Abstract
More than 150 million people worldwide are chronically infected with hepatitis C virus (HCV) and face higher risk of cirrhosis and hepatocellular carcinoma. Highly effective HCV treatments have recently been developed; however, they are costly and therefore poorly suited for application in resource-limited settings where HCV burden is high. Pegylated-interferon alpha (PEG-IFNα) and ribavirin (RBV) therapy is far less costly, but also less effective. Vitamin D supplementation has been proposed as an inexpensive adjuvant to treatment, however, prior epidemiological evidence on its effectiveness is inconsistent, with little data available among African Americans who naturally have lower vitamin D concentrations. We thus evaluated associations between baseline vitamin D status, measured by circulating 25-hydroxyvitamin D (25(OH)D), which is considered to be the best marker of vitamin D status in humans, and subsequent response to PEG-IFNα/RBV therapy in two large clinical trials that together included 1292 patients infected with HCV genotype 1. We used race-stratified logistic regression models to evaluate multivariable-adjusted associations of 25(OH)D with early virologic response (EVR; 2-log10 HCV RNA decline at week 12) and sustained virologic response (SVR). Among African Americans, we saw no associations. Among European Americans, we saw no association with low vitamin D (≤20 ng/mL) versus sufficient concentrations (20-<30 ng/mL). However, patients with 25(OH)D ≥30 ng/mL were actually less likely to attain EVR (OR = 0.64, 95% CI = 0.43–0.94) than those with sufficient concentrations, with a similar but non-significant association observed for SVR (OR = 0.49, 95% CI = 0.20–1.17).
Conclusion
Higher vitamin D status was not beneficially associated with responses to therapy; if anything, patients with higher vitamin D concentrations were less likely to attain SVR. Our data do not support a role for vitamin D supplementation as an adjuvant therapy for HCV.
Introduction
Worldwide 130 to 170 million people, or 2 to 3% of the global population, are chronically infected with hepatitis C virus (HCV) [1, 2]. In the absence of treatment, chronic HCV infection substantially elevates risk for cirrhosis and hepatocellular carcinoma [3, 4], and an estimated 500,000 people die annually from HCV-related liver diseases [5]. Pegylated-interferon alpha and ribavirin (IFNα/RBV) combination therapy results in sustained virologic response (SVR) in 40–50% of treatment naïve patients infected with HCV genotype 1 [6, 7], which accounts for ≥70% of HCV infections in the United States [8] and 90% of HCV infections among African Americans [9]. Direct-acting antiviral agents (DAAs) are far more effective for treating HCV genotype 1[10], but are also far more expensive than PEG-IFNα/RBV therapy, costing upwards of $84,000/patient [11, 12]. Cost is one of the greatest barriers to HCV treatment [13], especially in developing regions, including Africa and Asia, where the burden of HCV infection is highest [1, 14, 15], [16]. Predictors of poor virologic response to PEG-IFNα/RBV therapy include African ancestry [9, 17] and IFNL4 genotype [18–20], and a recent clinical trial implicated these factors as predictive of lower response rates to a commonly used DAA regimen (ledipasvir and sofosbuvir) as well [21].
Results from supplementation and observational studies suggest that vitamin D deficiency may adversely impact virologic response to PEG-IFNα/RBV therapy [22–24]. Vitamin D binds and activates the vitamin D receptor (VDR); VDR is a potent transcriptional regulator active in both the innate and adaptive immune system, including the interferon pathway, which is essential in HCV clearance [25, 26]. Additionally, vitamin D has been shown to inhibit HCV replication in cell culture [27–29]. However, prior findings on the relationship between vitamin D and response to HCV therapy have been inconsistent. Highlighting this lack of consensus, two recently published meta-analyses of the association of vitamin D status with SVR to PEG-IFNα/RBV therapy came to different conclusions [30, 31]. Prior studies have often been unable to adjust for potential confounding factors such as IFNL4 genotype, liver fibrosis stage, body mass index (BMI) and season of blood draw, and few studies have examined associations among individuals of African ancestry, who naturally have low vitamin D concentrations [32]. Finally, data on the temporal relationship between vitamin D and HCV are lacking [30, 31].
Here we conducted an observational study using data from two clinical trials with high quality patient data on known predictors of virologic response, Hepatitis C Antiviral Long-Term Treatment against Cirrhosis (HALT-C) and Viral Resistance to Antiviral Therapy of Chronic Hepatitis C (VIRAHEP-C), to assess whether pre-treatment vitamin D concentrations are independently associated with virologic response to PEG-IFNα/RBV among European American and African American patients.
Materials and Methods
HALT-C Study Population and Study Design
The design of the HALT-C trial (ClinicalTrials.gov Identifier: NCT00006164) has been described elsewhere [33, 34]. Briefly, 1145 HCV-positive patients who had failed previous interferon therapy and had an Ishak fibrosis score ≥3, but no history of hepatic decompensation, hepatocellular carcinoma, uncontrolled medical or psychiatric conditions, or contraindications to interferon treatment were enrolled in the lead-in phase of this study between August 2000 and August 2004 from 10 study centers in the United States. During the lead-in phase, patients received 180 μg of PEG-IFNα weekly and either 1000 or 1200 mg of oral RBV daily according to body weight of <75 kg or ≥75 kg, respectively. Patients with undetectable serum HCV RNA at week 20 continued therapy for a total 48 weeks, barring viral breakthrough or relapse, and were assessed for SVR 24 weeks after treatment was completed [34, 35]. Our study included all lead-in phase HALT-C participants of European and African American ancestry (n = 126 excluded) who were infected with HCV genotype 1 (n = 99 excluded) and had an available baseline blood sample for vitamin D measurement (n = 9 excluded); the final sample size was N = 911 (n = 166 African American; n = 745 European American).
VIRAHEP-C Study Population and Study Design
The design of the VIRAHEP-C Study (ClinicalTrials.gov Identifier: NCT00038974) has been described elsewhere [17]. Briefly, this study enrolled 401 treatment naïve patients with chronic HCV genotype 1 infection from eight study centers in the United States between July 2002 and December 2003 who identified themselves as “African American/black” or “Caucasian/white” and not as “both” or “other” [17]. Patients received 180 μg of PEG-IFNα weekly and either 1000 or 1200 mg of oral RBV daily according to body weight of <75 kg or ≥75 kg, respectively. Patients with detectable HCV RNA levels at week 24 were considered nonresponders and therapy was stopped. Patients with undetectable HCV RNA levels at week 24 continued therapy for another 24 weeks and were assessed for SVR 24 weeks after treatment was completed [17]. For the current analysis, we restricted the VIRAHEP-C population to those with available baseline serum for measuring vitamin D (n = 20 excluded). The final VIRAHEP-C analytic sample size was N = 381 (n = 184 African American; n = 197 European American).
Assessment of Virologic Response to Treatment
For patients enrolled in HALT-C, serum samples were tested with quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) at the University of Washington Virology Laboratory (Seattle, Washington) using the quantitative Roche COBAS Amplicor HCV Monitor Test, v.2.0 assay (sensitivity 600 IU/mL) and, if negative, using the Roche COBAS Amplicor HCV Test, v.2.0 assay (sensitivity 100 IU/mL) (Roche Molecular Systems, Branchburg, New Jersey) [35, 36]. HCV genotyping was determined with the INNO-LiPA HCV II kit (Siemens Medical Solutions Diagnostics, Tarrytown, New York).
For those enrolled in VIRAHEP-C, serum samples were tested at the SeraCare BioServices Laboratory (Gaithersburg, Maryland) using the quantitative Roche COBAS Amplicor HCV Monitor Test, v.2.0 assay (sensitivity 600 IU/mL) and, if negative using the Amplicor assay (sensitivity 50 IU/mL) (Roche Molecular Diagnostics, Alameda, CA). HCV genotyping was determined with the VERSANT HCV Genotype Assay (Bayer, Tarrytown, New York).
In both studies, serum HCV RNA level was measured at multiple time points including baseline, week 12 of treatment, end of treatment, and 24 weeks after treatment completion. In VIRAHEP-C serum HCV RNA level was also assessed at day 28 of treatment; prior studies have shown that this outcome is a strong predictor of SVR. In the main analyses, early virologic response (EVR) was defined as a ≥2-log10 decline in serum HCV RNA level at week 12 [37]; lack of EVR at week 12 has been found to accurately predict lack of SVR [6, 38]. In HALT-C, 38 participants had missing data on HCV RNA at week 12 and were thus excluded from the EVR analysis. In VIRAHEP-C only, day 28 change in HCV RNA level was defined as log10 (HCV RNA) baseline−log10 (HCV RNA) day 28; 14 VIRAHEP-C participants had missing data on HCV RNA at treatment day 28 and were thus excluded from this analysis. SVR was defined for both studies as the absence of detectable HCV RNA 24 weeks after treatment completion.
Laboratory Analyses
Baseline serum samples for 911 HALT-C patients and 381 VIRAHEP-C patients, as well as week 12 serum samples for 349 of the 381 VIRAHEP-C patients, were assayed at Heartland Assays, Inc. (Ames, Iowa). Measurement of 25(OH)D was conducted for each study by means of a direct, competitive chemiluminescence immunoassay using the DiaSorin LIAISON 25(OH)D TOTAL assay (DiaSorin, Inc., Stillwater, Minnesota), which is cospecific for 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 [39, 40]. For VIRAHEP-C patients with baseline and 12 week paired serum samples, aliquots were randomly ordered within batches. Quality control samples from a single pooled sample of patients from the HALT-C Study were randomly placed in each batch at a proportion of approximately 5%. Intra- and inter-assay coefficients of variation for blinded quality control samples were 6.5% and 2.8% in HALT-C and 5.1% and 3.6% in VIRAHEP-C, respectively.
As previously described, genotyping of IFNL4-ΔG/TT (rs368234815) was conducted in the Laboratory of Translational Genomics (NCI, Gaithersburg, Maryland) with a custom TaqMan allelic discrimination genotyping assay (Life Technologies, Foster City, California) [19, 20].
Statistical Analyses
Vitamin D status was analyzed both as a continuous (ng/mL) and categorical variable. First, we tested for differences in the distribution of baseline 25(OH)D between early responders and nonresponders, stratified by study and race, using the Brown-Mood test [41]. For the primary analyses, categories of vitamin D were defined according to the Institute of Medicine (IOM) cutpoints: deficiency (<12 ng/mL); inadequacy (12 to <20 ng/mL); sufficiency (20 to <30 ng/mL); and no consistent evidence for added benefit but potential concern for harm (≥30 ng/mL) [32, 42]. We also performed analyses based on the 25(OH)D categories used in the meta-analysis by García-Álvarez et al. (<20 ng/mL versus ≥20 ng/mL) that found evidence for an association between vitamin D status and SVR. In secondary analyses, we considered race and season-specific quartiles of vitamin D since HALT-C and VIRAHEP-C studies included sizable African American populations and baseline serum draw dates occurred during both summer (June-November) and winter (December-May) seasons [39].
Because decline in HCV RNA at treatment day 28 has been strongly associated with IFNL4 genotype [17], linear models were used to estimate unadjusted and multivariable-adjusted mean 28-day change in HCV RNA stratified by race and baseline 25(OH)D level. For binary outcomes, logistic regression models were also stratified by race, as African Americans are known to have both lower vitamin D concentrations and poorer response to pegylated IFNα/RBV treatment than European-American patients. We present study-specific and overall ORs from models adjusted for baseline age, sex, BMI, HCV RNA level, homeostatic model assessment of insulin resistance (HOMA) score, IFNL4 genotype, and the markers of liver disease severity which independently predicted virologic response in each study (albumin, AST/ALT ratio, and platelet count in HALT-C; and AST/ALT ratio in VIRAHEP-C). Additionally, data on treatment site was available in VIRAHEP-C and was included in multivariable models but did not meaningfully impact OR estimates. Results were similar following adjustment for additional markers of liver disease severity (e.g. Ishak stage).
For multivariable-adjusted models, we used Imputation and Variance Estimation Software (IVEware) to perform multiple imputations of missing values. [43]; to combine these results and generate valid statistical inferences, we used the SAS procedure PROC MIANALYZE. Finally, we used a random effects meta-analysis approach to obtain overall ORs and 95% CIs for HALT-C and VIRAHEP-C using Stata software. Study heterogeneity was assessed with I2 [44].
To better understand the impact of liver disease and season of baseline blood draw on virologic response, we conducted the following secondary analyses: 1) examined associations separately among patients with cirrhosis, defined as Ishak stage 5 or 6, and those with fibrosis, defined as an Ishak stage 1 through 4; 2) stratified by season of baseline blood draw. To evaluate the longitudinal relationship between 25(OH)D and HCV RNA levels we calculated the change in 25(OH)D concentration for 349 patients in VIRAHEP-C with paired baseline and week 12 serum samples and estimated the mean change in 25(OH)D concentration stratified by EVR status and race. We used a paired t-test to assess whether there was a change in individual 25(OH)D concentration from baseline to week 12. Since 12-week change in 25(OH)D concentration was approximately normally distributed, we used an unpaired t-test to compare the mean change in 25(OH)D between responders and nonresponders to treatment at week 12.
All tests were two-sided, and a P-value<0.05 was considered statistically significant. Analyses were performed using SAS software (release 9.3, SAS Institute, Cary, North Carolina), IVEware (version 0.1, Survey Research Center, University of Michigan) and Stata software (version 14.0, StataCorp, College Station, Texas).
Human Subjects
The HALT-C and VIRAHEP-C study protocols conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institutional review boards of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the participating HALT-C trial sites (University of California-Irvine/VA Medical Center-Long Beach; University of Southern California; University of Colorado; University of Connecticut; NIDDK Liver Disease Section; Massachusetts General Hospital; University of Massachusetts; University of Michigan; Saint Louis University; University of Texas Southwestern; Virginia Commonwealth University) and the participating VIRAHEP-C trial sites (University of California, San Francisco; University of Miami School of Medicine; Rush University; University of Maryland School of Medicine; University of Michigan Medical Center; New York-Presbyterian Medical Center; University of North Carolina) and written consent was obtained from all patients.
Results
Patient Characteristics
Demographic and baseline clinical factors differed according to varying study enrollment criteria in the two trials (Table 1). For example, the HALT-C study was restricted to patients with advanced fibrosis or cirrhosis who had previously failed interferon treatment whereas those in the VIRAHEP-C study were treatment naïve. Accordingly, cirrhosis was much more prevalent in HALT-C than in VIRAHEP-C (36.4% vs 7.1%), and a lower proportion of HALT-C patients as compared with VIRAHEP-C attained SVR (13.3% vs 39.1%) (Table 1). VIRAHEP-C study enrolled approximately equal numbers of African and European Americans, but only 18.2% of the HALT-C patients were African American.
Table 1. Baseline characteristics by study.
| Study | ||
|---|---|---|
| Baseline characteristic | HALT-C (n = 911) | VIRAHEP-C (n = 381) |
| Age (y), mean (SD) | 50.0 (7.2) | 48.2 (7.9) |
| Sex, n (%) | ||
| Male | 660 (72.4) | 247 (64.8) |
| Female | 251 (27.6) | 134 (35.2) |
| Race, n (%) | ||
| African American | 166 (18.2) | 184 (48.3) |
| European American | 745 (81.8) | 197 (51.7) |
| IFNL4 (rs368234815) genotype, n (column %) | ||
| TT/TT | 171 (18.8) | 92 (24.1) |
| ΔG/TT | 448 (49.2) | 152 (39.9) |
| ΔG/ΔG | 195 (21.4) | 89 (23.4) |
| Missing | 97 (10.6) | 48 (12.6) |
| Ishak stage, n (%) | ||
| ≤2 | 73 (8.0) | 240 (63.0) |
| 3 or 4 | 505 (55.4) | 111 (29.1) |
| 5 or 6 | 332 (36.4) | 27 (7.1) |
| Missing | 1 (0.1) | 3 (0.8) |
| BMI (kg/m2), n (%) | ||
| <18.5 | 1 (0.1) | |
| 18.5 to <25 | 158 (17.3) | 93 (24.4) |
| 25 to <30 | 369 (40.5) | 138 (36.2) |
| ≥30 | 384 (42.2) | 145 (38.1) |
| Missing | 0 (0) | 5 (1.3) |
| Season of baseline blood draw, n (%) | ||
| Summer | 452 (49.6) | 230 (60.4) |
| Winter | 459 (50.4) | 151 (39.6) |
| SVR, n (%) | ||
| Yes | 121 (13.3) | 149 (39.1) |
| No | 790 (86.7) | 232 (60.9) |
| 25(OH)D (ng/mL), mean (SD) | 21.5 (10.1) | 21.9 (11.0) |
| European Americans | 23.4 (9.8) | 27.7 (11.) |
| African Americans | 13.0 (6.1) | 15.7 (6.7) |
| HCV RNA level log10 (IU/mL), mean (SD) | 6.4 (0.5) | 6.3 (0.7) |
| Albumin (g/dL), mean (SD) * | 3.9 (0.4) | 4.1 (0.4) |
| AST/ALT ratio, mean (SD) | 0.85 (0.29) | 0.81 (0.30) |
| Bilirubin (mg/dL), mean (SD) † | 0.8 (0.4) | 0.7 (0.4) |
| Platelet count (x103/mm3), mean (SD) ‡ | 172 (65) | 214 (67) |
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; AST/ALT, aspartate transaminase and alanine transaminase ratio; BMI, body mass index; HCV, hepatitis C virus; SD, standard deviation; SVR, sustained virologic response
* 31% of participants in VIRAHEP-C were missing data on albumin
† 1% of participants in VIRAHEP-C were missing data on bilirubin
‡ 2% of participants in VIRAHEP-C were missing data on platelet count
Vitamin D Status in HALT-C and VIRAHEP-C
Despite the differences in the HALT-C and VIRAHEP-C study populations, vitamin D concentrations in the two studies were comparable. Overall, vitamin D deficiency (<12 ng/mL) was present in 17.2% of HALT-C participants and 19.4% of the VIRAHEP-C participants, and, in both the HALT-C and VIRAHEP-C studies, approximately 48% of patients had deficient or inadequate 25(OH)D concentrations, defined as <20 ng/mL, at baseline (S1 and S2 Tables). As expected, vitamin D status differed markedly by race. In HALT-C, vitamin D deficiency was found in 50.6% of African American patients compared to 9.8% of European Americans, and, in Virahep-C, those proportions were 33.7% and 6.1%, respectively (data not shown in table).
In both studies, lower concentrations of vitamin D were associated with most but not all markers of underlying liver disease, such as higher serum AST/ALT, higher measures of alkaline phosphatase and lower albumin (S1 and S2 Tables). In addition, vitamin D concentrations were inversely associated with Ishak fibrosis stage in HALT-C, although only when we compared vitamin D concentrations among patients with cirrhosis (Ishak 5–6) to those with fibrosis (Ishak 2–4), P-trend = 0.003; (more detailed comparisons are presented in S1 and S2 Tables).
Vitamin D Status and Treatment Response
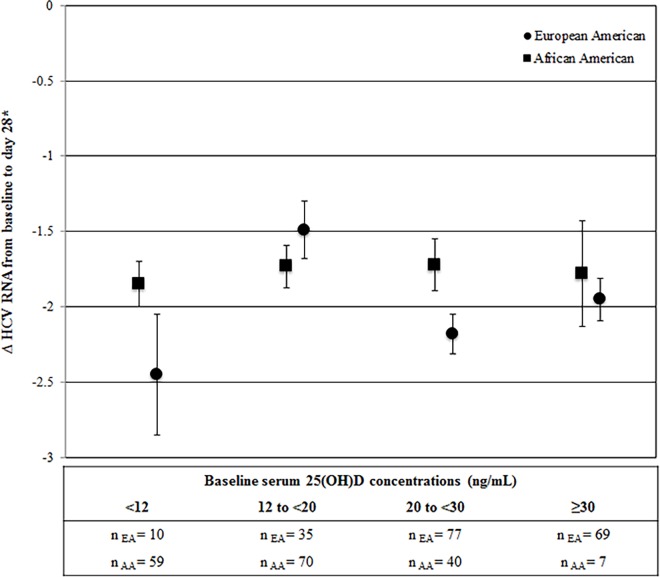
In VIRAHEP-C, HCV RNA levels were examined during the first 28 days of treatment (Fig 1 and S3 Table). Among African American participants, we saw a similar decrease in HCV RNA from baseline to day 28 (~1.8 log10 IU/Ml) with treatment across the four 25(OH)D categories. In European American participants, we did not observe a consistent relationship between 25(OH)D category and decrease in viral levels, as there were greater declines in HCV RNA among participants in the <12 and the 20 to <30 categories of vitamin D than for the intermediate category (12 to <20).
Fig 1. Adjusted mean ΔHCV RNA level (SE) from baseline to day 28 of PEG-IFNα/RBV treatment by baseline serum 25(OH)D status among European and African Americans in the VIRAHEP-C study (n = 191).
Mean ΔHCV RNA level was calculated as the change in log10 transformed viral load from baseline to day 28 of treatment [i.e. log10 (HCV RNA) day 28– log10 (HCV RNA) baseline] and adjusted for age (years), sex, IFNL4 genotype (ΔG/ΔG, ΔG/TT, TT/TT), BMI (kg/m2), baseline HCV RNA level (log10 transformed IU/mL), HOMA score, treatment site, and AST/ALT. Figure abbreviations: 25(OH)D, 25-hydroxyvitamin D; AA, African American; AST/ALT, aspartate transaminase and alanine transaminase ratio; BMI, body mass index; EA, European American; HCV, hepatitis C virus; HOMA, homeostasis model assessment; IOM, Institute of Medicine; LS, least squares; PEG-IFNα/RBV, pegylated interferon alpha and ribavirin; SE, standard error.
Early response to PEG-IFNα/RBV therapy, defined as ≥2-log10 decline in HCV RNA level from baseline to week 12, was achieved by 48% and 53% of patients in HALT-C and VIRAHEP-C, respectively. In both studies, EVR was associated with several previously established factors (European American ancestry, IFNL4-TT/TT genotype, lower Ishak fibrosis stage, lower HOMA or HOMA2 scores, lower measures of alkaline phosphatase, higher serum AST/ALT ratio, and higher platelet counts [data not shown]); however, higher baseline 25(OH)D was not associated with EVR (Table 2) or SVR (data not shown).
Table 2. Serum 25(OH)D concentrations (ng/mL) by early virologic response (≥ 2-log10 decline in HCV RNA level from baseline to week 12).
| Study | Race | Early virologic response | P* | |||
|---|---|---|---|---|---|---|
| Yes | No | |||||
| n | Median (IQR) | n | Median (IQR) | |||
| HALT-C † | European Americans | 373 | 21.9 (16.7–27.4) | 341 | 23.0 (16.2–30.0) | 0.261 |
| African Americans | 48 | 12.5 (8.9–17.1) | 111 | 11.5 (8.4–16.4) | 0.456 | |
| VIRAHEP-C ‡ | European Americans | 126 | 27.0 (21.1–34.4) | 71 | 25.9 (18.8–36.2) | 0.696 |
| African Americans | 76 | 13.7 (11.0–21.1) | 108 | 15.1 (10.7–20.1) | 0.370 | |
* P-value for Brown-Mood test, two-sided normal approximation
† n = 38 HALT-C participants did not have HCV RNA levels measured at a week 12 and were thus excluded from the EVR analysis
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; HCV, hepatitis C virus; IQR, interquartile range
Next, we evaluated the associations of vitamin D status with EVR and SVR. Among European Americans in both studies, a lower proportion of those attaining SVR had high baseline 25(OH)D, ≥30 ng/mL, as compared with those who failed treatment (9% vs. 22% and 33% vs. 38%, respectively) (data not shown in table). These associations persisted among European Americans in multivariable adjusted models, with comparable overall odds ratios for high vitamin D status, ≥30 ng/mL, as compared with sufficient vitamin D status, 20 to <30 ng/mL observed for EVR (OR = 0.64, 95% CI = 0.43–0.94) and SVR (OR = 0.49, 95% CI = 0.20–1.17), although there was significant heterogeneity between the two studies for SVR (HALT-C: OR = 0.31, 95% CI = 0.14–0.66; VIRAHEP-C: OR = 0.75, 95% CI = 0.37–1.55) and the overall association did not reach statistical significance (Table 3). We found no evidence of a dose-response relationship between vitamin D concentrations and rapid viral response at day 28 in VIRAHEP-C or SVR in either study (all P-trend>0.1; data not shown). For comparison with the meta-analysis by García-Álvarez et al., we also considered a cutpoint of 20 ng/mL, but were unable to replicate the reported association between vitamin D status and SVR (Table 3).
Table 3. Associations of baseline serum 25(OH)D status with response to PEG-IFNα/RBV treatment for HCV among European Americans.
| Baseline serum 25(OH)D concentrations (ng/mL) | ||||||
|---|---|---|---|---|---|---|
| IOM cut points | Meta-analysis cut points (31) | |||||
| <12 | 12 to <20 | 20 to <30 (Ref) | ≥30 | <20 | ≥20 (Ref) | |
| Early virologic response | ||||||
| HALT-C, n responders/n total * | 33/70 | 114/206 | 167/295 | 59/143 | 147/276 | 226/438 |
| Multivariable adjusted OR (95% CI) † | 0.96 (0.52–1.74) | 1.08 (0.72–1.62) | 1.00 | 0.60 (0.38–0.95) | 1.24 (0.88–1.74) | 1.00 |
| VIRAHEP-C, n responders/n total | 7/12 | 18/36 | 57/79 | 44/70 | 25/48 | 101/149 |
| Multivariable adjusted OR (95% CI) ‡ | 0.47 (0.10–2.18) | 0.23 (0.08–0.64) | 1.00 | 0.75 (0.33–1.72) | 0.31 (0.13–0.73) | 1.00 |
| Overall OR (95% CI) § | 0.87 (0.50–1.52) | 0.53 (0.12–2.45) ║ | 1.00 | 0.64 (0.43–0.94) | 0.66 (0.17–2.53) ║ | 1.00 |
| Sustained virologic response | ||||||
| HALT-C, n responders/n total | 7/73 | 34/221 | 59/306 | 10/145 | 41/294 | 69/451 |
| Multivariable adjusted OR (95% CI) † | 0.57 (0.23–1.38) | 0.80 (0.47–1.36) | 1.00 | 0.31 (0.14–0.66) | 0.99 (0.62–1.58) | 1.00 |
| VIRAHEP-C, n responders/n total | 7/12 | 13/36 | 46/79 | 33/70 | 20/48 | 79/149 |
| Multivariable adjusted OR (95% CI) ‡ | 1.16 (0.29–4.64) | 0.36 (0.15–0.91) | 1.00 | 0.75 (0.37–1.55) | 0.55 (0.26–1.17) | 1.00 |
| Overall OR (95% CI) § | 0.80 (0.32–1.98) | 0.59 (0.28–1.25) ║ | 1.00 | 0.49 (0.20–1.17) ║ | 0.80 (0.46–1.38) | 1.00 |
* n = 31 participants did not have HCV RNA levels measured at a week 12 and were thus excluded from the EVR analysis
† OR (95% CI) adjusted for age (years), sex, IFNL4 genotype (ΔG/ΔG, ΔG/TT, TT/TT), BMI (kg/m2), albumin (g/dL), AST/ALT, platelet count (x103/mm3), HOMA2 score, and baseline HCV RNA level (log10 transformed)
‡ OR (95% CI) adjusted for age (years), sex, IFNL4 genotype (ΔG/ΔG, ΔG/TT, TT/TT), BMI (kg/m2) AST/ALT, HOMA score, baseline HCV RNA level (log10 transformed), and treatment site
§ Overall multivariable ORs estimated from a random-effects meta-analysis
║ Significant study heterogeneity (I2>50%) for the pair of multivariable ORs
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; AST/ALT, aspartate transaminase and alanine transaminase ratio; BMI, body mass index; CI, confidence interval; HCV, hepatitis C virus; HOMA, homeostasis model assessment; OR, odds ratio; PEG-IFNα/RBV, pegylated interferon alpha and ribavirin
Among African Americans, vitamin D status, defined using either IOM cut-points or a 20 ng/mL cutpoint, was not associated with virologic response (all P-values>0.05) (Table 4). In secondary analyses, we found similar results using season- and race-specific quartiles of vitamin D (S4 Table).
Table 4. Associations of baseline serum 25(OH)D status with response to PEG-IFNα/RBV treatment for HCV among African Americans.
| Baseline serum 25(OH)D concentrations (ng/mL) | ||||||
|---|---|---|---|---|---|---|
| IOM cut points | Meta-analysis cut points (31) | |||||
| <12 | 12 to <20 | 20 to <30 (Ref) | ≥30 | <20 | ≥20 (Ref) | |
| Early virologic response | ||||||
| HALT-C, n responders/n total * | 22/80 | 18/57 | 7/19 | 1/3 | 40/137 | 8/22 |
| Multivariable adjusted OR (95% CI) † | 0.52 (0.15–1.73) | 0.63 (0.19–2.13) | 1.00 | 0.87 (0.05–16.16) | 0.58 (0.20–1.67) | 1.00 |
| VIRAHEP-C, n responders/n total | 26/62 | 30/74 | 18/41 | 2/7 | 56/136 | 20/48 |
| Multivariable adjusted OR (95% CI) ‡ | 0.91 (0.31–2.68) | 0.64 (0.23–1.77) | 1.00 | 0.84 (0.12–5.72) | 0.77 (0.32–1.84) | 1.00 |
| Overall OR (95% CI) § | 0.71 (0.32–1.59) | 0.64 (0.29–1.39) | 1.00 | 0.85 (0.17–4.22) | 0.68 (0.35–1.34) | 1.00 |
| Sustained virologic response | ||||||
| HALT-C, n responders/n total | 8/84 | 2/60 | 1/19 | 0/3 | 10/144 | 1/22 |
| Multivariable adjusted OR (95% CI) † | 0.83 (0.07–9.48) | 0.19 (0.01–3.39) | 1.00 | — | 0.77(0.07–8.17) | 1.00 |
| VIRAHEP-C, n responders/n total | 20/62 | 18/74 | 12/41 | 0/7 | 38/136 | 12/48 |
| Multivariable adjusted OR (95% CI) ‡ | 0.96 (0.32–2.89) | 0.45 (0.15–1.33) | 1.00 | — | 0.82 (0.32–2.06) | 1.00 |
| Overall OR (95% CI) § | 0.94 (0.34–2.56) | 0.40 (0.14–1.11) | 1.00 | — | 0.81 (0.34–1.92) | 1.00 |
* n = 7 participants did not have HCV RNA levels measured at a week 12 and were thus excluded from the EVR analysis
† OR (95% CI) adjusted for age (years), sex, IFNL4 genotype (ΔG/ΔG, ΔG/TT, TT/TT), BMI (kg/m2), albumin (g/dL), AST/ALT, platelet count (x103/mm3), HOMA2 score, and baseline HCV RNA level (log10 transformed)
‡ OR (95% CI) adjusted for age (years), sex, IFNL4 genotype (ΔG/ΔG, ΔG/TT, TT/TT), BMI (kg/m2) AST/ALT, HOMA score, baseline HCV RNA level (log10 transformed), and treatment site
§ Overall multivariable ORs estimated from a random-effects meta-analysis
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; AST/ALT, aspartate transaminase and alanine transaminase ratio; BMI, body mass index; CI, confidence interval; HCV, hepatitis C virus; HOMA, homeostasis model assessment; OR, odds ratio; PEG-IFNα/RBV, pegylated interferon alpha and ribavirin
Similar associations were observed when we adjusted more comprehensively for markers of liver disease, in unadjusted models (data not shown) and in models that were stratified by severity of fibrosis. Excluding patients with cirrhosis (i.e. Ishak stage of 5 or 6) did not meaningfully alter the associations between vitamin D and virologic response (Table 5) among European Americans, although our statistical power was limited in VIRAHEP-C due to a smaller sample size. In season-stratified analyses, observed associations between high vitamin D status and worse response to treatment were stronger for samples collected in the summer but not the winter months (Table 5).
Table 5. Associations of baseline serum 25(OH)D status with response to HCV-treatment in the HALT-C and VIRAHEP-C studies stratified by Ishak stage and season among European Americans.
| Baseline serum 25(OH)D concentrations (ng/mL) | |||
|---|---|---|---|
| <20 | 20 to <30 (Ref) | ≥30 | |
| EVR | |||
| Overall | |||
| HALT-C multivariable adjusted OR (95% CI) * | 1.05 (0.72–1.52) | 1.00 | 0.60 (0.38–0.95) |
| VIRAHEP-C multivariable adjusted OR (95% CI) † | 0.27 (0.11–0.70) | 1.00 | 0.76 (0.33–1.74) |
| Ishak stage 1–4 (fibrosis) | |||
| HALT-C multivariable adjusted OR (95% CI) * | 1.03 (0.64–1.66) | 1.00 | 0.48 (0.27–0.83) |
| VIRAHEP-C multivariable adjusted OR (95% CI) † | 0.31 (0.10–0.95) | 1.00 | 0.66 (0.26–1.72) |
| Ishak stage 5 or 6 (cirrhosis) | |||
| HALT-C multivariable adjusted OR (95% CI) * | 1.12 (0.59–2.12) | 1.00 | 0.84 (0.37–1.90) |
| VIRAHEP-C multivariable adjusted OR (95% CI) † | — | — | — |
| Summer (June to November) | |||
| HALT-C multivariable adjusted OR (95% CI) * ‡ | 1.49 (0.81–2.76) | 1.00 | 0.47 (0.26–0.83) |
| VIRAHEP-C multivariable adjusted OR (95% CI) † | 0.14 (0.03–0.67) | 1.00 | 0.44 (0.15–1.29) |
| Winter (December to May) | |||
| HALT-C multivariable adjusted OR (95% CI) * ‡ | 0.83 (0.49–1.38) | 1.00 | 1.27 (0.53–3.02) |
| VIRAHEP-C multivariable adjusted OR (95% CI) † | 0.59 (0.13–2.69) | 1.00 | 1.16 (0.15–9.01) |
| SVR | |||
| Overall | |||
| HALT-C multivariable adjusted OR (95% CI) * | 0.74 (0.45–1.22) | 1.00 | 0.31 (0.14–0.66) |
| VIRAHEP-C multivariable adjusted OR (95% CI) † | 0.49 (0.22–1.11) | 1.00 | 0.76 (0.37–1.57) |
| Ishak stage 1–4 (fibrosis) | |||
| HALT-C multivariable adjusted OR (95% CI) * | 0.83 (0.46–1.51) | 1.00 | 0.33 (0.17–0.77) |
| VIRAHEP-C multivariable adjusted OR (95% CI) † | 0.48 (0.18–1.23) | 1.00 | 0.69 (0.31–1.54) |
| Ishak stage 5 or 6 (cirrhosis) | |||
| HALT-C multivariable adjusted OR (95% CI) * | 0.58 (0.22–1.55) | 1.00 | 0.16 (0.02–1.38) |
| VIRAHEP-C multivariable adjusted OR (95% CI) † | — | — | — |
| Summer (June to November) | |||
| HALT-C multivariable adjusted OR (95% CI) * | 0.85 (0.38–1.90) | 1.00 | 0.22 (0.09–0.57) |
| VIRAHEP-C multivariable adjusted OR (95% CI) † | 0.45 (0.13–1.52) | 1.00 | 0.69 (0.29–1.62) |
| Winter (December to May) | |||
| HALT-C multivariable adjusted OR (95% CI) * | 0.71 (0.36–1.40) | 1.00 | 0.65 (0.15–2.84) |
| VIRAHEP-C multivariable adjusted OR (95% CI) † | 0.90 (0.21–3.97) | 1.00 | 0.63 (0.09–4.39) |
* OR (95% CI) adjusted for age (years), sex, IFNL4 genotype (ΔG/ΔG, ΔG/TT, TT/TT), BMI (kg/m2), albumin (g/dL), AST/ALT, platelet count (x103/mm3), HOMA2 score, and baseline HCV RNA level (log10 transformed)
† OR (95% CI) adjusted for age (years), sex, IFNL4 genotype (ΔG/ΔG, ΔG/TT, TT/TT), BMI (kg/m2) AST/ALT, HOMA score, baseline HCV RNA level (log10 transformed), and treatment site
‡ All P-value for heterogeneity < 0.05
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; AST/ALT, aspartate transaminase and alanine transaminase ratio; BMI, body mass index; CI, confidence interval; EVR, early virologic response; HCV, hepatitis C virus; HOMA, homeostasis model assessment; IOM, Institute of Medicine; OR, odds ratio; SVR, sustained virologic response
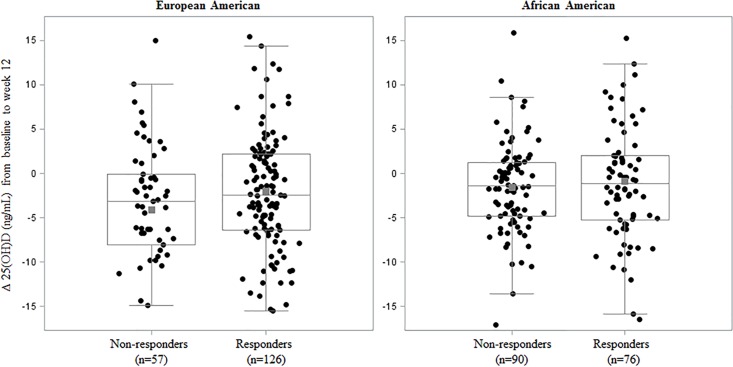
Finally, we considered the longitudinal relationship between 25(OH)D and EVR in VIRAHEP-C subjects with paired baseline and week 12 serum samples. Overall, serum 25(OH)D concentration decreased by an average of 2.7 and 1.2 ng/mL from baseline to week 12 among European (P-value for paired t-test<0.001) and African (P-values for paired t-test = 0.009) Americans, respectively. As illustrated in Fig 2, decreases in 25(OH)D concentrations appeared greater among non-responders than responders; however, these differences were not statistically significant in European Americans (mean 25(OH)D change = -2.00 ± 7.26 ng/mL and -4.09 ± 7.97 ng/mL for early and non-responders, respectively; P value for unpaired t-test = 0.08) or in African Americans (mean 25(OH)D change = -0.85 ± 6.80 ng/mL and -1.53 ± 5.07 ng/mL for early and non-responders, respectively; P value for unpaired t-test = 0.47) (Fig 2).
Fig 2. Race-specific distribution of change in vitamin D, 25(OH)D (ng/mL), from baseline to week 12 stratified by early virologic response status in the VIRAHEP-C study.
Individual change in vitamin D was calculated as the change in serum 25(OH)D (ng/mL) at week 12 of treatment compared with baseline [i.e. 25(OH)D week12−25(OH)D baseline] and was adjusted for age (years), sex, IFNL4 genotype (ΔG/ΔG, ΔG/TT, TT/TT), BMI (kg/m2), AST/ALT, HOMA score, baseline HCV RNA level (log10 transformed IU/mL), and treatment site. These adjusted values are plotted as black circles. The grey square represents the adjusted sample mean, and the horizontal grey lines indicate the adjusted sample median and interquartile range.
Discussion
We examined the association between vitamin D status, as measured by serum 25(OH)D, and response to PEG-IFNα/RBV treatment for chronic HCV genotype 1 infection in two large, high quality studies. In contrast to the hypothesis, our data do not provide evidence for an association between higher serum 25(OH)D and higher likelihood of response to treatment. If anything, the patients with higher vitamin D concentrations had a worse response. Furthermore, we saw no association between vitamin D status and response to treatment among African Americans, despite their lower measured concentrations of vitamin D.
Several previous studies have examined the association between vitamin D status and response to PEG-IFNα/RBV therapy; the results have, however, been inconsistent, as evidenced by two recently published and conflicting meta-analyses on the subject [30, 31]. The meta-analysis by Kitson et al. considered 2,605 patients from 11 studies and concluded that baseline vitamin D status was not associated with SVR [30]. Unlike Kitson et al., the meta-analysis by García-Álvarez et al. included studies involving patients co-infected with human immunodeficiency virus (HIV) but did not include published abstracts [31]. This meta-analysis included data from 1,406 patients from 11 studies, only two of which overlapped with Kitson et al. and three of which were related to the same Italian cohort of approximately 200 patients [45–48], and concluded that low vitamin D status (i.e. <20 ng/mL) was associated with lower odds (OR = 0.53, 95% CI = 0.31–0.91) of attaining SVR following PEG-IFNα/RBV therapy when HCV genotypes 1, 2, 3 and 4 were combined [31]. The authors later reported that this association was no longer significant after excluding two of the three Italian studies [49]. The discordant findings of these meta-analyses may also be due to differences in search methods [45] and definitions of vitamin D status. In the current analysis of data from HALT-C and VIRAHEP-C, we did not replicate the association observed by García-Álvarez et al. for low vitamin D status (i.e. <20 ng/mL) and SVR among European Americans (OR = 0.80, 95% CI = 0.46–1.38) or African Americans (OR = 0.81, 95% CI = 0.34–1.92).
Previous studies of the association between vitamin D status and SVR have varied considerably in their ability to adjust for potential confounders including known clinical and genetic predictors of virologic response to PEG-IFNα/RBV therapy as well as demographic and environmental determinants of vitamin D status [30, 31], and they often lacked extensive clinical data on underlying liver disease. A major strength of our study is the high quality patient data that was collected by both the HALT-C and VIRAHEP-C trials. Accordingly, we examined potential confounding by demographic factors (i.e. age and sex), IFNL4 genotype, which was not available in previous studies, and a comprehensive suite of systematically measured clinical markers of liver disease. Moreover, only one previous study considered the association of vitamin D and SVR separately among African Americans [50]; this cross-sectional study by Weintraub et al., which included 106 African Americans and 65 whites who were chronically infected with HCV genotype 1, did not adjust for potential confounding factors and pretreatment serum was not available for all patients. Similar to our findings, Weintraub et al., found no evidence of an association between vitamin D status and SVR following PEG-IFNα/RBV therapy among African Americans. Contrary to our findings, Weintraub et al. reported that higher vitamin D status was associated with higher rates of SVR among white patients [50].
Our finding that high vitamin D status, ≥30 ng/mL, is associated with significantly lower odds of early virologic response to treatment, particularly for treatment that commenced during summer months, is unexpected but is also in line with a growing body of evidence that indicates that very high vitamin D concentrations may have unexpected and adverse health effects [32, 42]. The 2011 IOM report on calcium and vitamin D described potential U- or reverse J-shaped associations of vitamin D with all-cause mortality, several cancers, cardiovascular risk, falls and fractures [32]. One prior study of vitamin D status and SVR following PEG-IFNα/RBV therapy for chronic HCV genotype 1 infection reported significantly higher prevalence of vitamin D concentrations <30 ng/ among those who achieved SVR than those who did not [51]. At the same time, our finding is at odds with two small supplementation studies, neither of which was placebo controlled, that found evidence for higher rates SVR rates among those receiving vitamin D supplementation [22, 23]. Additionally, our data conflict with the observation that patients have higher vitamin D concentrations once they attain SVR than they did before therapy [24] as we found a general decline in vitamin D concentrations as well as HCV RNA levels from baseline to treatment week 12. Moreover, HCV RNA levels at baseline were not lower among those with higher vitamin D concentrations, as would be expected if vitamin D had a direct antiviral effect.
There are several potential explanations for our unexpected findings. First, unmeasured or poorly measured variables associated with higher vitamin D concentrations as well as lower odds of response to treatment may account for the observed association. Second, it could be a spurious finding owing to chance, although we note consistent findings for high vitamin D status in our two contributing studies. It is also possible to obtain varying results owing to the random draws obtained when imputing missing data; however, we observed similar results for analyses using indicator variables for missing data instead of imputation (data not shown). Finally, we speculate that very high rates of hydroxylation of vitamin D2 and D3 to 25(OH)D in the liver may reduce the effectiveness of PEG-IFNα/RBV therapy by disrupting the metabolic pathway of one or both drugs.
Our study had several limitations. First, our results are based on observational data and should therefore be interpreted with caution. Although serum samples were obtained at baseline prior to the start of study treatment, earlier interferon therapy in HALT-C, underlying liver disease, and unmeasured, poorly measured, or unknown confounders may have impacted baseline vitamin D status as well as subsequent response to therapy. Second, despite a large sample size of African Americans, we had limited statistical power to evaluate the association of high vitamin D status with response to PEG-IFNα/RBV therapy among this group of patients because less than 3% of African Americans in our study, as compared with 23% of European Americans, had 25(OH)D concentrations ≥ 30 ng/mL. We note however, that vitamin D concentrations are typically lower among African-Americans than European-Americans and so we would expect that only a very small proportion of African-Americans in the general population have vitamin D concentrations that are ≥ 30 ng/mL.
Conclusions
In conclusion, our study found no evidence that low vitamin D status as compared with vitamin D sufficiency, defined by the IOM as serum 25(OH)D between 20 to 30 ng/mL, was associated with response to PEG-IFNα/RBV treatment for chronic HCV genotype 1 infection among African or European Americans. Our data did, however, indicate that high vitamin D status, ≥30 ng/mL, was associated with lower odds of early virologic response among European Americans; however, this unexpected finding could be due to chance and should be interpreted with caution. While this study cannot comment directly on the efficacy of vitamin D supplementation as adjuvant for PEG-IFNα/RBV therapy, our data do not support the hypothesis that high pre-treatment vitamin D levels favorably impact response to HCV treatment.
Supporting Information
(PDF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
This work was supported by the Intramural Research Program of the National Institutes of Health, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department of Health and Human Services. Heartland Assays provided support in the form of salaries for author [RH], but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific role of this author is articulated in the ‘author contributions’ section. The VIRAHEP-C and HALT-C studies were conducted, respectively, by the VIRAHEP-C and HALT-C Investigators and supported by the National Institute of Diabetes and Digestive and Kidney Diseases. The data and samples from the VIRAHEP-C and HALT-C studies reported here were supplied by the National Institute of Diabetes and Digestive and Kidney Diseases Central Repositories. This manuscript was not prepared in collaboration with the VIRAHEP-C study group or the HALT-C study group and does not necessarily reflect the opinions or views of the VIRAHEP-C Trial and HALT-C Trial, the National Institute of Diabetes and Digestive and Kidney Diseases Central Repositories or the National Institute of Diabetes and Digestive and Kidney Diseases.
Data Availability
Due to legal restrictions, data are made available upon request from the NIDDK Central Repository for the HALT-C Study and the VIRAHEP-C Study. Researchers can access the Data Request Form at: https://www.niddkrepository.org/wayf/?next=/requests/data-request/. To contact the NIDDK Central Repository please visit https://www.niddkrepository.org/contact/ or e-mail niddk-cr@imsweb.com.
Funding Statement
This work was supported by the Intramural Research Program of the National Institutes of Health, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, and Department of Health and Human Services. Heartland Assays provided support in the form of salaries for author [RH], but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific role of this author is articulated in the ‘author contributions’ section.
References
- 1.Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17(2):107–15. 10.1111/j.1469-0691.2010.03432.x [DOI] [PubMed] [Google Scholar]
- 2.WHO. Hepatitis C 2002 [October 7, 2015]. Available from: http://www.who.int/csr/disease/hepatitis/whocdscsrlyo2003/en/index4.html.
- 3.But DY, Lai CL, Yuen MF. Natural history of hepatitis-related hepatocellular carcinoma. World J Gastroenterol. 2008;14(11):1652–6. 10.3748/wjg.14.1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan A, Yeh SH, Liu CJ, Cheung C, Chen PJ. Viral hepatocarcinogenesis: from infection to cancer. Liver Int. 2008;28(2):175–88. 10.1111/j.1478-3231.2007.01652.x [DOI] [PubMed] [Google Scholar]
- 5.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–128. 10.1016/S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL Jr., et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975–82. 10.1056/NEJMoa020047 [DOI] [PubMed] [Google Scholar]
- 7.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358(9286):958–65 [DOI] [PubMed] [Google Scholar]
- 8.Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341(8):556–62. 10.1056/NEJM199908193410802 [DOI] [PubMed] [Google Scholar]
- 9.Jeffers LJ, Cassidy W, Howell CD, Hu S, Reddy KR. Peginterferon alfa-2a (40 kd) and ribavirin for black American patients with chronic HCV genotype 1. Hepatology. 2004;39(6):1702–8. 10.1002/hep.20212 [DOI] [PubMed] [Google Scholar]
- 10.Butt AA, Kanwal F. Boceprevir and telaprevir in the management of hepatitis C virus-infected patients. Clin Infect Dis. 2012;54(1):96–104. 10.1093/cid/cir774 [DOI] [PubMed] [Google Scholar]
- 11.Hoofnagle JH, Sherker AH. Therapy for hepatitis C—the costs of success. N Engl J Med. 2014;370(16):1552–3. 10.1056/NEJMe1401508 [DOI] [PubMed] [Google Scholar]
- 12.Chan K, Lai MN, Groessl EJ, Hanchate AD, Wong JB, Clark JA, et al. Cost effectiveness of direct-acting antiviral therapy for treatment-naive patients with chronic HCV genotype 1 infection in the veterans health administration. Clin Gastroenterol Hepatol. 2013;11(11):1503–10. 10.1016/j.cgh.2013.05.014 [DOI] [PubMed] [Google Scholar]
- 13.McGowan CE, Monis A, Bacon BR, Mallolas J, Goncales FL, Goulis I, et al. A global view of hepatitis C: physician knowledge, opinions, and perceived barriers to care. Hepatology. 2013;57(4):1325–32. 10.1002/hep.26246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rao VB, Johari N, du Cros P, Messina J, Ford N, Cooke GS. Hepatitis C seroprevalence and HIV co-infection in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Infect Dis. 2015;15(7):819–24. 10.1016/S1473-3099(15)00006-7 [DOI] [PubMed] [Google Scholar]
- 15.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57(4):1333–42. 10.1002/hep.26141 [DOI] [PubMed] [Google Scholar]
- 16.Zoulim F, Liang TJ, Gerbes AL, Aghemo A, Deuffic-Burban S, Dusheiko F, et al. Hepatitis C virus treatment in the real world: optimising treatment and access to therapies. Gut. 2015;64:1824–33. 10.1136/gutjnl-2015-310421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conjeevaram HS, Fried MW, Jeffers LJ, Terrault NA, Wiley-Lucas TE, Afdhal N, et al. Peginterferon and ribavirin treatment in African American and Caucasian American patients with hepatitis C genotype 1. Gastroenterology. 2006;131(2):470–7. 10.1053/j.gastro.2006.06.008 [DOI] [PubMed] [Google Scholar]
- 18.O'Brien TR, Pfeiffer RM, Paquin A, Lang Kuhs KA, Chen S, Bonkovsky HL, et al. Comparison of Functional Variants in IFNL4 and IFNL3 for Association with Hepatitis C Virus Clearance. J Hepatol. 2015. 10.1016/j.jhep.2015.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prokunina-Olsson L, Muchmore B, Tang W, Pfeiffer RM, Park H, Dickensheets H, et al. A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nat Genet. 2013;45(2):164–71. 10.1038/ng.2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aka PV, Kuniholm MH, Pfeiffer RM, Wang AS, Tang W, Chen S, et al. Association of the IFNL4-DeltaG Allele With Impaired Spontaneous Clearance of Hepatitis C Virus. J Infect Dis. 2014;209(3):350–4. 10.1093/infdis/jit433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naggie S, Cooper C, Saag M, Workowski K, Ruane P, Towner WJ, et al. Ledipasvir and Sofosbuvir for HCV in Patients Coinfected with HIV-1. N Engl J Med. 2015;373(8):705–13. 10.1056/NEJMoa1501315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abu-Mouch S, Fireman Z, Jarchovsky J, Zeina AR, Assy N. Vitamin D supplementation improves sustained virologic response in chronic hepatitis C (genotype 1)-naive patients. World J Gastroenterol. 2011;17(47):5184–90. 10.3748/wjg.v17.i47.5184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bitetto D, Fabris C, Fornasiere E, Pipan C, Fumolo E, Cussigh A, et al. Vitamin D supplementation improves response to antiviral treatment for recurrent hepatitis C. Transpl Int. 2011;24(1):43–50. 10.1111/j.1432-2277.2010.01141.x [DOI] [PubMed] [Google Scholar]
- 24.Lange CM, Bojunga J, Ramos-Lopez E, von Wagner M, Hassler A, Vermehren J, et al. Vitamin D deficiency and a CYP27B1-1260 promoter polymorphism are associated with chronic hepatitis C and poor response to interferon-alfa based therapy. J Hepatol. 2011;54(5):887–93. 10.1016/j.jhep.2010.08.036 [DOI] [PubMed] [Google Scholar]
- 25.Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008;8(9):685–98. 10.1038/nri2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christakos S, Dhawan P, Benn B, Porta A, Hediger M, Oh GT, et al. Vitamin D: molecular mechanism of action. Ann N Y Acad Sci. 2007;1116:340–8. 10.1196/annals.1402.070 [DOI] [PubMed] [Google Scholar]
- 27.Matsumura T, Kato T, Sugiyama N, Tasaka-Fujita M, Murayama A, Masaki T, et al. 25-Hydroxyvitamin D3 suppresses hepatitis C virus production. Hepatology. 2012;56(4):1231–9. 10.1002/hep.25763 [DOI] [PubMed] [Google Scholar]
- 28.Gal-Tanamy M, Bachmetov L, Ravid A, Koren R, Erman A, Tur-Kaspa R, et al. Vitamin D: an innate antiviral agent suppressing hepatitis C virus in human hepatocytes. Hepatology. 2011;54(5):1570–9. 10.1002/hep.24575 [DOI] [PubMed] [Google Scholar]
- 29.Yano M, Ikeda M, Abe K, Dansako H, Ohkoshi S, Aoyagi Y, et al. Comprehensive analysis of the effects of ordinary nutrients on hepatitis C virus RNA replication in cell culture. Antimicrob Agents Chemother. 2007;51(6):2016–27. 10.1128/AAC.01426-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitson MT, Sarrazin C, Toniutto P, Eslick GD, Roberts SK. Vitamin D level and sustained virologic response to interferon-based antiviral therapy in chronic hepatitis C: a systematic review and meta-analysis. J Hepatol. 2014;61(6):1247–52. 10.1016/j.jhep.2014.08.004 [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Alvarez M, Pineda-Tenor D, Jimenez-Sousa MA, Fernandez-Rodriguez A, Guzman-Fulgencio M, Resino S. Relationship of vitamin D status with advanced liver fibrosis and response to hepatitis C virus therapy: a meta-analysis. Hepatology. 2014;60(5):1541–50. 10.1002/hep.27281 [DOI] [PubMed] [Google Scholar]
- 32.Ross AC, Taylor CL, Yaktine AL, DelValle HB. Dietary Reference Intakes for Calcium and Vitamin D. Institute of Medicine, 2011. [PubMed]
- 33.Lee WM, Dienstag JL, Lindsay KL, Lok AS, Bonkovsky HL, Shiffman ML, et al. Evolution of the HALT-C Trial: pegylated interferon as maintenance therapy for chronic hepatitis C in previous interferon nonresponders. Control Clin Trials. 2004;25(5):472–92. 10.1016/j.cct.2004.08.003 [DOI] [PubMed] [Google Scholar]
- 34.Di Bisceglie AM, Shiffman ML, Everson GT, Lindsay KL, Everhart JE, Wright EC, et al. Prolonged therapy of advanced chronic hepatitis C with low-dose peginterferon. N Engl J Med. 2008;359(23):2429–41. 10.1056/NEJMoa0707615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shiffman ML, Di Bisceglie AM, Lindsay KL, Morishima C, Wright EC, Everson GT, et al. Peginterferon alfa-2a and ribavirin in patients with chronic hepatitis C who have failed prior treatment. Gastroenterology. 2004;126(4):1015–23; discussion 947 [DOI] [PubMed] [Google Scholar]
- 36.Morishima C, Chung M, Ng KW, Brambilla DJ, Gretch DR. Strengths and limitations of commercial tests for hepatitis C virus RNA quantification. J Clin Microbiol. 2004;42(1):421–5 10.1128/JCM.42.1.421-425.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freedman ND, Curto TM, Lindsay KL, Wright EC, Sinha R, Everhart JE, et al. Coffee Consumption Is Associated With Response to Peginterferon and Ribavirin Therapy in Patients With Chronic Hepatitis C. Gastroenterology. 2011;140(7):1961–9. 10.1053/j.gastro.2011.02.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strader DB, Wright T, Thomas DL, Seeff LB, American Association for the Study of Liver D. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39(4):1147–71. 10.1002/hep.20119 [DOI] [PubMed] [Google Scholar]
- 39.Gallicchio L, Helzlsouer KJ, Chow WH, Freedman DM, Hankinson SE, Hartge P, et al. Circulating 25-hydroxyvitamin D and the risk of rarer cancers: Design and methods of the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010;172(1):10–20. 10.1093/aje/kwq116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagner D, Hanwell HE, Vieth R. An evaluation of automated methods for measurement of serum 25-hydroxyvitamin D. Clin Biochem. 2009;42(15):1549–56. 10.1016/j.clinbiochem.2009.07.013 [DOI] [PubMed] [Google Scholar]
- 41.Gibbons JD. Median Test, Brown–Mood Encyclopedia of Statistical Sciences: John Wiley & Sons, Inc.; 2004. [Google Scholar]
- 42.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–8. 10.1210/jc.2010-2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raghunathan TE, Lepkowski JM, Hoewyk JV, Solenberger P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Survey Methodology. 2001;271:85–95. [Google Scholar]
- 44.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in medicine. 2002;21(11):1539–58. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 45.Kitson MT, Sarrazin C, Toniutto P, Roberts SK. Relationship between vitamin D status and response to hepatitis C virus therapy. Hepatology. 2015. 10.1002/hep.27797 [DOI] [PubMed] [Google Scholar]
- 46.Bitetto D, Bortolotti N, Falleti E, Vescovo S, Fabris C, Fattovich G, et al. Vitamin A deficiency is associated with hepatitis C virus chronic infection and with unresponsiveness to interferon-based antiviral therapy. Hepatology. 2013;57(3):925–33. 10.1002/hep.26186 [DOI] [PubMed] [Google Scholar]
- 47.Falleti E, Bitetto D, Fabris C, Fattovich G, Cussigh A, Cmet S, et al. Vitamin D binding protein gene polymorphisms and baseline vitamin D levels as predictors of antiviral response in chronic hepatitis C. Hepatology. 2012;56(5):1641–50. 10.1002/hep.25848 [DOI] [PubMed] [Google Scholar]
- 48.Bitetto D, Fattovich G, Fabris C, Ceriani E, Falleti E, Fornasiere E, et al. Complementary role of vitamin D deficiency and the interleukin-28B rs12979860 C/T polymorphism in predicting antiviral response in chronic hepatitis C. Hepatology. 2011;53(4):1118–26. 10.1002/hep.24201 [DOI] [PubMed] [Google Scholar]
- 49.Pineda-Tenor D, Garcia-Alvarez M, Jimenez-Sousa MA, Fernandez-Rodriguez A, Resino S. Reply: To PMID 24975775. Hepatology. 2015;62(5):1643 10.1002/hep.27796 [DOI] [PubMed] [Google Scholar]
- 50.Weintraub SJ, Fleckenstein JF, Marion TN, Madey MA, Mahmoudi TM, Schechtman KB. Vitamin D and the racial difference in the genotype 1 chronic hepatitis C treatment response. Am J Clin Nutr. 2012;96(5):1025–31. 10.3945/ajcn.112.039974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kitson MT, Dore GJ, George J, Button P, McCaughan GW, Crawford DH, et al. Vitamin D status does not predict sustained virologic response or fibrosis stage in chronic hepatitis C genotype 1 infection. J Hepatol. 2013;58(3):467–72. 10.1016/j.jhep.2012.11.017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
Due to legal restrictions, data are made available upon request from the NIDDK Central Repository for the HALT-C Study and the VIRAHEP-C Study. Researchers can access the Data Request Form at: https://www.niddkrepository.org/wayf/?next=/requests/data-request/. To contact the NIDDK Central Repository please visit https://www.niddkrepository.org/contact/ or e-mail niddk-cr@imsweb.com.