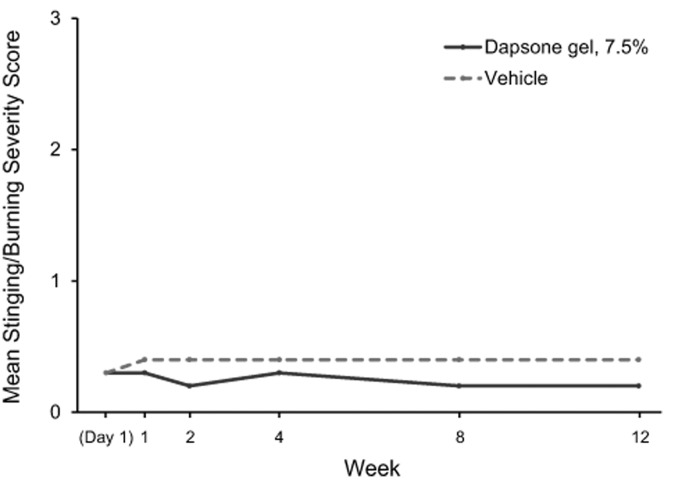
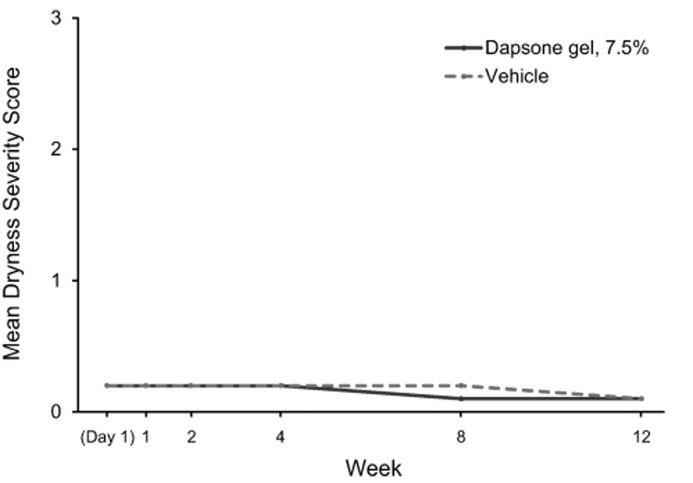
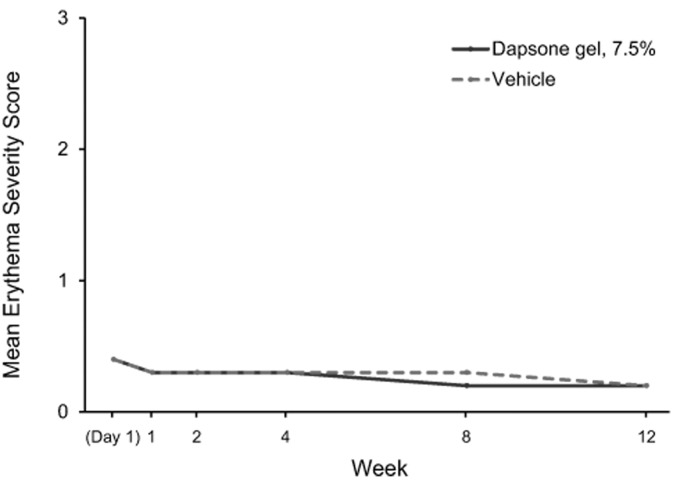
Tolerability. Dapsone gel, 7.5% and vehicle were generally well-tolerated. Mean dermal tolerability scores for stinging/burning, dryness, scaling, and erythema for each study visit from baseline to Week 12 are shown in Figure 3. Mean scores for patients who received dapsone gel, 7.5% and vehicle were similarly low (<0.5, with 0 indicating “none” and 1 indicating “mild”) at all time points. The proportion of patients with changes in dermal tolerability severity scores are shown in Table 5. The most frequently reported increase in severity for dermal tolerability assessments (stinging/burning, dryness, scaling, and erythema) was from “none” to “mild.” At all post-baseline visits, the incidence of increase in dermal tolerability scores to “severe” was one percent or less for the dapsone gel, 7.5% and vehicle groups.
Figures 3A-3D.
Dermal tolerability severity mean scores (0=none, 1=mild, 2=moderate, 3=severe) for (A) patient-assessed stinging/burning sensation, (B) investigator/evaluator-assessed dryness, (C) investigator/evaluator-assessed scaling, and (D) investigator/evaluator-assessed erythema.
TABLE 5.
Dermal tolerability: maximum severity and severity at Week 12 for patients whose severity score increased from baseline at any time point (safety population; N=4,336)
| PARAMETER, N (%) | DAPSONE GEL, 7.5% (N=2,161) | VEHICLE (N=2,175) | DAPSONE GEL, 7.5% (N=2,161) | VEHICLE (N=2,175) | DAPSONE GEL, 7.5% (N=2,161) | VEHICLE (N=2,175) | DAPSONE GEL, 7.5% (N=2,161) | VEHICLE (N=2,175) |
|---|---|---|---|---|---|---|---|---|
| MAXIMUM SEVERITY | None | Mild | Moderate | Severe | ||||
| Stinging/burning | - | - | 507 (23.5) | 686 (31.5) | 121 (5.6) | 249 (11.4) | 21 (1.0) | 48 (2.2) |
| Dryness | - | - | 383 (17.7) | 412 (18.9) | 44 (2.0) | 52 (2.4) | 4 (0.2) | 0 (0.0) |
| Scaling | - | - | 269 (12.4) | 324 (14.9) | 29 (1.3) | 44 (2.0) | 4 (0.2) | 2 (<0.1) |
| Erythema | - | - | 210 (9.7) | 246 (11.3) | 58 (2.7) | 79 (3.6) | 4 (0.2) | 2 (<0.1) |
| WEEK 12 SEVERITY | None | Mild | Moderate | Severe | ||||
| Stinging/burning | 315 (14.6) | 367 (16.9) | 251 (11.6) | 442 (20.3) | 28 (1.3) | 91 (4.2) | 4 (0.2) | 14 (0.6) |
| Dryness | 284 (13.1) | 269 (12.4) | 112 (5.2) | 163 (7.5) | 7 (0.3) | 8 (0.4) | 1 (<0.1) | 0 (0.0) |
| Scaling | 201 (9.3) | 246 (11.3) | 78 (3.6) | 106 (4.9) | 7 (0.3) | 4 (0.2) | 1 (<0.1) | 1 (<0.1) |
| Erythema | 160 (7.4) | 183 (8.4) | 82 (3.8) | 96 (4.4) | 16 (0.7) | 27 (1.2) | 0 (0.0) | 0 (0.0) |
Week 12 data were the last available time point during the post-baseline period. Data on stinging/burning were not reported for 52 patients at baseline and for 60 patients at Week 12; data for dryness, scaling, and erythema were not reported for 10 patients at baseline and for 68 patients at Week 12.
DISCUSSION
This pooled analysis of data from two large, multicenter, randomized, double-blind, vehicle-controlled trials comprising more than 4,000 patients demonstrated the efficacy, safety, and tolerability of once-daily administration of dapsone gel, 7.5% over 12 weeks of treatment. This pooled analysis confirms and extends these findings in a large cohort of adolescents and adults, with substantial representation of both males and females and caucasian and non-caucasian patients. The co-primary endpoints (GAAs success rates and mean reduction in inflammatory and noninflammatory lesion counts at Week 12) were statistically superior (p<0.001) for dapsone gel, 7.5% treatment compared with vehicle. The clinical improvement in acne severity was supported by substantial decreases in inflammatory, noninflammatory, and total lesion counts at Week 12. Significant differences (p<0.05) in favor of dapsone gel, 7.5% compared with vehicle appeared early in treatment: by Week 2 for decreases in mean inflammatory lesion count and by Week 4 for total lesion counts (percentage change from baseline).
An earlier pooled analysis of data from the dapsone gel, 5% trials (N=3,010) showed similar findings for GAAS success and reduction in inflammatory and noninflammatory lesions to that seen in this pooled analysis. However, using the GAAS, the baseline severity levels for patients in the current analysis differed from those in previous studies of dapsone gel, 5%.
Approximately one-third of the patients in the dapsone gel, 5% trials had mild acne16 while virtually all patients in the current analysis had moderate severity at baseline.
The time course for significantly greater GAAS success rates and improvements in inflammatory, noninflammatory, and total lesion counts overall was similar to results from two 12-week studies of dapsone gel, 5%. The time course for improvement in inflammatory and total lesion counts compares well with findings from a systematic review of onset of action for several other acne medications (adapalene, tretinoin, isotretinoin, benzoyl peroxide [BPO], adapalene/BPO, and clindamycin/BPO). In that systematic review, the time to a 25-percent decrease in inflammatory lesion counts was analyzed as a primary endpoint. This endpoint was achieved between Week 1 and Week 4 in the majority of cases.21 In this trial, a 25-percent decrease in inflammatory lesions with dapsone gel, 7.5% was observed at approximately Week 2, and was significantly greater than that for vehicle at Week 2 and all remaining time points in the study.
The overall incidence of adverse events was similar for patients who received dapsone gel, 7.5% and those who received vehicle. Most adverse events were mild to moderate in severity, most resolved without sequelae, and few adverse events resulted in discontinuations. The most common treatment-related adverse events were application site events that occurred at a similar rate in the dapsone gel, 7.5% and vehicle groups. During the 12 weeks of treatment, dapsone gel, 7.5% applied once daily had similar local tolerability ratings (stinging/ burning, dryness, scaling, and erythema) compared with vehicle.
Safety and dermal tolerability findings in the current study were generally similar to those shown in the large, randomized, controlled pivotal study of twice-daily dapsone gel, 5%.16
The two pivotal trials of dapsone gel, 7.5% were of relatively short duration (12 weeks). The maximal effects of dapsone gel, 7.5% may not have been achieved; improvements in acne severity and lesion count may continue beyond the 12 weeks. The change in total lesion count did not appear to plateau by Week 12. Of note, a longer term study demonstrated that treatment with dapsone gel, 5% was effective in reducing inflammatory and noninflammatory lesions for up to one year.17
CONCLUSION
Dapsone gel, 7.5% applied topically once daily is an effective, safe, and well-tolerated treatment for acne over 12 weeks. Dapsone gel, 7.5% applied once daily demonstrated similar local tolerability compared with vehicle and had a safety and tolerability profile similar to that of twice-daily dapsone gel, 5%. These findings confirm and extend the findings of safety and efficacy versus vehicle demonstrated in the analyses of data from the individual studies.18,19 Long-term treatment and good adherence to therapy are necessary for optimal acne management.7,9,22,23 Reducing the frequency of acne treatment application is an important strategy for improving treatment adherence.9,11,12 Once-daily dosing of dapsone gel, 7.5% may promote improved adherence with long-term use.
ACKNOWLEDGMENTS
Writing and editorial assistance was provided to the authors by Michael L. Pucci, PhD, of Peloton Advantage, Parsippany, New Jersey, and was funded by Allergan plc, Dublin, Ireland.
Footnotes
Disclosure:This study was sponsored by Allergan plc, Dublin, Ireland. Drs. Thiboutot, Kircik, McMichael, Cook-Bolden, and Tyring have served as investigators for Allergan plc, Dublin, Ireland. Drs. Berk, Chang-Lin, Lin, and Kaoukhov are employees of Allergan plc and may own stock in that company. Writing and editorial assistance were provided to the authors by Annette F. Skorupa, PhD, of Peloton Advantage, Parsippany, New Jersey, and were funded by Allergan plc. Neither honoraria nor other forms of payment were made for authorship.
REFERENCES
- 1.Landis ET, Davis SA, Taheri A, Feldman SR. Top dermatologic diagnoses by age. Dermatol Online J. 2014;20(4):22368. [PubMed] [Google Scholar]
- 2.Klassen AF, Newton JN, Mallon E. Measuring quality of life in people referred for specialist care of acne: comparing generic and disease-specific measures. J Am Acad Dermatol. 2000;43(2 Pt 1):229–233. doi: 10.1067/mjd.2000.105507. [DOI] [PubMed] [Google Scholar]
- 3.Dunn LK, O’Neill JL, Feldman SR. Acne in adolescents: quality of life, self-esteem, mood, and psychological disorders. Dermatol Online J. 2011;17(1):1. [PubMed] [Google Scholar]
- 4.Tanghetti EA, Kawata AK, Daniels SR, et al. Understanding the burden of adult female acne. J Clin Aesthet Dermatol. 2014;7(2):22–30. [PMC free article] [PubMed] [Google Scholar]
- 5.Kaymak Y, Taner E, Taner Y. Comparison of depression, anxiety and life quality in acne vulgaris patients who were treated with either isotretinoin or topical agents. Int J Dermatol. 2009;48(1):41–46. doi: 10.1111/j.1365-4632.2009.03806.x. [DOI] [PubMed] [Google Scholar]
- 6.Draelos ZD. Improving compliance in acne treatment: benzoyl peroxide considerations. Cutis. 2008;82(5 Suppl):17–20. [PubMed] [Google Scholar]
- 7.Dreno B, Thiboutot D, Gollnick H, et al. Large-scale worldwide observational study of adherence with acne therapy. Int J Dermatol. 2010;49(4):448–456. doi: 10.1111/j.1365-4632.2010.04416.x. [DOI] [PubMed] [Google Scholar]
- 8.Miyachi Y, Hayashi N, Furukawa F, et al. Acne management in Japan: study of patient adherence. Dermatology. 2011;223(2):174–181. doi: 10.1159/000332847. [DOI] [PubMed] [Google Scholar]
- 9.Koo J. How do you foster medication adherence for better acne vulgaris management? SKINmed. 2003;2(4):229–233. doi: 10.1111/j.1540-9740.2003.02037.x. [DOI] [PubMed] [Google Scholar]
- 10.Snyder S, Crandell I, Davis SA, Feldman SR. Medical adherence to acne therapy: a systematic review. Am J Clin Dermatol. 2014;15(2):87–94. doi: 10.1007/s40257-014-0063-y. [DOI] [PubMed] [Google Scholar]
- 11.Lott R, Taylor SL, O’Neill JL, et al. Medication adherence among acne patients: a review. J Cosmet Dermatol. 2010;9(2):160–166. doi: 10.1111/j.1473-2165.2010.00490.x. [DOI] [PubMed] [Google Scholar]
- 12.Marazzi P, Boorman GC, Donald AE, Davies HD. Clinical evaluation of double strength isotrexin versus benzamycin in the topical treatment of mild to moderate acne vulgaris. J Dermatolog Treat. 2002;13(3):111–117. doi: 10.1080/09546630260199460. [DOI] [PubMed] [Google Scholar]
- 13.Kellett N, West F, Finlay AY. Conjoint analysis: a novel, rigorous tool for determining patient preferences for topical antibiotic treatment for acne. A randomised controlled trial. Br J Dermatol. 2006;154(3):524–532. doi: 10.1111/j.1365-2133.2005.07047.x. [DOI] [PubMed] [Google Scholar]
- 14. [July 14, 2016]. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021794s000TOC.cfm Drug approval package: Aczone Gel, 5% (dapsone gel, 5%). September 13, 2007; Food and Drug Administration; Silver Spring, MD.
- 15. Aczone Gel, 5% [package insert]. Dublin, Ireland: Allergan plc; 2015.
- 16.Draelos ZD, Carter E, Maloney JM, et al. Two randomized studies demonstrate the efficacy and safety of dapsone gel, 5% for the treatment of acne vulgaris. J Am Acad Dermatol. 2007;56(3):439.e1–439.e10. doi: 10.1016/j.jaad.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Lucky AW, Maloney JM, Roberts J, et al. Dapsone gel 5% for the treatment of acne vulgaris: safety and efficacy of long-term (1 year) treatment. J Drugs Dermatol. 2007;6(10):981–987. [PubMed] [Google Scholar]
- 18.Eichenfield LF, Lain T, Frankel EH, et al. Efficacy and safety of once-daily dapsone gel 7.5% for treatment of adolescents and adults with acne vulgaris: second of two identically designed, large, multicenter, randomized, placebo-controlled trials [poster]. Presented at: the Annual Orlando Dermatology Aesthetic and Clinical Conference, January 15–18, 2016, Orlando, Florida. [PubMed]
- 19.Stein Gold LF, Jarratt MT, Bucko AD, et al. Efficacy and safety of once-daily dapsone gel 7.5% for treatment of adolescents and adults with acne vulgaris: first of two identically designed, large, multicenter, randomized, placebo-controlled trials [poster]. Presented at: the Annual Orlando Dermatology Aesthetic and Clinical Conference, January 15–18, 2016, Orlando, Florida. [PubMed]
- 20. Aczone Gel, 7.5% [package insert]. Dublin, Ireland: Allergan plc; 2016.
- 21.Jacobs A, Starke G, Rosumeck S, Nast A. Systematic review on the rapidity of the onset of action of topical treatments in the therapy of mild-to-moderate acne vulgaris. Br J Dermatol. 2014;170(3):557–564. doi: 10.1111/bjd.12706. [DOI] [PubMed] [Google Scholar]
- 22.Gollnick HP, Finlay AY, Shear N. Can we define acne as a chronic disease? If so, how and when? Am J Clin Dermatol. 2008;9(5):279–284. doi: 10.2165/00128071-200809050-00001. [DOI] [PubMed] [Google Scholar]
- 23.Zouboulis CC. Acne as a chronic systemic disease. Clin Dermatol. 2014;32(3):389–396. doi: 10.1016/j.clindermatol.2013.11.005. [DOI] [PubMed] [Google Scholar]