Abstract
Apicomplexa tick-borne hemoparasites, including Babesia bovis, Babesia microti, and Theileria equi are responsible for bovine and human babesiosis and equine theileriosis, respectively. These parasites of vast medical, epidemiological, and economic impact have complex life cycles in their vertebrate and tick hosts. Large gaps in knowledge concerning the mechanisms used by these parasites for gene regulation remain. Regulatory genes coding for DNA binding proteins such as members of the Api-AP2, HMG, and Myb families are known to play crucial roles as transcription factors. Although the repertoire of Api-AP2 has been defined and a HMG gene was previously identified in the B. bovis genome, these regulatory genes have not been described in detail in B. microti and T. equi. In this study, comparative bioinformatics was used to: (i) identify and map genes encoding for these transcription factors among three parasites’ genomes; (ii) identify a previously unreported HMG gene in B. microti; (iii) define a repertoire of eight conserved Myb genes; and (iv) identify AP2 correlates among B. bovis and the better-studied Plasmodium parasites. Searching the available transcriptome of B. bovis defined patterns of transcription of these three gene families in B. bovis erythrocyte stage parasites. Sequence comparisons show conservation of functional domains and general architecture in the AP2, Myb, and HMG proteins, which may be significant for the regulation of common critical parasite life cycle transitions in B. bovis, B. microti, and T. equi. A detailed understanding of the role of gene families encoding DNA binding proteins will provide new tools for unraveling regulatory mechanisms involved in B. bovis, B. microti, and T. equi life cycles and environmental adaptive responses and potentially contributes to the development of novel convergent strategies for improved control of babesiosis and equine piroplasmosis.
Author Summary
The tick-borne apicomplexan parasites Babesia and Theileria are responsible for costly and devastating diseases globally. Improved control is needed, but the biology of these parasites remains poorly understood. Significant gaps include better understanding of the mechanisms involved in control of gene expression and the events leading to parasite development among hosts, including the production of sexual stages in their definitive tick vector hosts. Similar to other better-studied eukaryotic cells, it is likely that regulatory genes coding for DNA binding proteins such as members of the Api-AP2, HMG, and Myb families play crucial roles as transcription factors in these processes, but these genes remain uncharacterized in these three related parasites. In this study, we describe the presence and genomic organization of these three types of genes in Babesia bovis, Babesia microti, and Theileria equi, highlighting the importance of the conservation of these genes and their possible contributions to parasite development through their different life stages. We also describe the occurrence of a previously unreported HMG gene in B. microti, an important emerging human pathogen; define the repertoire of eight conserved Myb genes; and describe the pattern of transcription of the regulatory AP2, HMG, and Myb genes in B. bovis intra-erythrocytic stages for the first time. It is expected that these findings will elicit additional research in this field and contribute to the development of converged intervention strategies for the improved control of these devastating and generally under-studied diseases.
Introduction
The tick-borne apicomplexan intraerythrocytic parasites Babesia bovis, Babesia microti, and Theileria equi cause similar potentially fatal acute hemolytic disease and persistent infections in bovines, humans, and equids, respectively. B. bovis and T. equi are mainly transmitted by Rhiphicephalus ticks, whereas B. microti is primarily transmitted by Ixodes scapularis [1]. Despite the use of tick control measures, the availability of live vaccines for preventing acute disease caused by B. bovis, as well as effective chemotherapeutics, bovine babesiosis and equine theileriosis remain poorly controlled globally. Both B. bovis and T. equi are responsible for large economic losses, while B. microti is responsible for public health concerns. These related apicomplexan parasites are able to cause persistent infections and have achieved a high degree of adaptation through millions of years of co-evolution within their tick and mammal hosts, resulting in the development of complex survival strategies. A practical consequence of these natural evolutionary processes is that the development of control measures against these parasites is extremely difficult to achieve [2]. Clearly, an improved understanding of the biology of Babesia and Theileria parasites is needed for designing novel and improved methods of control. However, important gaps of knowledge remain in our understanding of the biology of these parasites and the molecular mechanisms involved in interactions with their mammal and tick hosts [2]. Mining of genomes of B. bovis [3], B. microti [4] [5], and T. equi [6], based on known regulatory mechanisms used by eukaryotic cells, combined with current high-throughput research technologies such as transcriptomics, proteomics, metabolomics, gene editing, and transfection systems, can be employed to understand complex gene expression regulatory networks. Regulation of gene expression in eukaryotic cells can be achieved at the transcriptional level using both genetic and epigenetic mechanisms. Moreover, it is likely that the activity of transcription factors and DNA binding proteins, combined and acting in coordination with modulated chromatin organizations such as nucleosome positioning, essentially controls gene expression at different parasite life cycle stages [7]. In addition, gene expression can also be regulated at the post-transcriptional and translational levels. Key advances in understanding mechanisms involved in gene regulation have so far been achieved in the more studied Babesia-related Plasmodium, Theileria annulata, and Toxoplasma, among others [8]. These studies serve as a model for characterization of similar and generally conserved gene regulatory mechanisms in Babesia and closely related parasites.
Intriguingly, genomic and proteomic analysis initially performed in Plasmodium showed a paucity of genes encoding for recognizable and typical enhancers and transcription activators, such as transcription factors (TFs), despite the need for coordinated regulation of gene expression for parasite survival in dramatically different life stages [9–11]. These observations support the hypothesis of the evolution of unique transcription factors in Plasmodium parasites. These insights prompted recent investigations in Plasmodium and other related apicomplexans, leading to the identification and characterization of at least three well-characterized TFs: proteins encoded by the apicomplexan AP2 gene family (ApiAP2) and the Myb and HMG proteins [10]. This study describes the conservation of genes encoding for these three types of gene transcriptional regulators in B. bovis, B. microti, and T. equi parasites. The best characterized of these three factors is the AP2 gene family. This family is related to the Apetala 2 gene family originally identified in plants encoding for proteins that are involved in the regulation of transcription in many crucial cell stage developmental transitions. The plant AP2/ERF (Apetela2/Ethylene Response Factor) gene family [12] encodes a diverse family of proteins containing one or two ~60 amino acid conserved AP2 domains. Consistent with their function as nuclear transcriptional factors, the AP2 domains contain conserved structural motifs that are directly involved in DNA binding. Thus, the AP2 domains can bind DNA in a sequence-specific fashion. Initial studies suggested the involvement of an 18 amino acid core region that forms an amphipathic α-helix [13], but further structural studies demonstrated that Ap2 proteins use an antiparallel three-stranded β-sheet to make major groove contacts. The majority of the DNA contacts are made by arginine and tryptophan residues located in the β-sheet of the AP2 domains of the protein [14]. Further structural analysis in the Plasmodium falciparum PF14_0633 AP2 domain also revealed that a β-sheet fold binds the DNA major groove through base-specific and backbone contact. The role of the conserved α-helix is stabilization of the triple-stranded β-sheet [9, 15].
The AP2 proteins of plants contain one or two tandem-arranged AP2 domains, which are separated typically by a 25 amino acid conserved linker sequence. However, high sequence divergence was typically found among the regions of AP2 proteins that do not contain the otherwise conserved AP2 domains [16, 17]. Genes encoding for proteins containing AP2 domains were also annotated in the B. bovis, T. equi, and B. microti genomes, but a systematic and detailed characterization of these gene families was not reported for these parasites. In this study, bioinformatics tools are used to define the AP2 repertoire in T. equi and B. microti and to find possible AP2 correlates among B. bovis and Plasmodium parasites.
The Myb genes are evolutionarily conserved amongst eukaryotic cells. The Myb proteins, which are part of the tryptophan cluster family, are able to bind to DNA and typically regulate genes involved in cell proliferation and differentiation [17]. Myb proteins usually contain two or three distinct DNA binding domain located in their amino terminal regions. The Myb proteins generally contain three repeats of approximately 50 residues with three regularly spaced tryptophan residues [18].
The high mobility group (HMG) box (HMGB) are abundant, small (under 100 amino acids long), non-histone architectural chromosomal proteins. The HMGBs are also highly conserved across eukaryotic cells and have been implicated in a number of basic cellular functions such as DNA replication, transcription, and recombination, perhaps by modulating chromatin structure [19]. Importantly, Plasmodium HMGBs have been reported to be differentially expressed and co-related to the development of erythrocyte stages and gametocyte differentiation [20] [21], suggesting their role in parasite differentiation. Interestingly, secretory forms of Plasmodium HMG may be involved in triggering host inflammatory immune responses associated with malaria, since they can stimulate macrophages to release cytokines, such as TNF alpha [19], and so they were also implicated in pathogenic mechanisms.
Here, the organization and features of the AP2 gene family in B. bovis are described. In addition, we compare the features of the B. bovis AP2 genes with the previously undefined AP2 genes identified in the genome of the related T. equi and B. microti tick-borne intra-erythrocytic apicomplexans for the first time, and describe the pattern of expression of the AP2 genes within intra-erythrocyte stage parasites based on previously reported transcriptomic studies in a B. bovis T2bo strain virulent and attenuated pair [19]. Also discussed is the occurrence and characteristics of genes encoding for Myb and HMG DNA-binding proteins, the two additional classes of transcription factors that were previously identified and assigned significant roles in related parasites, but remained uncharacterized in B. bovis, T. equi, and B. microti.
The AP2 gene family in B. bovis, B. microti, and T. equi
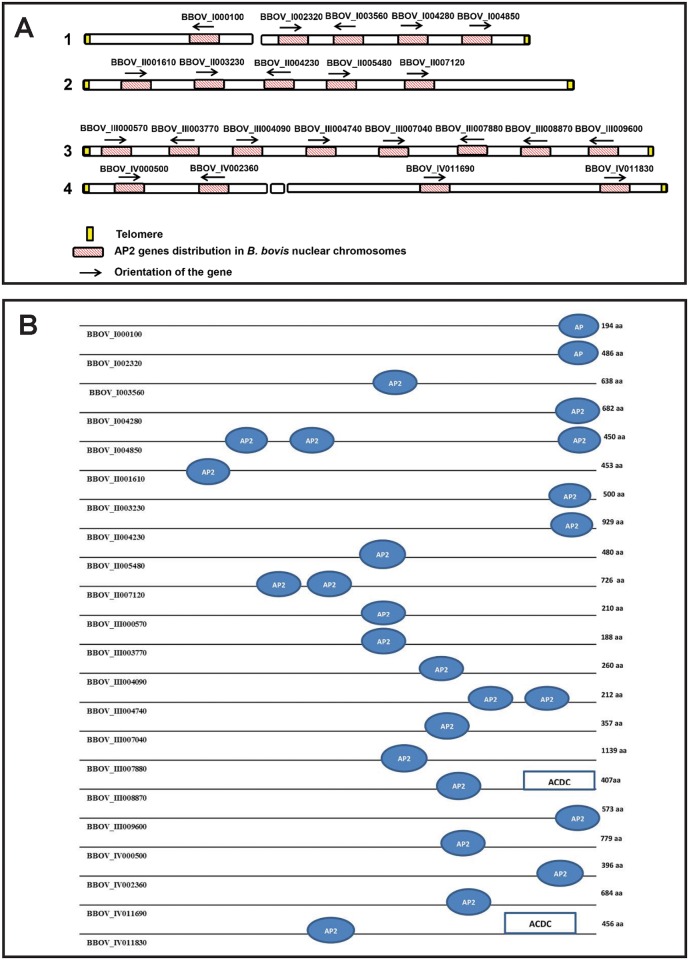
The presence of AP2 genes in apicomplexans was initially described by Balaji et al. [22], who first reported the identification of members of the AP2 gene family in the genomes of Plasmodium, Theileria, Cryptosporidium, and Toxoplasma. Initial genome characterization in the B. bovis T2Bo strain genome resulted in the annotation of 18 genes encoding for AP2 domain-containing proteins [3]. However, Oberstaller et al. [8], using a highly sensitive Hidden Marcov Model (HMM), recently identified four additional genes encoding for AP2 proteins, thus extending the number of genes encoding for AP2 domain-containing proteins to a total of 22. General features of the 22 B. bovis genes and their predicted proteins are shown in Table 1. Because AP2 proteins may have more than a single AP2 domain, the B. bovis AP2 proteins display a total of 26 known AP2 domains. Similar to what was found in other apicomplexan genomes, the AP2 genes are not organized in clusters but dispersed throughout the four chromosomes of B. bovis (Fig 1A and 1B and Table 1).
Table 1. Characteristics of AP2 genes identified in the B. bovis genome.
| Chromosome No. | Gene (Locus tag) | Annotation | Length(gDNA/cDNA/aa) | No. of Exons | No. of AP2 domains | Other conserved domains | PI-MW (kDa) |
|---|---|---|---|---|---|---|---|
| Chromosome 1 | BBOV_I000100 | hypothetical protein | 775bp/585bp/194aa | 4 | One (110–162) | _ | 10.23–22.85 |
| BBOV_I002320 | hypothetical protein | 1461bp/486aa | 1 | One (397–441) | _ | 5.62–65.12 | |
| BBOV_I003560 | hypothetical protein | 2076bp/638aa | 1 | One (421–460) | _ | 5.15–70.06 | |
| BBOV_I004280 | hypothetical protein | 2049bp/682aa | 1 | One (609–655) | _ | 6.08–77.30 | |
| BBOV_I004850 | hypothetical protein | 1366bp/450aa | 1 | Three (94–143/166–212/372–420) | _ | 6.62–52.12 | |
| Chromosome 2 | BBOV_II001610 | hypothetical protein | 1362bp/453aa | 1 | One (68–120) | _ | 5.72–50.22 |
| BBOV_II003230 | hypothetical protein | 1532bp/500aa | 1 | One (408–458) | -PBP1, -PAT1 Amelogenin | 7.02–57.40 | |
| BBOV_II004230 | hypothetical protein | 3339bp/929aa | 1 | One (823–873) | _ | 6.15–103.56 | |
| BBOV_II005480 | hypothetical protein | 1763bp/480aa | 1 | One (249–298) | _ | 5.68–54.23 | |
| BBOV_II007120 | hypothetical protein | 2181bp/726aa | 1 | Two (187–240/261–317) | - | 5.36–82.22 | |
| Chromosome 3 | BBOV_III000570 | hypothetical protein | 843bp/633bp/210aa | 5 | One (107–162) | _ | 9.53–24.92 |
| BBOV_III003770 | hypothetical protein | 567bp/188aa | 1 | One (65–120) | _ | 9.37–21.87 | |
| BBOV_III004090 | hypothetical protein | 882bp/260aa | 1 | One (187–235) | _ | 11.21–30.50 | |
| BBOV_III004740 | hypothetical protein | 707bp/639bp/212aa | 3 | Two (100–145/162–210) | _ | 9.67–24.27 | |
| BBOV_III007040 | hypothetical protein | 1074bp/357aa | 1 | One (202–252) | PRK12937 | 8.44–40.19 | |
| BBOV_III007880 | hypothetical protein | 3423bp/1139aa | 1 | One (537–590) | 6.43–130.70 | ||
| BBOV_III008870 | hypothetical protein | 2122bp/1712bp/407aa | 5 | One (211–264) | ACDC | 5.21–45.47 | |
| BBOV_III009600 | hypothetical protein | 2093bp/1722bp/573aa | 1 | One (515–564) | _ | 5.82–63.38 | |
| Chromosome 4 | BBOV_IV000500 | hypothetical protein | 2340bp/779aa | 1 | One (493–544) | _ | 5.71–86.15 |
| BBOV_IV002360 | hypothetical protein | 1650bp/396aa | 4 | One (270–321) | _ | 5.81–46.26 | |
| BBOV_IV011690 | hypothetical protein | 2055bp/684aa | 2 | One (528–583) | _ | 5.70–75.35 | |
| BBOV_IV011830 | hypothetical protein | 1371bp/456aa | 1 | One (170–221) | ACDC | 5.2–51.28 |
Fig 1. Schematic representation of the location of the 22 AP2 genes and the number and domains identified in the genome of B. bovis (data not presented to scale).
(A) The figure represents the genome localization of the B. bovis AP2 genes, distributed among the four chromosomes. Each gene orientation is indicated by black arrows. (B) Gene nomenclature, schematic representation of the number and location of the AP2 domains of the B. bovis AP2 proteins. The presence and location of the ACDC domains in the protein are also indicated.
Bioinformatics analysis performed on the predicted amino acid sequences of the B. bovis AP2 proteins shows that some contain other additional known functional domains (Table 1, Fig 1B), such as the ACDC domain (AP2 coincident domain present mostly at the C-terminus of the proteins), a conserved PBP1domain (PAB1-binding protein 1), which is also present in proteins interacting with a poly(A)-binding protein, and in the Topoisomerase II-associated protein (PAT1), a protein that facilitates accurate chromosome separation during cell division (Table 1). Consistently, and together with the AP2 domain, all these additional domains are known to function in a nuclear environment. Predicted intracellular localization and routing of B. bovis AP2 proteins into the cell nucleus is consistent with the lack of signal peptides in all the putative B. bovis AP2 proteins as determined by sequence analysis using the SMART programs (http://smart.embl-heidelberg.de/smart/set_mode.cgi?NORMAL=1). In addition, cellular localization predictions using the program Cello v2.5 (http://cello.life.nctu.edu.tw/) predicted an intranuclear subcellular localization for all B. bovis AP2 proteins.
The predicted molecular size and isoelectric points of the B. bovis AP2s are also highly diverse, ranging from ~21 to 103 kDa to 5.15 to 11.21 kDa (Table 1). In general, there appears to be an association between isoelectric points (pI) and size of the molecules, and, thus, molecules with higher pI are of a relatively smaller size than the ones with a lower pI (Table 1). This association is consistent with a previous study by Kiraga et al. [23], although its biological relevance remains unknown.
While 19 out of the 22 known B. bovis AP2 proteins contain a single AP2 domain, the genes BBOV_II007120 and BBOV_III004740 contain two AP2 domains, and gene BBOV_I004850 has three AP2 domains (Table 1, Fig 1B). Similar to AP2 proteins in plants, two of the three domains in the putative protein encoded by BBOV_I004850 are separated by 25 amino acids in the amino terminal part of the molecule, whereas the third domain is distally localized, separated by 160 amino acids from the second domain and 30 amino acids apart from the C-terminal end of the molecule. The AP2 protein encoded by gene BBOV_II007120 contains the two AP2 domains separated by 21 amino acids, whereas the two AP2 domains of the protein encoded by gene BBOV_III004740 are just 17 amino acids apart. It is possible that proteins containing multiple AP2 domains are able to bind to distinct DNA regions either separately or simultaneously, thus adding increasing functional versatility for these molecules. In general, and consistent with what was found for other AP2 proteins, there is low sequence identity or similarity among the AP2 proteins, and, thus, their similarities are just restricted to the conserved 60 amino acid domain [8, 22, 24]. The percent identities found among the full AP2 proteins after their alignment is shown in S1 Table. The alignment and the identity results were obtained by using Clustal omega (http://www.ebi.ac.uk/Tools/msa/clustalo/). The more significantly related AP2 proteins are BBOV_I004850 and BBOV_II005480, sharing 25.59% identity (S1 Table), followed by BBOV_I000100 and BBOV_III003770, with 23.68% identity (S1 Table). Overall, these data suggest that, with few exceptions, the B. bovis AP2 proteins are not highly related in sequence outside the AP2 domains.
Alignments of the AP2 domain among all B. bovis revealed 100% identity between the AP2 domains in BBOV_III003770 and BBOV_I003560, suggesting the possibility of shared DNA binding specificities. Interestingly, the highly related domains BBOV_I004850.3 and BBOV_I004850.2 (sharing 51.02% identity) are both localized in the same protein (gene BBOV_I004850).
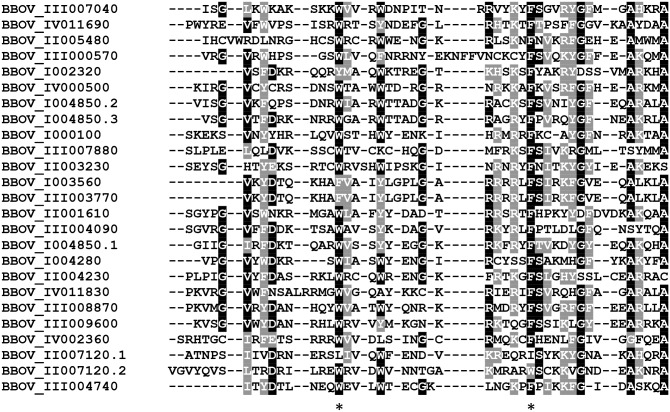
Alignments among all B. bovis AP2 domains (Fig 2) show that certain amino acid residues have a high degree of sequence conservation and may be functionally required in the B. bovis AP2 proteins. For instance, and similar to what was found for other apicomplexan AP2 proteins, all B. bovis AP2 domains contain highly conserved W and F residues (labeled with asterisks in Fig 2). It is known that these positional conserved residues are likely to help stabilize hydrophobic interactions between the AP2 domain and its recognized DNA target [22]. Consistently, and as described in more detail below, these residues are also conserved in the AP2 domains identified in the B. bovis related intra-erythrocytic apicomplexan B. microti and T. equi (S1 and S2 Figs). In addition, other amino acids are also highly conserved (Fig 2) among the B. bovis AP2 domains.
Fig 2. Sequence alignment of all AP2 domains found in the 22 B. bovis AP2 proteins.
Gene denominations are indicated on the left. Numerations (1–3) indicate the number of domains in each protein. Similar and identical amino acid residues are indicated in gray and black font, respectively. The [*] indicates conservation of W and F amino acids residues among the B. bovis AP2 domains.
Just 20 AP2 genes were annotated as containing AP2 domains in the published T. equi genome [6]. However, using further bioinformatics analysis, we found that genes BEWA_041620 and BEWA_018840 also contain AP2 domains. Thus, we propose that T. equi contains at least 22 AP2 genes. The organization and orientation of such genes into the four nuclear T. equi chromosomes are depicted in Fig 3A and S2 Table. Similar to what was observed for B. bovis, the T. equi AP2 genes are scattered among all four chromosomes (Fig 3A). As it was found for B. bovis, the AP2 genes of T. equi may also contain 1, 2 or 3 AP2 domains.
Fig 3.
(A) Schematic representation of the location of the 22 AP2 genes identified in the genome of T. equi (data not presented in scale). (B) Schematic representation of the location of the 21 AP2 genes identified in the genome of B. microti (data not presented in scale).
Similar searches performed on the published B. microti genome [5] resulted in the identification of 21 AP2 genes (S3 Table). All the Ap2 domain-containing genes present in the B. microti genome were previously annotated as such, except gene BBM_III08920 coding for a protein with a single previously unnoticed Ap2 domain, which is reported here for the first time. Fig 3B describes the organization as well as the orientation of the 21 AP2 genes into the four chromosomes of B. microti. Similar to what was found for B. bovis, the T. equi and B. microti AP-2 proteins contain other conserved domains, such as the ACDC and the PBP1 domains (S2 and S3 Tables).
Sequence comparisons among all the Ap2 domains identified in the B. bovis, T. equi, and B. microti putative AP2 proteins (Table 2) revealed high levels of identity among some domains. The identity reaches 100% among domains from proteins BBOV_III008870 (B. bovis), and BEWA_010510 (T. equi), and BBM_III06770 (B. microti). Interestingly, the proteins encoded by the B. bovis gene BBOV_I004850 and the T. equi BEWA_011980 gene have three highly similar domains. They share 100% identity for their first domain, which is also highly conserved in the B. microti protein encoded by gene BBM_III05870.1 (95.74% identity). Additional domain similarities are described in Table 2.
Table 2. Identity percent of selected Ap2 domains among B. bovis, B. microti, and T. equi.
| B. bovis domain | T. equi domain | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BBOV_III008870 | BBOV_I004850.1 | BBOV_I004850.2 | BBOV_I004850.3 | BBOV_II005480 | BBOV_III007040 | BEWA_010510 | BEWA_011980.1 | BEWA_011980.2 | BEWA_011980.3 | BEWA_022490 | BEWA_007130 | ||
| T. equi domain | BEWA_010510 | 100 | |||||||||||
| BEWA_011980.1 | 100 | ||||||||||||
| BEWA_011980.2 | 93.88 | ||||||||||||
| BEWA_011980.3 | 83.33 | ||||||||||||
| BEWA_022490 | 93.88 | ||||||||||||
| BEWA_007130 | 94.12 | ||||||||||||
| B. microti domain | BBM_III06770 | 100 | 100 | ||||||||||
| BBM_III05870.1 | 95.74 | 95.74 | |||||||||||
| BBM_III05870.2 | 89.8 | 89.8 | |||||||||||
| BBM_I03085 | NH | 84 | NH | 84 | NH | ||||||||
NH, No significant homology
The functions and DNA-binding specificities of the B. bovis, B. microti, and T. equi AP2 domains remain unknown, and they will need to be defined experimentally. Remarkably, the specificity of binding of some AP2 proteins to certain short DNA target motifs (usually six to seven base-pairs long) appears to be quite conserved among distinct Plasmodium species and, furthermore, among other related apicomplexans [7, 25]. These findings suggest that Plasmodium binding specificity data together with bioinformatics analysis on the 5′ upstream gene coding regions could guide the design of future experiments aimed at establishing the DNA binding specificities of the AP2 proteins in the three parasites examined in this study.
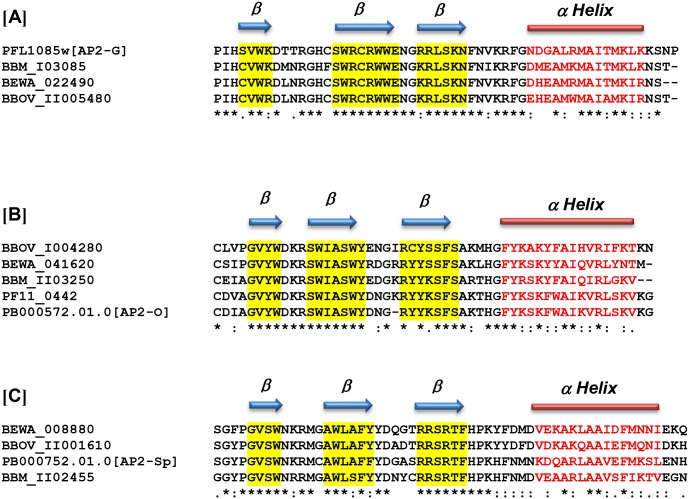
Recent research focused on the identification of specific AP2 proteins involved and required for regulating the expression of some stage-specific genes in Plasmodium [9, 10, 15, 16]. The related malaria parasites start differentiating into gametocytes while the parasites are still replicating inside erythrocytes in mammalian hosts. This crucial step requires a developmental decision, resulting in parasites that continue to replicate asexually or to differentiate into non-dividing male or female gametocytes, a life cycle event that is required to assure generation of genetic diversity and further transmission of the parasite upon mosquito acquisition. It was recently demonstrated that this developmental transition in P. falciparum parasites is regulated by the activity of the AP2 protein identified as pfAP2-g (PFL1085w) (Fig 4 Panel A). It was thus postulated that pfAP2-G functions as a transcriptional switch, stimulating the commitment to sexual development in this parasite [26]. Recent studies also supported the role of AP2 factors as candidate regulators driving the commitment to merozoite production in T. annulata [27]. Using a combination of techniques including transcriptome analysis and phenotypic characterization of AP2 gene knock outs, Yuda et al. [28] identified the AP2-O transcription factor, which is involved in the formation of invasive kinetes in Plasmodium berghei and P. falciparum (PB000572.01.0 and PF11_0442) (Fig 4, Panel B). Orthologues of the AP2-O gene have been also identified in other Plasmodium spp parasites. In addition, the same study also defined the sequence of the DNA involved in the binding to the AP2-O as the six-base motif TAGCTA. In a different study, Yuda et al. [29] also identified AP2-Sp (PB000752.01.0) (Fig 4, Panel C), a protein that is required for the regulation of the expression of P. berghei sporozoites and also defined the sequence TGCATG as a cis-acting element that is specific for its binding to DNA. Interestingly, the Ap2 domains involved in the binding of all these functionally defined Plasmodium AP2s are found to be well conserved in B. bovis, B. microti, and T. equi AP2 proteins, as shown in Fig 4. Therefore, and based on the sequence similarities of the AP2 domains shown in Fig 4, we hypothesize that the proteins encoded by genes BBOV_II005480 (~72% identity), BBM_I03085 (~76% identity), and BEWA_022490 (~77%) are functionally equivalent to the Plasmodium G (AP2-G) protein (PFL1085w). This is supported by previous findings demonstrating that the divergent T. annulata AP2-G protein containing AP2 motifs that are orthologous with the P. falciparum AP2-G protein are able to bind identical GxGTACxC motifs [27]. Data in S4 Table illustrates the orthologous relationships of putative AP2-G motifs of Theileria and Babesia parasites. The recently identified AP2-G T. annulata TA13515 gene [27] encodes for an AP2 motif that is 77.36% identical to the motif encoded by the functionally defined AP-G PFL1085w gene. However, this motif is more related in identity to the putative AP-G proteins in Theileria parva, Theileria orientalis, T. equi, B. bovis, and B. microti addressed in this study. These findings further support the testing of these AP2 as candidates for modulators in the transition of these parasites into sexual stages. Consistently, we also hypothesize that the genes identified as BBOV_I004280 (~70% identity), BBM_II03250 (~79% identity), and BEWA_041620 (~74% identity) are the functional equivalents of the Plasmodium AP2 proteins PF11_0442 and PB000572.01.0, which are both involved in Plasmodium ookinete development. Similarly, the AP2 proteins encoded by genes BBOV_II001610 (~65% identity), BBM_II02455 (~68% identity), and BEWA_008880 (~65% identity) might also be functional equivalents of AP2- Sp PB00752.01.0, which is involved in sporozoite development in malaria (Fig 4). These domain homology-driven predictions could help in prioritizing and selecting candidates for functional testing of these hypotheses, leading to define B. bovis, B. microti, and T. equi regulation pathways involved in gametocyte, ookinete, and sporozoite development.
Fig 4. Alignments among B. bovis, B. microti, T equi and functionally defined Plasmodium Ap2 domains.
Alignments of: (A) B. bovis BBOV_II005480, B. microti BBM_I03085, and T. equi BEWA_022490 with P. berghei PB000752.01.0, a domain involved in the development of sexual-stage forms in Plasmodium (AP2-G). (B) AP2 domain of the protein encoded by the B. bovis AP2 gene BBOV_I004280, B. microti BBM_II03250, and T. equi BEWA_041620 with putative orthologues in P. berghei (PB00572.01) and P. falciparum, (PF11_0442). Both Plasmodium AP2 proteins are required for the development of ookinetes, and known as AP2-O. (C) B. bovis BBOV_II001610, B. microti BBM_II02455, and T. equi BEWA_008880 with P. berghei PB000752.01.0, a domain involved in the development of sporozoites (AP2-Sp). Predicted secondary structures for the domains of interest are depicted at the top of each of the alignments.
It is possible that the proteins containing these highly conserved domains share similar DNA binding specificities among these three parasites, but this will have to be confirmed experimentally. Full transcriptome analysis in the life cycle of these organisms is not yet available, and it will be needed in order to perceive the possible role of AP2 proteins influencing life cycle transitions in these parasites.
Conservation of genes encoding for the transcriptional regulators Myb and HMG among B. bovis, B. microti, and T. equi
The Myb proteins, which are highly conserved in eukaryotes, belong to the tryptophan cluster family and are also known to regulate gene expression. Similar to AP2 factors, Myb proteins are involved in differentiation and growth control by binding to DNA in a sequence-specific manner through a DNA-binding domain [10, 30]. Importantly, Myb proteins have been confirmed to be essential for parasite growth, cell cycle regulation, and progression in Plasmodium parasites [18]. Myb families containing eight genes each are present in the B. bovis, T. equi, and B. microti genomes (Table 3).
Table 3. Myb genes distribution among B. bovis, B. microti and T. equi genome.
| Chromosome | B. bovis | B. microti | T. equi |
|---|---|---|---|
| Chromosome 1 |
|
BEWA_021480 | |
| Chromosome 2 |
|
BBM_II03695 |
|
| Chromosome 3 | BBOV_III005430 |
|
|
| Chromosome 4 |
|
BEWA_009170 |
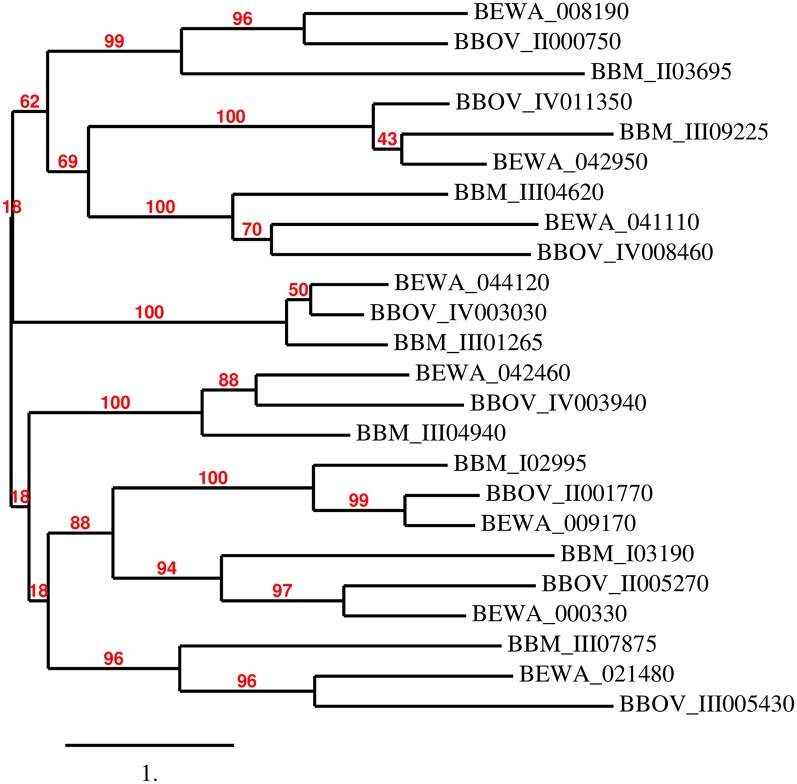
Interestingly, a full set of eight Myb genes appears to be well conserved in sequence among the three parasites, and the Myb proteins of these three parasites appear to have similar domain architectures (S3 Fig). Their phylogenetic relationships are shown in Fig 5 and their orthologous relationships confirmed by using Bidirectional Best Blast hit analysis [31]. The orthologous Myb proteins BBOV_II001770, BEWA_009170, and BBM_I02995 contain an additional DnaJ motif located at their N-terminus region, while the DNA binding domain typical of the Myb proteins is located in their C-terminus (S3 Fig). In general, Myb genes are unlinked and dispersed among these three parasites’ chromosomes. However, this is not the case for the T. equi Myb genes BEWA_008190 and BEWA_008180, which are contiguous in chromosome 3 of T. equi. Protein sequence comparisons revealed limited sequence identity among the Myb proteins encoded in each of these three parasites. The possible ortholog relationships among all Myb genes identified in these three parasites are illustrated in the phylogenetic tree shown in Fig 5. The highly conserved gene BBOV_IV003030 encodes for a Myb protein that is 60.43% identical to the one encoded by gene BEWA_044120 in T. equi, and 50% identical to the protein encoded by gene BBM_III01265 found in in the B. microti genome. It is thus possible that these three proteins are functional homologues.
Fig 5. Phylogenetic relationships among the putative Myb proteins of B. bovis, T. equi, and B. microti.
The unrooted phylogenetic tree was generated using the sequences of all the putative Myb proteins identified in B. bovis, B. microti, and T. equi with the program phylogeny.fr. The program calculated the branch support values in percent (%, red font) using an aLRT statistical test (http://www.phylogeny.fr/simple_phylogeny.cgi?workflow_id=ddf5cfc42f5b6c10f3df67f5152bf59a&tab_index=6&go_next=1#anchor).
In conclusion, these relationships indicate that a core of eight Myb genes is conserved among these three parasites, and perhaps this is also the case in other related apicomplexan parasites as well. Consistently, searches performed on the genome of T. annulata, T. parva, and T. orientalis revealed full conservation of the set of eight Myb genes in these classical Theileria parasites (S5 Table). The complement of eight Myb genes from B. bovis, B. microti, and T. equi grouped in the phylogenetic tree together with the three classical Theileria parasites is shown in S4 Fig. It is possible to infer from these data that an ancestor organism existing previous to speciation among Babesia and Theileria also contained an eight Myb gene family.
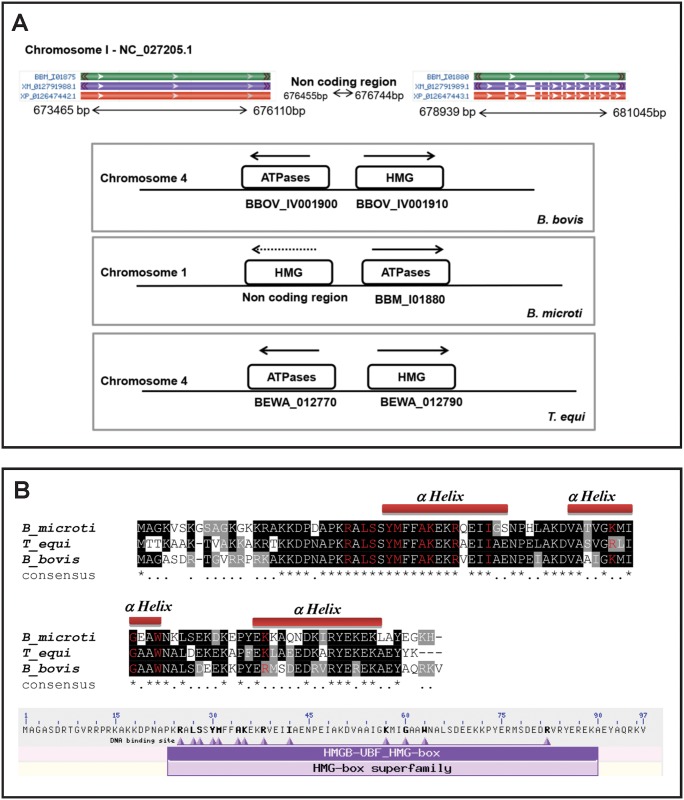
The high mobility group box proteins (HMG) is a group of DNA-binding transcription factors required for the maintenance of structural alterations in DNA during transcription. The HMG superfamily is divided into three families of proteins according to their functional motifs, known as HMGA, interacting with the AT hook; HMGN, involved with nucleosomes; and HMGB, containing one or several copies of HMG box DNA binding domain [20]. In contrast to the AP2 and Myb proteins, the HMG proteins have the ability to bind A-T—rich regions of DNA rather than sequence-specific targets, in a process mediated by basic amino-acid residues of the proteins [31]. There appears to be just one HMG gene in B. bovis (BBOV_IV001910) in chromosome 4. This HMG gene has been previously cloned and characterized in yeast and B. bovis [32, 33]. The size of the predicted protein, domain and secondary structure predictions, and sequence comparisons indicate that the B. bovis BBOV_IV001910 gene is similar to the Pf HMGB genes [20] and, thus, it can be considered as a member of the HMGB family. The binding specificity of the P. falciparum HMGB proteins to four-way DNA junctions was also previously established [20]. In addition, a single HMG gene copy in T. equi BEWA_012790 was found on chromosome 4. The B. bovis BBOV_IV001910 and the T. equi BEWA_012790 predicted proteins are 65% identical and contain just 92 amino acids and a single HMG domain, lacking the typical acidic C-terminal tail [20, 33]. This putative HMG gene is well conserved among apicomplexans [20] and in other cells but was not annotated as such in the B. microti genome. However, BLAST analysis of the B. microti genome with the BBOV_IV001910 sequence demonstrated the occurrence of a gene present in an unannotated region of the genome (http://protists.ensembl.org/Babesia_microti_strain_ri/Tools/Blast?db=core), encoding for a homologous HMG protein. This novel putative HMG gene is located in the ~2829bp non-coding region between bp 676455 and 676744 of chromosome 1 of B. microti (Fig 6A upper part). Furthermore, synteny among B. microti, B. bovis, and T. equi in genomic regions encoding this gene was identified (Fig 6A bottom part). Similar to B. bovis and T. equi, the non-coding region of B. microti, which contains the HMG domain, was found to be followed by gene BBM_I01880 (Fig 6A bottom part) encoding for a protein containing an AAA domain [cd00009], an ATP binding motif present in ATPases (Fig 6A bottom part). Furthermore, we also found conservation and consistent synteny of the HMG gene in other related apicomplexa (T. annulata, T. parva, P. falciparum, Plasmodium knowlesi, and Plasmodium vivax) (S5). In Fig 6B, the defining amino acids for the HMG domain are shown, as well as a sequence alignment of three putative HMG proteins and the predicted secondary structures of B. microti, B. bovis, and T. equi. Interestingly, the predicted secondary structures for the in silico translated HMG proteins of B. microti, B. bovis, and T. equi shows three identical alpha-helixes comprising all amino acids involved in the HMG domain (Fig 6B), identical to what was described for their Plasmodium HMGB homologues [20]. It is likely that this conserved secondary structure is essential for access of the HMG proteins to its DNA binding target and for effecting protein function. In P. falciparum, the HMG proteins are present in the nucleus and induce DNA bending [20]. However, the binding targets and exact functions of the Babesia and Theileria HMG proteins remain to be defined. Considering these observations, together with the facts that gene BBOV_IV001910 is relatively highly expressed in B. bovis erythrocyte stages, as described below and shown in Fig 7C, and that key residues defining the HMG domain are also fully conserved in the B. microti putative protein (Fig 6A and 6B), we propose that the region in chromosome 1 of B. microti represented in Fig 6A represents a novel HMG gene. If the presence of an HMG gene in B. microti is confirmed experimentally, then annotation in this region of chromosome 1 of B. microti should be revised.
Fig 6. Putative HMG proteins and genes of B. bovis, T. equi, and B. microti.
(A) Upper: Schematic representation of non-coding area in B. microti genome between 676455 bp and 676744 bp in chromosome 1. Lower: Synteny map for the HMG genes in the B. bovis, B. microti, and T. equi. (B) Upper: Alignment of the deduced amino acid sequences of HMG proteins identified in B. bovis (BBOV_IV001910), T. equi (BEWA_012790), and B. microti (no gene assignment). The residues in red represent the amino acids defining the HMG domain Lower: Location of the HMG domain and depiction of the amino acid residues defining the HMG domain (bold fonts) in the HMG protein encoded by the B. bovis gene BBOV_ IV001910.
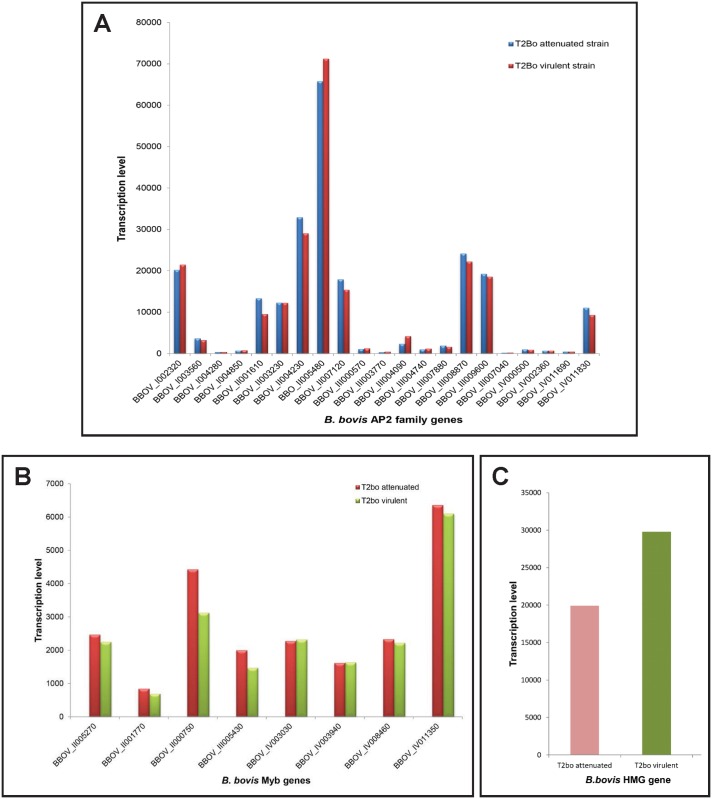
Fig 7. Normalized transcriptional profiles of B. bovis AP2 genes using microarray analysis in attenuated and virulent B. bovis strains.
(A) Microarray transcriptional levels expressed as relative transcription units log cpm (copy per million) are represented in the y-axis, and denominations for each B. bovis AP2 gene are represented in the x-axis. (B) Transcription profile of the eight Myb genes identified in the virulent and attenuated T2bo strains of B. bovis using microarray analysis. (C) Transcription profile of the BBOV_IV001910 HMG gene by microarray analysis in virulent and attenuated T2Bo strain of B. bovis. The transcriptome analysis was performed in triplicate on full blood stages of parasites developing in asynchronous cultures.
Pattern of transcription of the AP2, Myb, and HMG genes during blood stages of B. bovis
Studies in Plasmodium, T. annulata, and Toxoplasma indicated that most AP2 genes are differentially expressed during the life cycle of the parasites [27]. B. bovis parasites have a complex life cycle involving at least two distinct hosts, the mammal bovine and arthropod tick hosts. B. bovis parasites developing in the bovine hosts only invade and reproduce in erythrocytes, and it remains unclear whether they start committing into gametogenesis while residing in the erythrocyte. However, the life stages of the parasite developing in the definitive tick vector appear to be more diverse and complex, including sexual stages and sexual reproduction, in addition to the development of kinete and sporozoite stages. Furthermore, because of their trans-ovarian mode of transmission, Babesia parasites are able to survive in additional stages of the tick host (adult, egg, larva, and nymph, with each of these tick stages occurring in dramatically distinct physical surroundings). We propose that this feature reflects a high degree of plasticity for this parasite, which enables radical adaptive morphological transitions during changing temperatures, surviving the non-adaptive immune system of the tick and other variable environmental factors while replicating in the tick. Based on the known role of AP2 proteins in related apicomplexans, it is possible that these changes are correlated with unique patterns of expression of AP2 proteins, in order to fulfill their role as stage-specific transcriptional regulators. Analysis of the currently available transcriptome of B. bovis in the blood stages supports this notion, as, at least, expression of two of the AP2 genes, such as BBOV_II005480 and BBOV_II004230, are significantly elevated in blood stage parasites of attenuated and virulent B. bovis T2Bo strains, while some of the AP2 genes are silenced (Fig 7A). Interestingly, and as shown in Fig 4, sequence comparisons suggest that the AP2 gene BBOV_II005480, highly transcribed in blood stages of B. bovis, is a possible correlate of the P. falciparum gene AP2-G (PFL_1085w), which was shown to be involved in the transition of P. falciparum blood stage parasites into sexual forms [26, 34]. It was recently shown that PFAP2-G functions as a master regulator controlling sexual-stage differentiation decision in Plasmodium parasites [26].
It is currently unknown whether the B. bovis AP2 gene BBOV_II005480 is also involved in the regulation of the expression of genes involved in sexual stage transitions and whether such stage transition also occurs in blood-stage parasites of B. bovis. However, the general currently accepted paradigm is that commitment of B. bovis to sexual forms might start with the formation of pre-gametes while the parasites reside in the bovine hosts [35, 36], which would be associated with the high level of expression of the AP2 gene BBOV_II005480 gene in the blood stages of the parasite. It is possible that B. bovis blood-stage parasites need to be primed before developing into sexual stage while still developing into the mammalian host, but this remains unknown. Alternatively, it is also possible that the expression of the gene BBOV_II005480 in blood stages is required for functions unrelated to sexual stage development. Other AP2 genes found to be highly expressed in blood stage parasites include BBOV_II004230, BBOV_III008870, BBOV_I002320, and BBOV_III009600. Interestingly, levels of transcription for the putative gene AP2-O (BBov_I004280) are negligible in the blood stage, whereas the levels of transcript for the putative AP2-Sp gene, although higher than AP2-O (BBov_II001610), are also significantly lower than AP2-G (BBOV_II005480). It could be predicted that the levels of expression of both genes are elevated in tick stages of B. bovis, as its differentiation to kinete and sporozoite stages occurs in the tick.
Comparative multistage global transcriptome analysis, together with proteomic analysis, remains to be performed in order to fully understand the patterns of expression of the Babesia AP2 genes among its different life stages. Taken together, these studies should provide a framework for deciphering the gene regulation networks operating during the life cycle of B. bovis and may also contribute to the design of novel methods for the control of this parasite.
Myb transcript analysis performed on two distinct B. bovis strains (T2bo attenuated and virulent) shows that seven out of the eight gene members are transcribed at relatively low levels in B. bovis blood stages (Fig 7B), whereas the Myb gene BBOV_IV011350 appears to be expressed at significantly higher levels, and, thus, members of this family are also differentially expressed by the parasite. In addition, the HMG gene BBOV_IV001910 is also consistently and relatively highly expressed in the two distinct B. bovis strains analyzed (T2bo attenuated and virulent strains) (Fig 7C).
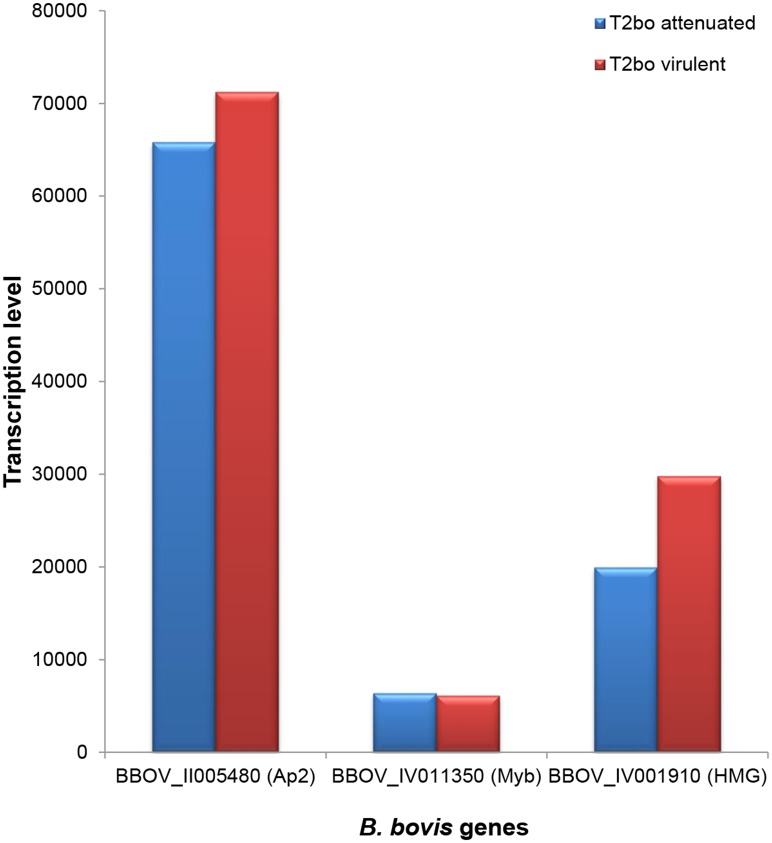
The relative high levels of expression of the AP2, Myb, and HMG genes in B. bovis blood stages can be compared in Fig 8. Transcripts of the AP2 gene BBOV_II005480 were detected at levels that are at least an order of magnitude higher than the Myb gene and at twice the levels of the highest expressed HMG gene BBOV_IV001910. The functional significance of these observations remains unknown and requires further study.
Fig 8. Comparison among the highest transcribed genes of the AP2, Myb, and HMG genes of B. bovis.
The Ap2 gene BBOV_II005480 shows the highest transcription levels compared to the highest transcribed genes of the Myb and HMG gene groups in B. bovis.
However, microarray data does not show significant differences in the level of expression of the genes analyzed in this study among the attenuated and virulent strain pairs so far analyzed. There is no experimental evidence supporting the hypothesis that differential expression of these regulatory genes has any correlation with the virulence phenotype of Babesia strains.
Concluding Remarks
Described here is the structure of the AP2 genes of B. bovis as well as the general organization of this family in the related T. equi and B. microti parasites. AP2 genes that are differentially expressed during the blood stages were identified and, based on domain sequence similarities, correlated with already functionally characterized Plasmodium AP2 proteins. A previously unknown gene family with an eight-gene core encoding for proteins, including the DNA binding domain that is characteristic for the transcription factors, known as Myb, was found conserved in B. bovis, B. microti, and T. equi. Remarkably, a conserved HMG gene was also described in these three parasites for the first time, although expression of the B. microti HMG gene identified in this study remains to be confirmed experimentally. The Myb and HMG genes of B. bovis might also be differentially expressed in the blood stages of the parasite. The pattern of expression of AP2, Myb, and HMG genes in multiple B. bovis, T. equi, and B. microti parasite stages should also be compared in order to start unraveling mechanisms involved in the regulation of gene expression in these parasites. Overall, the findings described in this study suggest conservation of regulatory genes in the face of large divergence of genome size, content and organization, and host specificities among these three apicomplexan parasites. Taking advantage of transfection and gene editing techniques, it is now possible to design KO and overexpression studies aimed at defining the resulting phenotype of mutated or genetically altered transfected parasites, leading to a correlation between gene and protein function for the AP2, HMG, and Myb proteins. In addition, experiments leading to the identification of the binding specificities for each of the B. bovis, B. microti, and T. equi AP2 proteins, as well as the Myb and HMG transcription factors, should also be performed. Finally, the ability to genetically manipulate genes encoding for transcription factors should result in a better understanding of the biology of these parasites and to the rational design of attenuated and non-tick transmissible parasite strains that can be used for the development of the next generation of live attenuated vaccines and chemotherapeutics. Conservation of key gene regulation mechanisms may lead to future development of novel converging control strategies that can be applied to apicomplexan parasites.
Key Learning Points
The functional domains and general architecture of the ApiAP2, Myb, and HMG proteins remain conserved among Babesia bovis, B. microti, and Theileria equi.
A repertoire of eight Myb genes is conserved among Babesia bovis, B. microti, and Theileria equi.
Transcriptome analysis suggests that the pattern of transcription of the regulatory AP2, HMG, and Myb genes in B. bovis is stage specific.
A new putative HMG gene is described for B. microti.
Defining the functional role of regulatory genes may contribute to the development of novel convergent strategies for improved control of babesiosis and equine piroplasmosis.
Key Papers in the Field
Yuda M, Iwanaga S, Shigenobu S, Mair GR, Janse CJ, Waters AP, et al. Identification of a transcription factor in the mosquito-invasive stage of malaria parasites. Molecular microbiology. 2009;71(6):1402–14. Epub 2009/02/18. doi: 10.1111/j.1365-2958.2009.06609.x. 19220746.
Yuda M, Iwanaga S, Shigenobu S, Kato T, Kaneko I. Transcription factor AP2-Sp and its target genes in malarial sporozoites. Molecular microbiology. 2010;75(4):854–63. Epub 2009/12/23. doi: 10.1111/j.1365-2958.2009.07005.x. 20025671.
Iwanaga S, Kaneko I, Kato T, Yuda M. Identification of an AP2-family protein that is critical for malaria liver stage development. PLoS ONE. 2012;7(11):e47557. Epub 2012/11/13. doi: 10.1371/journal.pone.0047557. 23144823; PubMed Central PMCID: PMCPmc3492389.
Tuteja R, Ansari A, Chauhan VS. Emerging functions of transcription factors in malaria parasite. Journal of biomedicine & biotechnology. 2011;2011:461979. Epub 2011/12/02. doi: 10.1155/2011/461979. 22131806; PubMed Central PMCID: PMCPmc3216465.
Pieszko M, Weir W, Goodhead I, Kinnaird J, Shiels B. ApiAP2 Factors as Candidate Regulators of Stochastic Commitment to Merozoite Production in Theileria annulata. PLoS Negl Trop Dis. 2015;9(8):e0003933. Epub 2015/08/15. doi: 10.1371/journal.pntd.0003933. 26273826; PubMed Central PMCID: PMCPmc4537280.
Supporting Information
Gene denominations are indicated on the left. Conserved residues are indicated in grey and black highlight.
(TIF)
Gene denominations are indicated on the left.
(TIF)
The three parasites’ Myb proteins have similar domain architectures.
(PDF)
The orthologous genes grouped into eight groups consisting of one gene from each organism.
(TIFF)
The black arrow indicates the orientation of the genes in the chromosome.
(TIFF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We are thankful to Dr. Audrey Lau and Jacob Laughery for the critical reading of the manuscript.
Funding Statement
This work was supported by United States Department of Agriculture Research Service Current Research Information System Project No. 5348-32000-028-00D, and Egyptian government, Ministry of High Education and Scientific Research scholarship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Scoles GA, Ueti MW. Vector ecology of equine piroplasmosis. Annual review of entomology. 2015;60:561–80. Epub 2015/01/08. 10.1146/annurev-ento-010814-021110 . [DOI] [PubMed] [Google Scholar]
- 2.Suarez CE, Noh S. Emerging perspectives in the research of bovine babesiosis and anaplasmosis. Vet Parasitol. 2011;180(1–2):109–25. Epub 2011/06/21. 10.1016/j.vetpar.2011.05.032 . [DOI] [PubMed] [Google Scholar]
- 3.Brayton KA, Lau AO, Herndon DR, Hannick L, Kappmeyer LS, Berens SJ, et al. Genome sequence of Babesia bovis and comparative analysis of apicomplexan hemoprotozoa. PLoS Pathog. 2007;3(10):e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornillot E, Hadj-Kaddour K, Dassouli A, Noel B, Ranwez V, Vacherie B, et al. Sequencing of the smallest Apicomplexan genome from the human pathogen Babesia microti. Nucleic acids research. 2012;40(18):9102–14. 10.1093/nar/gks700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornillot E, Dassouli A, Garg A, Pachikara N, Randazzo S, Depoix D, et al. Whole genome mapping and re-organization of the nuclear and mitochondrial genomes of Babesia microti isolates. PloS one. 2013;8(9):e72657 10.1371/journal.pone.0072657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kappmeyer LS, Thiagarajan M, Herndon DR, Ramsay JD, Caler E, Djikeng A, et al. Comparative genomic analysis and phylogenetic position of Theileria equi. BMC genomics. 2012;13(1):603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kensche PR, Maria Hoeijmakers WA, Toenhake CG, Bras M, Chappell L, Berriman M, et al. The nucleosome landscape of Plasmodium falciparum reveals chromatin architecture and dynamics of regulatory sequences. Nucleic acids research. 2015. Epub 2015/11/19. 10.1093/nar/gkv1214 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oberstaller J, Pumpalova Y, Schieler A, Llinas M, Kissinger JC. The Cryptosporidium parvum ApiAP2 gene family: insights into the evolution of apicomplexan AP2 regulatory systems. Nucleic acids research. 2014;42(13):8271–84. Epub 2014/06/25. 10.1093/nar/gku500 ; PubMed Central PMCID: PMCPmc4117751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Silva EK, Gehrke AR, Olszewski K, Leon I, Chahal JS, Bulyk ML, et al. Specific DNA-binding by apicomplexan AP2 transcription factors. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(24):8393–8. Epub 2008/06/11. 10.1073/pnas.0801993105 ; PubMed Central PMCID: PMCPmc2423414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuteja R, Ansari A, Chauhan VS. Emerging functions of transcription factors in malaria parasite. Journal of biomedicine & biotechnology. 2011;2011:461979 Epub 2011/12/02. 10.1155/2011/461979 ; PubMed Central PMCID: PMCPmc3216465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coleman BI, Duraisingh MT. Transcriptional control and gene silencing in Plasmodium falciparum. Cellular microbiology. 2008;10(10):1935–46. 10.1111/j.1462-5822.2008.01203.x [DOI] [PubMed] [Google Scholar]
- 12.Riechmann JL, Meyerowitz EM. The AP2/EREBP family of plant transcription factors. Biological chemistry. 1998;379(6):633–46. Epub 1998/08/01. . [DOI] [PubMed] [Google Scholar]
- 13.Okamuro JK, Caster B, Villarroel R, Van Montagu M, Jofuku KD. The AP2 domain of APETALA2 defines a large new family of DNA binding proteins in Arabidopsis. Proceedings of the National Academy of Sciences. 1997;94(13):7076–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen MD, Yamasaki K, Ohme‐Takagi M, Tateno M, Suzuki M. A novel mode of DNA recognition by a β‐sheet revealed by the solution structure of the GCC‐box binding domain in complex with DNA. The EMBO Journal. 1998;17(18):5484–96. 10.1093/emboj/17.18.5484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindner SE, De Silva EK, Keck JL, Llinás M. Structural determinants of DNA binding by a P. falciparum ApiAP2 transcriptional regulator. Journal of molecular biology. 2010;395(3):558–67. 10.1016/j.jmb.2009.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Silva EK, Gehrke AR, Olszewski K, León I, Chahal JS, Bulyk ML, et al. Specific DNA-binding by apicomplexan AP2 transcription factors. Proceedings of the National Academy of Sciences. 2008;105(24):8393–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jofuku KD, den Boer BG, Van Montagu M, Okamuro JK. Control of Arabidopsis flower and seed development by the homeotic gene APETALA2. The Plant cell. 1994;6(9):1211–25. Epub 1994/09/01. ; PubMed Central PMCID: PMCPmc160514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gissot M, Briquet S, Refour P, Boschet C, Vaquero C. PfMyb1, a Plasmodium falciparum transcription factor, is required for intra-erythrocytic growth and controls key genes for cell cycle regulation. Journal of molecular biology. 2005;346(1):29–42. Epub 2005/01/25. 10.1016/j.jmb.2004.11.045 . [DOI] [PubMed] [Google Scholar]
- 19.Kumar K, Singal A, Rizvi MM, Chauhan VS. High mobility group box (HMGB) proteins of Plasmodium falciparum: DNA binding proteins with pro-inflammatory activity. Parasitology international. 2008;57(2):150–7. Epub 2008/02/01. 10.1016/j.parint.2007.11.005 . [DOI] [PubMed] [Google Scholar]
- 20.Briquet S, Boschet C, Gissot M, Tissandie E, Sevilla E, Franetich JF, et al. High-mobility-group box nuclear factors of Plasmodium falciparum. Eukaryotic cell. 2006;5(4):672–82. Epub 2006/04/12. 10.1128/ec.5.4.672-682.2006 ; PubMed Central PMCID: PMCPmc1459676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Roch KG, Zhou Y, Blair PL, Grainger M, Moch JK, Haynes JD, et al. Discovery of gene function by expression profiling of the malaria parasite life cycle. Science. 2003;301(5639):1503–8. 10.1126/science.1087025 [DOI] [PubMed] [Google Scholar]
- 22.Balaji S, Babu MM, Iyer LM, Aravind L. Discovery of the principal specific transcription factors of Apicomplexa and their implication for the evolution of the AP2-integrase DNA binding domains. Nucleic acids research. 2005;33(13):3994–4006. Epub 2005/07/26. 10.1093/nar/gki709 ; PubMed Central PMCID: PMCPmc1178005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiraga J, Mackiewicz P, Mackiewicz D, Kowalczuk M, Biecek P, Polak N, et al. The relationships between the isoelectric point and: length of proteins, taxonomy and ecology of organisms. BMC Genomics. 2007;8:163 Epub 2007/06/15. 10.1186/1471-2164-8-163 ; PubMed Central PMCID: PMCPmc1905920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Painter HJ, Campbell TL, Llinas M. The Apicomplexan AP2 family: integral factors regulating Plasmodium development. Molecular and biochemical parasitology. 2011;176(1):1–7. Epub 2010/12/04. 10.1016/j.molbiopara.2010.11.014 ; PubMed Central PMCID: PMCPmc3026892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campbell TL, De Silva EK, Olszewski KL, Elemento O, Llinas M. Identification and genome-wide prediction of DNA binding specificities for the ApiAP2 family of regulators from the malaria parasite. PLoS Pathog. 2010;6(10):e1001165 Epub 2010/11/10. 10.1371/journal.ppat.1001165 ; PubMed Central PMCID: PMCPmc2965767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kafsack BF, Rovira-Graells N, Clark TG, Bancells C, Crowley VM, Campino SG, et al. A transcriptional switch underlies commitment to sexual development in malaria parasites. Nature. 2014;507(7491):248–52. Epub 2014/02/28. 10.1038/nature12920 ; PubMed Central PMCID: PMCPmc4040541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pieszko M, Weir W, Goodhead I, Kinnaird J, Shiels B. ApiAP2 Factors as Candidate Regulators of Stochastic Commitment to Merozoite Production in Theileria annulata. PLoS neglected tropical diseases. 2015;9(8):e0003933 Epub 2015/08/15. 10.1371/journal.pntd.0003933 ; PubMed Central PMCID: PMCPmc4537280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuda M, Iwanaga S, Shigenobu S, Mair GR, Janse CJ, Waters AP, et al. Identification of a transcription factor in the mosquito-invasive stage of malaria parasites. Molecular microbiology. 2009;71(6):1402–14. Epub 2009/02/18. 10.1111/j.1365-2958.2009.06609.x . [DOI] [PubMed] [Google Scholar]
- 29.Yuda M, Iwanaga S, Shigenobu S, Kato T, Kaneko I. Transcription factor AP2-Sp and its target genes in malarial sporozoites. Molecular microbiology. 2010;75(4):854–63. Epub 2009/12/23. 10.1111/j.1365-2958.2009.07005.x . [DOI] [PubMed] [Google Scholar]
- 30.Lipsick JS. One billion years of Myb. Oncogene. 1996;13(2):223–35. Epub 1996/07/18. . [PubMed] [Google Scholar]
- 31.Kun JF, Anders RF. A Plasmodium falciparum gene encoding a high mobility group protein box. Molecular and biochemical parasitology. 1995;71(2):249–53. Epub 1995/05/01. . [DOI] [PubMed] [Google Scholar]
- 32.Kolodrubetz D, Burgum A. Duplicated NHP6 genes of Saccharomyces cerevisiae encode proteins homologous to bovine high mobility group protein 1. The Journal of biological chemistry. 1990;265(6):3234–9. Epub 1990/02/25. . [PubMed] [Google Scholar]
- 33.Dalrymple BP, Peters JM. Characterization of a cDNA clone from the haemoparasite Babesia bovis encoding a protein containing an "HMG-Box". Biochemical and biophysical research communications. 1992;184(1):31–5. Epub 1992/04/15. . [DOI] [PubMed] [Google Scholar]
- 34.Sinha A, Hughes KR, Modrzynska KK, Otto TD, Pfander C, Dickens NJ, et al. A cascade of DNA-binding proteins for sexual commitment and development in Plasmodium. Nature. 2014;507(7491):253–7. Epub 2014/02/28. 10.1038/nature12970 ; PubMed Central PMCID: PMCPmc4105895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehlhorn H, Shein E. The piroplasms: life cycle and sexual stages. Advances in parasitology. 1984;23:37–103. Epub 1984/01/01. . [DOI] [PubMed] [Google Scholar]
- 36.Becker CA, Malandrin L, Larcher T, Chauvin A, Bischoff E, Bonnet SI. Validation of BdCCp2 as a marker for Babesia divergens sexual stages in ticks. Experimental parasitology. 2013;133(1):51–6. Epub 2012/10/30. 10.1016/j.exppara.2012.10.007 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gene denominations are indicated on the left. Conserved residues are indicated in grey and black highlight.
(TIF)
Gene denominations are indicated on the left.
(TIF)
The three parasites’ Myb proteins have similar domain architectures.
(PDF)
The orthologous genes grouped into eight groups consisting of one gene from each organism.
(TIFF)
The black arrow indicates the orientation of the genes in the chromosome.
(TIFF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)